Abstract
Background
The diagnosis of visceral leishmaniasis (VL) in patients with fever and a large spleen relies on showing Leishmania parasites in tissue samples and on serological tests. Parasitological techniques are invasive, require sophisticated laboratories, consume time, or lack accuracy. Recently, rapid diagnostic tests that are easy to perform have become available.
Objectives
To determine the diagnostic accuracy of rapid tests for diagnosing VL in patients with suspected disease presenting at health services in endemic areas.
Search methods
We searched MEDLINE, EMBASE, LILACS, CIDG SR, CENTRAL, SCI‐expanded, Medion, Arif, CCT, and the WHO trials register on 3 December 2013, without applying language or date limits.
Selection criteria
This review includes original, phase III, diagnostic accuracy studies of rapid tests in patients clinically suspected to have VL. As reference standards, we accepted: (1) direct smear or culture of spleen aspirate; (2) composite reference standard based on one or more of the following: parasitology, serology, or response to treatment; and (3) latent class analysis.
Data collection and analysis
Two review authors independently extracted data and assessed quality of included studies using the QUADAS‐2 tool. Discrepancies were resolved by a third author. We carried out a meta‐analysis to estimate sensitivity and specificity of rapid tests, using a bivariate normal model with a complementary log‐log link function. We analysed each index test separately. As possible sources of heterogeneity, we explored: geographical area, commercial brand of index test, type of reference standard, disease prevalence, study size, and risk of bias (QUADAS‐2). We also undertook a sensitivity analysis to assess the influence of imperfect reference standards.
Main results
Twenty‐four studies containing information about five index tests (rK39 immunochromatographic test (ICT), KAtex latex agglutination test in urine, FAST agglutination test, rK26 ICT, and rKE16 ICT) recruiting 4271 participants (2605 with VL) were included. We carried out a meta‐analysis for the rK39 ICT (including 18 studies; 3622 participants) and the latex agglutination test (six studies; 1374 participants). The results showed considerable heterogeneity. For the rK39 ICT, the overall sensitivity was 91.9% (95% confidence interval (95% CI) 84.8 to 96.5) and the specificity 92.4% (95% CI 85.6 to 96.8). The sensitivity was lower in East Africa (85.3%; 95% CI 74.5 to 93.2) than in the Indian subcontinent (97.0%; 95% CI 90.0 to 99.5). For the latex agglutination test, overall sensitivity was 63.6% (95% CI 40.9 to 85.6) and specificity 92.9% (95% CI 76.7 to 99.2).
Authors' conclusions
The rK39 ICT shows high sensitivity and specificity for the diagnosis of visceral leishmaniasis in patients with febrile splenomegaly and no previous history of the disease, but the sensitivity is notably lower in east Africa than in the Indian subcontinent. Other rapid tests lack accuracy, validation, or both.
15 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted in April 2019 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Keywords: Humans; Asymptomatic Infections; Africa, Eastern; Agglutination Tests; Agglutination Tests/methods; Antigens, Protozoan; Antigens, Protozoan/analysis; Biomarkers; Biomarkers/analysis; Chromatography, Affinity; Chromatography, Affinity/methods; Clinical Trials, Phase III as Topic; India; Latex Fixation Tests; Latex Fixation Tests/methods; Leishmaniasis, Visceral; Leishmaniasis, Visceral/diagnosis; Nepal; Protozoan Proteins; Protozoan Proteins/analysis; Sensitivity and Specificity
Plain language summary
Rapid diagnostic tests for visceral leishmaniasis
Visceral leishmaniasis (or kala‐azar) is caused by a parasite, results in fever, a large spleen and other health problems, occuring in India, Bangladesh and Nepal, east Africa, the Mediterranean region and Brazil. Without treatment people die, and proper treatment can result in cure, so diagnosis is important. Many of the tests that are used to determine if a person has visceral leishmaniasis are complicated, costly, painful and sometimes dangerous for the patients. Now rapid diagnostic tests that are safe and easy to perform are available.
This Cochrane review describes how accurate these rapid diagnostic tests are for diagnosing visceral leishmaniasis. We summarize those studies that evaluated the rapid tests in people who, according to their physicians, could have the disease. We only included studies in which the researchers had used established methods to distinguish the people with visceral leishmaniasis from those who did not have the disease.
We found 24 studies, which contained information about five different rapid tests. A total of 4271 people participated in these studies. One of the rapid tests (called the rK39 immunochromatographic test) gave correct, positive results in 92% of the people with visceral leishmaniasis and it gave correct, negative results in 92% of the people who did not have the disease. This test worked better in India and Nepal than in east Africa. In India and Nepal, it gave correct, positive results in 97% of the people with the disease. In east Africa, it gave correct, positive results in only 85% of the people with the disease.
A second rapid test (called latex agglutination test) gave correct, positive results in 64% of the people with the disease and it gave correct, negative results in 93% of the people without the disease. For the other rapid tests evaluated, there are too few studies to know how accurate they are.
Summary of findings
Summary of findings 1. rK39 immunochromatographic test for visceral leishmaniasis in the Indian subcontinent.
|
Population: Patients suspected to have visceral leishmaniasis disease 1 Setting: Health services in endemic areas of the Indian subcontinent 2 New test: rK39 immunochromatographic test 3 Reference standard: (1) direct smear test or culture of splenic aspirate; (2) composite reference standard based on one or more of the following: parasitology, serology, or response to treatment; or (3) latent class analysis 4 | ||||
| Pooled sensitivity: 0.97 (95% CI 0.90 to 1.00) | Pooled specificity : 0.90 (95% CI 0.76 to 0.98) | ||||
| Setting 5 | Positive predictive value6 | Negative predictive value6 | Number of participants (studies) | Quality of the evidence (QUADAS‐2) 7 |
| Peripheral health centre with a prior probability of disease of 40% | 87% | 98% | 1468 (6 studies) |
Risk of bias: none of the studies had a low risk of bias in all domains. One study had a high risk of bias (domain of flow and timing). Five studies had an unclear risk of bias (domains of index test or reference standard). Applicability: low concerns in all studies and in all domains. |
| Referral centre with a prior probability of disease of 60% | 94% | 95% | ||
|
Interpretation: When the rK39 ICT is used 1 in the Indian subcontinent, in a setting where the prior probability of VL among clinical suspects is 40%, which is typically seen in a peripheral health centre in an endemic area, the positive predictive value of the test is 87%. This means that out of 100 patients with a positive rK39 result, 87 would have VL (true positive result) and 13 would have another disease (false positive). The negative predictive value is 98%, meaning that out of 100 patients with a negative rK39 ICT result, 98 would have another disease (true negative) and 2 would have VL (false negative). When the same test is used in a setting with a prior probability of VL of 60%, which is more typical for a referral centre in an endemic area, the positive predictive value is 94% and the negative predictive value is 95%. A likelihood ratio is another way of expressing how informative a diagnostic test is: it indicates to what extent the rK39 ICT result changes the odds that a patient has VL. The likelihood ratio of a positive rK39 ICT result is 9.90, and the likelihood ratio of a negative test result is 0.03. This means that in the Indian subcontinent, a positive rK39 ICT result is a strong argument in favour of VL (ruling in) and that a negative rK39 ICT result is a strong argument against VL (ruling out). | ||||
| CI: confidence interval | ||||
| Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, Chappuis F, Rijal S. Rapid diagnostic tests for visceral leishmaniasis. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No.: CD009135. DOI: 10.1002/14651858.CD009135. | ||||
1. The rK39 immunochromatographic test must be used in combination with a clinical case definition (fever and splenomegaly for more than two weeks and no previous history of visceral leishmaniasis). Studies with mainly HIV‐positive patients were not included in the pooled analyses.
2. The results of the meta‐analysis showed considerable heterogeneity, which was partly explained by the geographic region.
3. This rapid diagnostic test has been developed specifically for field use. It is less invasive, less time‐consuming, and easier to perform than the alternative parasitological or serological tests.
4. Latent class analysis is a modelling technique that allows us to estimate the sensitivity and specificity of a set of diagnostic tests in situations in which there is no good reference standard.
5. Two hypothetical situations: a peripheral health centre and a referral centre with a different prior probability of disease
6. A narrative explanation of the predictive values is given in Appendix 3.
7. QUADAS‐2 is a tool for the assessment of the quality of diagnostic accuracy studies. The tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias, and the first three domains are also assessed in terms of concerns regarding applicability.
Summary of findings 2. rK39 immunochromatographic test for visceral leishmaniasis in east Africa.
|
Population: Patients suspected to have visceral leishmaniasis disease 1 Setting: Health services in endemic areas of east Africa 2 New test: rK39 immunochromatographic test 3 Reference standard: (1) direct smear test or culture of splenic aspirate; (2) composite reference standard based on one or more of the following: parasitology, serology, or response to treatment; or (3) latent class analysis 4 | ||||
| Pooled sensitivity: 0.85 (95% CI 0.75 to 0.93) ; Pooled specificity : 0.91 (95% CI 0.80 to 0.97) | ||||
| Setting 5 | Positive predictive value 6 | Negative predictive value6 | Number of participants (studies) |
Quality of the evidence (QUADAS‐2) 7 |
| Peripheral health centre with a prior probability of disease of 40% | 86% | 90% | 1692 (9 studies) |
Risk of bias: three studies had a low risk of bias across all domains; four studies had an unclear risk of bias (domains of index test or reference standard); two studies had a high risk of bias (domains of patient selection or flow and timing, or both). Applicability: one study with high concerns about applicability (domain of patient selection); low concerns for all other studies and all other domains. |
| Referral centre with a prior probability of disease of 60% | 93% | 81% | ||
|
Interpretation: When the rK39 ICT is used 1in east Africa, in a setting where the prior probability of VL is 40%, which is typically seen in a peripheral health centre in an endemic area, the positive predictive value of the test is 86%. This means that out of 100 patients with a positive rK39 ICT result, 86 would have VL (true positive result) and 14 would have another disease (false positive). The negative predictive value is 90%, meaning that out of 100 patients with a negative rK39 ICT result, 90 would have another disease (true negative) and 10 would have VL (false negative). When the same test is used in a setting with a prior probability of VL of 60%, which is more typical for a referral centre in an endemic area, the positive predictive value is 93% and the negative predictive value is 81%. In east Africa, the likelihood ratio of a positive rK39 ICT result is 9.58, and the likelihood of a negative rk39 ICT result is 0.16. This means that a positive rK39 ICT result is strong argument in favour of VL (ruling in), and that a negative rK39 ICT result is not an absolute argument against VL (does not allow to rule out VL completely). | ||||
| CI: confidence interval | ||||
| Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, Chappuis F, Rijal S. Rapid diagnostic tests for visceral leishmaniasis. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No.: CD009135. DOI: 10.1002/14651858.CD009135. | ||||
1. The rK39 immunochromatographic test must be used in combination with a clinical case definition (fever and splenomegaly for more than two weeks and no previous history of visceral leishmaniasis). Studies with mainly HIV‐positive patients were not included in the pooled analyses.
2. The results of the meta‐analysis showed considerable heterogeneity, which was partly explained by the geographic region.
3. This rapid diagnostic test has been developed specifically for field use. It is less invasive, less time‐consuming, and easier to perform than the alternative parasitological or serological tests.
4. Latent class analysis is a modelling technique that allows to estimate the sensitivity and specificity of a set of diagnostic tests in situations in which there is no good reference standard.
5. Two hypothetical situations: a peripheral health centre and a referral centre with a different prior probability of disease
6. A narrative explanation of the predictive values is given in Appendix 3.
7. QUADAS‐2 is a tool for the assessment of the quality of diagnostic accuracy studies. The tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias, and the first three domains are also assessed in terms of concerns regarding applicability.
Summary of findings 3. Latex agglutination test in urine for the diagnosis of visceral leishmaniasis.
|
Population: Patients suspected to have visceral leishmaniasis disease 1 Setting: Health services in endemic areas 2 New test: Latex agglutination test in urine 3 Reference standard: (1) direct smear test or culture of splenic aspirate; (2) composite reference standard based on one or more of the following: parasitology, serology, or response to treatment; or (3) latent class analysis 4 | ||||
| Pooled sensitivity : 0.64 (95% CI 0.41 to 0.86) ; Pooled specificity : 0.93 (95% CI 0.77 to 0.99) 5 | ||||
| Setting 6 | Positive predictive value | Negative predictive value | Number of participants (studies) |
Quality of the evidence (QUADAS‐2) 7 |
| Peripheral health centre with a prior probability of disease of 40% | 86% | 79% | 1374 (6 studies) |
Risk of bias: none of the studies had a low risk of bias in all domains. One study had a high risk of bias (domain of flow and timing). Five studies had an unclear risk of bias (domain of reference standard). Applicability: low concerns in all studies and in all domains. |
| Referral centre with a prior probability of disease of 60% | 93% | 63% | ||
| CI: confidence interval | ||||
| Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, Chappuis F, Rijal S. Rapid diagnostic tests for visceral leishmaniasis. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No.: CD009135. DOI: 10.1002/14651858.CD009135. | ||||
1. Studies with mainly HIV‐positive patients were not included in the pooled analyses.
2. The studies included in this review were conducted in Ethiopia, Kenya, Sudan, India and Nepal.
3. This rapid diagnostic test has been developed specifically for field use. It is less invasive, less time‐consuming, and easier to perform than the alternative parasitological or serological tests.
4. Latent class analysis is a modelling technique that allows to estimate the sensitivity and specificity of a set of diagnostic tests in situations in which there is no good reference standard.
5. The results of the meta‐analysis showed considerable heterogeneity.
6. Two hypothetical situations: a peripheral health centre and a referral centre with a different prior probability of disease.
7. QUADAS‐2 is a tool for the assessment of the quality of diagnostic accuracy studies. The tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias, and the first three domains are also assessed in terms of concerns regarding applicability.
Background
Target condition being diagnosed
Visceral leishmaniasis (VL), also known as kala‐azar, is a life‐threatening systemic disease caused by the obligate intracellular protozoan, Leishmania, and transmitted through the bites of phlebotomine sand flies (Herwaldt 1999). Leishmanial infection can cause a diverse spectrum of diseases, among which VL is the most severe form and which is almost always fatal without adequate, timely treatment. The species that causes VL isLeishmania donovani in Asia and eastern Africa, and L. infantum in Europe, North Africa and Latin America (Boelaert 2000). The geographical distribution of VL is often limited to well‐identified endemic foci, but it has also emerged as epidemics (Seaman 1996) and as one of the opportunistic infections in human immunodeficiency virus (HIV)‐positive patients (Alvar 2008; Pasquau 2005). VL is a neglected disease that affects the poorest and most vulnerable people in rural, remote settings where there is limited access to health care. The estimated incidence is 200,000 to 400,000 cases per year, of which more than 90% are reported from India, Bangladesh, Ethiopia, Sudan, South Sudan, and Brazil (Alvar 2012). The two main transmission modes of VL are anthroponotic and zoonotic. The human‐to‐human transmission is predominant in the Indian subcontinent and in eastern Africa, while the zoonotic transmission mode is found in the Mediterranean region and the Americas. The zoonotic transmission mode involves dogs as the main parasite reservoir (Chappuis 2007).
The pathogenesis of VL entails a complex interaction between the characteristics of the parasite and the host (Rittig 2000). The parasite life cycle involves the replication of the promastigote form in the gut of the female sand fly and is completed when she takes a blood meal on a non‐immune host. In the human body, the Leishmania promastigote evolves into an amastigote form (without flagellum), which colonizes the macrophages in the liver, spleen and bone marrow. The control of the infection depends on the characteristics of the host, especially an intact cell‐mediated immunity in the form of a T‐helper type 1 response, involving the secretion of interleukin 12 and interferon‐gamma. The failure of this cell‐mediated immunity in some, but not all, of the infected individuals ultimately leads to the clinical manifestations of VL disease (Murray 2005).
The main clinical manifestations of VL are fever of insidious onset, weakness, loss of appetite, weight loss, abdominal distension due to enlargement of the spleen or the liver or both, lymph node enlargement, and a low blood cell count (pancytopenia). In children, other symptoms include diarrhoea, coughing, abdominal pain, and growth retardation. In any age group, if left untreated, the disease will progress with time, causing debilitation, bleeding, susceptibility to secondary infection and, eventually, death. Anti‐leishmanial treatment is life‐saving but non‐response or relapse can occur. The pentavalent antimonials, sodium stibogluconate and meglumine antimoniate, despite their toxicity, have been the mainstay of VL treatment in many areas for decades. Alternatives include (liposomal) amphotericin B, paromomycin, and miltefosine. Combination therapy is the suggested way forward to increase treatment efficacy, prevent resistance, and reduce treatment duration and cost (van Griensven 2010). It is important that diagnostic tests for VL do not give false‐negative results because VL may be fatal. Neither should they give false‐positive results in order to prevent people without VL from receiving the toxic VL treatment.
As stated above, asymptomatic or subclinical infections also occur, and in cross‐sectional surveys in endemic areas, a significant proportion of the healthy individuals present with anti‐leishmanial antibodies. In longitudinal studies, the ratio between incident clinical cases and incident asymptomatic infections ranged from 1:5 in Kenya (Ho 1982) to 1:8 in Brazil (Evans 1992), 1:9 in Ethiopia (Hailu 2009), 1:8.9 in India and Nepal (Ostyn 2011), and 1:11 and 2.6:1 in Sudan (Zijlstra 1994). Furthermore, antibodies (seropositivity) in VL patients tend to persist for many years after cure (De Almeida 2006; Hailu 1990).
Index test(s)
Rapid diagnostic tests (RDTs) are defined as equipment‐free diagnostic devices that do not require highly skilled laboratory staff. The results of an RDT can be read easily within minutes, or at most an hour or two (PATH 2008). Most RDTs work by capturing either an antigen or an antibody on a solid surface and then attaching molecules to them that allow detection by the naked eye. The technology used is mainly immunochromatography with a dipstick or lateral flow format. These immunochromatographic tests (ICTs) are used for VL diagnosis in dipstick or cassette format, using protein isolated from Leishmania sp as the antigen. The recombinant form of the 39 amino‐acid‐sequence from L. chagasi is the most widely used and is known as rK39. Other recombinant antigens such as rK9, rK16, rK26 and rK28 have also been evaluated. Currently, there are several on‐going projects to develop a VL test based on antigen detection, for example, the latex agglutination test detecting a urinary leishmanial antigen.
The rK39 antigen was first used in an enzyme‐linked immunosorbent assay (ELISA) format, but the newer dipstick or strip formats are increasingly being used in the field or away from the main centres. These rK39 ICTs give an immediate result (typically between 10 and 20 minutes) and give a binary reading (positive or negative). The test procedure involves adding the patient’s blood or serum with diluent buffer on the strip. When present in the blood or serum, specific antibodies against rK39 antigens, which are bound to the strip, can be read with the naked eye in the test window. There is a limited number of commercially available RDTs for VL, such as the IT‐LEISH® (DiaMed AG, Switzerland – now Biorad, France), Kalazar Detect® (InBios International, USA), Onsite Leishmania Ab Rapid Test (CTK Biotech, USA) as well as a number of prototypes under various formats (Cunningham 2012).
rK39 ICTs cannot be used to diagnose VL in people with a past history of VL due to the persistence of antibodies after cure. In addition, to avoid diagnosis of asymptomatic infections, these tests must be applied in patients suspected to have VL, ie patients with prolonged fever and splenomegaly (WHO 2010).
Clinical pathway
Prior test(s)
The RDTs are meant to be used in patients with a clinical suspicion of VL without previous history of VL. According to the case definition proposed by the World Health Organization (WHO), VL is an illness with prolonged irregular fever, splenomegaly and weight loss as its main symptoms (WHO 2010).
Role of index test(s)
The RDTs were specifically developed for field use in VL‐endemic areas. If they are sufficiently accurate, they could be used for the early diagnosis of VL at both peripheral and central levels. A positive RDT result would then confirm the diagnosis of VL in clinically suspected patients and allow the start of treatment (WHO 2010).
Alternative test(s)
A definite VL diagnosis is provided by the demonstration of the parasite through a microscopic visualization from spleen, bone marrow or lymph node aspirates. These invasive procedures are not always feasible in field settings, and using them is by no means free of risk: spleen aspirates are the most sensitive (93% to 99%) but the aspiration carries a rare but fatal risk of bleeding (Zijlstra 1992). Splenic aspiration should only be performed if there is rapid access to blood transfusion in case of bleeding and if there are no contraindications. Clinical contraindications include signs of active bleeding, jaundice, pregnancy, a barely palpable spleen and a bad general condition. Biological contraindications include severe anaemia, a prolonged prothrombin time and a low platelet count (WHO 2010). Bone marrow and lymph node aspiration are safer, but both have lower sensitivity (Sarker 2004; Babiker 2007). Parasitological confirmation through culture or molecular techniques such as a polymerase chain reaction (PCR) is possible, but their complexity restricts their use as a routine diagnosis method. In addition, in endemic areas, a substantial proportion of healthy individuals have parasite DNA in the blood, which is detected by PCR (Bhattarai 2009).
Serological tests based on indirect fluorescence antibody, ELISA and Western blot were developed but their use is limited in the first‐line health services of endemic areas as they require a too sophisticated laboratory infrastructure. A more useful antibody detection test is the direct agglutination test (DAT), a semi‐quantitative method based on visual agglutinations obtained by the increased dilution of blood or serum mixed with stained, killed parasites in V‐shaped wells (Harith 1986). It has been extensively validated and used in the field. Nonetheless, it cannot be categorized as an RDT because it requires a degree of laboratory skills, facilities and equipment, and the result is ready only after overnight incubation (Boelaert 1999).
Rationale
Correctly diagnosing VL disease is crucial as the signs and symptoms of VL are not specific enough to differentiate the condition from chronic malaria, schistosomiasis or other systemic infections. VL should be suspected in patients presenting with fever and splenomegaly, but confirmation is needed. The classical method relying on microscopic smears from tissue aspirates (spleen, bone marrow, lymph node) are unsuitable for settings with limited resources. Other methods such as antibody detection are more feasible, most notably the DAT and the rK39 antigen‐based ICT. Both have been specifically developed in the past two decades for use in such contexts and have shown high diagnostic accuracy in most endemic areas (Boelaert 2004; Chappuis 2006).
The major drawbacks of these serological methods are linked to the antibodies that remain detectable after a cure and those that are due to past or present asymptomatic infection present in a sizeable proportion of residents of endemic areas. The use of a clinical case definition and adequate medical history taking should help to avoid false positives, yet it underscores the need for better VL diagnosis tests that are specific to acute stage disease. Ideally, the test should be highly sensitive, as VL is potentially fatal, and it should also be specific, as presumptive treatment cannot be fully justified with the current treatment regimens (den Boer 2006).
The RDTs for VL, mainly but not exclusively the rK39‐based ICTs, seem to be the current solution for field diagnosis in remote settings: their ease of use, convenience and cost make them potentially advantageous to increase patients' access to VL diagnosis and treatment. The current WHO recommendation for VL case management includes the use of the rK39‐based ICT as the basis for initiating treatment and it has also been adopted as the diagnostic tool in first‐line services in the VL elimination initiative in the Indian subcontinent (WHO 2005). In other areas, the RDTs have been used as the first test in diagnosis‐treatment algorithms that often incorporate other test(s), such as the DAT or tissue aspiration (Raguenaud 2007; Veeken 2003;).
Despite its operational advantages, some regional variability in rK39‐based ICT performance has been observed (Chappuis 2006). Other prototype tests have been proposed as RDTs, but their real value in clinical practice is unclear. Furthermore, in the published literature, many phase II studies (with a sub‐optimal, case‐control design) might overshadow the more limited information of better quality based on phase III studies (including consecutive patients suspected to have VL). Thus, it is of utmost importance to synthesize the available evidence in this rapidly evolving field. Knowing the diagnostic accuracy of these RDTs would help inform policy makers and stakeholders on how best to use them in VL control in diverse settings. The role of RDTs in the different epidemiological contexts could be better defined, giving clearer evidence on their accuracy, and this review aims to contribute to exactly this goal.
Objectives
To determine the diagnostic accuracy of rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease presenting at health services in endemic areas.
Secondary objectives
Investigation of sources of heterogeneity
We investigated differences in diagnostic accuracy in relation to test conditions (index and reference: commercial brand of index test, type of reference standard), geographical region (the Indian subcontinent, eastern Africa, Latin America and the Mediterranean region), disease prevalence in the sample, and study quality.
Methods
Criteria for considering studies for this review
Types of studies
Only original diagnostic accuracy studies of the phase III type (Zhou 2002) were included in the review, that is, prospective or retrospective cohort studies (meaning studies with patients who were consecutively enrolled, be it prospectively or retrospectively, and in which all patients were given the index test and reference standard), or studies that enrolled a random selection of patients from a series of patients. Randomized controlled trials in which patients were randomized to one of several index tests and all received the reference test could also be included.
Participants
Patients with a clinical suspicion of VL, that is, those who are febrile for more than two weeks, and present with splenomegaly; according to the WHO case definition (WHO 2010) presenting at health services in endemic areas.
We excluded studies with participants:
who were previously treated for VL (non‐responders or relapsed cases); and
who had signs and symptoms of other forms of leishmaniasis, such as post kala‐azar dermal leishmaniasis (PKDL).
Studies in which only a subgroup of participants was eligible for the review were included if it was possible to extract relevant data specific to that subgroup.
Studies of patients with HIV or other co‐infections were eligible for this review.
Index tests
All types of RDTs for VL, with results that are read out within one hour, regardless of the manufacturer. The RDTs could be assessed alone or in comparison with other tests.
Target conditions
The target condition was restricted to current clinical VL, and did not include asymptomatic leishmanial infection or VL in the past.
Reference standards
We accepted the following reference standards for the diagnosis of active VL (adapted from Boelaert 2007):
reference standard including direct smears or culture of splenic aspirate;
composite reference standard (Sullivan 2003) based on a combination of several tests. Test algorithms could include smears or culture of tissue aspirate, serology (other than the index test), or clinical arguments; and
latent class analysis (LCA) (Zhou 2002) based on one or more of the following: smears or culture of tissue aspirates, serology (other than the index test), clinical response to antimonial treatment; or specific clinical signs (pancytopenia, darkened skin). To be selected, the studies had to assess the conditional independence assumption between the tests, and, if conditional dependence was expected, they had to use appropriate statistical methods (Dendukuri 2001). More information about LCA is given in Appendix 1.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of their publication status (published, unpublished, in press, ongoing). No language or date limits were applied. We limited our searches to studies conducted in humans.
Electronic searches
To identify all relevant studies, we searched the following databases using the search terms and strategy described in Appendix 2: MEDLINE, EMBASE, LILACS, CIDG SR, the Cochrane Central Register of Controlled Trials (CENTRAL) published in The Cochrane Library, ISI Web of Knowledge (Science Citation Index Expanded), Medion, Arif, and CCT.
We also searched the metaRegister of Controlled Trials (mRCT) and the WHO Clinical Trials Registry Platform using "leishman*" and "diagnos* OR RDT*" as search terms.
MeSH and other search terms included: visceral leishmaniasis, kala‐azar, Leishmania donovani, Leishmania infantum,Leishmania chagasi, K39, immunoassay, chromatography, dipstick, immunochromatography, rapid diagnostic tests; RDT; antibody detection, serology.
Searching other resources
We checked the reference lists of the studies identified by the above methods. We also checked conference proceedings and reports from the WHO.
Data collection and analysis
Selection of studies
Two review authors assessed the titles and abstracts identified through the search strategy. All potentially relevant articles were retrieved in full and two authors (MB and TS or MB and KV) independently examined them for inclusion in the present review using an assessment form. Any discrepancies were resolved by discussion with a third author (JvG).
Data extraction and management
We extracted a standard set of data from each study, using a specifically modified data extraction form. If only a subgroup of participants in a study was eligible, we extracted the data for that particular subgroup.
Two authors independently extracted the data (MB and JvG). The discrepancies were resolved by discussion with a third author (KV).
For each study, data were extracted on:
| Study ID | First author, year of publication. |
| Clinical features and settings | Presenting signs and symptoms, clinical setting. |
| Participants | Sample size, summary statistics of age (mean or median), sex (% male or female), HIV status (% positive, if available), country. |
| Study design | Whether the sample was consecutive or random. Were consecutive patients enrolled retrospectively or prospectively? If the study evaluated more than one RDT, how were tests allocated to individuals, or did each individual receive all tests? |
| Target condition | Leishmania species, clinical features. |
| Reference standard | The reference standard test(s) used. Who performed the reference standard test(s) and where? How many observers or repeats were used? How were discrepancies between observers resolved? If not all patients received the reference tests, how many did not (and what proportion were they of the total)? If any participant received a different reference test, what reasons were stated for this, and how many participants were involved? |
| Index test | The commercial name, with batch number, if provided. Transport and storage conditions. Details of the test operators. |
| Index test results and reference standard results | Number of missing, not interpretable or doubtful results (for both index and reference tests). For each index test (and for each acceptable reference standard, if more than one reference standard was reported), the number of true and false positives, false and true negatives (2 x 2 contingency table); or for studies that used LCA as the reference standard: estimates and 95% confidence intervals (CI) for the prevalence and for the sensitivity and specificity of each index test. |
| Notes | Anything else of relevance |
Assessment of methodological quality
We first assessed the standard signalling questions of the QUADAS‐2 tool (Whiting 2011) and decided to omit one question (“If a threshold was used, was it pre‐specified?”) and not to add any specific signalling questions for this review.
The QUADAS‐2 tool comprises four domains: patient selection, index test, reference standard, and flow and timing. We considered that the risk of bias for a certain domain was low if all the signalling questions for that domain indicated a low risk of bias. If the answer to at least one signalling question for a certain domain was 'no', the risk of bias for that domain was considered to be high. If none of the answers was 'no', but the answer to at least one question was 'unclear', the risk of bias for that domain was considered to be unclear.
Two review authors (JVG and MB) then independently applied the tool to the included records. For each record, they assessed risk of bias and concerns about applicability. Several of the original studies included in this review were conducted by the review team. In order to ensure an objective assessment of all the included records, the judgements about eligibility, bias and applicability were entirely based on the published documents and not on unpublished background information. In addition, two of the three review authors who made the judgements about bias and applicability (JVG and KV) had not been involved in any of the original studies. In case of discrepant judgements, KV took the final decision.
Statistical analysis and data synthesis
The aim of the analysis was to estimate the diagnostic accuracy, that is, the sensitivity (Se) and specificity (Sp), of the index tests (RDTs for VL). We analysed each RDT separately and did not plan to make comparisons between different index tests.
The analysis was performed using R (R Development Team 2010) and WinBugs (Spiegelhalter 2003) because of these programs’ flexibility in model fitting, in line with recommendations in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy ‐ Chapter 10 Analysing and Presenting Results (Macaskill 2010). More technical detail on the statistical methods can be found in Appendix 1 and Menten 2013.
1) Outcome data
The statistical analysis was based on summary data as provided in the source publications: either the number of true and false positive or negative test results compared to the reference test, or the Sensitivity (Se) and Specificity (Sp) of the index tests and 95% CI or credible intervals obtained from latent class analysis.
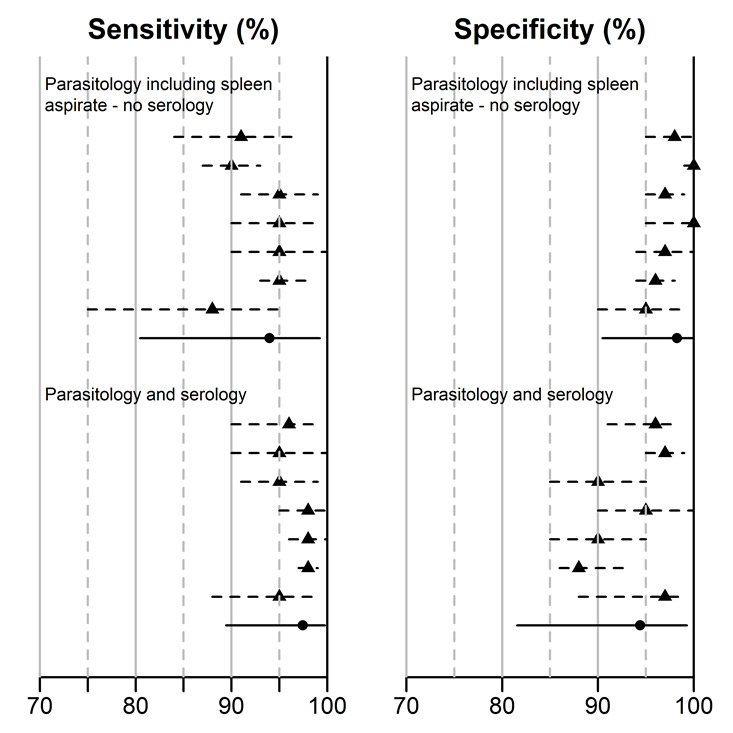
For those studies that reported data using several reference standards (Table 4), we extracted and summarized all 2 x 2 tables or Se and Sp estimates. The main analysis used a single 2 x 2 table or set of Se and Sp estimates for each study. The primary data for each study was selected based on a predefined ranking of reference standards: (1) latent class analysis, (2) parasitology and serology, (3) parasitology including spleen aspirate without serology, and (4) parasitology not including spleen aspirate without serology. The influence of the possible selection of alternative data sets was explored in sensitivity analyses.
1. Studies on rK39 ICT presenting two sets of Se and Sp estimates based on different reference standards: estimates included in primary analysis and sensitivity analysis.
| Study | Selected for primary analysis | Selected for sensitivity analysis |
| Boelaert 2004 ‐ LCA & Boelaert 2004 ‐ classic | LCA | Parasitology including spleen aspirate – no serology |
| Boelaert 2008 ‐ Ethiopia & Diro 2007 | LCA | Parasitology including spleen aspirate – no serology |
| Boelaert 2008 ‐ India & Sundar 2007 | LCA | Parasitology and serology |
| Cota 2013 ‐ composite 1 & Cota 2013 ‐ composite 2 * | Parasitology and serology | Parasitology not including spleen aspirate ‐ no serology |
| Machado de Assis 2012 & Machado de Assis 2011 | LCA | Parasitology not including spleen aspirate ‐ no serology |
| Veeken 2003 ‐ composite & Veeken 2003 ‐ spleen | Parasitology and serology | Parasitology including spleen aspirate – no serology |
* Cota 2013 describes HIV‐infected patients only and is not included in the formal meta‐analysis
2) Basic data presentation
The basic study results were summarized using coupled forest plots, presenting the crude Se and Sp estimates and 95% CIs as presented in the original publications. A summary receiver operating characteristic (ROC) plot was presented to assess the need for a ROC‐based analysis rather than the bivariate logistic normal model, planned and described below.
3) Basic statistical model
The primary analysis was performed using the bivariate model with a hierarchical approach (Reitsma 2005). The bivariate, rather than a ROC‐based, approach was chosen based on the prior experience of the author team in a VL diagnostic meta‐analysis (Chappuis 2006), where no threshold effects were observed. In addition, the rapid tests report the results as positive or negative, which implies a common cut‐off point for test positivity and which indicates, on prior grounds, that the bivariate model is the most appropriate. We determined the most appropriate link function for the bivariate model using the deviance information criterion (DIC, Verde 2010) and graphical assessment of model fit. Based on this analysis, we selected the complementary log‐log (cloglog) function (see Appendix 1) as providing the best and most parsimonious fit to the data. All results are presented on the probability scale allowing direct comparison with results obtained using the logit link function.
For the studies estimating Se and Sp compared to a reference standard, at the lower level the cell counts in the 2 x 2 tables extracted from each study were modelled using binomial distributions (Macaskill 2010).
For the studies estimating Se and Sp through LCA, the cloglog transforms of the Se and Sp and their standard errors were derived from the estimates and CIs in the source publications. They were then entered in the lower level of the hierarchical model using the normal distribution.
The Se and Sp, irrespective of their source, were, in the higher level of the hierarchical model, presumed to come from the same bivariate normal, that is, the model proposed corresponds to a hybrid approach of the bivariate model of Reitsma (Reitsma 2005) and standard random effects meta‐analyses (Whitehead 2002). This model was estimated using Markov Chain Monte Carlo (MCMC) methods as implemented by WinBugs with uniform ]0,1[ priors for the index test Se and Sp. The WinBugs code for the model can be found in Appendix 1.
4) Model summary
Results from the models were provided as point estimates of test Se and Sp and joint 95% CI and prediction regions. The confidence regions and prediction regions were plotted on the Se versus 1‐Sp diagram (that is, the ROC space), together with the crude or model‐based point estimates of Se and Sp, or both, for each study. In tables, we presented marginal CIs obtained from these confidence regions.
Investigations of heterogeneity
The amount of between‐study heterogeneity was graphically assessed in coupled forest plots.
To explain the between‐study variability, we assessed the following possible sources of heterogeneity:
geographical area: Indian subcontinent, eastern Africa, Latin America, Mediterranean region (note: this is related to the Leishmania species diversity and distribution: L. donovani complex in Asia and eastern Africa, and L. infantum in the Mediterranean region and Latin America);
commercial brand or type of test: categories determined based on extracted data;
type of reference standard: (a) parasitology including spleen aspirate – no serology; (b) parasitology not including spleen aspirate – no serology; (c) parasitology and serology; (d) latent class analysis;
disease prevalence (in the sample): below versus equal to or above the median (65%) across all studies; and
quality assessment: a first indicator of quality was based on the QUADAS‐2 assessment. Here, we used an overall, study‐level assessment of the risk of bias. If the risk of bias in a study was low in all four QUADAS‐2 domains, we defined the overall risk of bias in that study as 'low'. If the risk of bias was high in at least one of the QUADAS‐2 domains, we defined the overall risk of bias as 'high'. In all other cases, the overall risk of bias was labelled 'uncertain'. A second indicator of quality was the size of the study (below versus equal to or above median [250]), because in order to ensure reasonably precise estimates of sensitivity and specificity, investigators are expected to consider sample size issues during the planning of the study (Bachmann 2006; Peeling 2007). Larger studies may have been subjected to closer scrutiny and may have recruited a more representative sample of the clinical suspect population. Yet, the precision of Se and Sp estimates in original studies does not only depend on sample size, but also on disease prevalence (Deeks 2005).
We informally assessed the influence of these factors by presenting summary Sp and Se for subgroups of studies (categories of covariate). In a formal analysis, these sources were then individually added as fixed‐effect predictors of Se and Sp in the statistical model described above. We assessed the estimates and 95% CI of the effects of each of the predictors on Se and Sp. If warranted by the results of these analyses and the amount of studies available, we planned to consider developing this model further using multiple predictors or assuming separate bivariate normal distributions in different subgroups of studies.
Sensitivity analyses
Analysis using secondary reference standards
As a sensitivity analysis to the choice of the set of estimates of a given study, we made an alternative selection of the reference standard for those studies presenting results for two reference standards (Table 4).
Analysis allowing for imperfect reference standards
The model described above estimated the Se and Sp by comparison to a reference standard presuming that this reference standard was perfect, that is, that its Se and Sp were both equal to 1. However, in VL, it can be expected that reference standards have less than perfect Se or Sp, or both.
To allow for possible imperfect reference standards, we used the following approach:
during data extraction, each study was classified according to the type of reference standard: (a) parasitology including spleen aspirate – no serology; (b) parasitology not including spleen aspirate – no serology; (c) parasitology and serology; or (d) latent class analysis;
for these reference standards, expert opinion for the Se and Sp was elicited from seven experts (three of the authors FC, MB and SR, and four from the WHO technical expert panel on leishmaniasis);
the expert opinion on the reference test Se and Sp was then entered as priors, using the beta distribution, in an extension of the bivariate model described above as a Bayesian sensitivity analysis (Greenland 2006).
The bivariate model was extended using a multinomial distribution for the cell counts in the 2 x 2 tables, as in the latent class analysis of diagnostic tests (Black 2002). The Se and Sp of the index test were modelled through a bivariate normal distribution as in the basic model described above. The Se and Sp of the reference tests were assumed to be equal across studies using the same reference standard, with informative priors for the Se and Sp derived from expert opinion as described above. Prevalences were estimated for each study separately by providing a uniform ]0,1[ for the study‐specific prevalences. Data from studies that performed LCA in the source publication were included as in the basic model, as they already allowed for uncertainty in the true disease status in the source publication. The model was fitted using MCMC methods. Care was taken in assessing the fit and identifiability of the model using posterior predictive checks (Gelman 1995). It could be expected that the model would remain identifiable through the use of informative priors on the Se and Sp of the reference test and the assumption that the Se and Sp of the index test across studies arose from the same normal bivariate distribution (Enoe 2000), but, if needed, further constraints on the model parameters could be added (for example, by constraining the Sp of some of the reference standards to 1).
The results from this model were compared with those obtained in the primary analysis, described above. We also contrasted the assumptions of our model against the assumption of perfect ascertainment underlying the primary analysis approach. The performance of the model was evaluated through simulation studies. Details are given in Appendix 1 and Menten 2013.
Assessment of structure of hierarchical model
We compared the primary results with those obtained using the standard logit link function for hierarchical model. In addition, we relaxed the assumption of normally distributed random‐effects, by using the t‐distribution instead of the normal distribution.
Assessment of reporting bias
It is well known that reporting or publication bias in studies of diagnostic test accuracy may be difficult to detect and that formal tests of funnel plot asymmetry are biased (Macaskill 2010). We did not formally test for publication bias but explored the relationship between the logit Se, logit Sp, and log diagnostic odds ratio and the effective sample size (Deeks 2005) using funnel plots. However, the presence or absence of funnel plot asymmetry was not interpreted as definite proof of the presence or absence of publication bias.
Results
Results of the search
The date of the search was 3 December 2013. The search identified 1758 records, each record corresponding to a published article (Figure 1). After screening titles and abstracts, 1648 irrelevant records were removed. We were unable to obtain the full‐text article of one record and excluded one other article because it was written in Chinese language and no translators were available. We retrieved the full text of the remaining 108 records and excluded another 87 for the following reasons: publication of the same study in more than one record (we kept the record that was published most recently and excluded the others; n = 4); no original research (n = 6); index test not a rapid test (n = 1); target condition not clinical visceral leishmaniasis (n = 5); not a phase III diagnostic accuracy study (n = 66); and reference standard not according to criteria (n = 5; Figure 1 and Characteristics of excluded studies). The remaining 21 records were included in this review.
1.
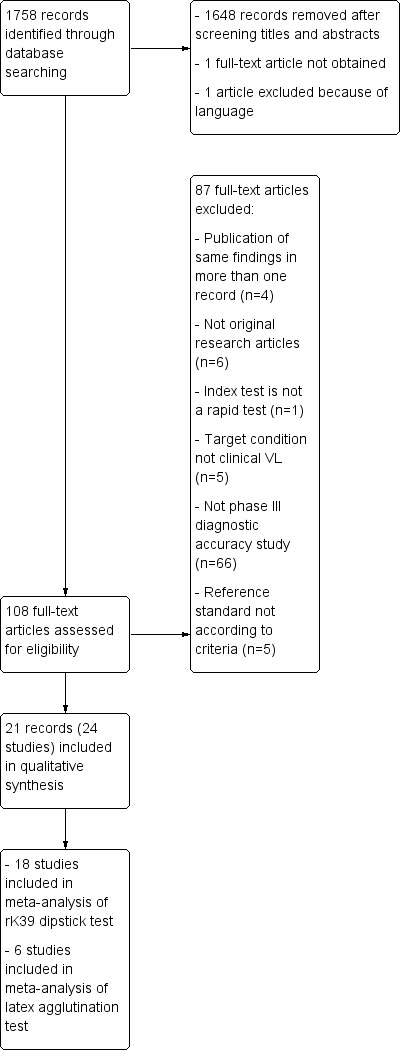
Study flow diagram showing the process of selection of records and studies for the review and for the meta‐analyses
Several records reported multi‐country, multicentre or stratified data. If a ‘study’ is defined as a phase III design leading to a sensitivity and specificity estimate of one or more RDTs in a specific patient population, then three of the 21 included records contained results of more than one unique study. One article described RDT performance in patient populations in five countries (Boelaert 2008, henceforth distinguished as Boelaert 2008 ‐ Ethiopia; Boelaert 2008 ‐ India; Boelaert 2008 ‐ Kenya; Boelaert 2008 ‐ Nepal; Boelaert 2008 ‐ Sudan); Ter Horst et al. reported data separately for HIV‐positive and HIV‐negative patient populations (ter Horst 2009 ‐ HIV neg; ter Horst 2009 ‐ HIV pos); and Diro 2007 included data from clinically suspected patients presenting at the study site and from people identified through active case finding in the community. Furthermore, in one record, two different brands of the rK39 immunochromatographic test (ICT) were evaluated in the same population (Chappuis 2005 ‐ DiaMed; Chappuis 2005 ‐ InBios), and we treated this record as two different studies. On the other hand, three patient populations were presented in more than one record. Boelaert 2008 ‐ Ethiopia re‐analysed the same patient population data as Diro 2007, but using a different reference standard. Similarly, Boelaert 2008 ‐ India described the same patient population as Sundar 2007 but again, analysed it with a different reference standard. This also occurred in Machado de Assis 2011 and Machado de Assis 2012. We have treated these records as one single study in each country with a primary and a secondary analysis (see below). Altogether, the 21 included records contained information about 25 unique studies. We excluded the study based on active case finding (part of Diro 2007) from the review as it did not comply with the eligibility criteria of our protocol and, therefore, we finally included 24 studies.
These 24 studies included 4271 participants of whom 2606 were classified as having VL. Four studies used a reference standard including direct smears or culture of splenic aspirate, or both, 11 used a composite reference standard, three used latent class analysis, and six presented two sets of accuracy estimates using two different reference standard categories (Table 4).
The 24 studies contained information about five index tests: the rK39 ICT, the latex agglutination test in urine, the FAST agglutination test, an rK26 ICT and an rKE16 ICT. Six studies assessed the accuracy of more than one type of index test in the same patient population: four studies evaluated the rK39 ICT and the latex agglutination test; one study evaluated the rK39 and the rKE16 ICT; and one study evaluated the rK39 ICT, the rK26 ICT, and the latex agglutination test (Sundar 2007). Overall, the rK39 ICT test was evaluated in 20 studies including a total of 3806 participants of whom 2370 had VL. The latex agglutination test was evaluated in seven studies, which corresponds to 1459 participants including 873 people with VL. The FAST agglutination screening test was evaluated in two studies with a total of 148 participants including 69 with VL. The rK26 ICT test was assessed in one study with 352 participants of whom 282 had VL, and the rKE16 test was evaluated in one study with 219 participants of whom 131 had VL.
For the meta‐analysis of the accuracy of the rK39 ICT test, 18 out of 20 studies were included (Table 5). At this stage, we excluded two studies because they only described HIV‐positive patients (ter Horst 2009 ‐ HIV pos and Cota 2013). In all the other studies using the rK39 ICT test, the included patients were HIV negative or the HIV status was unknown but considered of no importance in the study population. Six out of the 18 included studies generated more than one set of sensitivity and specificity estimates by using different reference standards in a primary and a secondary analysis. We selected one of the two estimates for the primary meta‐analysis and explored the effects of this choice in the sensitivity analysis (Table 4).
2. Overall Analysis Summary.
| Number of studies | Se (95% CI) | Sp (95% CI) | |
| rK39 immunochromatographic test | |||
| Overall | 18 | 91.9 (84.8 to 96.5) | 92.4 (85.6 to 96.8) |
| Indian subcontinent | 6 | 97.0 (90.0 to 99.5) | 90.2 (76.1 to 97.7) |
| East Africa | 9 | 85.3 (74.5 to 93.2) | 91.1 (80.3 to 97.3) |
| Latex agglutination test | |||
| Overall | 6 | 63.6 (40.9 to 85.6) | 92.9 (76.7 to 99.2) |
For the meta‐analysis of the accuracy of the latex agglutination test, we included six studies (Table 5). We excluded one study because it described mostly HIV‐positive patients (Vilaplana 2004).
Methodological quality of included studies
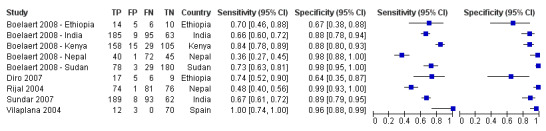
The QUADAS‐2 tool was used to assess the quality of the 21 included records in terms of the risk of bias and of concerns about applicability. Figure 2 summarizes the overall methodological quality and Figure 3 gives the ratings for each of the included records. In the domain of patient selection, we had high concerns about the risk of bias and the applicability of one record (Veeken 2003 ‐ composite) because the inclusion of participants was conditional on the availability of stored serum samples and of diagnostic information from another serological test that could have correlated with the index test. In another record (Cota 2013), we had high concerns about the applicability to the review question because a considerable proportion of the study population (21%) had a previous history of VL. In the domains of the index test and the reference standard, the risk of bias was unclear for 14 of the 21 included records. The most frequent underlying reason was that the publication did not report whether the index test results were interpreted without knowledge of the reference standard and vice versa. We considered that there was a high risk of bias in those studies that used bone marrow aspirate smear tests and culture with or without clinical information as a reference standard due to the low sensitivity of these techniques (Kilic 2008 and Cota 2013 ‐ composite 2). In the domain of flow and timing, we considered that there was a high risk of bias in five records, because not all patients were included in the analysis (Rijal 2004; ter Horst 2009; Veeken 2003; and Peruhype‐Magalhaes 2012) or because not all patients received the same reference standard (Sundar 1998).
2.
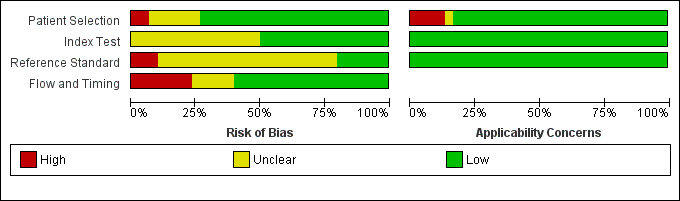
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
Footnote
Figure 2 represents studies but our quality assessment was done per record. Some of the records include more than one study. Figure 2 shows all the estimates used in this review, including the estimates for primary and sensitivity analyses of the same study.
3.
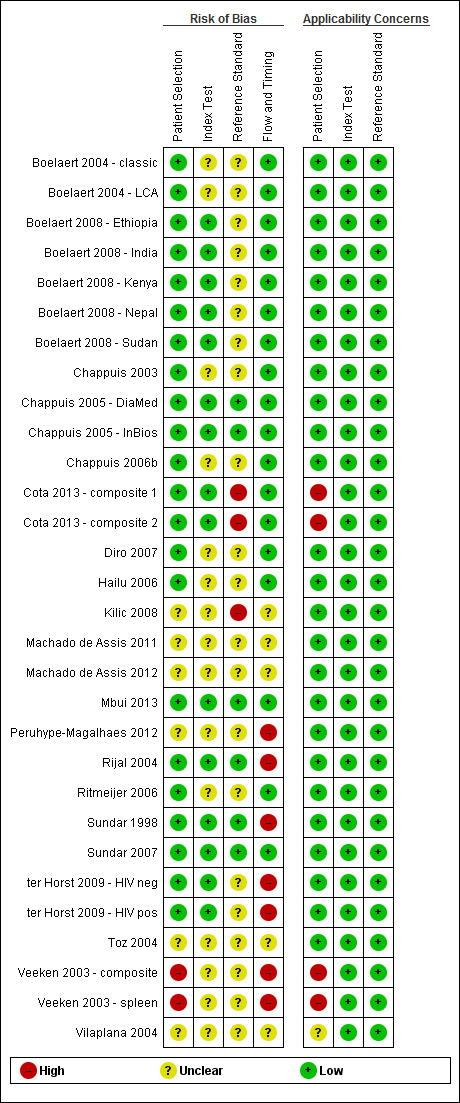
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Footnote
Figure 3 represents studies but our quality assessment was done per record. Some of the records include more than one study. Figure 3 shows all the estimates used in this review, including the estimates for primary and sensitivity analyses of the same study.
Findings
rK39‐based rapid diagnostic tests
Data summary
Twenty studies reported Se and Sp estimates for the rK39 ICT. Six of these studies gave two sets of Se and Sp estimates, based on alternative reference standards (Table 4). A forest plot of the 26 available Se/Sp estimates is given in Figure 4. Figure 5 presents the available Se/Sp pairs according to the reference standard used; the results referring to the same data analysed with different reference standards are connected with a line.
4.
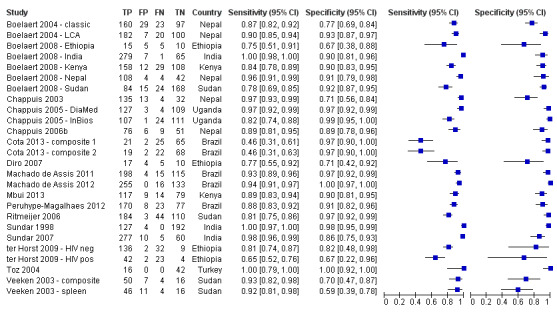
rK39 ICT: forest plot of all the available estimates of sensitivity and specificity (n = 20 studies; 26 sets of estimates)
Footnote
For studies that use latent class analysis, the counts of true positive, false positive, false negative and true negative results are imputed from the Se and Sp estimates and the overall sample size. The estimates and confidence intervals are subsequently calculated from these imputed values.
5.
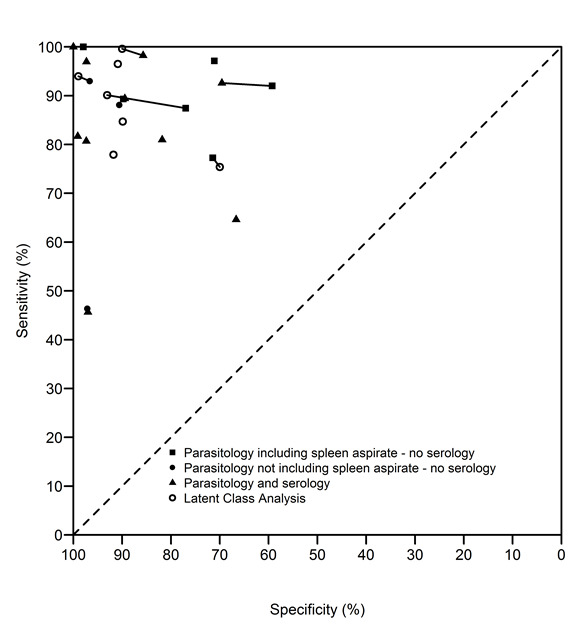
rK39 ICT: summary of sensitivity‐specificity pairs according to the reference standard (n = 20 studies; 26 sets of estimates). The type of reference standard is classified as: (a) parasitology including spleen aspirate – no serology; (b) parasitology not including spleen aspirate – no serology; (c) parasitology and serology; or (d) latent class analysis. The data points that are connected with a line refer to the same data analysed with different reference standards.
Meta‐analysis
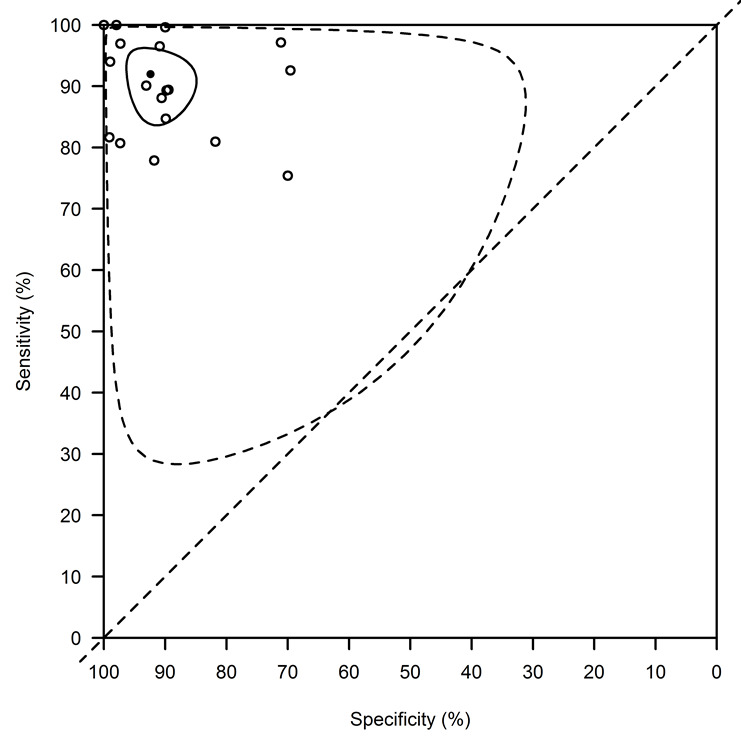
A formal meta‐analysis was performed on 18 of the 26 available data points: studies including mainly HIV‐infected patients were reported separately (ter Horst 2009 ‐ HIV pos, Cota 2013 ‐ composite 1 and Cota 2013 ‐ composite 2) and for the five remaining studies with multiple Se and Sp estimates, we used one set of estimates for the primary analysis (Table 4). Combining data from all studies without accounting for possible covariates explaining heterogeneity and using the bivariate model (Reitsma 2005), the average (95% CI) Se was 91.9% (84.8 to 96.5) and the average Sp was 92.4% (85.6 to 96.8)
The 95% prediction interval (PI) for the diagnostic accuracy that would be observed in a new study was (52.3 to 100)% for the Se and (54.9 to 100)% for the Sp (Figure 6).
6.
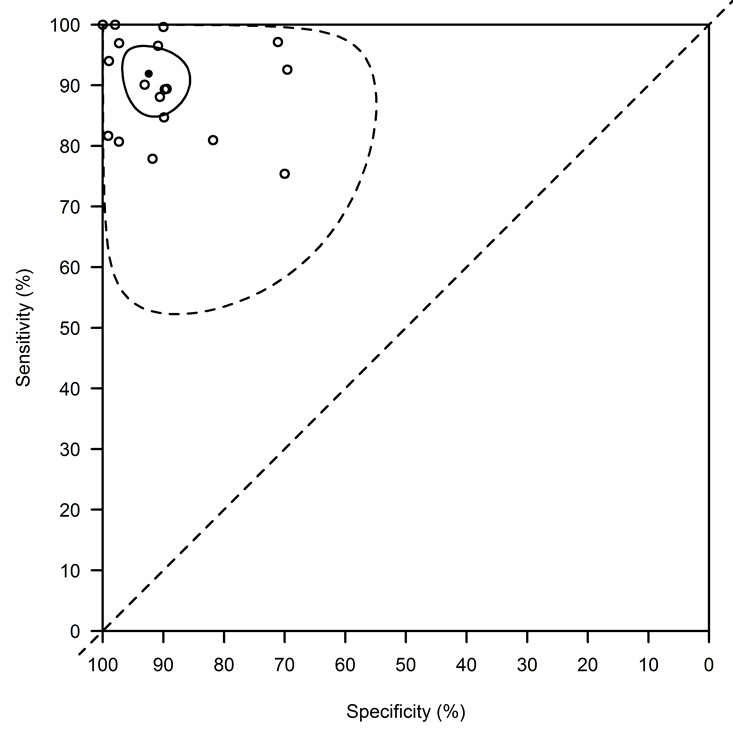
rK39 ICT: basic summary using the bivariate normal model with a complementary log‐log link function. This analysis combines data from all studies without accounting for covariates. The average sensitivity is 91.9% (95% confidence interval: 84.8, to 96.5) and the average specificity is 92.4% (95% confidence interval: 85.6, to 96.8). The confidence region is indicated with a full line, the prediction region with a dotted line.
Of note is the fact that, in contrast to what is presumed in the HROC model, the estimated correlation between the transformed Se and Sp was positive: 0.16 (95% CI: ‐0.40 to 0.65). Given these observations, we only report results from the bivariate model using the complementary log‐log, and not from the ROC‐based analysis.
Assessment of heterogeneity
A summary of the heterogeneity assessment through meta‐analysis can be found in Figure 7 and Table 6.
7.
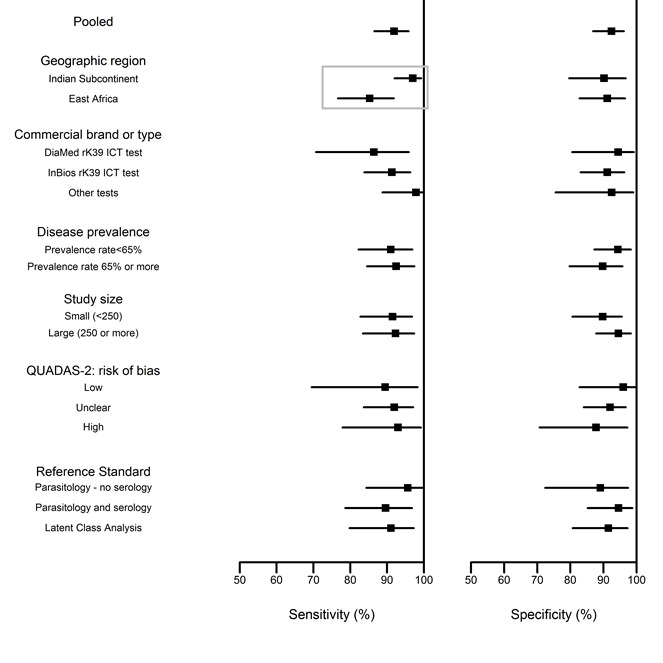
rK39 ICT; summary of the heterogeneity assessment through meta‐analysis: sensitivity and specificity estimates by covariates. Rectangles indicate significant differences.
3. Heterogeneity assessment of the rK39 ICT meta‐analysis.
| Sensitivity | Specificity | |||
| Estimate | (95% CI) | Estimate | (95% CI) | |
| Geographic region | ||||
| Indian subcontinent | 97.0 | (90.0 to 99.5) | 90.2 | (76.1 to 97.7) |
| East Africa | 85.3 | (74.5 to 93.2) | 91.1 | (80.3 to 97.3) |
| Commercial brand | ||||
| DiaMed | 86.4 | (70.7 to 95.9) | 94.4 | (80.5 to 99.2) |
| InBios | 91.3 | (83.8 to 96.3) | 91.2 | (83.1 to 96.3) |
| Other | 97.8 | (88.7 to 99.9 | 92.5 | (75.5 to 99.0) |
| Disease prevalence in sample | ||||
| Low (< 65%) | 91.0 | (82.2 to 96.9) | 94.4 | (87.2 to 98.3) |
| High (≥ 65%) | 92.4 | (84.5 to 97.4) | 89.8 | (79.7 to 95.8) |
| Study size | ||||
| Small (< 250) | 91.5 | (82.7 to 96.7) | 89.8 | (80.6 to 95.6) |
| Large (≥ 250) | 92.3 | (83.4 to 97.4) | 94.5 | (87.8 to 98.3) |
| QUADAS‐2: risk of bias | ||||
| Low | 89.5 | (69.5 to 98.3) | 96.0 | (82.8 to 99.7) |
| Unclear | 91.9 | (83.7 to 97.1) | 92.0 | (84.0 to 96.7) |
| High | 93.0 | (77.9 to 99.1) | 87.8 | (70.7 to 97.2) |
| Reference standard | ||||
| Parasitology ‐ no serology * | 95.6 | (84.3 to 99.6) | 89.1 | (72.4 to 97.4) |
| Parasitology and serology | 89.6 | (78.6 to 96.7) | 94.6 | (85.2 to 98.7) |
| Latent class analysis | 91.0 | (79.8 to 97.2) | 91.5 | (80.7 to 97.3) |
* The categories "parasitology including spleen aspirate ‐ no serology" and "parasitology not including spleen aspirate ‐ no serology" were combined because the latter category contained only one study.
Geographic region
We assessed the difference in diagnostic accuracy effect between East Africa and the Indian subcontinent. There were nine data points from East Africa (two from Ethiopia, two from Kenya, three from Sudan, and two from Uganda), and six from the Indian subcontinent (two from India and four from Nepal). Three studies from other regions (two from Brazil and one from Turkey) were not considered in this analysis. The Se was significantly lower in East Africa (85.3%; 95% CI: 74.5 to 93.2) than in the Indian subcontinent (97.0%; 95% CI: 90.0 to 99.5). There was no significant difference in Sp: the Sp (95% CI) in east Africa was 91.1% (80.3 to 97.3) and in the Indian subcontinent 90.2% (76.1 to 97.7) (Table 1 and Table 2). Confidence and prediction regions by geographic region are given in Figure 8.
8.
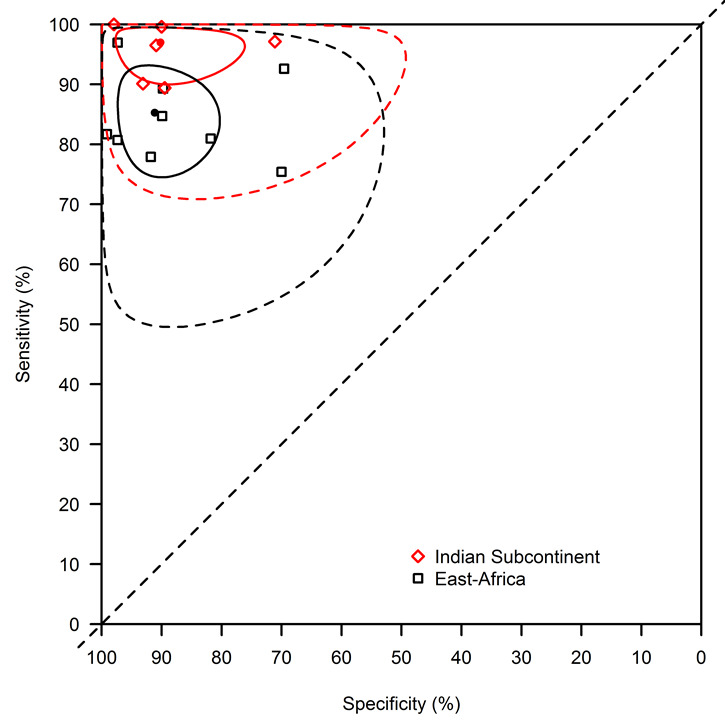
rK39 ICT: summary of the meta‐regression model with effects for geographic region using the bivariate normal model with a complementary log‐log link function. The sensitivity was significantly lower in East Africa (85.3%; 95% confidence interval: 74.5 to 93.2) than in the Indian subcontinent (97.0%; 95% confidence interval: 90.0 to 99.5). There was no significant difference in specificity: the specificity (95% confidence interval) in East Africa was 91.1% (80.3 to 97.3) and in the Indian subcontinent 90.2% (76.1 to 97.7). The confidence region is indicated with a full line, the prediction region with a dotted line.
Commercial brand or type of test
The majority of studies used the InBios rK39 ICT test (11 studies); four assessed the DiaMed rK39 ICT test; and three evaluated other tests (DiaMed DUAL‐IT L/M ICT test, Amrad ICT rK39 ICT test, and Arista Biologicals rK39 ICT test). There were no significant differences in diagnostic accuracy between commercial brands.
Disease prevalence
We separated studies by estimated prevalence of VL in the study sample: nine studies reported prevalence rates < 65% and nine reported prevalence rates ≥ 65%. There were no significant differences in diagnostic accuracy between studies with low and high prior probability of disease in the patient sample.
Quality assessment
We used study size and risk of bias according to QUADAS‐2 as indicators of the quality of the primary studies.
Study size
We categorized studies according to the total number of study participants (true cases + non‐cases) in small (< 250) and large (≥ 250) studies. There were 10 small and eight large studies. There were no significant differences in diagnostic accuracy between small and large studies, but there was a trend for larger studies to show a higher Sp estimate.
QUADAS‐2: risk of bias
Studies were categorized according to the risk of bias assessed with QUADAS‐2: low risk (three studies), high risk (four studies), and unclear risk of bias (11 studies). There were no significant differences among these categories, but there was a trend for studies with high risk of bias to show a low Sp and studies with low risk of bias to show a higher Sp.
Type of reference standard
Studies were categorized according to the reference standard used in the primary publication. There were no significant differences in accuracy among these categories. Further exploration of the influence of the reference standard is assessed in the sensitivity analysis below.
Diagnostic accuracy in HIV‐positive participants
Two studies evaluated the rK39 ICT in HIV‐positive participants and were not included in the meta‐analysis.
The first study reported on the accuracy of the DiaMed rK39 ICT in 71 HIV‐positive participants in Ethiopia (ter Horst 2009 ‐ HIV pos). Based on a composite reference standard that combined direct parasitological examination of spleen aspirate with DAT serology, 65 participants had a diagnosis of VL. In this setting, the sensitivity of the rK39 ICT was 64.6% and the specificity 66.7%.
The second study evaluated the InBios rK39 ICT in 113 HIV‐infected people in Brazil. Data were extracted according to two different reference standards: (1) the decision of an adjudication committee after clinical follow‐up and taking into account all available information (including parasitology and serology) (Cota 2013 ‐ composite 1; VL prevalence: 40.7%); and (2) direct smear and culture of bone marrow aspirate (Cota 2013 ‐ composite 2; VL prevalence: 36.9%). The choice of the reference standard was of little influence. The Se of the rK39 ICT was low: 45.7% with the first and 46.3% with the second reference standard; and the Sp was high: 97.0% with the first and 97.1% with the second reference standard.
Sensitivity analyses
We performed sensitivity analyses of the influence of the choice of reference standard and possible biases resulting from imperfect reference standards. Given the impact of geographic region on the diagnostic accuracy of rK39 ICT, we corrected the sensitivity analyses for region effects and limited the analyses to 15 studies performed in the Indian subcontinent and East Africa. An overview of the results of the sensitivity analyses is given in Table 7.
4. Sensitivity analysis results of the rK39 ICT meta‐analysis.
| Sensitivity | Specificity | |||
| Estimate | (95% CI) | Estimate | (95% CI) | |
| Main Analysis | ||||
| Indian subcontinent | 97.0 | (90.0 to 99.5) | 90.2 | (76.1 to 97.7) |
| East Africa | 85.3 | (74.5 to 93.2) | 91.1 | (80.3 to 97.3) |
| Alternative Analysis Set | ||||
| Indian subcontinent | 96.1 | (88.9 to 99.2) | 86.7 | (69.2 to 99.2) |
| East Africa | 85.2 | (75.0 to 92.7) | 90.1 | (77.0 to 97.4) |
| Bayesian analysis allowing for imperfect reference standards: expert priors ‐ using main analysis set | ||||
| Indian subcontinent | 97.3 | (91.9 to 99.5) | 93.7 | (74.9 to 99.7) |
| East Africa | 86.7 | (77.5 to 94.0) | 95.7 | (84.3 to 99.8) |
| Bayesian analysis allowing for imperfect reference standards: vague priors ‐ using main analysis set | ||||
| Indian subcontinent | 97.3 | (91.9 to 99.5) | 93.0 | (77.8 to 99.3) |
| East Africa | 86.1 | (77.2 to 93.3) | 94.3 | (83.8 to 99.6) |
Analysis using secondary reference standards
As a sensitivity analysis to the choice of the set of estimates of a given study, we made an alternative selection of the reference standard for those studies presenting results for two reference standards (Table 4). The estimated Sp was slightly lower compared to the main analysis set: 86.7% versus 90.2% for the Indian subcontinent and 90.1% versus 91.1% for east Africa. This may be related to a lower Se of the reference standards included in this analysis, which would result in an underestimation of the Sp of the index test. Estimated Se was similar in the sensitivity and main analysis.
Analysis allowing for imperfect reference standards
In the studies available for this sensitivity analysis, two types of reference standards were used: parasitology including spleen aspirate – no serology; (three studies) and a combined reference standard of parasitology and serology (six studies), in addition to the six studies analysed using latent class analysis. We elicited the opinion from seven VL experts on the diagnostic accuracy and possible variation across the two reference standards (Figure 9). We used these expert opinions as prior information in a Bayesian statistical model to estimate the diagnostic accuracy of the rK39 ICT. This analysis indicated that due to lack of sensitivity of the reference standard, the Sp of rK39 ICT may be somewhat underestimated. The estimated Sp correcting for this bias was 93.7% in the Indian subcontinent, and 95.7% in east Africa, compared to 90.2% and 91.1% assuming perfect reference standards. The estimates of Se did not change significantly when we corrected for imperfect reference standards. In this model, the Se of spleen aspirate was estimated to be 96.0% (95% CI: 92.5 to 98.8) and the Sp was estimated to be 99.4% (95% CI: 98.1 to 99.9). The estimated Se of the combined reference standard of spleen parasitology and a serological test was estimated to be 98.6% (95% CI: 97.1 to 99.4) and the estimated Sp was 96.5% (95% CI: 91.5 to 99.2).
9.
Opinion from seven experts on visceral leishmaniasis regarding the diagnostic accuracy and possible variation of two reference standards: parasitology including spleen aspirate without serology and any combination of parasitology with serology. These expert opinions were used as prior information in a Bayesian statistical model to estimate the diagnostic accuracy of the rK39 ICT.
Similar results were obtained when we used non‐informative priors instead of expert opinion to estimate the Bayesian model.
Assessment of structure of hierarchical model
Using the more standard logit link, in comparison to the better fitting complementary log‐log link increased the DIC from 171.3 to 199.0. The logit link resulted in similar estimates of Se and Sp: the average (95% CI) Se was 91.9% (83.6 to 96.2) and the average Sp was 92.4% (84.9 to 96.3). However, the remaining heterogeneity was considerably larger resulting in wider prediction regions: 28.3 to 99.7% for Se and 31.1 to 99.7% for Sp (Figure 10) compared to the primary analysis.
10.
rK39 ICT: basic model summary using the bivariate logistic normal model. This analysis combines data from all studies without accounting for covariates. The average sensitivity is 91.9% (95% confidence interval: 83.6, to 96.2) and the average specificity is 92.4% (95% confidence interval: 84.9, to 96.3). The confidence region is indicated with a full line, the prediction region with a dotted line. In this analysis, the prediction intervals are wide: 28.3 to 99.7% for the sensitivity and 31.1 to 99.7% for the specificity.
Using a bivariate t‐distribution for the random‐effects did not improve model fit compared to the normal model.
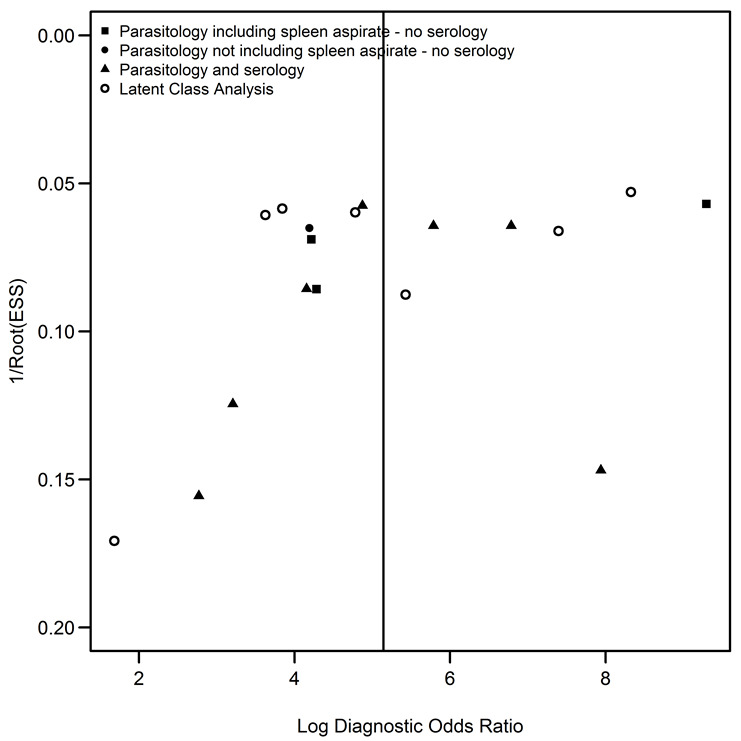
Assessment of reporting bias
Reporting bias was assessed through the robust funnel plot proposed by Deeks 2005. This robust funnel plot presents the log of the diagnostic odds ratio by a robust measure of the study size. No indication of reporting bias was observed in this plot (Figure 11).
11.
rK39 ICT; assessment of reporting bias: funnel plot for log diagnostic odds ratio
Latex agglutination test
Data summary
Seven studies were identified that reported Se or Sp results of the latex agglutination test in urine (KAtex). For two of these studies, two sets of Se and Sp estimates were available. A forest plot of the nine available Se/Sp estimates is given in Figure 12.
12.
Latex agglutination test: forest plot of all the available estimates of sensitivity and specificity (n = 7 studies; 9 sets of estimates).
Meta‐analysis
One study was not pooled with the others in the formal meta‐analysis because, contrary to the other studies, this study included a majority of HIV‐positive participants (Vilaplana 2004). For the two studies that presented Se/Sp estimates using LCA and a reference standard on the same data (Boelaert 2008 ‐ Ethiopia and Diro 2007, Boelaert 2008 ‐ India and Sundar 2007), the results from the two analysis approaches were very similar. We used the results from LCA for the primary meta‐analysis.
Combining data from six studies without accounting for possible covariates explaining heterogeneity, and using the bivariate normal model with complementary log‐log link, the average (95% CI) Se was 63.6% (40.9 to 85.6) and the average Sp was 92.9% (76.7 to 99.2) (Figure 13 and Table 3). The 95% prediction intervals were very wide: (16.5 to 99.6)% for the Se and (41.3 to 100)% for the Sp (Figure 13). The estimated correlation between the transformed Se and Sp was ‐0.39 (95% CI: ‐0.90 to 0.70).
13.
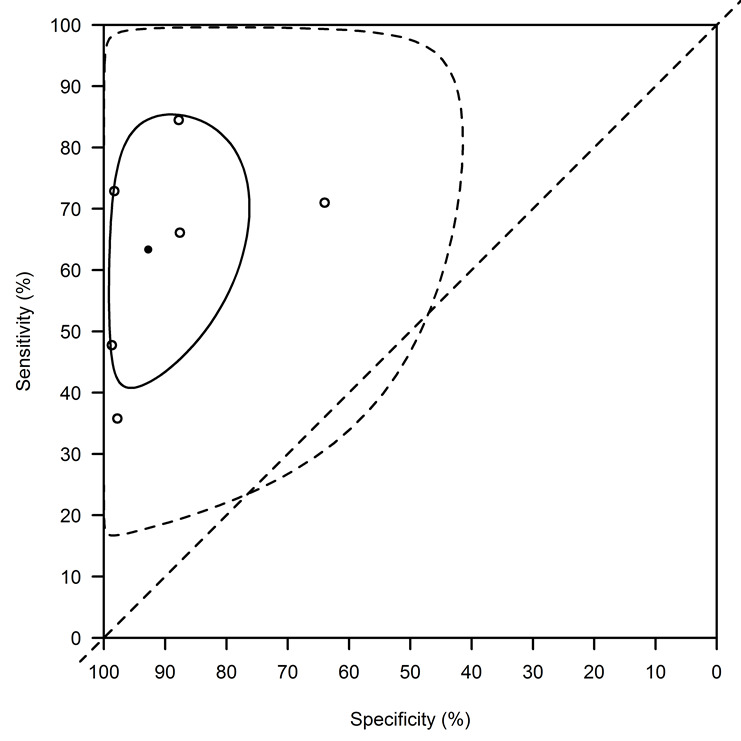
Latex agglutination test: basic summary using the bivariate normal model with a complementary log‐log link function. This analysis combines data from all studies without accounting for covariates. The average sensitivity (95% confidence interval) is 63.6% (40.9 to 85.6) and the average specificity is 92.9% (76.7 to 99.2). The confidence region is indicated with a full line, the prediction region with a dotted line. The 95% prediction intervals are very wide: 16.5% to 99.6% for the sensitivity and 41.3% to 100% for the specificity.
Assessment of heterogeneity
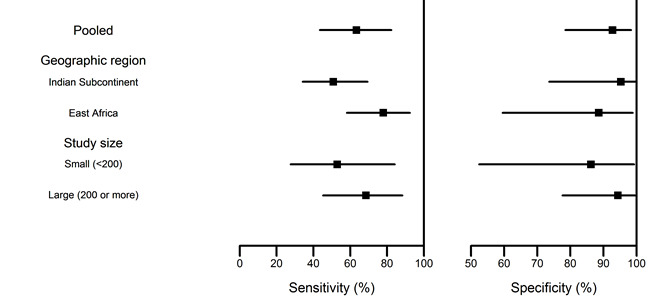
Of the predefined sources of heterogeneity, geographic region, disease prevalence, and study size showed sufficient variability across studies to warrant a meta‐regression. All studies used the KAtex latex agglutination test manufactured by Kalon biological. Five of the six studies had an unclear risk of bias. All studies in east Africa reported prevalences of VL < 65% and all studies in the Indian subcontinent reported prevalences ≥ 65%. Consequently, the effects of region and disease prevalence in the sample could not be separated. There were no significant differences in diagnostic accuracy of the latex agglutination test among regions or study sizes (Figure 14 and Table 8).
14.
Latex agglutination test; summary of the heterogeneity assessment through meta‐analysis: sensitivity and specificity estimates by covariates.
5. Heterogeneity assessment of the Latex agglutination test meta‐analysis.
| Sensitivity | Specificity | |||
| Estimate | (95% CI) | Estimate | (95% CI) | |
| Geographic region | ||||
| Indian subcontinent | 50.8 | (34.1 to 69.3) | 95.3 | (73.6 to 99.8) |
| East Africa | 77.9 | (58.2 to 92.3) | 88.6 | (59.5 to 92.3) |
| Study size | ||||
| Small (< 200) | 52.9 | (27.6 to 84.1) | 86.2 | (52.5 to 99.2) |
| Large (≥ 200) | 68.5 | (45.2 to 88.2) | 94.5 | (49.0 to 100.0) |
Sensitivity analysis
As a sensitivity analysis to the choice of the set of estimates of a given study, we made an alternative selection of the reference standard for those studies presenting results for two reference standards. The results were very similar to the primary analysis: the average (95% CI) Se was 63.4% (40.8 to 85.4) and the average Sp was 92.8% (76.3 to 99.2) (Table 9).
6. Sensitivity analysis results of the Latex agglutination test meta‐analysis.
| Sensitivity | Specificity | |||
| Estimate | (95% CI) | Estimate | (95% CI) | |
| Main Analysis | 63.6 | (40.9 to 85.6) | 92.9 | (76.7 to 99.2) |
| Alternative Analysis Set | 63.4 | (40.8 to 85.4) | 92.8 | (76.3 to 99.2) |
Diagnostic accuracy in HIV‐positive participants
One study assessed the accuracy of the latex agglutination test in a population of 85 mostly HIV‐positive participants (93%) in Spain (Vilaplana 2004). According to a reference standard based on culture or direct parasitological examination of bone marrow aspirate, 12 participants were classified as having VL. The estimated Se and Sp of the KAtex test were very high: the Se was 100% (12/12; 95% CI: 74 to 100) and the Sp was 96% (70/73; 95% CI: 88 to 99).
FAST
Two studies assessed the accuracy of the Fast agglutination screening test (FAST), one in Ethiopia (Hailu 2006) and one in Turkey (Kilic 2008). Both studies were small (89 participants in Ethiopia and 59 in Turkey). The prevalence of VL in the study population was 51% in Ethiopia and 41% in Turkey. The reference standard was the culture or direct parasitological examination of spleen or lymph node samples (Ethiopia) or of bone marrow samples (Turkey). In Ethiopia, the estimated Se of FAST was 91.1% and the Sp 70.5%. In Turkey, the estimated Se was 95.8% and the Sp 85.7%.
rK26‐based rapid diagnostic test
One study assessed the accuracy of an rK26‐based rapid diagnostic test manufactured by InBios International (Seattle, Washington, USA) in a population of 352 patients with suspected VL in India (Sundar 2007). According to a reference standard combining direct parasitological examination of spleen aspirate with DAT serology and response to VL treatment, 282 participants had a diagnosis of VL. The sensitivity of the rK26 ICT was 21.3% and the specificity 100%.
rKE16 immunochromatographic test
One study evaluated an rKE16‐based ICT (Signal KA, Span Diagnostics, India) in Kenya (Mbui 2013). This study included 219 patients suspected to have VL. Based on a reference standard consisting of direct smear examination of a splenic aspirate sample, 131 participants were classified as having VL. The Se of the rKE16 ICT was 77.1% and the Sp 95.5%.
Discussion
Summary of main results
The rK39 ICT, developed in 1998, is the rapid diagnostic test for VL that has been most thoroughly evaluated so far. We retrieved 21 publications that corresponded to our inclusion criteria for the review, and from these, ultimately 18 independent sets of sensitivity (Se) and specificity (Sp) estimates could be included in a formal meta‐analysis of diagnostic accuracy of the rK39 ICT. In patients with febrile splenomegaly and no previous history of VL, the overall sensitivity of the rK39 ICT was 91.9% (95% CI 84.8 to 96.5) and the specificity 92.4% (95% CI 85.6 to 96.8). Sensitivity was significantly lower in East Africa (84.3%; 95% CI 74.5 to 93.2) than in the Indian subcontinent (97.0%; 95% CI 90.0 to 99.5), but there was no significant difference in specificity between geographic regions (Table 1, Table 2, and Appendix 3). This assessment of heterogeneity did not include the Latin American and Mediterranean region as the number of studies from those regions was too low. There was no significant difference according to prevalence of disease in the sample or manufacturer of the rK39 ICT, but the number of estimates in some categories of the latter was low (11/18 InBios, 4/18 DiaMed, and 3/18 other brands). Generally, care should be taken when interpreting this heterogeneity assessment due to the limited number of studies included in the meta‐analysis. Apart from the lack of power, several risk factors may be correlated inducing confounding between different covariates.
For the KAtex test, a latex agglutination test in urine, when used in a clinical setting in patients with febrile splenomegaly, overall sensitivity was 63.6% (95% CI 40.9 to 85.6) and specificity 92.9 % (95% CI 76.7 to 99.2) (Table 3). Seven studies were included in the review and six in the meta‐analysis. Because of the limited number of estimates, no complete heterogeneity assessment could be made. There were no significant differences in diagnostic accuracy of the KAtex test among regions or study sizes, but the number of included studies was too small to allow for definite conclusions.
The number of studies addressing three other rapid diagnostic tests (FAST, rK26 ICT, and rKE16 ICT) was too low to allow for a meta‐analysis. Our conclusions do not apply to HIV‐positive patients. In summary, completeness of evidence was most satisfactory for the rK39 ICT, only partially complete for the latex agglutination test in urine, and too incomplete for the other three tests.
The methodological quality of the evidence on the rK39 ICT and the latex agglutination test was acceptable, although we had concerns about the risk of bias in some studies (see below).
Strengths and weaknesses of the review
Our review protocol explicitly set out to select clinical evaluations in a phase III design, based on consecutively recruited patients all suspect for VL, as this type of evidence level is required for making policy recommendations for clinical practice. Therefore, we had to exclude many records using a phase II (case control) design. However, in some records, the study design was not clearly reported, which made it difficult to distinguish between a phase III and a case control design. Because of this poor reporting, we may have missed some true phase III studies.
We believe that the procedures used for study selection were quite exhaustive, as we did not impose any a priori language barriers (excluding only one study in Chinese ex‐post), and we searched several databases including regional ones such as LILACS. Therefore, we believe that the information presented here reflects the body of evidence accumulating over the past 15 years rather well. In addition, assessment of possible publication bias did not indicate that smaller studies without favourable results for the RDTs were less likely to be reported.
Most important was the striking heterogeneity in methods across the studies. Although the included studies all complied with the criteria for an acceptable reference standard set out in our protocol, there was a remarkable variety in the reference standards that were used. These reference standards were based on different tests, done in different orders, with different cut‐off values, and making different use of clinical information. We formally assessed the risk of bias in each record using the revised QUADAS‐2 instrument. In five out of the 21 included records, we had clear concerns related to the patient and sample flow and the timing. For 14 records, the risk of bias was difficult to assess in the domains of the index test and the reference standard mainly because authors failed to report explicitly if and how blinding was applied. When we categorized records into low (n = 3), unclear (n = 11), and high (n = 7) risk of bias, there was no significant difference in Se and Sp of the rK39 ICT, but high‐risk studies tended to have a higher sensitivity and a lower specificity. One possible explanation is that the reference standards in studies classified as at high risk of bias were of sub‐optimal sensitivity. This could result in a selection of cases being primarily severe cases which may be easy to diagnose with rapid diagnostic tests, while the control group may contain some undiagnosed true VL cases (falsely negative in the reference standard).
We tried to assess the influence of this quality in study design on the estimates of accuracy in a sensitivity analysis in the case of the rK39 ICT. Neither the use of a secondary analysis set that was based on a different reference standard, nor an analysis allowing for an imperfect reference standard affected the main conclusions of the meta‐analysis on the rK39 ICT, although the secondary analysis using an alternative reference standard in five studies tended to give a lower specificity estimate. This may be related to a lower Se of the reference standards included in this analysis, which would result in an underestimation of the Sp of the index test.
Finally, the findings seemed sufficiently homogeneous to allow for a meaningful summary estimate for the specificity of rK39 ICT as well as the Se and Sp of the latex agglutination test in urine. However, the sensitivity of rK39 ICT was different according to geographic region, and seems substantially lower in East Africa than in the Indian subcontinent. This finding is in line with an earlier meta‐analysis conducted by our team (Chappuis 2006) and with a multi‐country study conducted by WHO/TDR (data not included) (Cunningham 2012). Several hypotheses have been raised to explain this lower sensitivity in east Africa: a lower level of circulating antibodies, different age group, or parasitological differences (Bhattacharyya 2013; Harhay 2011).
Applicability of findings to the review question
Our review focused on clinical studies (phase III) of diagnostic accuracy, recruiting participants representative for the spectrum of patients encountered in clinical practice in whom a rapid diagnostic test for VL would be warranted. In summary, those patients would be in line with the WHO case definition and defined as “patients with a clinical suspicion of VL, that is, those who are febrile for more than two weeks and have splenomegaly, presenting at health services in endemic areas.” (Source: protocol). Patients who were previously treated for VL (non‐responders or relapsed cases), and those who had signs and symptoms of other forms of leishmaniasis, such as post kala‐azar dermal leishmaniasis (PKDL) were excluded. All clinical studies included in the review clearly corresponded with these inclusion criteria. Nevertheless, the settings of these clinical studies varied: some were conducted in tertiary care centres, and some in smaller hospitals of an intermediary level. Few studies recruited patients at the first‐contact point, the most peripheral level of the health service, ie health centres or health posts. Although the level of health service leads to variable prior probability of disease in the study sample, and as such is of influence in diagnostic accuracy studies, we believe that our findings are applicable across the several levels of the health system as an analysis of heterogeneity according to disease prevalence did not reveal any difference in the Se or Sp estimates.
Our review does not apply to HIV‐positive patients, and caution should be taken in areas with high HIV‐prevalence. Because HIV‐status is known to influence diagnostic accuracy (ter Horst 2009 ‐ HIV neg; ter Horst 2009 ‐ HIV pos; Alvar 2008), we originally intended to analyse the accuracy of the RDTs in HIV‐positive and HIV‐negative patients separately. Unfortunately, the number of included studies of HIV‐positive people was too low (n = 3) for a separate analysis or a comparison. Furthermore, the information from the Mediterranean region and from Latin America was too limited to draw robust conclusions. Finally, it was not possible to precisely evaluate the importance of the type of sample (serum, plasma or blood; fresh or frozen urine) or the manufacturer or version of a certain test, because these parameters did not vary enough among the included studies. In particular, Cunningham et al. pointed to important variations of sensitivity in a head‐to‐head comparison of five brands of RDT where two tests based on rK16 antigen performed substantially less well in east Africa and Brazil (Cunningham 2012).
In summary, we conclude that for current practice of VL control, there is one rapid diagnostic test, the rK39 ICT, which has been sufficiently evaluated, showing high sensitivity and specificity on average, but with a notably lower sensitivity in east Africa compared to the Indian subcontinent. In the Indian subcontinent, the rK39 ICT clearly complies with the normative requirements put forward before, of a sensitivity above 95% and a specificity above 90% (Boelaert 2007). In east Africa, the sensitivity of the rK39 ICT does not comply with these criteria, and can therefore not be used as a stand‐alone test to reliably rule out VL in a patient who is suspected to have VL. This does not mean that the use of an rK39 ICT is precluded, but it should be embedded in a test algorithm with a clear instruction on what to do in case of a negative rK39 ICT (second test, referral, come back after two weeks for repeat testing or other).
For the latex agglutination test, although there were only six studies included in the meta‐analysis, we can conclude that the low sensitivity makes it unsuitable for use in current clinical practice, though it does not preclude that its performance could be further improved in the future.
No recommendations can be made concerning the FAST test, the rK26 ICT or the rKE16 ICT because of paucity of evidence.
Authors' conclusions
Implications for practice.
The rK39 ICT can be clearly recommended as a rapid diagnostic test for use in clinical care in the Indian subcontinent in patients with febrile splenomegaly and no previous history of VL.
In east Africa, the rK39 ICT can replace the DAT and other diagnostic tests as the basis for the therapeutic decision in patients suspected to have VL. However, because of the low sensitivity of the rK39 ICT, a negative test result does not rule out VL. Therefore, additional actions are needed in case of a negative result (eg second or different test, referral, coming back after two weeks for repeat testing or other). Too little evidence has accrued so far from other regions to make specific recommendations.
It is important to remember that this antibody‐based test has to be used in combination with a clinical case definition (fever of more than two weeks, splenomegaly and no previous history of VL) that needs to be strictly adhered to.
The sensitivity of the KAtex latex agglutination test in urine is too low to recommend it for standard practice guidelines for detection of VL in similar settings.
Implications for research.
Although this review yielded solid evidence for recommending the rK39 ICT for clinical practice in the Indian subcontinent and East Africa, it would be helpful to conduct in the future more head‐to‐head comparisons of available tests by region and by test format, as was done by Cunningham et al. (Cunningham 2012), on a regular basis, as this would inform policy makers for their purchasing policies and quality assurance.
Several rapid diagnostic tests such as the latex agglutination test and the FAST should be further developed and evaluated before any recommendation on wide‐scale use can be made. Furthermore, better tests are needed, ie tests that are more specific for the acute stage of disease and more sensitive in all geographic regions, especially east Africa.
Last but not least, phase III clinical prospective studies are an essential element in the research and development process of a diagnostic device because they provide the basis for clinical guidelines. More studies of this type are needed, although in the case of VL, they are more complex in terms of reference standards. In addition, further standardization of evaluation methodology and a broader awareness of the QUADAS‐2 and STARD criteria by investigators would be beneficial for the quality of future evidence in this field.
Acknowledgements
We thank Anne‐Marie Trooskens from the Institute of Tropical Medicine in Antwerp for expert administrative support and Vittoria Lutje, Information Specialist of the Cochrane Infectious Diseases Group for the literature searches.
The editorial base of the Cochrane Infectious Diseases Group (CIDG) is funded by UKaid from the UK Government for the benefit of low‐ and middle‐income countries.
Appendices
Appendix 1. Statistical methods
A short summary of some less common statistical methods used in this review is given below. A more extensive description of the model used to correct for imperfect reference standards can be found in Menten 2013.
1) Latent class analysis (LCA)
LCA is a modelling technique that can be used in situations in which there is no good reference standard. It assumes that the true disease status in a study population is unknown (or latent). The LCA model estimates the sensitivity and specificity of a set of diagnostic tests (A, B, C, …) on the basis of observed frequencies in test patterns (ABC+++, ABC++‐, ABC+‐+,…). As such, the LCA model provides a model‐based estimate of the gold standard classification; ie the best way to group study participants in diseased or non‐diseased.
The basic latent class model assumes that the observed variables are conditionally independent. This means that there should be no associations between the results of the diagnostic tests within each category of the latent variable (disease status). If this assumption does not hold, more advanced techniques (eg based on Bayesian statistical methodology) can be used. To be selected for this review, studies using LCA had to assess the conditional independence assumption between the diagnostic tests, and if conditional dependence was expected, they had to use appropriate statistical methods to take this into account. If a study was selected, the sensitivity and specificity estimates derived from the final LCA model were included in this review.
More information can be found in Hui 1980, Black 2002, Branscum 2005, and Baughman 2008 among other references.
2) The complementary Log‐Log function
In the bivariate model a "link" function g(y) is used to allow the use of the Normal (Gaussian) distribution to model the underlying study‐specific sensitivity (Se) and specificity (Sp) of each study included in the meta‐analysis. The standard link function used in the bivariate model is the logit link:
g(y) = log (y/(1‐y))
An alternative link function is the complementary log log (cloglog) link:
g(y) = log(‐log(1‐y))
Both link functions transform Se and Sp, which are in the interval [0,1], to any real number between minus infinity and plus infinity.
The advantage of the cloglog link with our data is that it approaches infinity less quickly when y approaches 1 and consequently it mitigates the influence of studies that report 100% Se or Sp. This also reduces the inflation of the random‐effects standard deviations as is apparent from comparison of Figure 6 and Figure 10. With the logit link, the prediction region extends to below the line of no diagnostic value (Se + Sp < 1), while the study which reports the lowest diagnostic value has Se = 0.75 and Sp = 0.70 (Figure 10). On the other hand, some observed data with high Se and Sp are not contained within the prediction region. The prediction region of the model with the cloglog link contains all observed data points, while not extending far beyond the studies with lowest observed Se and Sp (Figure 6). This is reflected in a lower deviance information criterion (DIC), a measure of model fit, for the cloglog model formulation compared to the logit formulation. The model with the lowest DIC shows the best fit to the data.
3) WinBUGS code for the primary model
WinBUGS is a statistical software for Bayesian analysis using Markov chain Monte Carlo methods. WinBUGS (or its recent open‐source version OpenBUGS) provides a flexible Bayesian framework for model fitting. It can be used to fit both the bivariate and HSROC models.
Below is the code to fit the basic bivariate model, allowing for data from studies that use a reference standard and for studies that use latent class analysis.
Assuming there are N=N1+N2 studies:
for the N1 studies that use a reference standard, the data extracted is:
NDiseased[i] and NNotDiseased[i]: the (true) number of diseased and non‐diseased subjects in study i
TP[i], TN[i]: the number of true positives and true negatives in study i
for the N2 studies that use latent class analysis, the data extracted is:
Y1[i] and Y2[i]: the estimates of g(Se) and g(Sp) obtained from the results of the LCA reported in the publication for study i
W1[i] and W2[i]: the estimates of the standard error of Y1[i] and Y2[i] for study i
with g() the link function (logit or cloglog)
The code is as follows:
model{
# Binomials for Studies that Use a Reference Standard
# (where the reference standard is presumed to be perfect)
for(i in 1:N1){
TP[i] ˜ dbin(SE[i],NDiseased[i])
TN[i] ˜ dbin(SP[i],NNotDiseased[i])
}
# Normals for Studies that Use LCA
for(i in 1:N2){
Y1[i] ˜ dnorm(alpha[i+N1,1],W1[i])
Y2[i] ˜ dnorm(alpha[i+N1,2],W2[i])
}
# Bivariate normals for g(Se) and g(Sp)
for(i in 1:N){
# Implement the link function g()
# If logit is used
logit(SE[i]) <‐ alpha[i,1]
logit(SP[i]) <‐ alpha[i,2]
OR
# If cloglog is used
SE[i] <‐ 1‐exp(‐exp(alpha[i,1]))
SP[i] <‐ 1‐exp(‐exp(alpha[i,2]))
# Specify the bivariate normal
alpha[i,1:2] ˜ dmnorm(mu[],R[,])
}
# Specify Non‐Informative Priors
# for means:
mu[1] ˜ dnorm(0.0,.37)
mu[2] ˜ dnorm(0.0,.37)
# for variance‐covariance matrix
R[1:2,1:2] ˜ dwish(Omega[,], 2) }
Note: Prior for Omega are provided as data:
Omega = matrix(c(.001,0,0,.001),nrow=2,byrow=T)
Appendix 2. Search strategy
Detailed search strategy
| Search set | MEDLINE | EMBASE |
| 1 | Exp Leishmaniasis, visceral [MeSH] | Exp Visceral leishmaniasis [Emtree] |
| 2 | Exp Leishmania donovani [MeSH] | Exp Leishmania donovani [Emtree] |
| 3 | Exp Leishmania infantum [MeSH] | Exp Leishmania infantum [Emtree] |
| 4 | Kala azar OR kala‐azar ti, ab | Exp Leishmania chagasi [Emtree] |
| 5 | Leishmania chagasi ti, ab | Kala azar OR kala‐azar ti, ab |
| 6 | Visceral leishmania* ti, ab | Visceral leishmania* ti, ab |
| 7 | 1‐6/OR | 1‐6/OR |
| 8 | Rapid diagnostic test* ti, ab | Rapid diagnostic test* ti, ab |
| 9 | RDT ti, ab | RDT ti, ab |
| 10 | Antigen* detect* ti, ab | Antigen detection [Emtree] |
| 11 | Antibod* detect* ti, ab | Antibody detection [Emtree] |
| 12 | Latex Fixation Tests [MeSH] | Latex agglutination test [Emtree] |
| 13 | Lateral flow test ti, ab | Lateral flow test ti, ab |
| 14 | Enzyme‐Linked Immunosorbent Assay [MeSH] | Latex fixation test* ti, ab |
| 15 | Serodiagnostic test* ti, ab | Enzyme‐linked immunosorbent assay [Emtree] |
| 16 | ELISA ti, ab | ELISA ti, ab |
| 17 | Direct agglutination test* ti, ab | Agglutination test [Emtree] |
| 18 | Dipstick* ti, ab | Dipstick* ti, ab |
| 19 | K39 antigen, Leishmania [Substance Name] | Test strip [Emtree] |
| 20 | K26 antigen, Leishmania [Substance Name] | K39 Or rK39 ti, ab |
| 21 | K39 Or rK39 ti, ab | K26 or rK26 ti, ab |
| 22 | Strip test* ti, ab | Serodiagnosis [Emtree] |
| 23 | Reagent kits, diagnostic [MeSH] | Immunoblotting [Emtree] |
| 24 | Immunoblotting [MeSH] | 8‐12/OR |
| 25 | Serological tests [MeSH] | 7 AND 24 |
| 26 | 8‐25/OR | Limit 25 to humans |
| 27 | 7 AND 26 | |
| 28 | Limit 27 to humans | |
| 27 | ||
| 28 |
Appendix 3. Interpretation of the clinical relevance of the findings of this review ‐ predictive values and likelihood ratios
What follows is an interpretation of the clinical relevance of the findings of this review regarding the rK39 immunochromatographic test (ICT) when it is used to diagnose visceral leishmaniasis (VL) among patients with febrile splenomegaly and no previous history of VL.
Indian subcontinent
When the rK39 ICT is used in the Indian subcontinent, in a setting where the prior probability of VL among clinical suspects is 40%, which is typically seen in a peripheral health centre in an endemic area, the positive predictive value of the test is 87%. This means that out of 100 patients with a positive rK39 result, 87 would have VL (true positive result) and 13 would have another disease (false positive). The negative predictive value is 98%, meaning that out of 100 patients with a negative rK39 ICT result, 98 would have another disease (true negative) and 2 would have VL (false negative).
When the same test is used in a setting with a prior probability of VL of 60%, which is more typical for a referral centre in an endemic area, the positive predictive value is 94% and the negative predictive value is 95%.
A likelihood ratio is another way of expressing how informative a diagnostic test is: it indicates to what extent the rK39 ICT result changes the odds that a patient has VL. The likelihood ratio of a positive rK39 ICT result is 9.90, and the likelihood ratio of a negative test result is 0.03. This means that in the Indian subcontinent, a positive rK39 ICT result is a strong argument in favour of VL (ruling in) and that a negative rK39 ICT result is a strong argument against VL (ruling out).
East Africa
When the rK39 ICT is used in east Africa, in a setting where the prior probability of VL is 40%, which is typically seen in a peripheral health centre in an endemic area, the positive predictive value of the test is 86%. This means that out of 100 patients with a positive rK39 ICT result, 86 would have VL (true positive result) and 14 would have another disease (false positive). The negative predictive value is 90%, meaning that out of 100 patients with a negative rK39 ICT result, 90 would have another disease (true negative) and 10 would have VL (false negative).
When the same test is used in a setting with a prior probability of VL of 60%, which is more typical for a referral centre in an endemic area, the positive predictive value is 93% and the negative predictive value is 81%.
In east Africa, the likelihood ratio of a positive rK39 ICT result is 9.58, and the likelihood of a negative rk39 ICT result is 0.16. This means that a positive rK39 ICT result is strong argument in favour of VL (ruling in), and that a negative rK39 ICT result is not an absolute argument against VL (does not allow to rule out VL completely).
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 rK39 immunochromatographic test | 26 | 5544 |
| 2 KAtex | 9 | 1848 |
| 3 FAST | 2 | 148 |
| 4 rK26 immunochromatographic test | 1 | 352 |
| 5 rK39 Primary Analysis | 16 | 3574 |
| 6 rKE16 immunochromatographic test | 1 | 219 |
1. Test.
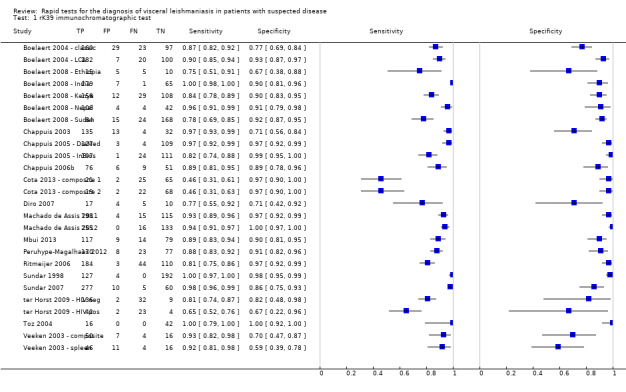
rK39 immunochromatographic test.
2. Test.
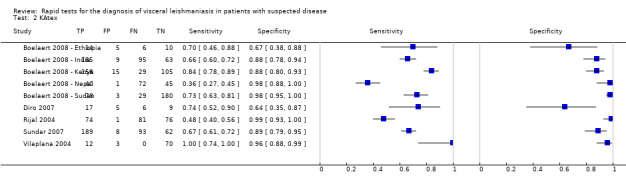
KAtex.
3. Test.
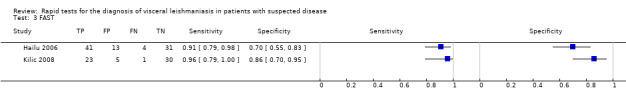
FAST.
4. Test.
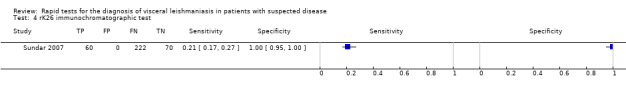
rK26 immunochromatographic test.
5. Test.
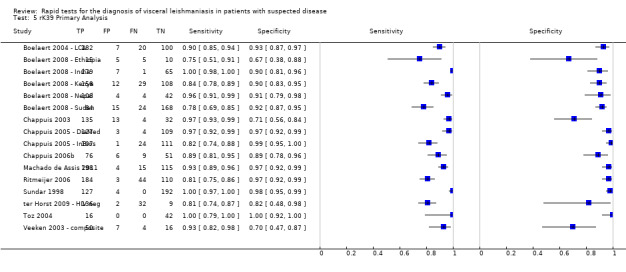
rK39 Primary Analysis.
6. Test.

rKE16 immunochromatographic test.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boelaert 2004 ‐ classic.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 310 Age (reported for 181 new VL cases): median 25, interquartile range 13‐36 Sex (reported for 181 new VL cases): male:female ratio 1.7:1 Presenting signs and symptoms: fever of 14 days or more and splenomegaly Frequency of VL: 59% HIV: not reported Clinical setting: tertiary care centre (B.P. Koirala Institute of Health Sciences in Dharan) Country: Nepal Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test Brand: InSure Rapid test for Visceral Leishmaniasis, InBios International, Washington, USA Sample: serum |
||
| Target condition and reference standard(s) |
Target condition: clinical VL (3/310 participants were relapse cases) Sample: bone marrow; if negative, possible and no malaria: spleen Technique: direct smear (Giemsa stain) Definition of VL: bone marrow or spleen parasitology positive Definition of non‐VL: bone marrow negative, and spleen negative or spleen aspirate not done Reference standard category: parasitology including spleen aspirate ‐ no serology |
||
| Flow and timing | one participant was excluded because of a missing serum sample | ||
| Comparative | |||
| Notes | Same study as Boelaert 2004 ‐ LCA but different analysis | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Boelaert 2004 ‐ LCA.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 310 Age (reported for 181 new VL cases): median 25, interquartile range 13‐36 Sex (reported for 181 new VL cases): male:female ratio 1.7:1 Presenting signs and symptoms: fever of 14 days or more and splenomegaly Frequency of VL: 59% HIV: not reported Clinical setting: tertiary care centre (B.P. Koirala Institute of Health Sciences in Dharan) Country: Nepal EndemicLeishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test Brand: InSure Rapid test for Visceral Leishmaniasis, InBios International, Washington, USA Sample: serum |
||
| Target condition and reference standard(s) |
Target condition: clinical VL (3/310 participants were relapse cases) Approach: latent class analysis Tests included in latent class analysis: rK39 immunochromatographic test, pancytopenia, IFAT, DAT, formol‐gel test, and parasitology (direct smear; Giemsa stain) of bone marrow or spleen samples |
||
| Flow and timing | One participant was excluded because of a missing serum sample | ||
| Comparative | |||
| Notes | Same study as Boelaert 2004 ‐ classic but different analysis | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Boelaert 2008 ‐ Ethiopia.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 38 Age: median 25 years; range 16‐49. No children ≤ 12 years Sex: 95% men Presenting signs and symptoms: fever of 2 weeks or more, and splenomegaly or lymphadenopathy or both Pregnant women were excluded. Patients with a thick‐film‐positive malaria episode were excluded. Estimated frequency of VL: 57% HIV: not tested Clinical setting: university hospital (Kahsay Abera Hospital in Humera and Gondar College of Medical Sciences) Country: Ethiopia Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; Sample: serum Type: latex agglutination test in urine; Brand: KAtex, Kalon Biological Ltd, Guildford, UK; Sample: fresh urine |
||
| Target condition and reference standard(s) |
Target condition: clinical VL Approach: latent class analysis Tests included in latent class analysis: rK39 immunochromatographic test, latex agglutination test, DAT, and parasitology (culture and direct smear (Giemsa stain)) of lymph node, bone marrow or spleen sample |
||
| Flow and timing | Three participants were excluded because of missing data. | ||
| Comparative | |||
| Notes | Same study as Diro 2007 but different analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Boelaert 2008 ‐ India.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 352 Age (computed on 260/352 participants): median 15 years; range 2‐65; 44% of the study population was ≤ 12 years old Sex (computed on 260/352 participants): 55% men Presenting signs and symptoms: fever (more or less than 2 weeks), and splenomegaly or lymphadenopathy or both No children < 2 years old; no pregnant women Estimated frequency of VL: 80% HIV: not reported Clinical setting: research centre (Kala‐Azar Medical Research and Training Centre in Muzaffarpur) Country: India Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; Sample: serum Type: latex agglutination test in urine; Brand: KAtex, Kalon Biological Ltd, Guildford, UK; Sample: fresh urine |
||
| Target condition and reference standard(s) |
Target condition: clinical VL Approach: latent class analysis Tests included in latent class analysis: rK39 immunochromatographic test, latex agglutination test, DAT, and parasitology (direct smear (Giemsa stain)) of lymph node, bone marrow or spleen sample |
||
| Flow and timing | No withdrawals | ||
| Comparative | |||
| Notes | Same study as Sundar 2007 but different analysis | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Boelaert 2008 ‐ Kenya.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 308 Age: median 13 years; range 1‐75; 45% of the study population was ≤12 years old Sex: 65% men Presenting signs and symptoms: fever for 2 weeks or more, and splenomegaly or lymphadenopathy or both; no children < 2 years old; no pregnant women; no patients with a thick‐film‐positive malaria episode Estimated frequency of VL: 61% HIV: not reported Clinical setting: hospital (Kabarnet hospital in Baringo district) Country: Kenya Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; Sample: serum Type: latex agglutination test in urine; Brand: KAtex, Kalon Biological Ltd, Guildford, UK; Sample: fresh urine |
||
| Target condition and reference standard(s) |
Target condition: clinical VL Approach: latent class analysis Tests included in latent class analysis: rK39 immunochromatographic test, latex agglutination test, DAT, and parasitology (direct smear (Giemsa stain)) of lymph node, bone marrow or spleen sample |
||
| Flow and timing | One participant was excluded because of missing data. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Boelaert 2008 ‐ Nepal.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 158 Age: median 23 years; range 2‐68; 25% of the study population was ≤ 12 years old Sex: 59% men Presenting signs and symptoms: fever for 2 weeks or more, and splenomegaly or lymphadenopathy or both; No children < 2 years old; no pregnant women Estimated frequency of VL: 71% HIV: not reported Clinical setting: tertiary care centre (B.P. Koirala Institute of Health Sciences in Dharan) Country: Nepal Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; Sample: serum Type: latex agglutination test in urine; Brand: KAtex, Kalon Biological Ltd, Guildford, UK; Sample: fresh urine |
||
| Target condition and reference standard(s) |
Target condition: clinical VL Approach: latent class analysis Tests included in latent class analysis: rK39 immunochromatographic test, latex agglutination test, DAT, and parasitology (direct smear (Giemsa stain)) of lymph node, bone marrow or spleen sample |
||
| Flow and timing | No withdrawals | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Boelaert 2008 ‐ Sudan.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 294 Age: median 20 years; range 2‐72; 36% of the study population was ≤12 years old Sex: 64% men Presenting signs and symptoms: fever for 2 weeks or more, and splenomegaly or lymphadenopathy or both No children < 2 years old; no pregnant women; no patients with a thick‐film‐positive malaria episode Estimated frequency of VL: 37% HIV: not reported Clinical setting: two health centres (Umalkhair and Tabarakelleh) Country: Sudan Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; Sample: serum Type: latex agglutination test in urine; Brand: KAtex, Kalon Biological Ltd, Guildford, UK; Sample: fresh urine |
||
| Target condition and reference standard(s) |
Target condition: clinical VL Approach: latent class analysis Tests included in latent class analysis: rK39 immunochromatographic test, latex agglutination, DAT, and parasitology (direct smear (Giemsa stain)) of lymph node, bone marrow or spleen sample |
||
| Flow and timing | Three participants were excluded because of missing data. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Chappuis 2003.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 195 Age (data available for 184 included participants): mean 23 years Sex (data available for 184 included participants): 59% men Presenting signs and symptoms: fever for 2 weeks or more and clinical splenomegaly Frequency of VL: 76% HIV: 0% Clinical setting: tertiary care centre (B.P. Koirala Institute of Health Sciences in Dharan) Country: Nepal Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: InSure Rapid Test for Visceral Leishmaniasis, InBios Interational, Washington, USA; Sample: serum | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: bone marrow; if bone marrow negative and spleen aspiration possible: spleen Technique: direct smear examination Reference standard category: parasitology including spleen aspirate ‐ no serology |
||
| Flow and timing | Eleven participants with an uncertain diagnosis were excluded. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Chappuis 2005 ‐ DiaMed.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 255 Age (among 131 VL patients): mean age 14 years (standard deviation 11) Age (among 112 non‐VL patients): mean age 16 years (standard deviation 13) Sex (among 131 VL patients): 73% men; Sex (among 112 non‐VL patients): 53% men Presenting signs and symptoms: fever for 2 weeks or more and either clinical splenomegaly or wasting syndrome Frequency of VL: 54% 6/112 non‐VL patients had previous history of treatment for VL HIV: not reported Clinical setting: a 120‐bed district hospital (Amudat Hospital) Country: Uganda Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test (dual test for visceral leishmaniasis and malaria); Brand: DUAL‐IT L/M dipstick, Diamed AG, Switzerland; Sample: blood | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology of spleen sample (direct smear, Giemsa stain), PCR of spleen sample, serology (DAT), and response to VL therapy Definition of VL: (1) positive spleen aspirate in two laboratories (by microscopic reading in local or regional laboratory and by PCR in reference laboratory) or (2) DAT >1:12800 and response to VL therapy Definition of non‐VL: (1) DAT titres of <1:1600; or (2) borderline DAT titres with negative spleen aspirate in two laboratories with no diagnosis of VL made during the following 6 months Reference standard category: combination of parasitology and serology |
||
| Flow and timing | Twelve participants were excluded: 2 persons died and 10 had discrepant results for the reference standard | ||
| Comparative | |||
| Notes | Same patient population as Chappuis 2005 ‐ InBios but different brand of rK39 immunochromatographic test. Considered as two studies in meta‐analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Chappuis 2005 ‐ InBios.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 255 Age (among 131 VL patients): mean age 14 years (standard deviation 11) Age (among 112 non‐VL patients): mean age 16 years (standard deviation 13) Sex (among 131 VL patients): 73% men; Sex (among 112 non‐VL patients): 53% men Presenting signs and symptoms: fever for 2 weeks or more and either clinical splenomegaly or wasting syndrome Frequency of VL: 54% 6/112 non‐VL patients had previous history of treatment for VL HIV: not reported Clinical setting: a 120‐bed district hospital (Amudat Hospital) Country: Uganda Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: InBios International, Washington, USA; Sample: plasma | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology of spleen sample (direct smear, Giemsa stain), PCR of spleen sample, serology (DAT), and response to VL therapy Definition of VL: (1) positive spleen aspirate in two laboratories (by microscopic reading in local or regional laboratory and by PCR in reference laboratory) or (2) DAT >1:12800 and response to VL therapy Definition of non‐VL: (1) DAT titres of <1:1600; or (2) borderline DAT titres with negative spleen aspirate in two laboratories with no diagnosis of VL made during the following 6 months Reference standard category: combination of parasitology and serology |
||
| Flow and timing | Twelve participants were excluded: 2 persons died and 10 had discrepant results for the reference standard | ||
| Comparative | |||
| Notes | Same patient population as Chappuis 2005 ‐ DiaMed but different brand of rK39 immunochromatographic test. Considered as two studies in meta‐analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Chappuis 2006b.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 154 Age (among 85 VL patients): mean age 22 years (standard deviation 13) Age (among 57 non‐VL patients): mean age 26 (standard deviation 15) Sex (among 85 VL patients): 57% men Age (among 57 non‐VL patients): 65% men Presenting signs and symptoms: fever for 2 weeks or more and clinical splenomegaly All patients received malaria treatment 12/142 participants had previous history of VL Frequency of VL: 60% HIV: not tested Clinical setting: 15‐bed first‐referral government hospital (Rangeli District Hospital) Country: Nepal Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: InSure One‐Step Rapid Test for Visceral Leishmaniasis, InBios International, Washington, USA; Sample: serum | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology of bone marrow sample (direct smear) and serology (DAT) and response to therapy Definition of VL: (1) positive bone marrow aspirate during initial evaluation or follow‐up; or (2) DAT titre ≥1:3200 and absence of response to antimalarial treatment but successful response to anti‐leishmanial therapy Definition of non‐VL: negative bone marrow aspirate with (1) DAT titre < 1:3200 or with (2) positive DAT but a definite cure with non‐VL specific therapy Reference standard category: combination of parasitology and serology |
||
| Flow and timing | Twelve patients were excluded: 4 with incomplete diagnostic work‐up and 8 with an uncertain diagnosis | ||
| Comparative | |||
| Notes | Exclusion of patients with previous history of VL had no significant effect on Se and Sp estimates (data not reported). Data on accuracy of latex agglutination test were incomplete and not included. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Cota 2013 ‐ composite 1.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 113 Age (VL cases): mean 41.0 years (standard deviation 10.8) Age (non‐VL cases): mean 40.0 years (standard deviation 9.8) Sex (VL cases): 24% men (11 men out of 46) Sex (non‐VL cases): 42% men (28 men out of 67) Presenting signs and symptoms: clinical suspicion of VL, ie more than 14 days of fever or splenomegaly or cytopenia Previous VL diagnosis: 24 out of 113 participants had a previous history of VL Frequency of VL: 41% HIV: 100% Clinical setting: HIV cohort in reference hospital in Belo Horizonte (Eduardo de Menezes Hospital, Fundação Hospitalar do Estado de Minos Gerais) Country: Brazil Endemic Leishmania species:L. infantum |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; sample: serum | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Samples: bone marrow aspirate and venous blood Techniques: direct smear and culture of bone marrow aspirate; PCR on peripheral blood; and three serological tests: index test, indirect fluorescent antibody test (IFI, Bio‐Manguinhos) and direct agglutination test (prototype kit) Definition of VL and non‐VL: decided after clinical follow‐up by an adjudication committee with four members. They evaluated results of all tests available, including tests performed for other diagnostic possibilities Reference standard category: parasitology and serology |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Another estimate of Se and Sp based on latent class analysis in the same study population was not included because there was no information about the assessment of the conditional independence assumption (explained in the Criteria for considering studies for this review and in Appendix 1). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Cota 2013 ‐ composite 2.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 113 Age (VL cases): mean 41.0 years (standard deviation 10.8) Age (non‐VL cases): mean 40.0 years (standard deviation 9.8) Sex (VL cases): 24% men (11 men out of 46) Sex (non‐VL cases): 42% men (28 men out of 67) Presenting signs and symptoms: clinical suspicion of VL, ie more than 14 days of fever or splenomegaly or cytopenia Previous VL diagnosis: 24 out of 113 participants had a previous history of VL Frequency of VL: 37% HIV: 100% Clinical setting: HIV cohort in reference hospital in Belo Horizonte (Eduardo de Menezes Hospital, Fundação Hospitalar do Estado de Minos Gerais) Country: Brazil Endemic Leishmania species:L. infantum |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; sample: serum | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Samples: bone marrow aspirate Techniques: direct smear and culture Reference standard category: parasitology not including spleen aspirate ‐ no serology |
||
| Flow and timing | Two patients were not included in the analysis because bone marrow aspiration was not done | ||
| Comparative | |||
| Notes | Another estimate of Se and Sp based on latent class analysis in the same study population was not included because there was no information about the assessment of the conditional independence assumption (explained in the Criteria for considering studies for this review and in Appendix 1). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Diro 2007.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 38 Age: median 25 years; range 16‐49. No children ≤ 12 years Sex: 95% men Presenting signs and symptoms: fever of 2 weeks or more, and splenomegaly or lymphadenopathy or both Pregnant women and people with a previous history of VL treatment were not included. Patients with a thick‐film‐positive malaria episode were excluded. Frequency of VL: 61% HIV: not tested Clinical setting: university hospital (Kahsay Abera Hospital in Humera and Gondar College of Medical Sciences) Country: Ethiopia Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; Sample: serum Type: Latex agglutination test in urine; Brand: KAtex, Kalon Biologicals Ltd, Guildford, UK; Sample: fresh urine |
||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: spleen or lymph node aspirate Technique: parasitology: culture or direct smear (Giemsa stain) Reference standard category: parasitology including spleen aspirate ‐ no serology |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Findings in context of active case detection were not included in this review. Same study as Boelaert 2008 ‐ Ethiopia but different analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Hailu 2006.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 103 Age: not reported Sex: not reported Presenting signs and symptoms: fever for 2 weeks or more and splenomegaly Not clear whether VL relapse suspects were excluded Frequency of VL: 51% (45/89) HIV: not tested Clinical setting: health centres in rural northern Ethiopia (Humera and Gondar) Country: Ethiopia Endemic Leishmania species:L. donovani |
||
| Index tests | Type: FAST; Brand: not applicable; Sample: not reported | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: lymph node or spleen Technique: direct smear (Giemsa stain) or culture Reference standard category: parasitology including spleen aspirate ‐ no serology |
||
| Flow and timing | Fourteen patients were excluded, probably because the FAST results could not be read under field conditions. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Kilic 2008.
| Study characteristics | |||
| Patient sampling | Consecutive enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 59 Age (data available for 24 VL patients): median age 7 years; 22/24 cases <14 years Sex: 71% men Presenting signs and symptoms: prolonged fever + splenomegaly or hepatomegaly + anaemia/pancytopenia Frequency of VL: 41% HIV: not reported Clinical setting: tertiary care centre Country: Turkey Endemic Leishmania species:L. infantum |
||
| Index tests | Type: FAST; Brand: not applicable; Sample: serum | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: bone marrow Technique: direct smear (Giemsa stain) and culture Reference standard category: parasitology not including spleen aspirate ‐ no serology |
||
| Flow and timing | No information about exclusion | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Machado de Assis 2011.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 332 Age: median 13 years; range 1 month ‐ 76 years Sex: 58% men Presenting signs and symptoms: fever + coming from endemic area + one of the following: splenomegaly, hepatomegaly, anaemia, leucopenia, and thrombocytopenia Frequency of VL: 64% HIV: people with known immunodeficiency were excluded Clinical setting: two university hospitals and two research centres in four different states Country: Brazil Endemic Leishmania species:L. infantum |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: IT‐LEISH, DiaMed Latino‐America S.A, Cressier sur Morat, Switzerland; Sample: blood | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: bone marrow Technique: culture or direct smear (Giemsa stain); at least 2 smears per patient Definition of VL: positive parasitological test Definition of non‐VL: negative parasitological test and firm diagnosis of another disease Reference standard category: parasitology not including spleen aspirate ‐ no serology |
||
| Flow and timing | People with missing data or uncertain diagnosis were not mentioned | ||
| Comparative | |||
| Notes | Same study as Machado de Assis 2012 but different analysis. Same study as Peruhype‐Magalhaes 2012 but different brand of index test. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Machado de Assis 2012.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 404 Age: median 13 years; range 1 month ‐ 77 years; standard deviation 17 years Sex: 58% men Presenting signs and symptoms: fever + coming from endemic area + one of the following: splenomegaly, hepatomegaly, anaemia, leucopenia, and thrombocytopaenia Frequency of VL: 67% HIV: people with known immunodeficiency were excluded Clinical setting: two university hospitals and two research centres in four different states Country: Brazil Endemic Leishmania species:L. infantum |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: IT‐LEISH, DiaMed Latino‐America S.A, Cressier sur Morat, Switzerland; Sample: finger prick sample of blood | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: bone marrow Approach: latent class analysis Tests included in latent class analysis: rK39 immunochromatographic test, rK39‐ELISA, IFAT, DAT, microscopy of bone marrow sample |
||
| Flow and timing | People with missing data or inconclusive test results were not mentioned | ||
| Comparative | |||
| Notes | Same study as Machado de Assis 2011 but different analysis. Same study as Peruhype‐Magalhaes 2012 but different brand of index test. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Mbui 2013.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL ≥ 5 years with suspected VL and presenting at outpatient department | ||
| Patient characteristics and setting |
Sample size: 249 (total number of participants) Age (VL cases): median 16 years (interquartile range 10‐25 years) Age (non‐VL cases): median 16.5 years (interquartile range 11‐25 years) Sex (VL cases): 66.4% men Sex (non‐VL cases): 79.6% men Presenting signs and symptoms: fever for 2 weeks or more + clinical splenomegaly + malaria ruled out by a negative rapid test (Paracheck) Frequency of VL: 59.8% HIV: 0.5% (195 participants were tested; 1 non‐VL case was HIV positive) Clinical setting: Kimalel Health Centre, Baringo district and Kacheliba Kala‐azar Treatment Centre, Pokot North district Country: Kenya Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test; Brand: DiaMed‐IT LEISH, DiaMed AG, Switzerland; Sample: serum Type: rKE16 immunochromatographic test; Brand: Signal KA, Span Diagnostics Ltd, India; Sample: serum |
||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: spleen aspirate Technique: direct smear examination (Giemsa stain) Definition of VL: positive splenic aspirate Definition of non‐VL: negative splenic aspirate Reference standard category: parasitology including spleen aspirate ‐ no serology |
||
| Flow and timing | Thirty participants were excluded: 27 had contra‐indications for spleen aspiration and 3 had an inconclusive smear reading | ||
| Comparative | |||
| Notes | Unclear if study population includes people with previous history of VL | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Peruhype‐Magalhaes 2012.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 278 Age: mean 12 years; range 1 month ‐ 77 years Sex: 58% (161/278) men Presenting signs and symptoms: fever + coming from endemic area + one of the following: splenomegaly, hepatomegaly, anaemia, leucopenia, and thrombocytopaenia Frequency of VL: 69% (193/278) HIV: people with known immunodeficiency were excluded Clinical setting: two university hospitals and two research centres in four different states Country: Brazil Endemic Leishmania species:L. infantum |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: Kalazar Detect, InBios International, Washington, USA; Sample: stored serum | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: bone marrow Technique: culture or direct smear (Giemsa stain); at least 2 smears per patient Definition of VL: positive parasitological test Definition of non‐VL: negative parasitological test and firm diagnosis of another disease Reference standard category: parasitology not including spleen aspirate ‐ no serology |
||
| Flow and timing | Unclear why there are 54 participants less than in Machado de Assis 2011 | ||
| Comparative | |||
| Notes | Same study population as Machado de Assis 2011 and Machado de Assis 2012. Evaluation of a new index test on stored serum samples. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
Rijal 2004.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 269 Age (155 VL patients): median age 23 (interquartile range 13‐26) Age (77 non‐VL patients): median age 20 (interquartile range 10‐30) Sex: not reported Presenting signs and symptoms: fever for 2 weeks or more and clinical splenomegaly Frequency of VL: 67% HIV: done but not reported; at least 3 HIV‐positive participants among non‐VL patients Clinical setting: tertiary care centre (B.P. Koirala Institute of Health Sciences in Dharan) Country: Nepal Endemic Leishmania species:L. donovani |
||
| Index tests | Type: latex agglutination test in urine; Brand: KAtex, Kalon Biological Ltd, Aldershot, UK; Sample: frozen urine | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology (direct smear, Giemsa stain) of bone marrow or spleen aspirate and serology (DAT) Definition of VL: parasitology positive Definition of non‐VL: parasitology negative and DAT ≤1:3200 Reference standard category: combination of parasitology and serology |
||
| Flow and timing | Thirty‐seven patients were not included: the urine samples of 8 patients were lost; 1 person left against medical advice; and 28 had an uncertain diagnosis (parasitology negative and DAT positive) | ||
| Comparative | |||
| Notes | Not clear whether VL relapse suspects were excluded | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
Ritmeijer 2006.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 356 Age: not reported Sex: not reported Presenting signs and symptoms: fever for more than 2 weeks + malaria ruled out + wasting + splenomegaly or lymphadenopathy Frequency of VL: 67% (228/341) HIV: not reported Clinical setting: three Kala‐azar treatment centres in eastern and southern Sudan, supported by Médecins sans frontières Country: Sudan Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: DiaMed‐IT Leish, DiaMed AG, Cressier sur Morat, Switzerland; Sample: blood | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology (direct smear, Giemsa stain) of lymph node or spleen aspirate sample and serology (DAT) Definition of VL: parasitology positive or DAT positive ≥1:6400 Definition of non‐VL: DAT≤1/400 or borderline DAT with negative aspirate Reference standard category: combination of parasitology and serology |
||
| Flow and timing | Fifteen patients were excluded because of an uncertain diagnosis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Sundar 1998.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 323 Age: mean age of VL patients: 25 years (standard error 1); among non‐VL patients, age is reported in subgroups: 73 patients with documented other infections: mean age 26 (standard error 2); 83 patients with presumed other infections: mean age 23 (standard error 2); and 40 patients without final diagnosis: mean age 25 years (standard error 2) Sex: 69% men Frequency of VL: 39% Presenting signs and symptoms: persistent fever with or without splenomegaly and suspected VL Some patients had been empirically treated with antimalarial or antibacterial agents before referral, without response HIV: not reported Clinical setting: kala‐azar units in Varanasi and Muzaffarpur, linked to research centre Country: India Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: not applicable;Sample: blood | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology (direct smear Giemsa stain) of spleen aspirate sample and diagnosis of other diseases Reference standard category: parasitology including spleen aspirate ‐ no serology |
||
| Flow and timing | Reference standard was conditional on result of index test. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
Sundar 2007.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 352 Age: median 15 years; interquartile range 2‐65 Sex: 55% men Presenting signs and symptoms: fever for 2 weeks or more and splenomegaly No children < 1 year old; no pregnant women; no people with past kala‐azar history Frequency of VL: 80% HIV: known HIV infection exclusion criterion Clinical setting: two research centres, in Muzaffarpur and Patna Country: India Endemic Leishmania species:L. donovani |
||
| Index tests |
Type: rK39 immunochromatographic test; Brand: InBios International, Washington, USA; Sample: serum or blood Type: rK26 immunochromatographic test; Brand: InBios International, Washington, USA; Sample: serum or blood Type: latex agglutination test in urine; Brand: KAtex, Kalon Biologicals, Aldershot, UK; Sample: fresh urine |
||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology (direct smear, Giemsa stain) of spleen aspirate sample and serology (DAT) and clinical diagnosis / follow‐up / response to therapy Definition of VL: (1) spleen parasitology positive; or (2) clinical diagnosis and DAT positive ≥ 1:3200 and response to anti‐leishmanial therapy Definition of non‐VL: spleen parasitology negative and alternative diagnosis and no VL during follow‐up of six months Reference standard category: combination of parasitology and serology |
||
| Flow and timing | No withdrawals explained. | ||
| Comparative | |||
| Notes | Same study as Boelaert 2008 ‐ India but different analysis and additional test (rK26 immunochromatographic test). Group of 100 healthy endemic controls not included in this review. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
ter Horst 2009 ‐ HIV neg.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 179 Age: reported for larger population (n = 699): mean age is 25,4 (standard deviation 10,1) Sex: reported for larger population (n = 699): ratio men:women is 15:1 Presenting signs and symptoms: clinical VL case definition (not specified) No patients with previous history of VL treatment. Frequency of VL: 94% HIV: all HIV negative Clinical setting: kala‐azar treatment centre supported by Médecins sans frontières (Kahsay Abera Hospital in Humera) Country: Ethiopia Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: DiaMed AG, Switzerland; Sample: blood | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology (direct smear, Giemsa stain) of spleen aspirate sample and serology (DAT) Definition of VL: (1) spleen parasitology positive; or (2) DAT positive ≥ 1:3200 Definition of non‐VL: (1) DAT ≤ 1:400; or (2) DAT≤ 1:3200 and spleen parasitology negative Reference standard category: combination of parasitology and serology |
||
| Flow and timing | Many patients with unknown HIV status were not included. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
ter Horst 2009 ‐ HIV pos.
| Study characteristics | |||
| Patient sampling | Consecutive and prospective enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 71 Age: reported for larger population (n = 699): mean age is 25.4 (standard deviation 10.1) Sex: reported for larger population (n = 699): ratio men:women is 15:1 Presenting signs and symptoms: clinical VL case definition (not specified) No patients with previous history of VL treatment. Frequency of VL: 92% HIV: all HIV positive Clinical setting: kala‐azar treatment centre supported by Médecins sans frontières (Kahsay Abera Hospital in Humera) Country: Ethiopia Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: DiaMed AG, Switzerland; Sample: blood | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology (direct smear, Giemsa stain) of spleen aspirate sample and serology (DAT) Definition of VL: (1) spleen parasitology positive; or (2) DAT positive ≥ 1:3200 Definition of non‐VL: (1) DAT ≤ 1:400; or (2) DAT≤ 1:3200 and spleen parasitology negative Reference standard category: combination of parasitology and serology |
||
| Flow and timing | Many patients with unknown HIV status were not included. | ||
| Comparative | |||
| Notes | Not included in meta‐analysis because this is the only evaluation of the rK39 immunochromatographic test in a population of HIV‐positive patients. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
Toz 2004.
| Study characteristics | |||
| Patient sampling | Consecutive enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 58 Age: only reported for 13 cases with all tests positive: 12 children between 1 and 15 years and 1 adult of 48 years Sex: not reported Frequency of VL: 28% Presenting signs and symptoms: ≥1 of the following signs: fever, anaemia, thrombocytopenia, leucopenia, splenomegaly, hepatomegaly, and weight loss HIV: not reported Clinical setting: various regional hospitals Country: Turkey Endemic Leishmania species:L. infantum |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: InBios International, Washington, USA; Sample: serum or plasma | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology (direct smear, Giemsa stain) of lymph node or bone marrow aspirate sample and serology (IFAT) Definition of VL: (1) lymph node or bone marrow parasitology positive; or (2) IFAT positive ≥ 1:128 Definition of non‐VL: parasitology and IFAT negative Reference standard category: combination of parasitology and serology |
||
| Flow and timing | No information about excluded patients. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Veeken 2003 ‐ composite.
| Study characteristics | |||
| Patient sampling | Retrospective selection of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 77 Age: median 11; range 4‐66 years Sex: 53% men Presenting signs and symptoms: fever, and splenomegaly or wasting Frequency of VL: 70% HIV: not reported Clinical setting: kala‐azar treatment centre at Um el Kher supported by Médecins sans frontières Country: Sudan Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: Amrad ICT, Brookdale, Australia; Sample: serum | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Combination of parasitology (direct smear, Giemsa stain) of spleen aspirate sample and serology (DAT) Definition of VL: spleen parasitology positive or DAT positive ≥ 1:6400 Definition of non‐VL: all other patients Reference standard category: combination of parasitology and serology |
||
| Flow and timing | Only people with stored serum and available results of DAT and parasitological test of spleen aspirate were included. According to the protocol by Médecins sans frontières, splenic aspiration was done in patients with suspected VL who were critically ill and in those with borderline DAT titres. | ||
| Comparative | |||
| Notes | Same study as Veeken 2003 ‐ spleen but different analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| High | |||
Veeken 2003 ‐ spleen.
| Study characteristics | |||
| Patient sampling | Retrospective selection of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 77 Age: median 11; range 4‐66 years Sex: 53% men Presenting signs and symptoms: fever, and splenomegaly or wasting Frequency of VL: 65% HIV: not reported Clinical setting: kala‐azar treatment centre at Um el Kher supported by Médecins sans frontières Country: Sudan Endemic Leishmania species:L. donovani |
||
| Index tests | Type: rK39 immunochromatographic test; Brand: Amrad ICT, Brookdale, Australia; Sample: serum | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: spleen Technique: parasitology (direct smear, Giemsa stain) Reference standard category: parasitology including spleen aspirate ‐ no serology |
||
| Flow and timing | Only people with stored serum and available results of DAT and parasitological test of spleen aspirate were included. According to the protocol by Médecins sans frontières, splenic aspiration was done in patients with suspected VL who were critically ill and in those with borderline DAT titres. | ||
| Comparative | |||
| Notes | Same study as Veeken 2003 ‐ composite but different analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| High | |||
Vilaplana 2004.
| Study characteristics | |||
| Patient sampling | Consecutive enrolment of patients with suspected VL | ||
| Patient characteristics and setting |
Sample size: 89 Age: not reported Sex: not reported Presenting signs and symptoms: not specified (suggestive clinical signs and symptoms of VL) Frequency of VL: 14% (12/85) HIV: 100% of cases and 91% of non‐cases Clinical setting: not reported Country: Spain Endemic Leishmania species:L. infantum |
||
| Index tests | Type: latex agglutination test in urine; Brand: KAtex, Kalon Biological Ltd, Aldershot, UK | ||
| Target condition and reference standard(s) |
Target condition: clinical VL Sample: bone marrow Technique: parasitology: culture or direct smear (Giemsa stain) Reference standard category: parasitology not including spleen aspirate ‐ no serology |
||
| Flow and timing | Three patients are not included in the analysis | ||
| Comparative | |||
| Notes | Not included in meta‐analysis because only evaluation of latex agglutination test in population with mainly HIV‐positive patients. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
DAT: direct agglutination test ELISA: enzyme‐linked immuno‐sorbent assay FAST: fast agglutination screening test IFAT: indirect fluorescence antibody test LCA: latent class analysis PCR: polymerase chain reaction Se: sensitivity Sp: specificity VL: visceral leishmaniasis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abeijon 2013 | index test is not a rapid test |
| Ahsan 2010 | not phase III diagnostic accuracy study |
| Akhoundi 2010 | not phase III diagnostic accuracy study |
| Al‐Nahhas 2003 | target condition is not clinical visceral leishmaniasis |
| Al‐Nahhas 2008 | not phase III diagnostic accuracy study |
| Alam 2008 | not phase III diagnostic accuracy study |
| Alborzi 2006 | not phase III diagnostic accuracy study |
| Amato 2009 | not phase III diagnostic accuracy study |
| Arya 1997 | not original research article |
| Arya 2001 | not original research article |
| Attar 2001 | not phase III diagnostic accuracy study |
| Azazy 2004 | not phase III diagnostic accuracy study |
| Bagchi 1998 | not phase III diagnostic accuracy study |
| Bern 2000 | not phase III diagnostic accuracy study |
| Brandonisio 2002 | not phase III diagnostic accuracy study |
| Canavate 2011 | not phase III diagnostic accuracy study |
| Carvalho 2003 | not phase III diagnostic accuracy study |
| Cruz 2006 | not phase III diagnostic accuracy study |
| de Assis 2008 | publication of the same study in more than one record |
| Delgado 2001 | not phase III diagnostic accuracy study |
| Edrissian 2003 | not phase III diagnostic accuracy study |
| El‐Safi 2003 | not phase III diagnostic accuracy study |
| Ferreira Dourado 2007 | not original research article |
| Gavgani 2008 | not phase III diagnostic accuracy study |
| Goswami 2003 | not phase III diagnostic accuracy study |
| Goswami 2007 | not phase III diagnostic accuracy study |
| Goswami 2012 | not phase III diagnostic accuracy study |
| Gupta 1994 | not phase III diagnostic accuracy study |
| Hatam 2009 | not phase III diagnostic accuracy study |
| Hommel 2001 | not original research article |
| Iqbal 2002 | not phase III diagnostic accuracy study |
| Jelinek 1999 | target condition is not clinical visceral leishmaniasis |
| Khan 2009 | not phase III diagnostic accuracy study |
| Khan 2010 | not phase III diagnostic accuracy study |
| Kumar 2006 | not phase III diagnostic accuracy study |
| Lemos 2003 | not phase III diagnostic accuracy study |
| López Corbalán 2012 | not phase III diagnostic accuracy study |
| Mandal 2008 | reference standard is not according to criteria |
| Mansour 2009 | not phase III diagnostic accuracy study |
| Marty 2007 | not phase III diagnostic accuracy study |
| Mathur 2005 | not phase III diagnostic accuracy study |
| Matlashewski 2013 | reference standard is not according to criteria |
| Mbui 2011 | publication of the same study in more than one record |
| Mohapatra 2010 | not phase III diagnostic accuracy study |
| Monno 2009 | not phase III diagnostic accuracy study |
| Moura 2013 | reference standard is not according to criteria |
| Mueller 2013 | not phase III diagnostic accuracy study |
| Ozerdem 2009 | reference standard is not according to criteria |
| Ozkan 2008 | not phase III diagnostic accuracy study |
| Pappas 1983 | not phase III diagnostic accuracy study |
| Pappas 1984 | not phase III diagnostic accuracy study |
| Pappas 1984a | not phase III diagnostic accuracy study |
| Pattabhi 2010 | not phase III diagnostic accuracy study |
| Qu 1987 | target condition is not clinical visceral leishmaniasis |
| Qu 2000 | not phase III diagnostic accuracy study |
| Riera 2004 | not phase III diagnostic accuracy study |
| Rouf 2009 | not phase III diagnostic accuracy study |
| Saghrouni 2009 | not phase III diagnostic accuracy study |
| Saha 2011 | not phase III diagnostic accuracy study |
| Salam 2008 | not phase III diagnostic accuracy study |
| Salam 2011 | target condition is not clinical visceral leishmaniasis |
| Salotra 2005 | not original research article |
| Sarkari 2005 | not phase III diagnostic accuracy study |
| Sarker 2003 | not phase III diagnostic accuracy study |
| Schallig 2002 | not phase III diagnostic accuracy study |
| Schoone 2001 | not phase III diagnostic accuracy study |
| Scott 1991 | not phase III diagnostic accuracy study |
| Senaldi 1996 | not phase III diagnostic accuracy study |
| Shamsuzzaman 2003 | not phase III diagnostic accuracy study |
| Sharma 2009 | target condition is not clinical visceral leishmaniasis |
| Silva 2005 | not phase III diagnostic accuracy study |
| Singh 2009 | not phase III diagnostic accuracy study |
| Singh 2010 | not phase III diagnostic accuracy study |
| Sinha 2008 | not phase III diagnostic accuracy study |
| Srivastava 1988 | not phase III diagnostic accuracy study |
| Sundar 2002 | reference standard is not according to criteria |
| Sundar 2002a | not phase III diagnostic accuracy study |
| Sundar 2002b | not original research article |
| Sundar 2005 | not phase III diagnostic accuracy study |
| Sundar 2005a | publication of the same study in more than one record |
| Sundar 2006 | not phase III diagnostic accuracy study |
| Sundar 2006a | not phase III diagnostic accuracy study |
| Teran‐Angel 2010 | not phase III diagnostic accuracy study |
| Walton 1986 | not phase III diagnostic accuracy study |
| Walton 1987 | publication of the same study in more than one record |
| Welch 2008 | not phase III diagnostic accuracy study |
| Zijlstra 2001 | not phase III diagnostic accuracy study |
Differences between protocol and review
For the assessment of the quality of the included records, we planned and started to work with QUADAS. When QUADAS‐2 became available, we switched to this new tool (Whiting 2011). In the review, we only report the information obtained using QUADAS‐2.
In order to avoid loss of information, we used a somewhat wider interpretation of the composite reference standard. For the sake of clarity, we slightly reformulated the accepted reference standards in the section Criteria for considering studies for this review.
In the statistical modelling, we used a complementary log‐log, rather than a logit link function in the bivariate model. The choice was based on an improved fit of the model to the data as described in the findings section.
Contributions of authors
Marleen Boelaert, Joris Menten, François Chappuis and Suman Rijal performed previous work that was the foundation of the current study. Marleen Boelaert, Joris Menten and Temmy Sunyoto conceived and designed the review. Marleen Boelaert, Temmy Sunyoto, Kristien Verdonck and Johan van Griensven screened the retrieved papers against the inclusion criteria. Marleen Boelaert, Johan van Griensven and Kristien Verdonck extracted data and appraised the quality of the papers. Data management for the review was carried out by Joris Menten, Kristien Verdonck and Temmy Sunyoto. Joris Menten did the statistical analysis. Marleen Boelaert, Joris Menten, Temmy Sunyoto and Kristien Verdonck wrote the first draft of the review. All authors contributed to the final manuscript. Marleen Boelaert is the guarantor of the review.
Sources of support
Internal sources
Institute of Tropical Medicine, Antwerp, Belgium.
External sources
-
Belgian Development Cooperation, Belgium.
Grant to Temmy Sunyoto
-
Department of Economy, Science and Innovation of the Flemish Government, Belgium.
Funding for the Clinical Trials Unit at the Institute of Tropical Medicine, Antwerp
Declarations of interest
Marleen Boelaert, Joris Menten, François Chappuis and Suman Rijal are authors of studies included in this review. There are no other known conflicts of interest.
Unchanged
References
References to studies included in this review
Boelaert 2004 ‐ classic {published data only}
- Boelaert M, Rijal S, Regmi S, Singh R, Karki B, Jacquet D, et al. A comparative study of the effectiveness of diagnostic tests for visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 2004;70(1):72‐7. [PubMed] [Google Scholar]
Boelaert 2004 ‐ LCA {published data only}
- Boelaert M, Rijal S, Regmi S, Singh R, Karki B, Jacquet D, et al. A comparative study of the effectiveness of diagnostic tests for visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 2004;70(1):72‐7. [PubMed] [Google Scholar]
Boelaert 2008 ‐ Ethiopia {published data only}
- Boelaert M, El‐Safi S, Hailu A, Mukhtar M, Rijal S, Sundar S, et al. Diagnostic tests for kala‐azar: a multi‐centre study of the freeze‐dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(1):32‐40. [DOI] [PubMed] [Google Scholar]
Boelaert 2008 ‐ India {published data only}
- Boelaert M, El‐Safi S, Hailu A, Mukhtar M, Rijal S, Sundar S, et al. Diagnostic tests for kala‐azar: a multi‐centre study of the freeze‐dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(1):32‐40. [DOI] [PubMed] [Google Scholar]
Boelaert 2008 ‐ Kenya {published data only}
- Boelaert M, El‐Safi S, Hailu A, Mukhtar M, Rijal S, Sundar S, et al. Diagnostic tests for kala‐azar: a multi‐centre study of the freeze‐dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(1):32‐40. [DOI] [PubMed] [Google Scholar]
Boelaert 2008 ‐ Nepal {published data only}
- Boelaert M, El‐Safi S, Hailu A, Mukhtar M, Rijal S, Sundar S, et al. Diagnostic tests for kala‐azar: a multi‐centre study of the freeze‐dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(1):32‐40. [DOI] [PubMed] [Google Scholar]
Boelaert 2008 ‐ Sudan {published data only}
- Boelaert M, El‐Safi S, Hailu A, Mukhtar M, Rijal S, Sundar S, et al. Diagnostic tests for kala‐azar: a multi‐centre study of the freeze‐dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(1):32‐40. [DOI] [PubMed] [Google Scholar]
Chappuis 2003 {published data only}
- Chappuis F, Rijal S, Singh R, Acharya P, Karki BM, Das ML, et al. Prospective evaluation and comparison of the direct agglutination test and an rK39‐antigen‐based dipstick test for the diagnosis of suspected kala‐azar in Nepal. Tropical Medicine & International Health : TM & IH 2003;8(3):277‐85. [DOI] [PubMed] [Google Scholar]
Chappuis 2005 ‐ DiaMed {published data only}
- Chappuis F, Mueller Y, Nguimfack A, Rwakimari JB, Couffignal S, Boelaert M, et al. Diagnostic accuracy of two rK39 antigen‐based dipsticks and the formol gel test for rapid diagnosis of visceral leishmaniasis in northeastern Uganda. Journal of Clinical Microbiology 2005;43(12):5973‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chappuis 2005 ‐ InBios {published data only}
- Chappuis F, Mueller Y, Nguimfack A, Rwakimari JB, Couffignal S, Boelaert M, et al. Diagnostic accuracy of two rK39 antigen‐based dipsticks and the formol gel test for rapid diagnosis of visceral leishmaniasis in northeastern Uganda. Journal of Clinical Microbiology 2005;43(12):5973‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chappuis 2006b {published data only}
- Chappuis F, Rijal S, Jha UK, Desjeux P, Karki BM, Koirala S, et al. Field validity, reproducibility and feasibility of diagnostic tests for visceral leishmaniasis in rural Nepal. Tropical Medicine & International Health : TM & IH 2006;11(1):31‐40. [DOI] [PubMed] [Google Scholar]
Cota 2013 ‐ composite 1 {published data only}
- Cota GF, Sousa MR, Freitas Nogueira BM, Gomes LI, Oliveira E, Assis TS, et al. Comparison of parasitological, serological, and molecular tests for visceral leishmaniasis in HIV‐infected patients: a cross‐sectional delayed‐type study. American Journal of Tropical Medicine and Hygiene 2013;89:570‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cota 2013 ‐ composite 2 {published data only}
- Cota GF, Sousa MR, Freitas Nogueira BM, Gomes LI, Oliveira E, Assis TS, et al. Comparison of parasitological, serological, and molecular tests for visceral leishmaniasis in HIV‐infected patients: a cross‐sectional delayed‐type study. American Journal of Tropical Medicine and Hygiene 2013;89:570‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diro 2007 {published data only}
- Diro E, Techane Y, Tefera T, Assefa Y, Kebede T, Genetu A, et al. Field evaluation of FD‐DAT, rK39 dipstick and KATEX (urine latex agglutination) for diagnosis of visceral leishmaniasis in northwest Ethiopia. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(9):908‐14. [DOI] [PubMed] [Google Scholar]
Hailu 2006 {published data only}
- Hailu A, Schoone GJ, Diro E, Tesfaye A, Techane Y, Tefera T, et al. Field evaluation of a fast anti‐Leishmania antibody detection assay in Ethiopia. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006;100(1):48‐52. [DOI] [PubMed] [Google Scholar]
Kilic 2008 {published data only}
- Kilic S, Taylan Ozkan A, Babur C, Tanir G, Schallig H. Evaluation of serological tests for the diagnosis of visceral leishmaniasis [Visseral Leishmaniasis Tan›s›nda Serolojik Testlerin De¤erlendirilmesi]. Turkish Journal of MedicalSciences 2008;38(1):13‐9. [Google Scholar]
Machado de Assis 2011 {published data only}
- Assis TS, Braga AS, Pedras MJ, Oliveira E, Barral A, Siqueira IC, et al. Multi‐centric prospective evaluation of rk39 rapid test and direct agglutination test for the diagnosis of visceral leishmaniasis in Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011;105(2):81‐5. [DOI] [PubMed] [Google Scholar]
Machado de Assis 2012 {published data only}
- Machado de Assis TS, Rabello A, Werneck GL. Latent class analysis of diagnostic tests for visceral leishmaniasis in Brazil. Tropical Medicine and International Health 2012;17(10):1202‐7. [DOI] [PubMed] [Google Scholar]
Mbui 2013 {published data only}
- Mbui J, Wasunna M, Balasegaram M, Laussermayer A, Juma R, Njenga SN, et al. Validation of two rapid diagnostic tests for visceral leishmaniasis in Kenya. PLoS Neglected Tropical Diseases 2013;7(9):e2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peruhype‐Magalhaes 2012 {published data only}
- Peruhype‐Magalhaes V, Machado‐de‐Assis TS, Rabello A. Use of the Kala‐Azar Detect(R) and IT‐LEISH(R) rapid tests for the diagnosis of visceral leishmaniasis in Brazil. Memorias do Instituto Oswaldo Cruz. 2012;107:951‐2. [DOI] [PubMed] [Google Scholar]
Rijal 2004 {published data only}
- Rijal S, Boelaert M, Regmi S, Karki BM, Jacquet D, Singh R, et al. Evaluation of a urinary antigen‐based latex agglutination test in the diagnosis of kala‐azar in eastern Nepal. Tropical Medicine & International Health : TM & IH 2004;9(6):724‐9. [DOI] [PubMed] [Google Scholar]
Ritmeijer 2006 {published data only}
- Ritmeijer K, Melaku Y, Mueller M, Kipngetich S, O'keeffe C, Davidson RN. Evaluation of a new recombinant K39 rapid diagnostic test for Sudanese visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 2006;74(1):76‐80. [PubMed] [Google Scholar]
Sundar 1998 {published data only}
- Sundar S, Reed SG, Singh VP, Kumar PC, Murray HW. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet 1998;351(9102):563‐5. [DOI] [PubMed] [Google Scholar]
Sundar 2007 {published data only}
- Sundar S, Singh RK, Bimal SK, Gidwani K, Mishra A, Maurya R, et al. Comparative evaluation of parasitology and serological tests in the diagnosis of visceral leishmaniasis in India: a phase III diagnostic accuracy study. Tropical Medicine & International Health : TM & IH 2007;12(2):284‐9. [DOI] [PubMed] [Google Scholar]
ter Horst 2009 ‐ HIV neg {published data only}
- ter Horst R, Tefera T, Assefa G, Ebrahim AZ, Davidson RN, Ritmeijer K. Field evaluation of rK39 test and direct agglutination test for diagnosis of visceral leishmaniasis in a population with high prevalence of human immunodeficiency virus in Ethiopia. American Journal of Tropical Medicine and Hygiene 2009;80(6):929‐34. [PubMed] [Google Scholar]
ter Horst 2009 ‐ HIV pos {published data only}
- ter Horst R, Tefera T, Assefa G, Ebrahim AZ, Davidson RN, Ritmeijer K. Field evaluation of rK39 test and direct agglutination test for diagnosis of visceral leishmaniasis in a population with high prevalence of human immunodeficiency virus in Ethiopia. American Journal of Tropical Medicine and Hygiene 2009;80(6):929‐34. [PubMed] [Google Scholar]
Toz 2004 {published data only}
- Toz SO, Chang KP, Ozbel Y, Alkan MZ. Diagnostic value of rK39 dipstick in zoonotic visceral leishmaniasis in Turkey. Journal of Parasitology 2004;90(6):1484‐6. [PUBMED: 15715249] [DOI] [PubMed] [Google Scholar]
Veeken 2003 ‐ composite {published data only}
- Veeken H, Ritmeijer K, Seaman J, Davidson R. Comparison of an rK39 dipstick rapid test with direct agglutination test and splenic aspiration for the diagnosis of kala‐azar in Sudan. Tropical Medicine & International Health : TM & IH 2003;8(2):164‐7. [DOI] [PubMed] [Google Scholar]
Veeken 2003 ‐ spleen {published data only}
- Veeken H, Ritmeijer K, Seaman J, Davidson R. Comparison of an rK39 dipstick rapid test with direct agglutination test and splenic aspiration for the diagnosis of kala‐azar in Sudan. Tropical Medicine & International Health : TM & IH 2003;8(2):164‐7. [DOI] [PubMed] [Google Scholar]
Vilaplana 2004 {published data only}
- Vilaplana C, Blanco S, Dominguez J, Gimenez M, Ausina V, TUral C, et al. Noninvasive method for diagnosis of visceral leishmaniasis by a latex agglutination test for detection of antigens in urine samples. Journal of Clinical Microbiology 2004; Vol. 42, issue 4:1853‐4. [DOI] [PMC free article] [PubMed]
References to studies excluded from this review
Abeijon 2013 {published data only}
- Abeijon C, Campos‐Neto A. Potential non‐invasive urine‐based antigen (protein) detection assay to diagnose active visceral leishmaniasis. PLoS Neglected Tropical Diseases 2013;7:e2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahsan 2010 {published data only}
- Ahsan MM, Islam MN, Mollah AH, Hoque MA, Hossain MA, Begum Z, et al. Evaluation of latex agglutination test (KAtex) for early diagnosis of kala‐azar. Mymensingh Medical Journal 2010;19(3):335‐9. [PubMed] [Google Scholar]
Akhoundi 2010 {published data only}
- Akhoundi B, Mohebali M, Babakhan L, Edrissian GH, Eslami MB, Keshavarz H, et al. Rapid detection of human Leishmania infantum infection: a comparative field study using the fast agglutination screening test and the direct agglutination test. Travel Medicine and Infectious Disease 2010;8(5):305‐10. [DOI] [PubMed] [Google Scholar]
Alam 2008 {published data only}
- Alam M, Shamsuzzaman A, Musa A, Khan A, Mahmud M, Hossain M, et al. Antigen detection from urine of Kala‐azar cases by latex agglutination test. Mymensingh Medical Journal 2008;17(1):22‐7. [PubMed] [Google Scholar]
Alborzi 2006 {published data only}
- Alborzi A, Rasouli M, Nademi Z, Kadivar MR, Pourabbas B. Evaluation of rK39 strip test for the diagnosis of visceral leishmaniasis in infants. Eastern Mediterranean Health Journal = La revue de sante de la Mediterranee orientale = al‐Majallah al‐sihhiyah li‐sharq al‐mutawassit 2006;12(3‐4):294‐9. [PubMed] [Google Scholar]
Al‐Nahhas 2003 {published data only}
- Al‐Nahhas S, Shabaan M, Hammoud L, Al‐Taweel A, Al‐Jorf S. Visceral leishmaniasis in the Syrian Arab Republic: early detection using rK39. Eastern Mediterranean Health Journal = La revue de sante de la Mediterranee orientale = al‐Majallah al‐sihhiyah li‐sharq al‐mutawassit 2003;9(4):856‐62. [PubMed] [Google Scholar]
Al‐Nahhas 2008 {published data only}
- Al‐Nahhas SA, Al‐Taweel AA, Al‐Taweel MA. Assessment of the direct agglutination test, fast agglutination screening test, and rK39 dipstick test for the sero‐diagnosis of visceral leishmaniasis in Syria. Saudi Medical Journal 2008;29(9):1250‐4. [PubMed] [Google Scholar]
Amato 2009 {published data only}
- Amato Neto V, Amato VS, Tuon FF, Gakiya E, Marchi CR, Souza RM, et al. False‐positive results of a rapid K39‐based strip test and Chagas disease. International Journal of Infectious Diseases : IJID : official publication of the International Society for Infectious Diseases 2009;13(2):182‐5. [DOI] [PubMed] [Google Scholar]
Arya 1997 {published data only}
Arya 2001 {published data only}
Attar 2001 {published data only}
- Attar ZJ, Chance ML, el‐Safi S, Carney J, Azazy A, El‐Hadi M, et al. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Tropica 2001;78(1):11‐6. [DOI] [PubMed] [Google Scholar]
Azazy 2004 {published data only}
- Azazy AA. Detection of circulating antigens in sera from visceral leishmaniasis patients using dot‐ELISA. Journal of the Egyptian Society of Parasitology 2004;34(1):35‐43. [PUBMED: 15125515] [PubMed] [Google Scholar]
Bagchi 1998 {published data only}
- Bagchi AK, Tiwari S, Gupta S, Katiyar JC. The latex agglutination test: standardization and comparison with direct agglutination and dot‐ELISA in the diagnosis of visceral leishmaniasis in India. Annals of Tropical Medicine and Parasitology 1998;92(2):159‐63. [DOI] [PubMed] [Google Scholar]
Bern 2000 {published data only}
- Bern C, Jha SN, Joshi AB, Thakur GD, Bista MB. Use of the recombinant K39 dipstick test and the direct agglutination test in a setting endemic for visceral leishmaniasis in Nepal. American Journal of Tropical Medicine and Hygiene 2000;63(3‐4):153‐7. [DOI] [PubMed] [Google Scholar]
Brandonisio 2002 {published data only}
- Brandonisio O, Fumarola L, Maggi P, Cavaliere R, Spinelli R, Pastore G. Evaluation of a rapid immunochromatographic test for serodiagnosis of visceral leishmaniasis. European Journal of Clinical Microbiology & Infectious Diseases : official publication of the European Society of Clinical Microbiology 2002;21(6):461‐4. [DOI] [PubMed] [Google Scholar]
Canavate 2011 {published data only}
- Canavate C, Herrero M, Nieto J, Cruz I, Chicharro C, Aparicio P, et al. Evaluation of two rK39 dipstick tests, direct agglutination test, and indirect fluorescent antibody test for diagnosis of visceral leishmaniasis in a new epidemic site in highland Ethiopia. American Journal of Tropical Medicine and Hygiene 2011;84(1):102‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carvalho 2003 {published data only}
- Carvalho SF, Lemos EM, Corey R, Dietze R. Performance of recombinant K39 antigen in the diagnosis of Brazilian visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 2003;68(3):321‐4. [PubMed] [Google Scholar]
Cruz 2006 {published data only}
- Cruz I, Chicharro C, Nieto J, Bailo B, Canavate C, Figueras MC, et al. Comparison of new diagnostic tools for management of pediatric Mediterranean visceral leishmaniasis. Journal of Clinical Microbiology 2006;44(7):2343‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Assis 2008 {published data only}
- Machado de Assis T, Costa Braga AS, Junqueira Pedras M, Prado Barral A, Siqueira IC, Nery Costa CH, ET AL. Validation of the rapid immunochromatographic test IT‐LEISH® for the diagnosis of human visceral leishmaniasis [Validação do teste imunocromatográfico rápido IT‐LEISH® para o diagnóstico da leishmaniose visceral humana]. Epidemiologia e Serviços de Saúde, Brasília 2008;17(2):107‐16. [Google Scholar]
Delgado 2001 {published data only}
- Delgado O, Feliciangeli MD, Coraspe V, Silva S, Perez A, Arias J. Value of a dipstick based on recombinant RK39 antigen for differential diagnosis of American visceral leishmaniasis from other sympatric endemic diseases in Venezuela. Parasite (Paris, France) 2001;8(4):355‐7. [DOI] [PubMed] [Google Scholar]
Edrissian 2003 {published data only}
- Edrissian GH, Shamssi S, Mohebali M, Hajjaran H, Mamishi S, Desjeux P. Evaluation of rapid "dipstick rK39" test in diagnosis and serological survey of visceral leishmaniasis in humans and dogs in Iran. Archives of Iranian Medicine 2003;6(1):29‐31. [Google Scholar]
El‐Safi 2003 {published data only}
- El‐Safi SH, Abdel‐Haleem A, Hammad A, El‐Basha I, Omer A, Kareem HG, et al. Field evaluation of latex agglutination test for detecting urinary antigens in visceral leishmaniasis in Sudan. Eastern Mediterranean Health Journal = La revue de sante de la Mediterranee orientale = al‐Majallah al‐sihhiyah li‐sharq al‐mutawassit 2003;9(4):844‐55. [PubMed] [Google Scholar]
Ferreira Dourado 2007 {published data only}
- Ferreira Dourado Z, Delleon Silva H, Silveira‐Lacerda E, García‐Zapata MTA. Historical panorama of laboratorial diagnostic of visceral leishmaniasis until the appearance of immunochromatographic tests [Panorama histórico do diagnóstico laboratorial da leishmaniose visceral até o surgimento dos testes imunocromatográficos (rK39)]. Revista de Patología Tropical 2007;36(3):205‐14. [Google Scholar]
Gavgani 2008 {published data only}
- Gavgani AM, Vatan SK, Ghazanchaei A. KAtex antigen‐detection test as a diagnostic tool for latent visceral leishmaniasis cases. African Journal of Biotechnology 2008;7(7):852‐9. [Google Scholar]
Goswami 2003 {published data only}
- Goswami RP, Bairagi B, Kundu PK. K39 strip test‐‐easy, reliable and cost‐effective field diagnosis for visceral leishmaniasis in India. Journal of the Association of Physicians of India 2003;51:759‐61. [PubMed] [Google Scholar]
Goswami 2007 {published data only}
- Goswami RP, Rahman M, Guha SK. Utility of K39 strip test in visceral leishmaniasis (VL) and HIV co‐infected patients: an early report from Eastern India. Journal of the Association of Physicians of India 2007; Vol. 55:154‐5. [PubMed]
Goswami 2012 {published data only}
- Goswami RP, Das S, Ray Y, Rahman M. Testing urine samples with rK39 strip as the simplest non‐invasive field diagnosis for visceral leishmaniasis: an early report from eastern India. Journal of Postgraduate Medicine 2012;58:180‐4. [DOI] [PubMed] [Google Scholar]
Gupta 1994 {published data only}
- Gupta S, Srivastava JK, Pal A, Katiyar JC, Saxena KC, Dhawan BN, et al. Direct agglutination test and dot‐ELISA in the serodiagnosis of visceral leishmaniasis (Kala‐azar) ‐ a comparative study. Serodiagnosis and Immunotherapy in Infectious Disease 1994;6:154‐8. [Google Scholar]
Hatam 2009 {published data only}
- Hatam GR, Ghatee MA, Hossini SM, Sarkari B. Improvement of the newly developed latex agglutination test (Katex) for diagnosis of visceral lieshmaniasis. Journal of Clinical Laboratory Analysis 2009;23(4):202‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hommel 2001 {published data only}
- Hommel M, Sarkari B, Carney J, Chance ML. Katex for the diagnosis of human visceral leishmaniasis [Le Katex pour le diagnostic de la leishmaniose viscerale humaine.]. Médecine Tropicale: Revue du Corps de Santé Colonial 2001;61(6):503‐5. [PUBMED: 11980401] [PubMed] [Google Scholar]
Iqbal 2002 {published data only}
- Iqbal J, Hira PR, Saroj G, Philip R, Al‐Ali F, Madda PJ, et al. Imported visceral leishmaniasis: diagnostic dilemmas and comparative analysis of three assays. Journal of Clinical Microbiology 2002;40(2):475‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jelinek 1999 {published data only}
- Jelinek T, Eichenlaub S, Loscher T. Sensitivity and specificity of a rapid immunochromatographic test for diagnosis of visceral leishmaniasis. European Journal of Clinical Microbiology & Infectious Diseases : official publication of the European Society of Clinical Microbiology 1999;18(9):669‐70. [DOI] [PubMed] [Google Scholar]
Khan 2009 {published data only}
- Khan AM, Pandey K, Kumar V, Dutta P, Das P, Mahanta J. Sample survey for indigenous cases of kala‐azar in Assam by rk39 dipstick test. The Indian Journal of Medical Research 2009; Vol. 129, issue 3:327‐8. [PubMed]
Khan 2010 {published data only}
- Khan MG, Alam MS, Podder MP, Itoh M, Jamil KM, Haque R, et al. Evaluation of rK‐39 strip test using urine for diagnosis of visceral leishmaniasis in an endemic area in Bangladesh. Parasites & Vectors 2010;3:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2006 {published data only}
- Kumar R, Pai K, Kumar P, Pandey HP, Sundar S. Sero‐epidemiological study of kala‐azar in a village of Varanasi district, India. Tropical Medicine & International Health : TM & IH 2006;11(1):41‐8. [DOI] [PubMed] [Google Scholar]
Lemos 2003 {published data only}
- Lemos EM, Carvalho SF, Corey R, Dietze R. Evaluation of a rapid test using recombinant k39 antigen in the diagnosis of visceral leishmaniasis in Brazil [Avaliacao do teste rapido utilizando o antigeno recombinante k39 no diagnostico da leishmaniose visceral no Brasil]. Revista da Sociedade Brasileira de Medicina Tropical 2003;36 Suppl 2:36‐8. [DOI] [PubMed] [Google Scholar]
López Corbalán 2012 {published data only}
- López Corbalán JC, Seguí Ripoll JM, Estebán Fernández JMª. Urinary antigen as an effective and safe diagnosis of visceral leishmaniasis [Antígeno urinario como diagnóstico eficaz y seguro de leishmaniasis visceral]. Atención Farmacéutica 2012;14(4):298‐9. [Google Scholar]
Mandal 2008 {published data only}
- Mandal J, Khurana S, Dubey ML, Bhatia P, Varma N, Malla N. Evaluation of direct agglutination test, rk39 Test, and ELISA for the diagnosis of visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 2008;79(1):76‐8. [PubMed] [Google Scholar]
Mansour 2009 {published data only}
- Mansour D, Abass EM, Mahamoud A, Harith A. Qualitative and semi‐quantitative comparison of an rK39 strip test and direct agglutination test for detection of anti‐Leishmania donovani antibodies in the Sudan. Iranian Journal of Immunology : IJI 2009;6(4):208‐15. [PubMed] [Google Scholar]
Marty 2007 {published data only}
- Marty P, Delaunay P, Fissore C, Fichoux Y. Mediterranean leishmaniasis caused by Leishmania infantum. Update on the utility of the IT‐Leish and ID‐Pagia leishmaniasis tests [La leishmaniose mediterraneenne due a Leishmania infantum Mise au point‐‐interets des tests de diagnostic rapide: IT‐Leish et ID‐Pagia leishmaniasis.]. Médecine Tropicale: Revue du Corps de Santé Colonial 2007;67(1):79‐85. [PubMed] [Google Scholar]
Mathur 2005 {published data only}
- Mathur P, Samantaray J, Chauhan NK. Evaluation of a rapid immunochromatographic test for diagnosis of kala‐azar & post kala‐azar dermal leishmaniasis at a tertiary care centre of north India. The Indian Journal of Medical Research 2005;122(6):485‐90. [PubMed] [Google Scholar]
Matlashewski 2013 {published data only}
- Matlashewski G, Das V N, Pandey K, Singh D, Das S, Ghosh A K, et al. Diagnosis of visceral leishmaniasis in Bihar India: comparison of the rK39 rapid diagnostic test on whole blood versus serum. PLoS Neglected Tropical Diseases 2013;7:e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mbui 2011 {published data only}
- Mbui J, Wasunna M, Kirigi G, Kinoti D, Riongoita M, Tour R, et al. Validation of two rapid tests for diagnosis of visceral leishmaniasis in Kenya. Tropical Medicine and International Health 2011;Conference: 7th European Congress on Tropical Medicine and International Health Barcelona Spain. Conference Start: 20111003 Conference End: 20111006. Conference Publication: (var.pagings). 16:283. [Google Scholar]
Mohapatra 2010 {published data only}
- Mohapatra TM, Singh DP, Sen MR, Bharti K, Sundar S. Compararative evaluation of rK9, rK26 and rK39 antigens in the serodiagnosis of Indian visceral leishmaniasis. Journal of Infection in Developing Countries 2010;4(2):114‐7. [DOI] [PubMed] [Google Scholar]
Monno 2009 {published data only}
- Monno R, Giannelli G, Rizzo C, Vito D, Fumarola L. Recombinant K39 immunochromatographic test for diagnosis of human leishmaniasis. Future Microbiology 2009;4(2):159‐70. [DOI] [PubMed] [Google Scholar]
Moura 2013 {published data only}
- Moura AS, Lopes HM, Mourao MV, Morais MH. Performance of a rapid diagnostic test for the detection of visceral leishmaniasis in a large urban setting. Revista da Sociedade Brasileira de Medicina Tropical 2013;46:589‐93. [DOI] [PubMed] [Google Scholar]
Mueller 2013 {published data only}
- Mueller YK, Kolaczinski JH, Koech T, Lokwang P, Riongoita M, Velilla E, et al. Clinical epidemiology, diagnosis and treatment of visceral leishmaniasis in the Pokot endemic area of Uganda and Kenya. American Society of Tropical Medicine and Hygiene 2013;90(1):33‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozerdem 2009 {published data only}
- Ozerdem D, Eroglu F, Genc A, Demirkazik M, Koltas IS. Comparison of microscopic examination, rK39, and PCR for visceral leishmaniasis diagnosis in Turkey. Parasitology Research 2009;106(1):197‐200. [DOI] [PubMed] [Google Scholar]
Ozkan 2008 {published data only}
- Ozkan AT, Yalcinkaya T, Kilic S, Babur C, Schallig HD. Investigation of Leishmania infantum seropositivity in HIV/AIDS patients [HIV/AIDS hastalarinda Leishmania infantum seropozitifliginin arastirilmasi]. Mikrobiyoloji Bulteni 2008;42(1):113‐7. [PubMed] [Google Scholar]
Pappas 1983 {published data only}
- Pappas MG, Hajkowski R, Hockmeyer WT. Dot enzyme‐linked immunosorbent assay (Dot‐ELISA): a micro technique for the rapid diagnosis of visceral leishmaniasis. Journal of Immunological Methods 1983;64(1‐2):205‐14. [DOI] [PubMed] [Google Scholar]
Pappas 1984 {published data only}
- Pappas MG, Hajkowski R, Cannon LT Sr, Hockmeyer WT. Dot enzyme‐linked immunosorbent assay (Dot‐ELISA): comparison with standard ELISA and complement fixation assays for the diagnosis of human visceral leishmaniasis. Veterinary Parasitology 1984;14(3‐4):239‐49. [DOI] [PubMed] [Google Scholar]
Pappas 1984a {published data only}
- Pappas MG, Hajkowski R, Hockmeyer WT. Standardization of the dot enzyme‐linked immunosorbent assay (Dot‐ELISA) for human visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 1984;33(6):1105‐11. [DOI] [PubMed] [Google Scholar]
Pattabhi 2010 {published data only}
- Pattabhi S, Whittle J, Mohamath R, El‐Safi S, Moulton GG, Guderian JA, et al. Design, development and evaluation of rK28‐based point‐of‐care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Neglected Tropical Diseases 2010;4(9):e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qu 1987 {published data only}
- Qu JQ, Bao YF. Dot‐ELISA using monoclonal antibodies for identification of Leishmania donovani. Chinese Medical Journal 1987;100(10):823‐6. [PubMed] [Google Scholar]
Qu 2000 {published data only}
- Qu JQ, Guan LR, Shulidan I, Zuo XP, Chai JJ, Chen SB, et al. Rapid screening with a recombinant antigen (rK39) for diagnosis of visceral leishmaniasis using dipstick. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi = Chinese Journal of Parasitology & Parasitic Diseases 2000;18(3):155‐8. [PubMed] [Google Scholar]
Riera 2004 {published data only}
- Riera C, Fisa R, Lopez P, Ribera E, Carrio J, Falco V, et al. Evaluation of a latex agglutination test (KAtex) for detection of Leishmania antigen in urine of patients with HIV‐Leishmania coinfection: value in diagnosis and post‐treatment follow‐up. European Journal of Clinical Microbiology & Infectious Diseases : official publication of the European Society of Clinical Microbiology 2004;23(12):899‐904. [DOI] [PubMed] [Google Scholar]
Rouf 2009 {published data only}
- Rouf MA, Rahman ME, Islam MN, Islam MN, Ferdous NN, Hossain MA. Sensitivity, specificity and predictive values of immunochromatographic strip test in diagnosis of childhood kala‐azar. Mymensingh Medical Journal 2009;18(1 Suppl):S1‐5. [PubMed] [Google Scholar]
Saghrouni 2009 {published data only}
- Saghrouni F, Gaied‐Meksi S, Fathallah A, Amri F, Ach H, Guizani I, et al. Immunochromatographic rK39 strip test in the serodiagnosis of visceral leishmaniasis in Tunisia. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(12):1273‐8. [DOI] [PubMed] [Google Scholar]
Saha 2011 {published data only}
- Saha S, Goswami R, Pramanik N, Guha S K, Saha B, Rahman M, et al. Easy test for visceral Leishmaniasis and post‐Kala‐azar Dermal Leishmaniasis. Emerging Infectious Diseases 2011;17:1304‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2008 {published data only}
- Salam MA. Imunochromatographic strip test detection of anti‐rK39 antibody for the diagnosis of kala‐azar in an endemic zone of Bangladesh. Pakistan Journal of Medical Sciences 2008;24(4):497‐501. [Google Scholar]
Salam 2011 {published data only}
- Salam MA, Khan MG, Mondal D. Urine antigen detection by latex agglutination test for diagnosis and assessment of initial cure of visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011;105(5):269‐72. [DOI] [PubMed] [Google Scholar]
Salotra 2005 {published data only}
- Salotra P, Singh R. Rapid & reliable diagnostic tests for visceral leishmaniasis. The Indian Journal of Medical Research 2005;122(6):464‐7. [PubMed] [Google Scholar]
Sarkari 2005 {published data only}
- Sarkari B, Chance M, Hommel M. A capture ELISA for the diagnosis of visceral leishmaniasis using a monoclonal antibody against a leishmanial urinary antigen. Iranian Biomedical Journal 2005;9(3):117‐22. [Google Scholar]
Sarker 2003 {published data only}
- Sarker CB, Momen A, Jamal MF, Siddiqui NI, Siddiqui FM, Chowdhury KS, et al. Immunochromatographic (rK39) strip test in the diagnosis of visceral leishmaniasis in Bangladesh. Mymensingh Medical Journal 2003;12(2):93‐7. [PubMed] [Google Scholar]
Schallig 2002 {published data only}
- Schallig HD, Canto‐Cavalheiro M, Silva ES. Evaluation of the direct agglutination test and the rK39 dipstick test for the sero‐diagnosis of visceral leishmaniasis. Memorias do Instituto Oswaldo Cruz 2002;97(7):1015‐8. [DOI] [PubMed] [Google Scholar]
Schoone 2001 {published data only}
- Schoone GJ, Hailu A, Kroon CC, Nieuwenhuys JL, Schallig HD, Oskam L. A fast agglutination screening test (FAST) for the detection of anti‐Leishmania antibodies. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(4):400‐1. [DOI] [PubMed] [Google Scholar]
Scott 1991 {published data only}
- Scott JM, Shreffler WG, Ghalib HW, Asad A, Siddig M, Badaro R, et al. A rapid and simple diagnostic test for active visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 1991;44(3):272‐7. [DOI] [PubMed] [Google Scholar]
Senaldi 1996 {published data only}
- Senaldi G, Xiao‐su H, Hoessli DC, Bordier C. Serological diagnosis of visceral leishmaniasis by a dot‐enzyme immunoassay for the detection of a Leishmania donovani‐related circulating antigen. Journal of Immunological Methods 1996;193(1):9‐15. [DOI] [PubMed] [Google Scholar]
Shamsuzzaman 2003 {published data only}
- Shamsuzzaman AK, Hossain MA, Musa AK, Hasan MU, Dhar DK. A preliminary report on culture of Leishmania donovani in Mymensingh Medical College and evaluation of new immuno‐chromatography test (ICT). Mymensingh Medical Journal 2003;12(1):51‐4. [PubMed] [Google Scholar]
Sharma 2009 {published data only}
- Sharma NL, Mahajan VK, Negi AK, Verma GK. The rK39 immunochromatic dipstick testing: a study for K39 seroprevalence in dogs and human leishmaniasis patients for possible animal reservoir of cutaneous and visceral leishmaniasis in endemic focus of Satluj river valley of Himachal Pradesh (India). Indian Journal of Dermatology, Venereology, and Leprology 2009;75(1):52‐5. [DOI] [PubMed] [Google Scholar]
Silva 2005 {published data only}
- Silva ES, Schoone GJ, Gontijo CM, Brazil RP, Pacheco RS, Schallig HD. Application of direct agglutination test (DAT) and fast agglutination screening test (FAST) for sero‐diagnosis of visceral leishmaniasis in endemic area of Minas Gerais, Brazil. Kinetoplastid Biology and Disease 2005;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2009 {published data only}
- Singh DP, Sundar S, Mohapatra TM. The rK39 strip test is non‐predictor of clinical status for kala‐azar. BMC Research Notes 2009;2:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2010 {published data only}
- Singh DP, Goyal RK, Singh RK, Sundar S, Mohapatra TM. In search of an ideal test for diagnosis and prognosis of kala‐azar. Journal of Health, Population, and Nutrition 2010;28(3):281‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2008 {published data only}
- Sinha PK, Bimal S, Pandey K, Singh SK, Ranjan A, Kumar N, et al. A community‐based, comparative evaluation of direct agglutination and rK39 strip tests in the early detection of subclinical Leishmania donovani infection. Annals of Tropical Medicine and Parasitology 2008;102(2):119‐25. [DOI] [PubMed] [Google Scholar]
Srivastava 1988 {published data only}
- Srivastava L, Singh VK. Diagnosis of Indian kala‐azar by dot enzyme‐linked immunosorbent assay (dot‐ELISA). Annals of Tropical Medicine and Parasitology 1988;82(4):331‐4. [DOI] [PubMed] [Google Scholar]
Sundar 2002 {published data only}
- Sundar S, Sahu M, Mehta H, Gupta A, Kohli U, Rai M, et al. Noninvasive management of Indian visceral leishmaniasis: clinical application of diagnosis by K39 antigen strip testing at a kala‐azar referral unit. Clinical Infectious Diseases : an official publication of the Infectious Diseases Society of America 2002;35(5):581‐6. [DOI] [PubMed] [Google Scholar]
Sundar 2002a {published data only}
- Sundar S, Pai K, Sahu M, Kumar V, Murray HW. Immunochromatographic strip‐test detection of anti‐K39 antibody in Indian visceral leishmaniasis. Annals of Tropical Medicine and Parasitology 2002;96(1):19‐23. [DOI] [PubMed] [Google Scholar]
Sundar 2002b {published data only}
- Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clinical and Diagnostic Laboratory Immunology 2002;9(5):951‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sundar 2005 {published data only}
- Sundar S, Agrawal S, Pai K, Chance M, Hommel M. Detection of leishmanial antigen in the urine of patients with visceral leishmaniasis by a latex agglutination test. American Journal of Tropical Medicine and Hygiene 2005;73(2):269‐71. [PubMed] [Google Scholar]
Sundar 2005a {published data only}
Sundar 2006 {published data only}
- Sundar S, Singh RK, Maurya R, Kumar B, Chhabra A, Singh V, et al. Serological diagnosis of Indian visceral leishmaniasis: direct agglutination test versus rK39 strip test. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006;100(6):533‐7. [DOI] [PubMed] [Google Scholar]
Sundar 2006a {published data only}
- Sundar S, Maurya R, Singh RK, Bharti K, Chakravarty J, Parekh A, et al. Rapid, noninvasive diagnosis of visceral leishmaniasis in India: comparison of two immunochromatographic strip tests for detection of anti‐K39 antibody. Journal of Clinical Microbiology 2006;44(1):251‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teran‐Angel 2010 {published data only}
- Teran‐Angel G, Rodriguez V, Silva R, Zerpa O, Schallig H, Ulrich M, et al. Non invasive diagnostic tools for visceral leishmaniasis: a comparison of the immunoserological tests DAT, rK26 and rK39 [Herramientas no invasivas en Venezuela: comparacion entre las pruebas inmunoserologicas DAT, rK26 y rK39 en el diagnostico de leishmaniasis visceral.]. Biomedica : Revista del Instituto Nacional de Salud 2010;30(1):39‐45. [PubMed] [Google Scholar]
Walton 1986 {published data only}
- Walton BC, Pappas MG, Sierra M Jr, Hajkowski R, Jackson P, Custodio R. Field use of the Dot‐ELISA test for visceral leishmaniasis in Honduras. Bulletin of the Pan American Health Organization 1986;20(2):147‐56. [PubMed] [Google Scholar]
Walton 1987 {published data only}
- Walton BC, Pappas MG, Sierra M, Hajkowski R, Jackson P, Custodio R. Field use of the DOT‐ELISA test for visceral leishmaniasis in Honduras [La prueba DOT‐ELISA para la detección de campo de la leishmaniasis visceral en Honduras]. Boletín de la Oficina Sanitaria Panamericana 1987;102(1):19‐28. [Google Scholar]
Welch 2008 {published data only}
- Welch RJ, Anderson BL, Litwin CM. Rapid immunochromatographic strip test for detection of anti‐K39 immunoglobulin G antibodies for diagnosis of visceral leishmaniasis. Clinical and Vaccine Immunology : CVI 2008;15(9):1483‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zijlstra 2001 {published data only}
- Zijlstra EE, Nur Y, Desjeux P, Khalil EA, El‐Hassan AM, Groen J. Diagnosing visceral leishmaniasis with the recombinant K39 strip test: experience from the Sudan. Tropical Medicine & International Health : TM & IH 2001;6(2):108‐13. [DOI] [PubMed] [Google Scholar]
Additional references
Alvar 2008
- Alvar J, Aparicio P, Aseffa A, Boer M, Canavate C, Dedet JP, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clinical Microbiology Reviews 2008;21(2):334‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvar 2012
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS One 2012;7(5):e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Babiker 2007
- Babiker ZO, Davidson R, Mazinda C, Kipngetich S, Ritmeijer K. Utility of lymph node aspiration in the diagnosis of visceral leishmaniasis in Sudan. American Journal of Tropical Medicine and Hygiene 2007;76(4):689‐93. [PubMed] [Google Scholar]
Bachmann 2006
- Bachmann LM, Puhan MA, ter Riet G, Bossuyt PM. Sample sizes of studies on diagnostic accuracy: literature survey. BMJ (Clinical research ed.) 2006;332(7550):1127‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baughman 2008
- Baughman AL, Bisgard KM, Cortese MM, Thompson WW, Sanden GN, Strebel PM. Utility of composite reference standards and latent class analysis in evaluating the clinical accuracy of diagnostic tests for pertussis. Clinical and Vaccine Immunology : CVI 2008;15(1):106‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharyya 2013
- Bhattacharyya T, Boelaert M, Miles MA. Comparison of visceral leishmaniasis diagnostic antigens in African and Asian Leishmania donovani reveals extensive diversity and region‐specific polymorphisms.. PLoS Neglected Tropical Diseases 2013;7(2):e2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattarai 2009
- Bhattarai NR, Auwera G, Khanal B, Doncker S, Rijal S, Das ML, et al. PCR and direct agglutination as Leishmania infection markers among healthy Nepalese subjects living in areas endemic for Kala‐Azar. Tropical Medicine & International Health : TM & IH 2009;14(4):404‐11. [DOI] [PubMed] [Google Scholar]
Black 2002
- Black MA, Craig BA. Estimating disease prevalence in the absence of a gold standard. Statistics in Medicine 2002;21(18):2653‐69. [DOI] [PubMed] [Google Scholar]
Boelaert 1999
- Boelaert M, Safi S, Jacquet D, Muynck A, Stuyft P, Ray D. Operational validation of the direct agglutination test for diagnosis of visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 1999;60(1):129‐34. [DOI] [PubMed] [Google Scholar]
Boelaert 2000
- Boelaert M, Criel B, Leeuwenburg J, Damme W, Ray D, Stuyft P. Visceral leishmaniasis control: a public health perspective. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(5):465‐71. [DOI] [PubMed] [Google Scholar]
Boelaert 2004
- Boelaert M, Rijal S, Regmi S, Singh R, Karki B, Jacquet D, et al. A comparative study of the effectiveness of diagnostic tests for visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 2004;70(1):72‐7. [PubMed] [Google Scholar]
Boelaert 2007
- Boelaert M, Bhattacharya S, Chappuis F, Safi SH, Hailu A, Mondal D, et al. Evaluation of rapid diagnostic tests: visceral leishmaniasis. Nature Reviews Microbiology November 2007;5(11):S30‐S39. [DOI] [PubMed] [Google Scholar]
Branscum 2005
- Branscum AJ, Gardner IA, Johnson WO. Estimation of diagnostic‐test sensitivity and specificity through Bayesian modeling. Preventive Veterinary Medicine 2005;68(2‐4):145‐63. [DOI] [PubMed] [Google Scholar]
Chappuis 2006
- Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta‐analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ (Clinical Research Ed.) 2006;333(7571):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chappuis 2007
- Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control?. Nature Reviews Microbiology 2007;5(11):873‐82. [DOI] [PubMed] [Google Scholar]
Cunningham 2012
- Cunningham J, Hasker E, Das P, Safi S, Goto H, Mondal D, et al. A global comparative evaluation of commercial immunochromatographic rapid diagnostic tests for visceral leishmaniasis. Clinical Infectious Diseases : an official publication of the Infectious Diseases Society of America 2012;55(10):1312‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Almeida 2006
- Almeida Silva L, Romero HD, Prata A, Costa RT, Nascimento E, Carvalho SF, et al. Immunologic tests in patients after clinical cure of visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 2006;75(4):739‐43. [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(7):882‐93. [DOI] [PubMed] [Google Scholar]
den Boer 2006
- Boer M, Davidson RN. Treatment options for visceral leishmaniasis. Expert Review of Anti‐infective Therapy 2006;4(2):187‐97. [DOI] [PubMed] [Google Scholar]
Dendukuri 2001
- Dendukuri N, Joseph L. Bayesian approaches to modeling the conditional dependence between multiple diagnostic tests. Biometrics 2001;57(1):158‐67. [DOI] [PubMed] [Google Scholar]
Enoe 2000
- Enoe C, Georgiadis MP, Johnson WO. Estimation of sensitivity and specificity of diagnostic tests and disease prevalence when the true disease state is unknown. Preventive Veterinary Medicine 2000;45(1‐2):61‐81. [DOI] [PubMed] [Google Scholar]
Evans 1992
- Evans TG, Teixeira MJ, McAuliffe IT, Vasconcelos I, Vasconcelos AW, Sousa Ade A, et al. Epidemiology of visceral leishmaniasis in northeast Brazil. Journal of Infectious Diseases 1992;166(5):1124‐32. [DOI] [PubMed] [Google Scholar]
Gelman 1995
- Gelman A, Carlin JC, Stern H, Rubin DB. Bayesian Data Analysis. New York: Chapman and Hall, 1995. [Google Scholar]
Greenland 2006
- Greenland S. Bayesian perspectives for epidemiological research: I. Foundations and basic methods. International Journal of Epidemiology 2006;35:765‐75. [DOI] [PubMed] [Google Scholar]
Hailu 1990
- Hailu A. Pre‐ and post‐treatment antibody levels in visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84(5):673‐5. [DOI] [PubMed] [Google Scholar]
Hailu 2009
- Hailu A, Gramiccia M, Kager PA. Visceral leishmaniasis in Aba‐Roba, south‐western Ethiopia: prevalence and incidence of active and subclinical infections. Annals of Tropical Medicine and Parasitology 2009;103(8):659‐70. [DOI] [PubMed] [Google Scholar]
Harhay 2011
- Harhay MO, Olliaro PL, Vaillant M, Chappuis F, Lima MA, Ritmeijer K, et al. Who is a typical patient with visceral leishmaniasis? Characterizing the demographic and nutritional profile of patients in Brazil, East Africa, and South Asia. American Journal of Tropical Medicine and Hygiene 2011;84(4):543‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harith 1986
- Harith AE, Kolk AH, Kager PA, Leeuwenburg J, Muigai R, Kiugu S, et al. A simple and economical direct agglutination test for serodiagnosis and sero‐epidemiological studies of visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1986;80(4):583‐6. [DOI] [PubMed] [Google Scholar]
Herwaldt 1999
- Herwaldt BL. Leishmaniasis. Lancet 1999;354(9185):1191‐9. [DOI] [PubMed] [Google Scholar]
Ho 1982
- Ho M, Siongok TK, Lyerly WH, Smith DH. Prevalence and disease spectrum in a new focus of visceral leishmaniasis in Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 1982;76(6):741‐6. [DOI] [PubMed] [Google Scholar]
Hui 1980
- Hui SL, Walter SD. Estimating the error rates of diagnostic tests. Biometrics 1980;36(1):167‐71. [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0. The Cochrane Collaboration, 2010. [Google Scholar]
Menten 2013
- Menten J, Boelaert M, Lesaffre E. Bayesian meta‐analysis of diagnostic tests allowing for imperfect reference standards. Statistics in Medicine 2013;32(30):5398‐413. [DOI] [PubMed] [Google Scholar]
Murray 2005
- Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet 2005;366(9496):1561‐77. [DOI] [PubMed] [Google Scholar]
Ostyn 2011
- Ostyn B, Gidwani K, Khanal B, Picado A, Chappuis F, Singh SP, et al. Incidence of symptomatic and asymptomatic Leishmania donovani infections in high‐endemic foci in India and Nepal: a prospective study. PLoS Neglected Tropical Diseases 2011;5(10):e1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasquau 2005
- Pasquau F, Ena J, Sanchez R, Cuadrado JM, Amador C, Flores J, et al. Leishmaniasis as an opportunistic infection in HIV‐infected patients: determinants of relapse and mortality in a collaborative study of 228 episodes in a Mediterreanean region. European Journal of Clinical Microbiology 2005;24(6):411‐8. [DOI] [PubMed] [Google Scholar]
PATH 2008
- PATH. RDT Info. Current information on rapid diagnostic tests. www.rapid‐diagnostics.org/index.htm (accessed 16 August 2010).
Peeling 2007
- Peeling RW, Smith PG, Bossuyt PMM. A guide for diagnostic evaluations. Nature Reviews Microbiology 2007;4(9 Suppl):S2‐S6. [DOI] [PubMed] [Google Scholar]
R Development Team 2010 [Computer program]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2010.
Raguenaud 2007
- Raguenaud ME, Jansson A, Vanlerberghe V, Deborggraeve S, Dujardin JC, Orfanos G, et al. Epidemiology and clinical features of patients with visceral leishmaniasis treated by an MSF clinic in Bakool region, Somalia, 2004‐2006. PLoS Neglected Tropical Diseases 2007;1(1):e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
Rittig 2000
- Rittig MG, Bogdan C. Leishmania‐host‐cell interaction: complexities and alternative views. Parasitology Today 2000;16(7):292‐7. [DOI] [PubMed] [Google Scholar]
Sarker 2004
- Sarker CB, Alam KS, Jamal MF, Rahman S, Huq MH, Musa AK, et al. Sensitivity of splenic and bone marrow aspirate study for diagnosis of kala‐azar. Mymensingh Medical Journal 2004;13(2):130‐3. [PubMed] [Google Scholar]
Seaman 1996
- Seaman J, Mercer AJ, Sondorp E. The epidemic of visceral leishmaniasis in western Upper Nile, southern Sudan: course and impact from 1984 to 1994. International Journal of Epidemiology 1996;25(4):862‐71. [DOI] [PubMed] [Google Scholar]
Spiegelhalter 2003 [Computer program]
- Spiegelhalter D, Thomas A, Best N, Lunn D. WinBUGS User Manual Version 1.4. Cambridge: MRC Biostatistics Unit, 2003.
Sullivan 2003
- Sullivan Pepe M. The Statistical Evaluation of Medical tests for Classification and Prediction. Oxford: Oxford University Press, 2003. [Google Scholar]
van Griensven 2010
- Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M. Combination therapy for visceral leishmaniasis. Lancet Infectious Diseases 2010;10(3):184‐94. [DOI] [PubMed] [Google Scholar]
Veeken 2003
- Veeken H, Ritmeijer K, Seaman J, Davidson R. Comparison of an rK39 dipstick rapid test with direct agglutination test and splenic aspiration for the diagnosis of kala‐azar in Sudan. Tropical Medicine and International Health 2003;8(2):164‐7. [DOI] [PubMed] [Google Scholar]
Verde 2010
- Verde PE. Meta‐analysis of diagnostic test data: a bivariate Bayesian modeling approach. Statistics in Medicine 2010;29(30):3088‐102. [PUBMED: 21170904] [DOI] [PubMed] [Google Scholar]
Whitehead 2002
- Whitehead, A. Meta‐Analysis of Controlled Clinical Trials. Chichester: John Wiley & Sons, 2002. [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. Regional strategic framework for elimination of kala‐azar from the South‐east Asia region (2005‐2015). New Delhi: WHO Regional Office for South‐East Asia 2005.
WHO 2010
- World Health Organization. Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control ofLeishmaniases, Geneva, 22‐26 March 2010. WHO technical report series 2010, issue 949.
Zhou 2002
- Zhou XH, McClish DK, Obuchowski NA. Statistical Methods in Diagnostic Medicine. New York: John Wiley, 2002. [Google Scholar]
Zijlstra 1992
- Zijlstra EE, Ali MS, el‐Hassan AM, el‐Toum IA, Satti M, Ghalib HW, et al. Kala‐azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1992;86(5):505‐7. [DOI] [PubMed] [Google Scholar]
Zijlstra 1994
- Zijlstra EE, el‐Hassan AM, Ismael A, Ghalib HW. Endemic kala‐azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post‐kala‐azar dermal leishmaniasis. American Journal of Tropical Medicine and Hygiene 1994;51(6):826‐36. [DOI] [PubMed] [Google Scholar]


