Abstract
Mature astrocytes exhibit a linear current-to-voltage K+ membrane conductance (passive conductance) and an extremely low membrane resistance (Rm) in situ. The combination of these electrophysiological characteristics establishes a highly negative and stable membrane potential that is essential for basic functions, such as K+ spatial buffering and neurotransmitter uptake. However, astrocytes are coupled extensively in situ. It remains to be determined whether the observed passive behavior and low Rm are attributable to the intrinsic properties of membrane ion channels or to gap junction coupling in functionally mature astrocytes. In the present study, freshly dissociated hippocampal tissues were used as a new model to examine this basic question in young adult animals. The morphologically intact single astrocytes could be reliably dissociated from animals postnatal day 21 and older. At this animal age, dissociated single astrocytes exhibit passive conductance and resting membrane potential similar to those exhibited by astrocytes in situ. To precisely measure the Rm from single astrocytes, dual-patch single-astrocyte recording was performed. We show that dissociated single astrocytes exhibit a low Rm similarly to syncytial coupled astrocytes. Functionally, the symmetric expression of high-K+ conductance enabled rapid change in the intracellular K+ concentrations in response to changing K+ drive force. Altogether, we demonstrate that freshly dissociated tissue preparation is a highly useful model for study of the functional expression and regulation of ion channels, receptors, and transporters in astrocytes and that passive behavior and low Rm are the intrinsic properties of mature astrocytes.
Keywords: astrocytes, passive membrane conductance, membrane resistance, patch-clamp recording, hippocampus
the characteristic linear current-to-voltage (I-V) membrane conductance (passive conductance) was first described in the seminal neuroglia study by Stephen Kuffler and colleagues (Kuffler et al. 1966). In the early 1990s, the existence of a glial subtype showing the same electrophysiological characteristic was confirmed by two pioneer patch-clamp studies from white matter and gray matter, and these glial cells were termed “passive astrocytes” (Berger et al. 1991; Steinhauser et al. 1992). Since then, passive conductance has been emerging as a general feature of astrocytes from low to high species, including humans (Han et al. 2013; Schroder et al. 2000; Zayas-Santiago et al. 2014). In the hippocampus, the passive astrocyte becomes the only electrophysiological phenotype after the third postnatal week; therefore, the loss of rectifying channel conductances appears to be an indication of functional maturation of astrocytes (Kafitz et al. 2008; Zhou et al. 2006).
As for the nature of passive behavior of membrane conductance, it remains to be directly determined whether the conductance signifies the properties of membrane ion channels or is otherwise caused by extensive cell coupling. Recordings from both excised outside-out astrocyte membrane patches and astrocytes in situ in the absence of connexin43/30 all favored the view that gap junctional conductance is unlikely to be the cause of passive membrane behavior (Schools et al. 2006; Wallraff et al. 2006). However, inhibition of gap junctions in olfactory ensheathing cells did convert the passive conductance to a rectifying conductance (Rela et al. 2010). Therefore, a definitive answer to this question is essential for our in-depth understanding of the molecular identity of the channels and the role of passive conductance in basic astrocyte function (Walz 2000).
In the present study, a modified cell isolation protocol has been achieved with a gentle enzymatic and mechanic treatment of brain tissues, and the yielded viable astrocytes are identified based on the wholeness of the intact domain territory that can be readily confirmed by astrocytic marker sulforhodamine-101 (SR-101) (Nimmerjahn et al. 2004). In addition, this method yields dissociated tissue blocks containing variable astrocytes from single to multiple cells; thus this study model should be highly useful for physiological and pharmacological analysis of basic membrane ionic activity and gap junctional communication in the future. We show that the passive behavior and the low membrane resistance are intrinsic properties of membrane ion channels, and the symmetrically expressed high-K+ conductance enables rapid change in transmembrane K+ gradient in functionally mature hippocampal astrocytes.
MATERIALS AND METHODS
Animals.
All the experimental procedures were performed in accordance with a protocol approved by the Animal Care and Use Committees of The Ohio State University. All the experiments were performed from the C57BL/6J male and female mice at postnatal days (P) 21–28.
Preparation of acute hippocampal slices.
Hippocampal slices were prepared as described previously. Briefly, brains were rapidly removed from skulls and placed into ice-cold oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (aCSF) slice cutting solution with reduced Ca2+ and increased Mg2+ (in mM: 125 NaCl, 3.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 0.1 CaCl2, 3 MgCl2, and 10 glucose). Coronal hippocampal slices (250 μm) were cut at 4°C with a Vibratome (Pelco 1500) and transferred to the oxygenated standard aCSF (in mM: 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3.5 KCl, 2 CaCl2, 1 MgCl2, and 10 glucose, osmolality 295 ± 5 mosM; pH 7.3–7.4), recovering at room temperature for at least 1 h before recording or SR-101 incubation.
Fresh dissociation of hippocampal tissues.
The method used in this study was modified from a protocol we have reported previously (Wang et al. 2013). Coronal hippocampal slices at 250-μm thickness were sectioned and incubated in oxygenated aCSF. For tissue dissociation, one to three slices were transferred from standard aCSF to oxygenated Ca2+-free aCSF at 34°C supplemented with 10 μM astrocytic marker SR-101 for 30 min. After incubation, the CA1 regions were dissected out from slices, cut into small pieces (1 mm2), and then transferred into a 1.5-ml Eppendorf tube containing oxygenated aCSF supplemented with 24 U/ml papain and 0.8 mg/ml l-cysteine for a short (7-min) incubation at 25°C. After papain digestion, the loosened tissues were gently triturated five to seven times into a cell suspension and transferred into the recording chamber mounted on the microscope. In each cell suspension, up to 40 small tissue blocks were yielded that contained single to up to 15 astrocytes. The number of astrocytes and their domain territories in each tissue block can be readily visualized based on SR-101 staining (Fig. 1). An important difference in cell selection criterion is to choose those astrocytes with their domain territories largely intact. The dissociated cells were allowed 3–5 min to touch down to the bottom of the chamber before a constant aCSF perfusion was switched on at a flow rate of 2 ml/min. The whole cell recordings were made at least 10 min after the settlement of cells to the recording chamber. Morphologically intact single dissociated astrocytes were identified by SR-101 fluorescence. The dissociated cell suspensions were discarded 2 h after papain digestion, and the above procedure was repeated to obtain a new dissociated cell suspension in the same day's experiment.
Fig. 1.
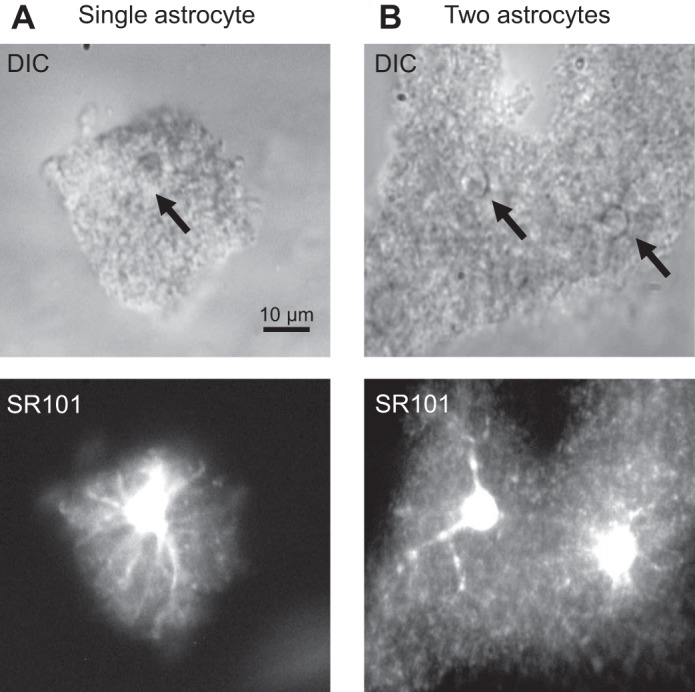
Morphology of astrocytes in freshly dissociated hippocampal tissues. A and B, top: differential interference contrast (DIC) images of 2 freshly dissociated hippocampal blocks. The block in A was identified as a single astrocyte based on the existence of a single, glial-like soma with diameter <10 μm and well-preserved domain territory, and the block in B contained 2 astrocytes whose identification was based on the same criteria (B). A and B, bottom: the initial morphological cell identification could be readily confirmed by the respective sulforhodamine-101 (SR-101) staining.
Imaging acquisition.
A fluorescent imaging system (Polychrome V system; Till Photonics, Munich, Germany) was used for identification of astrocytes from freshly dissociated tissues and vitalization of SR-101 staining (Fig. 1). This system was also used for high-resolution visualization of small glial soma for placing electrodes onto individual astrocytes for dual-patch single-astrocyte recording (Ma et al. 2014).
Electrophysiology.
For brain slice recording, individual hippocampal slices were transferred to the recording chamber mounted on an Olympus BX51WI microscope, with constant perfusion of oxygenated aCSF (2.0 ml/min). Astrocytes located in the CA1 region were visualized using an infrared differential interference contrast video camera. Whole cell patch-clamp recordings were performed using a MultiClamp 700A amplifier and pClamp 9.2 software (Molecular Devices, Sunnyvale, CA). Borosilicate glass pipettes (Warner Instrument) were pulled from a micropipette puller (model P-87; Sutter Instrument). The recording electrodes had a resistance of 3–7 MΩ when filled with the electrode solution containing (in mM) 140 K-gluconate or KCl, 13.4 Na-gluconate, 0.5 CaCl2, 1.0 MgCl2, 5 EGTA, 10 HEPES, 3 Mg-ATP, and 0.3 Na-GTP (280 ± 5 mosM). In the experiments presented in Fig. 5, the intracellular K+ was fully substituted by Na+. In the experiments presented in Figs. 3 and 5E, the bath K+ concentrations were altered by equimolar substitution of K+ by Na+.
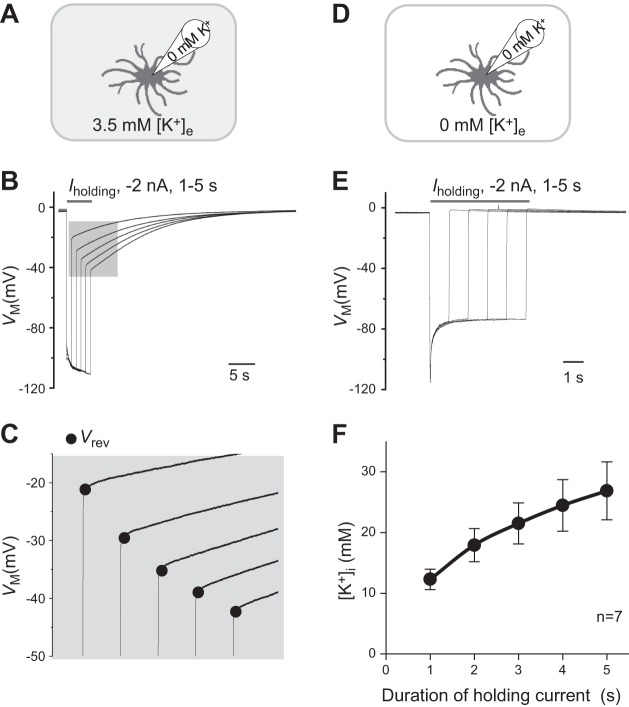
Fig. 5.
Passive conductance enables a rapid change in transmembrane K+ concentrations. A: illustration of experimental paradigm where the K+ ions in the electrode were fully replaced by Na+ ions while the bath K+ remained at physiological [K+]e of 3.5 mM. B: single astrocyte recorded under the condition shown in A in current-clamp mode; a −2-nA holding current (Iholding) pulse was applied at incremental durations from 1 to 5 s. In between these Iholding pulses, the cell was maintained at Iholding = 0 for Vm recovery back to resting levels. The longer the duration of the Iholding pulse, the larger the induced maximal Vm hyperpolarization. Also, the longer the duration of the Iholding pulse, the more negative the reversal potential (Vrev), indicating more accumulation of K+ inside astrocytes (see expanded scale in C). D: illustration of experimental paradigm where both the electrode and bath K+ ions were substituted by Na+ ions. E. under the experimental condition shown in D, Vrev returned to the resting potential level immediately, indicating a high selectivity of passive conductance to K+. F: according to the Goldman-Hodgkin-Katz equation, the estimated intracellular K+ concentrations ([K+]i), corresponding to the Vrev values, are plotted against the Iholding pulse durations. Values are means ± SE.
Fig. 3.
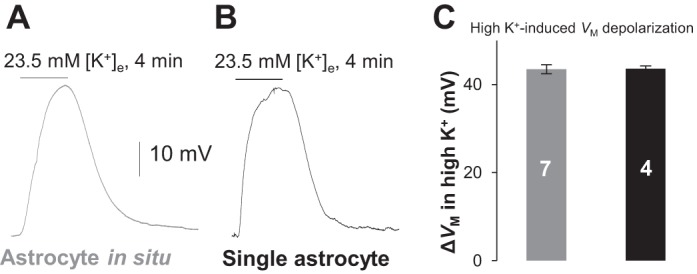
Functional K+ channels in single astrocytes are well-preserved after tissue dissociation. A and B: Vm recordings from a syncytial coupled astrocyte (left) and a single astrocyte (right) that responded to elevation of bath K+ concentration ([K+]e) from 3.5 to 23.5 mM with similar Vm depolarization amplitude. C: high K+-induced Vm depolarization (ΔVm) was comparable between single and syncytial coupled astrocytes. Values are means ± SE; numerals on bars indicate the number of observations.
The membrane potential (Vm) was recorded under current-clamp mode in pClamp 9.2 software. The liquid junction potential was compensated for before the establishment of the cell-attached mode in all recordings. In current-clamp recording, the input resistance (Rin) was measured by “resistance test” (a 63 pA/600 ms pulse) before and after recording. Recordings with initial Rin >25 MΩ or in which Rin varied more than 10% during recording were discarded.
The procedure for dual-patch single-astrocyte recording was detailed in our previous report (Ma et al. 2014). Briefly, the Polychrome V system was used for high-resolution cell visualization. A pair of PatchStar micromanipulators (Scientifica, Uckfield, UK) was used for automatic loading of two electrodes to the top of the brain slice or dissociated tissue. Other electrophysiological hardware and software were the same as for single-electrode recording described above. All the experiments were conducted at room temperature.
Chemical reagents.
SR-101 was purchased from Invitrogen (New York, NY). All other chemicals and salts used in intracellular and extracellular solutions were purchased from Sigma-Aldrich.
Data analyses.
The intracellular K+ concentrations ([K+]i) were calculated from the Goldman-Hodgkin-Katz (GHK) equation in the following form:
where [x]i and [x]e are intracellular and extracellular ion concentrations, respectively, and E is the voltage across the membrane. For astrocytes, PK is 1, PCl is assumed to be 0, and PNa is 0.015 (Stephan et al. 2012).
The patch-clamp recording data were analyzed using Clampfit 9.0 (Molecular Devices) and Origin 8.0 (OriginLab, Northampton, MA). Results are means ± SE. Statistical analysis was performed using Student's t-test. Significance level was set at P < 0.05.
RESULTS
Astrocytes in freshly dissociated tissues show intact cell morphology and domain territories.
Severing of the peripheral processes is a major concern for the use of freshly isolated astrocytes in electrophysiology study, because the processes are the primary cellular locations where ion channels, receptors, and transporters perform cellular functions (Kimelberg et al. 2000). In our improved tissue dissociation protocol, the dissociated hippocampal tissues from young adult animals contained single to multiple astrocytes that could be readily identified by SR-101 staining (Fig. 1). Guided by SR-101 staining, viable astrocytes inside the tissues could be readily identified on the basis of somatic shape and the well-preserved spatial domain that matched closely to astrocytes in situ (Bushong et al. 2002). The isolated single astrocytes were morphologically similar to those reported previously with a similar cell dissociation protocol (Haseleu et al. 2013). These cell selection criteria were used in the following study.
Single dissociated astrocytes exhibit the same passive conductance as astrocytes in situ.
In gap junction-coupled olfactory ensheathing cells, gap junction inhibition altered a linear membrane conductance to a rectifying conductance (Rela et al. 2010), indicating that gap junction coupling is able to modify the rectification characteristics of membrane ion channels. To definitively answer the nature of passive behavior of membrane conductance, the whole cell patch-clamp recording was made from single dissociated astrocytes. First, the dissociated single astrocytes showed a comparable whole cell membrane potential to astrocytes in situ: −78.42 ± 1.85 mV (n = 11) in single astrocytes vs. −78.06 ± 0.67 mV (n = 11) in astrocytes in situ (P > 0.05; Fig. 2D), suggesting that dissociation procedure itself exerted barely detectable damage to the functional membrane K+ channels. Second, the passive behavior was not significantly altered in freshly dissociated astrocytes compared with astrocytes in situ (Fig. 2, A and B). We next used the rectification index (RI) (Wang et al. 2013) to quantitatively compare whether removal of gap junction coupling altered the passive behavior of astrocyte conductance. The RI value in single astrocytes, 0.89 ± 0.01 (n = 4), was comparable to that of astrocytes in situ, 0.93 ± 0.02 (n = 14) (P = 0.177; Fig. 2E). Altogether, freshly dissociated single astrocytes show comparable membrane potential and RI; thus the passive behavior should primarily reflect the intrinsic property of membrane ion channels, instead of an epiphenomenon resulting from cell coupling.
Fig. 2.
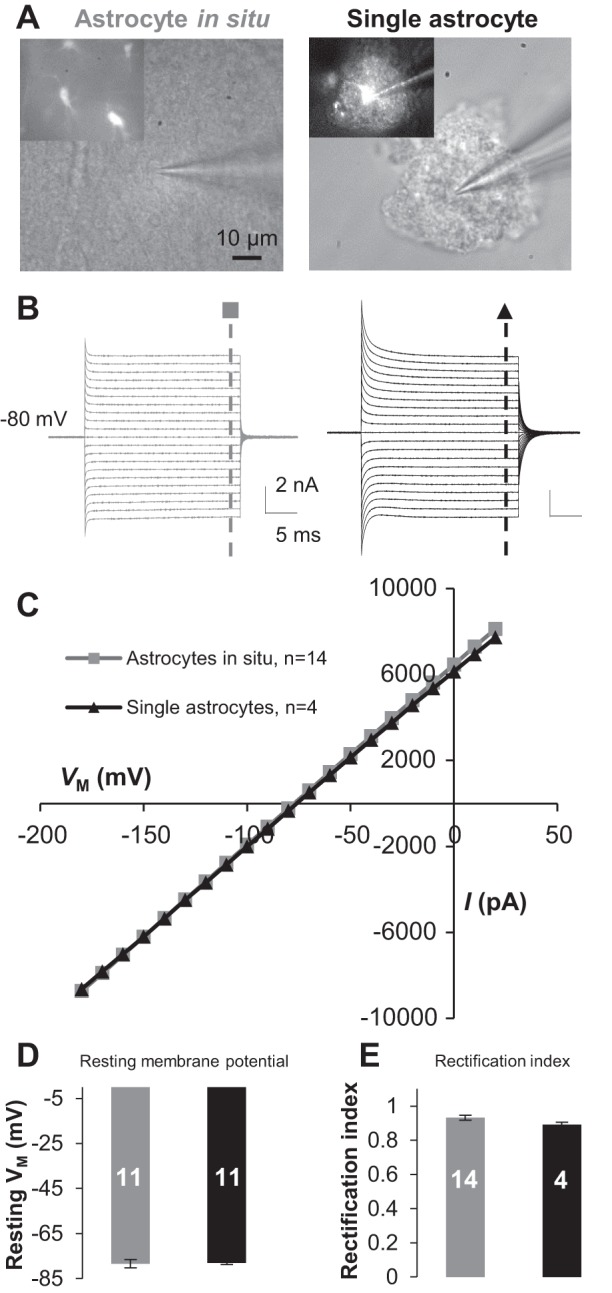
Freshly dissociated single astrocytes exhibit the same passive conductance and resting membrane potential (Vm) as astrocytes do in situ. A: DIC images were obtained during patch recording from a syncytial coupled astrocyte (left) and a single astrocyte in freshly dissociated tissue preparation (right). B and C: the astrocytes in A showed a similar passive membrane conductance (B) with comparable amplitudes in current-voltage (I-Vm) plots (C). D: the single astrocytes showed Vm comparable to that of the astrocytes in situ. E: the rectification index (RI) values were comparable between single and syncytial coupled astrocytes. Values are means ± SE; numerals on bars indicate the number of observations.
Single dissociated astrocytes show intact K+ channel function.
To further confirm that the activity of functional K+ channels remains largely intact in freshly dissociated astrocytes, we compared the response of Vm to high-K+ bath application. As shown in Fig. 3, in response to the elevation of bath K+ concentrations from 3.5 to 23.5 mM, single dissociated astrocytes and astrocytes in situ showed comparable amplitude in Vm depolarization: 43.55 ± 0.64 mV (n = 4) vs. 43.44 ± 1.02 mV (n = 7) (P > 0.05). These results further show that viable astrocytes with unchanged K+ channel activity can be readily dissociated using our improved method.
Gap junctional coupling has little contribution to an astrocyte's low membrane resistance.
An extremely low Rm causes ∼80% of voltage error in astrocyte voltage-clamp recording in situ (Ma et al. 2014; Park et al. 2013; Zhou et al. 2009). We have shown that single astrocytes exhibit comparable whole cell current amplitude in both inward and outward directions (Fig. 1), suggesting a similarly low Rm in dissociated single astrocytes compared with syncytial coupled astrocytes. To precisely measure the voltage error as well as the Rm from single astrocytes and compare them with values for astrocytes in situ, we used dual-patch single-astrocyte recording (Ma et al. 2014) in the following experiment (Fig. 4A). With this method, one electrode is used for conventional voltage-clamp recording that records the command voltage (Vcom)-induced membrane currents (I), whereas the second electrode is set up in current-clamp mode (no holding currents) and records the Vcom-induced actual membrane potential (Vm) fall on the membrane (Fig. 4A) (Ma et al. 2014). The Vcom-induced Vm was comparable between single dissociated astrocytes and astrocytes in situ (n = 6; Fig. 4B), suggesting that in the absence of gap junctional coupling, the voltage-clamp quality was as similarly poor as for astrocytes in situ.
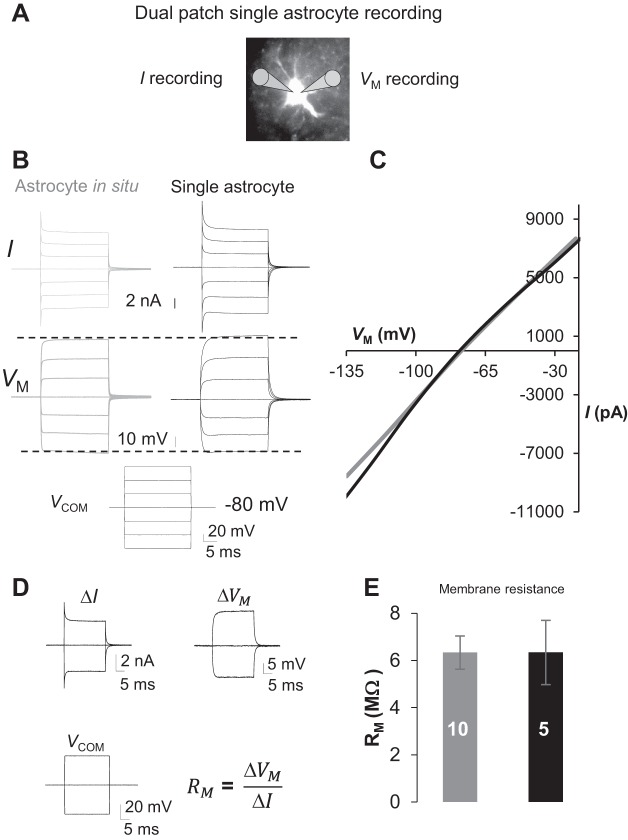
Fig. 4.
Poor voltage-clamping quality remains unchanged in single dissociated astrocytes due to the low membrane resistance (Rm). A: illustration of dual-patch single-astrocyte recording from a dissociated astrocyte; the electrode at left was set up for conventional voltage-clamp recording of command voltage (Vcom)-induced membrane current (I), and the electrode at right was set up in current-clamp mode to report the actual Vcom-induced drop in Vm. B: measured I and Vm from single and syncytial coupled astrocytes. C: measured Vm and I values shown in B were used in I-Vm plots, where the overall voltage-clamping quality did not show an improvement. D: dual-patch single-astrocyte recording for accurate Rm measurement. The changes in Vcom-induced current (ΔI) and measured actual current (ΔVm) were used for Rm analysis according to the equation shown in D. E: single astrocytes showed a low RM similar to that of syncytial coupled astrocytes. Values are means ± SE; numerals on bars indicate the number of observations.
We recently showed that an extremely low Rm is causal for the poor voltage-clamp quality and that dual-patch single-astrocyte recording allows more accurate analysis of Rm (Ma et al. 2014). Thus dual-patch single-astrocyte recording was used to precisely measure Rm in single dissociated astrocytes. A pair of ±40-mV voltages was used for simultaneous dual-patch measurement of I and Vm for Rm calculation (Fig. 4D). The results showed that the Rm in single astrocytes, 6.34 ± 1.36 (n = 5), was comparably as low as in syncytial coupled astrocytes, 6.33 ± 0.71 MΩ (n = 10) (P > 0.05). Thus, to a great extent, the passive behavior and low Rm reflect the intrinsic channel expression in astrocytes.
Passive conductance enables rapid change in transmembrane K+ gradient.
The passive conductance implies an abundant and combined expression of multiple leak-type K+ channels, such as inwardly rectifying Kir4.1, two-pore domain K+ channels, and Ca2+-activated K+ channels (Chever et al. 2010; Chu et al. 2010; Djukic et al. 2007; Hwang et al. 2014; Kucheryavykh et al. 2007; Longden et al. 2011; Olsen and Sontheimer 2008; Seifert et al. 2009; Skatchkov et al. 2006; Tong et al. 2014). The extremely low Rm shown in single astrocytes also suggests that the transmembrane K+ gradient could be easily altered in the event of a rapid change in transmembrane K+ driving force.
To demonstrate this, we created the conditions where the intracellular K+ ions were completely substituted by Na+ ions at the beginning of the experiment (Fig. 5A), and negative holding current (Iholding) pulses was applied to the recorded cells with an incremental duration from 1 to 5 s. The prolonged inward K+ drive force should result in an increasing accumulation of intracellular K+ that can be calculated by GHK equation from the reversal potential (Vrev) measured immediately after the release of Iholding pulses. As shown in Fig. 5B, the incremental Iholding pulses induced a duration-dependent negative shift in the maximal Vm hyperpolarization, and this was associated with a duration-dependent negative shift in Vrev (shaded areas, Fig. 5C). According to the GHK calculation, the resulting Vrev corresponded to an Iholding duration-dependent increase in intracellular K+ concentrations. For instance, 1- and 5-s negative Iholding resulted in a net intracellular K+ accumulation of 12.28 ± 1.66 and 26.84 ± 4.77 mM, respectively (n = 7; Fig. 5F). Thus a transient inward K+ driving force would be enough to induce a substantial accumulation of intracellular K+ ions. In the absence of extracellular K+ ions (Fig. 5D), release of negative Iholding returned the Vrev directly to the resting Vm (Fig. 5E). Therefore, the passive conductance enables highly efficient flux of K+ ions across astrocyte membrane.
DISCUSSION
In the present study, an improved cell isolation protocol was developed to yield morphologically and functionally intact astrocytes from a young adult brain. Aided by this technical advancement, we show that the passive behavior and low Rm primarily reflect the intrinsic property of the membrane ion channels of mature astrocytes. This new astrocyte model should be highly valuable for examining the basic functional properties of individual astrocytes without the influence from the syncytial coupling and unpredictable secondary effects from the surrounding neurons.
Freshly dissociated hippocampal tissues as a new model for study of astrocyte function.
The use of acutely isolated astrocytes has generally been considered a highly valuable model to gain insight into the basic property and function of astrocytes (Kimelberg et al. 2000). During development, astrocyte gene expression and function change dramatically (Cahoy et al. 2008; Sun et al. 2013). Thus, to understand the basic function of astrocytes in the adult brain, the very first step is to reliably learn the basic properties of astrocytes acutely dissociated from adult brain. However, successful isolation of morphologically and functionally intact astrocytes from adult brains remains technically challenging.
In the advent of SR-101 as an excellent astrocyte marker (Nimmerjahn et al. 2004), we aimed at a cell isolation protocol that would yield astrocytes with domain territories as well-preserved as their counterparts in situ. In the present study, we found that with reduced enzymatic treatment time and softened strength in mechanical tissue trituration, this improved cell dissociation method can reliably yield hippocampal tissues that contain single to multiple viable astrocytes. The morphology of astrocytes in the tissues can be clearly identified on the basis of SR-101 staining, and an important criterion for choosing astrocytes in the following functional study is the wholeness of the domain territory (Fig. 1).
At the functional levels, we have shown that the resting membrane potential and the overall K+ channel activity did not show obvious differences in freshly dissociated astrocytes compared with astrocytes in brain slices (Figs. 2 and 3), indicating the preservation of this essential functional property after tissue dissociation.
Passive behavior and low Rm are intrinsic properties of membrane ion channels.
A definitive answer to the nature of passive behavior of astrocyte membrane conductance is the very first step for further understanding of a series of questions relevant to astrocyte physiology, such as the molecular identity of K+ channels, the influence of syncytial coupling on the membrane potential of individual astrocytes, and how the passive behavior of K+ conductance is superior to the classic GHK outwardly rectifying leak-type K+ channels for K+ uptake and release (Hille 2001).
We have previously shown that after the third postnatal week, all recorded hippocampal astrocytes exhibit an identical passive conductance (Zhou et al. 2006), and the passive conductance remains in the majority of excised outside-out astrocyte membrane patches (Schools et al. 2006). Astrocytes with the absence of major gap junction channels connexin43/30 also show an unchanged passive behavior and minimally reduced membrane conductance (Wallraff et al. 2006). On the other hand, gap junction coupling does mask the rectifying conductance in olfactory ensheathing cells and glial fibrillary acidic protein (GFAP)-expressing cells in the subventricular zone (Liu et al. 2006; Rela et al. 2010). Thus the use of freshly dissociated astrocytes from hippocampus of P21 and older mice is critical to clarify this important issue.
In the present study, we have shown that the activation kinetics of the endogenous membrane ion channels should be the primary cause of the passive behavior of membrane conductance. Furthermore, in dual-patch single-astrocyte recordings, we have demonstrated directly that the low Rm is mainly attributable to the endogenous expression of high-K+ conductance. This finding is important to guide future discovery of additional ion channels that altogether give rise to a passive behavior of astrocyte membrane conductance.
In the central nervous system, spermine/spermidine is predominantly localized in astrocytes (Laube and Veh 1997). Studies from retinal Müller glia have shown that these endogenous polyamines block the outward rectification of Kir4.1 and also modify gap junction coupling of hippocampal astrocytes in situ (Benedikt et al. 2012; Skatchkov et al. 2000). Additionally, an additive inhibition of Kir4.1 and TASK-1 two-pore domain K+ channel could be achieved by their respective channel blockers in Müller glia (Kucheryavykh et al. 2008). These observations would be highly valuable for future identification and study of the K+ channels in freshly dissociated hippocampal astrocytes.
Symmetrically expressed K+ conductance enables rapid change in transmembrane K+ gradient.
The low Rm indicates a high-density expression of K+ conductance, and one of the functional implications would be for high-efficiency K+ uptake and release (Orkand et al. 1966). In the present study, our tissue dissociation protocol uncouples single astrocytes from the syncytium, which allows experimental substitution of intracellular ions. Under the experimental condition where the intracellular K+ was completely absent, a 1- to 5-s change in inward K+ driving force resulted in a substantial accumulation of K+ from 12.28 ± 1.66 (1 s) to 26.84 ± 4.77 mM (5 s). In the same study, we also have demonstrated that removal of extracellular K+ prevented the accumulation of K+ under the same inward K+ driving force. We therefore concluded from these observations that the astrocyte membrane is highly permeable to K+ and that an important functional role of the symmetrically expressed K+ conductance is for high-efficiency K+ flux across the astrocyte membrane.
GRANTS
This work was sponsored by National Institute of Neurological Disorders and Stroke Grant R01NS062784 (to M. Zhou) and start-up funds from The Ohio State University College of Medicine (to M. Zhou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.D., B.M., and M.Z. conception and design of research; Y.D., B.M., C.M.K., C.C.A., and W.W. performed experiments; Y.D. and B.M. analyzed data; Y.D., B.M., C.M.K., and M.Z. interpreted results of experiments; Y.D., B.M., and M.Z. prepared figures; Y.D., B.M., and M.Z. drafted manuscript; C.M.K., C.C.A., and M.Z. edited and revised manuscript; M.Z. approved final version of manuscript.
REFERENCES
- Benedikt J, Inyushin M, Kucheryavykh YV, Rivera Y, Kucheryavykh LY, Nichols CG, Eaton MJ, Skatchkov SN. Intracellular polyamines enhance astrocytic coupling. Neuroreport 23: 1021–1025, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Schnitzer J, Kettenmann H. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slice. J Neurosci 11: 3008–3024, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: 183–192, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chever O, Djukic B, McCarthy KD, Amzica F. Implication of Kir4.1 channel in excess potassium clearance: an in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J Neurosci 30: 15769–15777, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KC, Chiu CD, Hsu TT, Hsieh YM, Huang YY, Lien CC. Functional identification of an outwardly rectifying pH- and anesthetic-sensitive leak K+ conductance in hippocampal astrocytes. Eur J Neurosci 32: 725–735, 2010. [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27: 11354–11365, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12: 342–353, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseleu J, Anlauf E, Blaess S, Endl E, Derouiche A. Studying subcellular detail in fixed astrocytes: dissociation of morphologically intact glial cells (DIMIGs). Front Cell Neurosci 7: 54, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Cells. Sunderland, MA: Sinauer, 2001. [Google Scholar]
- Hwang EM, Kim E, Yarishkin O, Woo DH, Han KS, Park N, Bae Y, Woo J, Kim D, Park M, Lee CJ, Park JY. A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat Commun 5: 3227, 2014. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Meier SD, Stephan J, Rose CR. Developmental profile and properties of sulforhodamine 101-labeled glial cells in acute brain slices of rat hippocampus. J Neurosci Methods 169: 84–92, 2008. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Cai Z, Schools G, Zhou M. Acutely isolated astrocytes as models to probe astrocyte functions. Neurochem Int 36: 359–367, 2000. [DOI] [PubMed] [Google Scholar]
- Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 55: 274–281, 2007. [DOI] [PubMed] [Google Scholar]
- Kucheryavykh YV, Shuba YM, Antonov SM, Inyushin MY, Cubano L, Pearson WL, Kurata H, Reichenbach A, Veh RW, Nichols CG, Eaton MJ, Skatchkov SN. Complex rectification of Muller cell Kir currents. Glia 56: 775–790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol 29: 768–787, 1966. [DOI] [PubMed] [Google Scholar]
- Laube G, Veh RW. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 19: 171–179, 1997. [DOI] [PubMed] [Google Scholar]
- Liu X, Bolteus AJ, Balkin DM, Henschel O, Bordey A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia 54: 394–410, 2006. [DOI] [PubMed] [Google Scholar]
- Longden TA, Dunn KM, Draheim HJ, Nelson MT, Weston AH, Edwards G. Intermediate-conductance calcium-activated potassium channels participate in neurovascular coupling. Br J Pharmacol 164: 922–933, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Xu G, Wang W, Enyeart JJ, Zhou M. Dual patch voltage clamp study of low membrane resistance astrocytes in situ. Mol Brain 7: 18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 1: 31–37, 2004. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem 107: 589–601, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29: 788–806, 1966. [DOI] [PubMed] [Google Scholar]
- Park H, Han KS, Oh SJ, Jo S, Woo J, Yoon BE, Lee CJ. High glutamate permeability and distal localization of Best1 channel in CA1 hippocampal astrocyte. Mol Brain 6: 54, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rela L, Bordey A, Greer CA. Olfactory ensheathing cell membrane properties are shaped by connectivity. Glia 58: 665–678, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J Neurophysiol 96: 1383–1392, 2006. [DOI] [PubMed] [Google Scholar]
- Schroder W, Hinterkeuser S, Seifert G, Schramm J, Jabs R, Wilkin GP, Steinhauser C. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia 41, Suppl 6: S181–S184, 2000. [DOI] [PubMed] [Google Scholar]
- Seifert G, Huttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, Steinhauser C. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci 29: 7474–7488, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatchkov SN, Eaton MJ, Krusek J, Veh RW, Biedermann B, Bringmann A, Pannicke T, Orkand RK, Reichenbach A. Spatial distribution of spermine/spermidine content and K+-current rectification in frog retinal glial (Muller) cells. Glia 31: 84–90, 2000. [DOI] [PubMed] [Google Scholar]
- Skatchkov SN, Eaton MJ, Shuba YM, Kucheryavykh YV, Derst C, Veh RW, Wurm A, Iandiev I, Pannicke T, Bringmann A, Reichenbach A. Tandem-pore domain potassium channels are functionally expressed in retinal (Muller) glial cells. Glia 53: 266–276, 2006. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Berger T, Frotscher M, Kettenmann H. Heterogeneity in the membrane current pattern of identified glial cells in the hippocampal slice. Eur J Neurosci 4: 472–484, 1992. [DOI] [PubMed] [Google Scholar]
- Stephan J, Haack N, Kafitz KW, Durry S, Koch D, Hochstrate P, Seifert G, Steinhauser C, Rose CR. Kir4.1 channels mediate a depolarization of hippocampal astrocytes under hyperammonemic conditions in situ. Glia 60: 965–978, 2012. [DOI] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339: 197–200, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, Khakh BS. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci 17: 694–703, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26: 5438–5447, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia 31: 95–103, 2000. [DOI] [PubMed] [Google Scholar]
- Wang W, Putra A, Schools GP, Ma B, Chen H, Kaczmarek LK, Barhanin J, Lesage F, Zhou M. The contribution of TWIK-1 channels to astrocyte K+ current is limited by retention in intracellular compartments. Front Cell Neurosci 7: 246, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas-Santiago A, Agte S, Rivera Y, Benedikt J, Ulbricht E, Karl A, Davila J, Savvinov A, Kucheryavykh Y, Inyushin M, Cubano LA, Pannicke T, Veh RW, Francke M, Verkhratsky A, Eaton MJ, Reichenbach A, Skatchkov SN. Unidirectional photoreceptor-to-Muller glia coupling and unique K+ channel expression in Caiman retina. PLoS One 9: e97155, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol 95: 134–143, 2006. [DOI] [PubMed] [Google Scholar]
- Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci 29: 8551–8564, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]