Abstract
Brief bouts of sublethal ischemia have been shown to protect exposed tissue (ischemic conditioning) and tissues at remote sites (remote ischemic conditioning) against subsequent ischemic challenges. Given that the mechanisms of this protective phenomenon are multifactorial and epigenetic, we postulated that remote limb ischemic conditioning (RLIC) might enhance mechanisms responsible for neural plasticity, and thereby facilitate learning. Specifically, we hypothesized that conditioning of the nervous system with RLIC, achieved through brief repetitive limb ischemia prior to training, would facilitate the neurophysiological processes of learning, thus making training more effective and more long-lasting. Eighteen healthy adults participated in this study; nine were randomly allocated to RLIC and nine to sham conditioning. All subjects underwent seven consecutive weekday sessions and 2-wk and 4-wk follow-up sessions. We found that RLIC resulted in significantly greater motor learning and longer retention of motor performance gains in healthy adults. Changes in motor performance do not appear to be due to a generalized increase in muscle activation or muscle strength and were not associated with changes in serum brain-derived neurotrophic factor (BDNF) concentration. Of note, RLIC did not enhance cognitive learning on a hippocampus-dependent task. While future research is needed to establish optimal conditioning and training parameters, this inexpensive, clinically feasible paradigm might ultimately be implemented to enhance motor learning in individuals undergoing neuromuscular rehabilitation for brain injury and other pathological conditions.
Keywords: remote limb ischemic conditioning, motor learning, behavioral training
ischemic conditioning is an endogenous phenomenon in which exposure of a target organ or tissue to one or more brief episodes of sublethal ischemia results in protection of that organ against subsequent ischemia (Dirnagl et al. 2009; Gidday 2006; Iadecola and Anrather 2011; Stetler et al. 2014). Beneficial effects of ischemic conditioning were first demonstrated in the heart (Jennings et al. 1991; Murry et al. 1986). Intraorgan conditioning, achieved via intermittent occlusion of a remote coronary artery, resulted in reduced infarct size (Przyklenk et al. 1993). Subsequently, it has been found that the effects of ischemic conditioning are not confined to within an organ and can be transferred from one organ to another, a phenomenon called remote ischemic conditioning. With this approach, a brief period of tissue ischemia at one site induces widespread systemic protection of other tissues. For example, cardiac tissue has been protected via brief renal (McClanahan et al. 1993; Pell et al. 1998) and mesenteric (Gho et al. 1996; Patel et al. 2002) ischemia. Remote ischemic conditioning is advantageous because similar levels of protection can be achieved without exposing the target tissue to direct stress (Gidday 2006).
Remote limb ischemic conditioning (RLIC) (Kharbanda et al. 2002, 2009) is a clinically feasible method to deliver ischemic conditioning, since episodes of ischemia and reperfusion can be easily induced in a limb with a blood pressure cuff. A wealth of data exists indicating the cardio- and neuroprotective benefits of RLIC in animal models (Birnbaum et al. 1997; Gidday 2006; Oxman et al. 1997; Ren et al. 2008; Tapuria et al. 2008). The clinical utility of ischemic conditioning was extended when it was discovered that the magnitude of protective effects is relatively similar regardless of whether the conditioning is provided before injury (preconditioning), during the injurious event (periconditioning), or shortly after the injury (postconditioning) (Laskey 2005; Saxena et al. 2010; Staat et al. 2005; Zhao 2013; Zhao et al. 2003).
In humans, the cardioprotective effects of RLIC have been demonstrated repeatedly (Ali et al. 2007; Botker et al. 2010; Brevoord et al. 2012; Cheung et al. 2006; Hausenloy et al. 2007) and the neuroprotective effects are beginning to emerge (Hougaard et al. 2014). RLIC is safe and well-tolerated in critically ill patients with subarachnoid hemorrhage (Koch et al. 2011), and brief repetitive bilateral arm ischemia performed twice daily for 300 consecutive days was found to significantly reduce the incidence of stroke in patients with intracranial arterial stenosis compared with disease-matched control subjects (Meng et al. 2012). Interestingly, chronic remote limb hypoperfusion that occurs as a result of untreated peripheral vascular disease also produces a neuroprotective effect in people with acute stroke as measured by clinical outcome and infarct volume (Connolly et al. 2013).
The exact mechanisms by which RLIC affords cardio- and neuroprotection remain unclear, but the robust protection obtained in preclinical studies is strongly indicative of a pleiotropic response. There is substantial evidence that supports active mechanisms involving inflammation, oxidative stress, apoptosis, excitotoxicity, metabolism, and glial and vascular function (Brooks and Andrews 2013; Hausenloy and Yellon 2008, 2011; Hess et al. 2013; Ishida et al. 1997; Koch 2013; Lim et al. 2010; Liu et al. 2009; Saxena et al. 2010; Vinten-Johansen and Shi 2013). Mounting data support the theory that multiple humoral factors may be involved (Dickson et al. 1999; Koch 2010; Koch et al. 2014) and that the conditioning stimulus triggers myriad changes in gene expression (both up- and downregulation) secondary to complex epigenetic modulations of the genome (Brand and Ratan 2013; Stapels et al. 2010; Stowell et al. 2010; Thompson et al. 2015), resulting in transient or long-lasting changes in phenotype. In fact, there appear to be two distinct windows of protection, the first of which manifests immediately after ischemic conditioning and lasts for 2–3 h and the second of which appears 12 h after ischemic conditioning and lasts for 48–72 h (Hausenloy and Yellon 2009, 2011; Kuzuya et al. 1993). The multifactorial and epigenetic nature of the mechanisms opens the possibility that RLIC might induce some of the same systemic and neural mechanisms responsible for neural plasticity, and thereby facilitate learning.
The purpose of this first-in-humans trial was to test whether RLIC, administered via repetitive blood pressure cuff inflation/deflation, could enhance motor and cognitive learning in a homogeneous group of neurologically intact human adults. This proof-of-concept study specifically probes for systemwide facilitation of learning that can be explained by the RLIC-induced generation of humoral factors, rather than focal facilitation of learning that could be attributed to cortical reorganization resulting from altered sensorimotor inputs that occur with ischemic nerve block. We hypothesized that combining RLIC with training would facilitate the neurobiological processes of learning and make training more effective and more long-lasting. We included measures of muscle activation, muscle strength, and serum brain-derived neurotrophic factor (BDNF) in an attempt to elucidate how RLIC influences learning. We show that RLIC robustly facilitates motor learning; RLIC could therefore have great potential for enhancing outcomes in a wide range of clinical applications.
MATERIALS AND METHODS
Experimental design.
This study used a repeated-measures design with nine total sessions to examine the effects of RLIC combined with motor and cognitive training on learning in neurologically intact adults (Fig. 1). This study was approved by the Washington University Human Research Protection Office and was conducted in compliance with the Helsinki Declaration. All participants provided informed consent prior to beginning the study and were compensated for their time.
Fig. 1.
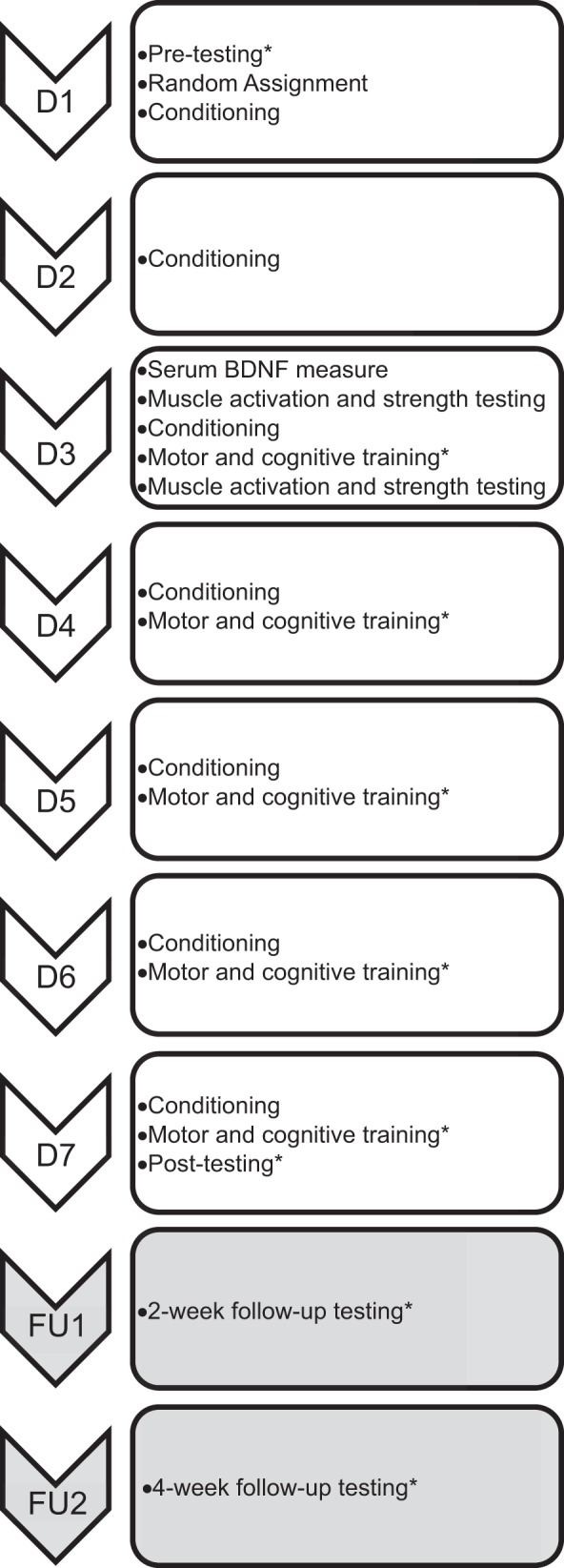
Experimental design. There were 9 total sessions: 7 consecutive weekday sessions (D1–D7) and 2 follow-up sessions (FU1, FU2) 2 and 4 wk after D7. *Items were completed in a randomized order. BDNF, brain-derived neurotrophic factor.
Participants.
Eighteen neurologically intact adults were recruited for participation in this study. Participants were included if they were 1) 18 yr of age or older and 2) had sufficient cognitive skills to actively participate, determined during a standardized interview. Exclusion criteria were determined by self-report and included 1) history of neurological condition, balance impairment, or vestibular disorder; 2) history of attentional disorders that could affect learning (ADD, ADHD); 3) history of sleep apnea (Drager et al. 2010; Yang et al. 2013); 4) history of lower extremity condition, injury, or surgery that could compromise performance on the motor task; 5) any extremity soft tissue, orthopedic, or vascular condition or injury that may contraindicate RLIC (uncontrolled hypertension, peripheral vascular disease, hematological disease, severe hepatic or renal dysfunction); 6) any cognitive, sensory, or communication problem that would prevent completion of the study; 7) current use of medication for systemic inflammation or spasticity or treatment with selective serotonin reuptake inhibitors, which could decrease nervous system excitability (Koch and Gonzalez 2013); 8) current weight lifting or interval training exercise, which could alter serum neurotrophic factors and confound the effects of RLIC (Ding et al. 2011; Yarrow et al. 2010); and 9) current substance abuse or dependence. These criteria were established to ensure a relatively homogeneous sample that optimized our potential to detect an effect of RLIC on learning.
Order of experiment.
This experiment included seven consecutive weekday sessions (D1–D7) and two follow-up sessions (FU1, FU2), shown in Fig. 1. During the first session (D1), participants provided informed consent and demographic data. Each participant then completed pretest measurements of 1) serum BDNF concentration, 2) the motor task, 3) the cognitive task, 4) finger flexor muscle activation, and 5) grip strength. Serum BDNF concentration was measured first to avoid the confounding effects of physical activity on BDNF concentrations. The sequence of pretest motor task performance, cognitive task performance, and muscle activation/grip strength performance was randomized. After pretesting, participants were randomly assigned to the RLIC or sham conditioning group via a random numbers generator in MATLAB. Participants were blinded to their group assignment, meaning that they were not told whether they were receiving RLIC or sham conditioning. Once randomized, participants underwent one set of RLIC or sham conditioning. During the second session (D2), which took place the following day, participants underwent RLIC or sham conditioning.
Participants returned for the next five consecutive weekdays for sessions that included conditioning plus training (D3–D7). These sessions consisted of RLIC or sham conditioning, immediately followed by 15–20 min of both motor and cognitive training, in a randomized order. Details of motor and cognitive training are outlined below. Five sessions that included conditioning plus training were selected because this period of time is adequate to assess learning but is unlikely to result in performance plateaus and does not present an excessive time burden for participants (McNevin et al. 2003; Schaefer and Lang 2012; Wulf et al. 2003). Learning on the motor and cognitive tasks as well as changes in muscle activation, muscle strength, and serum BDNF concentration were evaluated through posttests at the end of the seventh session (D7). Finally, two follow-up sessions (FU1, FU2) took place 2 and 4 wk after session D7. Follow-up sessions, consisting of assessments of performance on the motor and cognitive tasks, were included to evaluate retention.
Remote limb ischemic and sham conditioning.
RLIC was achieved via blood pressure cuff inflation to 200 mmHg on the nondominant upper extremity (Botker et al. 2010; Hausenloy et al. 2007; Koch et al. 2011; Meng et al. 2012). Sham conditioning was achieved via blood pressure cuff inflation to 10 mmHg below the individual's diastolic blood pressure, also on the nondominant upper extremity (Koch and Gonzalez 2013). The nondominant extremity was determined by self-report and was chosen as a practical matter, in order to ensure consistency in administration. Periodically, a pulse oximeter was placed on the index finger of the conditioned arm in order to confirm that ischemia was being achieved in participants in the RLIC group (oxygen saturation reading of 0 or “error”) and that ischemia was not occurring in participants in the sham conditioning group (oxygen saturation reading equivalent to preconditioning measure). In addition to periodic oxygen saturation checks, visual inspection of the conditioned limb was also done intermittently to ensure that the color and temperature of the limb remained unchanged in participants undergoing sham conditioning whereas the distal limb of participants in the RLIC group was cool and pale, indicating that ischemia was occurring. One set of RLIC or sham conditioning consisted of five cycles of alternating 5 min of inflation and 5 min of deflation. These are the timing and dose of RLIC that were shown to produce effects in human cardio- and neuroprotective trials (Botker et al. 2010; Hougaard et al. 2014) and are well tolerated by participants. One set of RLIC or sham conditioning was performed in sessions D1–D7. The goal of including conditioning without training during the first two sessions (D1, D2) was to prepare the central nervous system for subsequent motor and cognitive training. To assess the effect of participant blinding, participants were asked to identify whether they believed that they had been assigned to the RLIC or sham conditioning group upon completion of the study.
We monitored participants for pain and/or adverse cardiovascular effects before, during, and after RLIC or sham conditioning. Treatment was terminated if 1) pain levels were >6 on a 10-point numerical pain rating scale, 2) oxygen saturation levels were <80%, 3) heart rate was <40 or >110 beats/min, or 4) systolic blood pressure was <80 mmHg or >150 mmHg. For the purpose of safety monitoring, oxygen saturation, heart rate, and blood pressure were measured on the arm not undergoing conditioning.
Motor task.
The motor task used for this study was a stability platform balance task (Lafayette Instrument model 16030L) (Cherry et al. 2014; Taubert et al. 2010). The stability platform itself features integrated electronic tilt angle measurements (angle measurement resolution 1.0°), experimenter-selectable balance thresholds (angle limit setting resolution 1.0°), digital angle readouts, platform re-zero ability, and built-in timing functions for test and rest timing (timing resolution 0.001 s). This task engages multiple systems of the brain including the vestibular system, visual system, motor system, somatosensory system, and cognitive centers. Successful performance on the stability platform task requires that a subject anticipate changes in posture and coordinate muscle activation in response to self-induced perturbations. The stability platform task was selected because decreased dynamic balance is a significant contributor to fall risk (Toraman and Yildirim 2010) and maintaining stability during dynamic activities is required for daily activities such as walking and reaching. Moreover, we chose this motor task that engages a broad range of brain systems in order to distinguish the systemic neuroplastic effects of RLIC from focal changes in the sensorimotor cortex that could result from brief periods of limb deafferentation. Participants were instructed to stand on the platform with feet facing forward and to keep the platform level for as many seconds as possible during a 30-s trial. Performance was quantified by measuring the cumulative amount of time (to the tenth of a second) that a participant maintained the stability platform ±3° of horizontal during each trial. Participants performed 90 total trials including 5 pretest trials during D1, 15 trials on each during D3–D7, and 5 trials during each of the two follow-up sessions (FU1, FU2). The final five trials during D7 (trials 76–80) served as the posttest measure of performance on the stability platform. Trials were separated by 30 s of rest. A hand rail was positioned in front of the stability platform for safety. Use of the handrail was permitted for initial balance and positioning; however, no upper extremity support was allowed during a trial. After each trial on the stability platform, participants were told how many seconds they maintained the platform in balance. No feedback was given regarding balance or postural strategies, allowing participants to develop their own techniques and adapt their movement strategies based on trial and error.
Cognitive task.
An associative recognition task was used to measure hippocampus-based learning (Bunge et al. 2004; Duzel et al. 2003). Participants were given a study period during which they were shown 60 “target” nonword-image pairs in a random order (stimulus presentation = 750 ms, interstimulus interval = 1,000 ms). Participants were instructed to associate and remember the 60 target pairs. After the study period, participants completed a trial in which they were shown 60 pairs in a random order and were instructed to indicate whether each pair was a target pair or a novel “foil” pair by pressing the 1 or 2 key, respectively, on a standard keyboard (stimulus presentation + response time = 750 ms, interstimulus interval = 1,000 ms). Foil pairs were pairs in which the nonword, the image, or both were not target items or in which the nonword and the image were incorrectly paired target items. The number of target pairs included in a trial ranged from 15 to 35 pairs. This range was established to increase task difficulty and prevent participants from discerning a pattern of the number of “target” and “foil” responses for a given trial. Each participant performed one pretest (D1), five training (D3–D7), and two follow-up (FU1, FU2) trials of the associative recognition task. The trial in D7 served as the posttest measure of performance on the associative recognition task. The participants were not given a study period before the follow-up assessments and thus were required to remember target pairs for approximately 2 and 4 wk.
Instructions, stimuli, and feedback were presented electronically on a laptop computer screen with E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Nonwords (e.g., “piction”) were displayed in size 18 Courier New font on the left side of the screen while the image of a familiar object (e.g., apple, chair) appeared on the right side of the screen. Nonwords were selected from a database of nonwords provided by the English Lexicon Project (Balota et al. 2007). All nonwords were pronounceable and five to seven letters in length and had no more than two syllables. Images were selected from a bank of Microsoft Clip Art. All images were single items, considered to be familiar to most American adults.
Performance on the associative recognition task was evaluated with a measure of discriminability (Pr) and reaction time (RT) (Snodgrass and Corwin 1988). Pr reflects the degree of accuracy with which a participant is able to discriminate between target pairs and foil pairs. Higher values indicate greater discriminability. RT was defined as the amount of time between stimulus presentation and subject response, averaged across all correct responses. Response times shorter than 150 ms were considered anticipatory responses and were excluded from RT analysis. Participants received immediate feedback about the accuracy of each response as the word(s) “correct,” “incorrect,” or “no response detected” appeared on the screen for 500 ms after each stimulus. These variables were computed with the following formulas (Snodgrass and Corwin 1988; Wolf et al. 2011):
| (1) |
where hit rate = (0.5 + no. of correct targets)/(1 + no. of total targets) and false alarm rate = (0.5 + no. of incorrect foils)/(1 + no. of total foils).
| (2) |
Data from two participants (1 in each group) were excluded from cognitive task analysis. Reasons for exclusion were 1) an abundance of anticipatory responses during all trials, indicating a lack of task engagement (sham conditioning participant), and 2) limited command of the English language (RLIC participant).
Measures of muscle activation and strength.
To distinguish the effects of learning from more generalized increases in muscle activation and strength, we measured finger flexor electromyographic (EMG) activity and grip strength of the upper extremity. We chose measures of the upper extremity in order to dissociate the effects of RLIC alone from neuromuscular changes in the lower extremity that may occur as a consequence of motor training on the stability platform. The dominant side was selected because the muscles of the dominant upper extremity did not experience direct limb ischemia and that arm was not explicitly trained by other tasks. Muscle activation of the long finger flexors was measured during three trials of maximal isometric force measurements of grip strength. Participants were instructed to squeeze a handheld dynamometer with as much force as possible and to maintain that force for ∼5 s, until the auditory cue “relax” was given. Approximately 10 s of rest was given between trials to prevent muscle fatigue. Muscle activation and strength measurements were taken for each participant at the pretest, before and after RLIC/sham conditioning in session D3 (within-session changes), and at posttest in session D7 (across-sessions changes).
Muscle activation was measured via EMG with surface electrodes positioned over the flexor digitorum superficialis and profundus muscle bellies on the dominant arm (Janda et al. 1987). EMG was recorded with a sampling rate of 1,000 Hz and a tethered EMG system (Noraxon U.S.A.). EMG data were processed off-line in MATLAB R2012a (MathWorks, Natick, MA) with custom-written software. Signals were full-wave rectified and smoothed with a second-order Butterworth low-pass filter with a cutoff frequency of 20 Hz (Farina et al. 2004; Reaz et al. 2006). EMG data were quantified by averaging the amplitude of the EMG activation during the middle 500 ms of each trial. The three trials were then averaged to yield a single value representing mean finger flexor muscle activation for a given measurement time. EMG electrodes remained in place on the participant's forearm between assessments in D3 (within session) for consistency. Grip strength performance was quantified as kilograms of force generated with the dominant hand when squeezing the handheld dynamometer (JAMAR hydraulic hand dynamometer, model SMP-5030J1, Sammons Preston) set on the third rung. Three grip strength trials were averaged to obtain a single score for a given session (Hamilton et al. 1994; Mathiowetz et al. 1984).
Serum BDNF.
We measured serum BDNF because animal models of intermittent hypoxia have shown increases in BDNF measured directly in the respiratory and nonrespiratory motor nuclei in the cervical spinal cord (Baker-Herman et al. 2004; Lovett-Barr et al. 2012; Mitchell et al. 2001; Satriotomo et al. 2012; Wilkerson and Mitchell 2009). Additionally, BDNF is the most commonly used serum marker in human learning studies at the present time (Cunha et al. 2010; Edelmann et al. 2014). A 10-ml blood withdrawal via aseptic technique from the antecubital vein was performed by a trained patient care technician from the Washington University Clinical Research Unit. Blood samples were processed by staff at the Washington University CORE Laboratory. Blood was allowed to clot at room temperature for 30 min after collection (BD Vacutainer) and was then centrifuged (Jouan GR422) for 15 min at 2,780 g. The separated sample was stored in a −80°C freezer until analysis. Serum BDNF concentration was detected in an antibody sandwich format with a BDNF enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems). BDNF values are the concentration (ng/ml) in the serum of each sample. Each participant underwent a blood draw at pretest, before conditioning in session D3, and at posttest in session D7. A comparison of pretest and D3 values allowed us to evaluate the effects of 2 days of conditioning alone on serum BDNF concentration, while comparing values from pretest and posttest allowed us to evaluate the effects of repeated sessions of conditioning plus training on serum BDNF concentration.
Data analysis.
Data were managed and stored in a secure REDCap database (Harris et al. 2009), and statistical analyses were done with SPSS Statistics 21 (IBM, Armonk, NY). In this proof-of-concept study, the criterion for statistical significance was set at α ≤ 0.05. We also examined our results at a more conservative level of 0.017 (0.05/3) to account for multiple comparisons. Actual P values are reported in the text, and interpretations are made based upon the original α-level; notations are made, however, when there is a discrepancy between significance at the original α-level and the adjusted α-level. Data were normally distributed. A repeated-measures ANOVA was used to analyze pain data, with a within-subject factor of time (7 levels: sessions D1–D7) and a between-subject factor of conditioning group (RLIC vs. sham conditioning). Mauchly's test of sphericity was performed to test the assumption of sphericity.
The analysis used to test our hypotheses involved computing change scores and their 95% confidence intervals (CIs) to quantify performance improvement from training (D7 − D1) and retention of training effects (FU1 − D7 and FU2 − D7) on the motor and cognitive tasks. Group differences between change scores were tested with independent-sample t-tests. Our hypothesis that RLIC enhances motor and cognitive learning would be supported by a significant between-group difference and 95% CIs that do not contain 0. Our hypothesis that RLIC enhances retention would be supported by significant between-group differences and 95% CIs for the RLIC group that do contain 0.
Change scores and 95% CIs for muscle activation and muscle strength measures were calculated within sessions (posttest D3 − pretest D3) and across sessions (D7 − D1), with group differences tested by independent-sample t-tests. We hypothesized that RLIC could enhance muscle activation and muscle strength both within and across sessions, as was found in a study using intermittent respiratory hypoxia (Trumbower et al. 2012). A significant group difference would support this hypothesis. The same approach was also used to test whether RLIC influenced serum BDNF levels with changes from D3 − D1 evaluating an initial conditioning effect of RLIC and changes from D7 − D1 evaluating an overall across-sessions effect. We hypothesized that individuals in the RLIC group would experience increases in serum BDNF levels from pretest (D1) to D3 and posttest (D7) beyond those experienced by individuals in the sham conditioning group; this hypothesis would be supported by positive change score values with 95% CIs that do not contain 0 in the RLIC group. The overall statistical approach was chosen because our specific hypotheses were about magnitude of change over time, i.e., comparison of slopes, and not about comparison of absolute magnitudes. This approach allowed us to decrease the number of independent-sample t-tests and reduce irrelevant post hoc comparisons as we directly tested our hypotheses. Values in results are means [95% CI] unless otherwise indicated.
RESULTS
Participants.
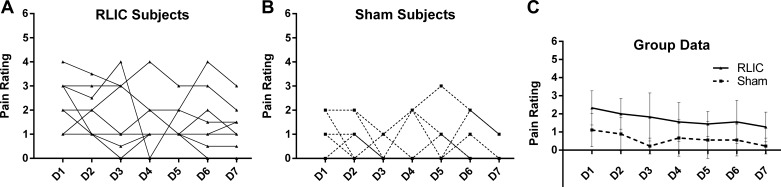
All 18 of the recruited adults were enrolled between May and August 2014 (Table 1). Half of the participants (n = 9) were randomized to the RLIC group and half to the sham conditioning group. Most participants (15/18) reported that they believed that they were in the RLIC group; one of the three subjects who reported being in the sham conditioning group was in the RLIC group. There were no significant demographic differences between groups that underwent RLIC vs. those that underwent sham conditioning (Table 1). All participants completed all nine sessions of the experiment with no serious adverse events, and no sessions had to be terminated for any reason. No periods of blood pressure cuff inflation were missed, paused, or terminated for any participant during any session. The results of a numerical pain rating scale are presented in Fig. 2. Pain scores are shown for individual participants in the RLIC group (Fig. 2A) and the sham conditioning group (Fig. 2B) as well as group averages (Fig. 2C). Individual ratings of pain were variable; no person rated pain higher than a 4/10. The RLIC group experienced slightly greater pain than the sham conditioning group, as expected (main effect of group; F = 49.335, df = 1, P < 0.001), and pain decreased across conditioning sessions (main effect of time; F = 3.714, df = 6, P = 0.002) for both groups. There was no group × interaction effect (F = 0.563, df = 6, P = 0.759). Mauchly's test of sphericity indicated that the assumption of sphericity was not violated [χ2(20) = 24.573, P = 0.239].
Table 1.
Demographic data
| Participants |
|||
|---|---|---|---|
| Characteristics | RLIC group (n = 9) | Sham conditioning group (n = 9) | Group differences (P value) |
| Age, yr | 23.1 ± 3.9 | 26.4 ± 5.9 | 0.426 |
| Sex (female/male) | 6/3 | 7/2 | 0.599 |
| Dominant side (right/left) | 7/2 | 8/1 | 0.527 |
| Height, cm | 165.9 ± 7.6 | 167.9 ± 8.4 | 0.934 |
| Weight, kg | 62.1 ± 9.3 | 68.2 ± 18.5 | 0.266 |
| Race | 0.717 | ||
| Caucasian | 6 | 6 | |
| African American | 1 | 2 | |
| Asian | 2 | 1 | |
Values represent counts or means ± SD.
Fig. 2.
Pain ratings for individual participants in the RLIC group (A) and the sham conditioning group (B) and mean pain ratings for each group (C). Error bars represent SD. Pain rating scale ranged from 0 (no pain or discomfort) to 10 (extreme pain and discomfort). The threshold for terminating remote limb ischemic conditioning (RLIC) or sham conditioning was a pain/discomfort rating of 6; thus the y-axis is set from 0 to 6. There was a significant difference in pain ratings between groups (RLIC > sham conditioning) at each time point, and both groups had decreased average pain rating over time.
Motor learning and retention.
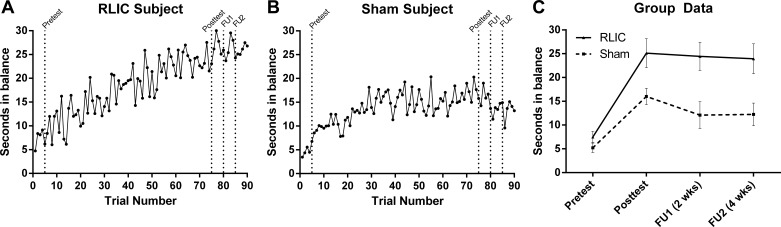
RLIC had a robust effect on motor learning (Fig. 3). Individualized learning curves are provided for a RLIC participant (Fig. 3A) and a sham conditioning participant (Fig. 3B). While both groups (Fig. 3C) improved on the trained motor task over time (RLIC: 17.6 [15.7, 19.5] s; sham conditioning: 10.8 [9.4, 12.2] s), those who underwent RLIC experienced significantly greater improvements in motor learning from pretest to posttest, compared with those who underwent sham conditioning [t(16) = −6.49, P < 0.001]. Moreover, RLIC group improvements were retained over time at the first (−0.7 [−1.7, 3.0] s) and second (−1.2 [−1.0, 3.3] s) follow-ups. In the sham conditioning group, improvements were not fully retained at both follow-ups (−3.9 [−2.0, −5.8] s and −3.8 [−2.0, −5.6] s, respectively). The RLIC group had significantly greater retention than the sham conditioning group 2 wk after intervention [FU1: t(16) = 2.474, P = 0.025] and was approaching significance at the 4 wk follow-up [FU2: t(16) = 2.124, P = 0.05]. When the adjusted α-level of α ≤ 0.017 was applied, the RLIC group did not have statistically greater retention than the sham conditioning group at FU1 or FU2.
Fig. 3.
Motor task performance. A and B: individual training data for a participant in the RLIC group (A) and a participant in the sham conditioning group (B). C: group data at pretest (D1), at posttest (D7), and at 2 and 4 wk follow-up sessions (FU1, FU2); data points represent the average of 5 trials, and error bars represent SD. Individuals in the RLIC group had significantly greater improvements in motor performance from pretest to posttest, and these improvements were retained at 2 and 4 wk follow-up.
Cognitive learning and retention.
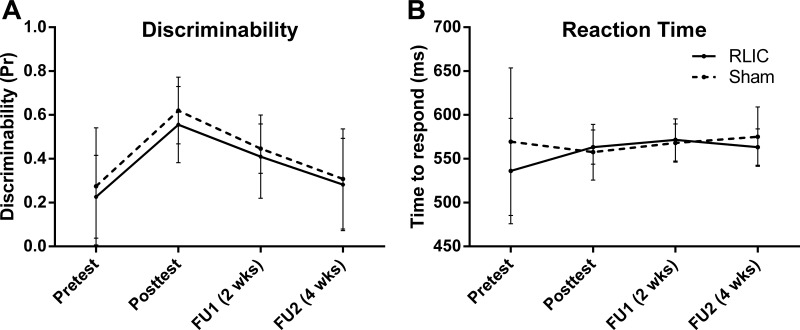
Data from the cognitive test are shown in Fig. 4. Overall, discriminability scores were low (chance = 0; all correct = 1) at the pretest and improved by the posttest (Fig. 4A). Both groups had improved discriminability on the associative recognition task from pretest to posttest (RLIC: 0.33 [0.13, 0.53]; sham conditioning: 0.35 [0.15, 0.54]). RLIC did not, however, enhance cognitive learning as measured by discriminability [t(14) = 0.14, P = 0.892]. RTs were highly variable and did not change over the course of training (Fig. 4B; RLIC: 27.2 [−25.46, 79.83] ms; sham conditioning: −11.9 [−87.43, 63.70] ms), and there was no effect of RLIC on RT [t(14) = −1.00, P = 0.333].
Fig. 4.
Cognitive task performance. Mean discriminability (A) and reaction time (B) for each group at pretest (D1), at posttest (D7), and at 2 and 4 wk follow-up sessions (FU1, FU2). Error bars represent SD. RLIC did not enhance cognitive learning.
Muscle activation and strength.
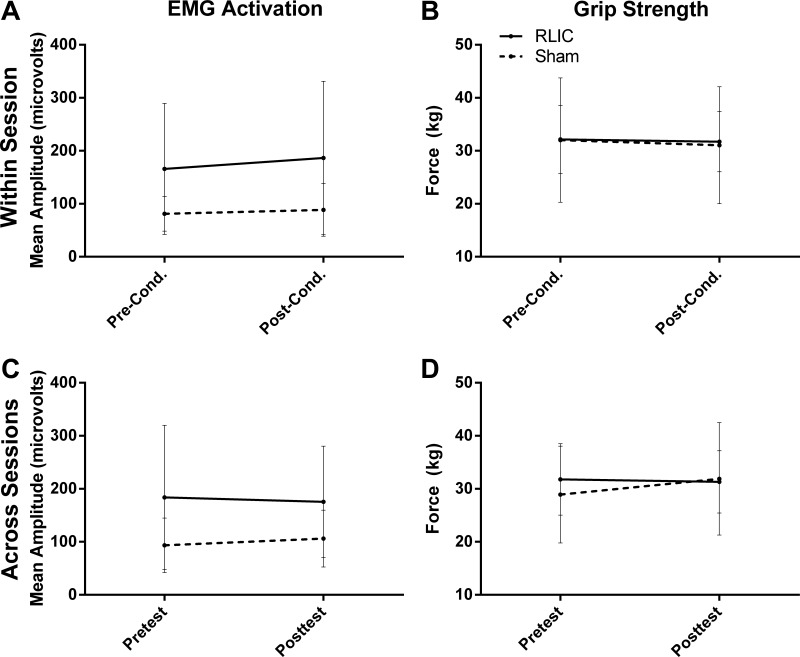
RLIC alone did not result in increased muscle activation or muscle strength (Fig. 5). Assessment of muscle activation and grip strength within a session (D3 pre- and D3 postconditioning) showed that neither the RLIC group nor the sham conditioning group increased muscle activation (RLIC: 20.51 [−2.64, 43.66] μV; sham conditioning: 7.33 [−16.92, 31.59] μV) or grip strength (RLIC: −0.45 [−2.44, 1.53] kg; sham conditioning: −0.94 [−2.85, 0.96] kg) and that there was no effect of RLIC [Fig. 5, A and B; muscle activation: t(16) = −0.91, P = 0.378; grip strength: t(16) = −0.41, P = 0.688]. Similarly, assessment of muscle activation across sessions (D1 pretest–D7 posttest) showed that neither the RLIC group nor the sham conditioning group increased over time (RLIC: −8.27 [−65.48, 48.93] μV; sham conditioning: 12.59 [−27.08, 52.26] μV) and that there was no facilitative effect of RLIC [Fig. 5C; t(16) = 0.69, P = 0.499]. Across-sessions measures of grip strength did not change in the RLIC group (−0.45 [−2.66, 1.75] kg), but the sham conditioning group showed a small increase (2.94 [0.31, 5.57] kg) leading to a statistically significant group difference [Fig. 5D; t(16) = 2.28, P = 0.037]. This difference in the sham conditioning group became nonsignificant when the adjusted α-level of α ≤ 0.017 was applied.
Fig. 5.
Mean finger flexor muscle activation and grip strength for each group. Error bars represent SD. A and B: RLIC did not enhance EMG activation (A) or grip strength (B) within a session (before and after conditioning in session D3). C and D: RLIC did not enhance EMG activation (C) or grip strength (D) across sessions (pretest vs. posttest).
Serum BDNF.
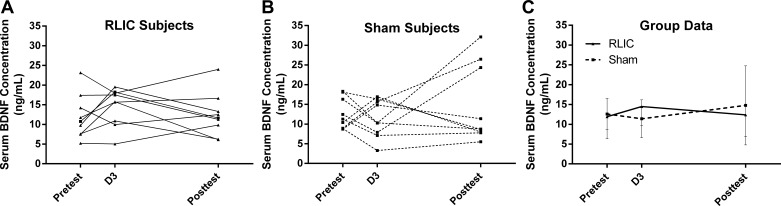
Neither participants in the RLIC group nor participants in the sham conditioning group had increased serum BDNF concentration either from D1 to D3 (RLIC: 2.56 [−1.57, 6.70] ng/ml; sham conditioning: −1.18 [−5.76, 3.39] ng/ml) or from D1 pretest to D7 posttest (RLIC: −2.10 [−6.50, 2.31] ng/ml; sham conditioning: 3.37 [−4.89, 11.64] ng/ml). Participants who underwent RLIC treatment did not have significantly different serum BDNF concentration change scores compared with those who received sham conditioning at either time point [t(16) = −1.40, P = 0.180; t(16) = 1.35, P = 0.197]. Individual data (Fig. 6, A and B) are provided along with group data (Fig. 6C) to highlight the within-subject variability of BDNF levels.
Fig. 6.
Serum BDNF concentrations. A and B: individual BDNF values are shown for participants in the RLIC group (A) and the sham conditioning group (B). C: group data are shown at pretest (D1), before conditioning or training in D3, and at posttest (D7). RLIC did not result in an increase in serum BDNF concentration compared with sham conditioning, either after 2 days of conditioning alone or after repeated sessions of conditioning + training.
DISCUSSION
Our results indicate that combining RLIC with motor training results in significantly improved motor learning and retention in healthy adults. These changes in motor performance were not due to a generalized increase in muscle activation and/or muscle strength; nor were they associated with distinct changes in serum BDNF concentrations after conditioning or after training, relative to baseline. Despite our robust findings in the motor domain, RLIC did not enhance cognitive learning on a hippocampus-dependent memory task. To the best of our knowledge this study represents the first time that RLIC has been used to promote human motor learning. This paradigm is inexpensive and clinically feasible and could be easily implemented to enhance motor learning in individuals undergoing neuromuscular rehabilitation for brain injury and other conditions.
RLIC enhanced motor learning and retention.
It was initially thought that the mechanisms through which RLIC affords protection to tissues involved neural pathways and an afferent signal from the remote organ that stimulated the efferent limb of the reflex in distant tissues (Gho et al. 1996). This hypothesis was disputed, however, by a subsequent study in a porcine model that showed that RLIC led to protection within a transplanted heart that did not have intact afferent nerves (Konstantinov 2005). Additional support for the theory that RLIC-facilitated protection is mediated by humoral factors was provided by a study that found similar protection against infarction following coronary artery ligation in rabbits that underwent RLIC themselves and those that were pretreated with plasma from donor rabbits who underwent RLIC (Shimizu et al. 2009). While the exact nature of signal transduction from remote tissue to target organ remains to be fully elucidated, the prevailing theory is that RLIC liberates one or more humoral factors that circulate to have multitissue protective effects (Hausenloy and Yellon 2009). The fact that we demonstrated a robust enhancement of motor learning in an activity that did not directly involve the use of the conditioned limb indicates that the mechanisms through which RLIC enhances neuroplasticity and motor learning are also likely to be humoral in nature.
Deafferentation, induced by upper limb ischemia, has been shown to cause reorganization of the contralateral motor cortex (Brasil-Neto et al. 1993; McNulty et al. 2002). This deafferentation-induced reorganization can be purposefully modulated (Ziemann et al. 1998a), and it has been hypothesized that it is due to a decrease in local GABA concentrations leading to a release of latent cortiocortical projections from tonic inhibition (Levy et al. 2002; Ziemann et al. 1998b). Short-term deprivation of sensory input by ischemic nerve block also elicits functional changes in the homotopic regions of the cortex contralateral to the deafferented one through transcallosal interactions and GABAergic transmission (Werhahn et al. 2002). We cannot rule out that some of same mechanisms may be involved; it is not likely, however, that they are the exact same mechanisms because there are significant differences between the protocols that were used in the limb deafferentation studies and ours. Most importantly, those studies investigated neuroplasticity in the cortical regions that corresponded to the deafferented limb or in the analogous motor region of the contralateral hemisphere, while we assessed motor learning in terms of performance on a standing dynamic balance task. Because performance on our stability platform balance task does not require the direct use of the conditioned upper extremity, it is extremely unlikely that the changes in balancing performance were a consequence of motor reorganization that resulted from cortical deafferentation that occurred during RLIC. Another key difference between the limb deafferentation studies and ours is that those studies used a single prolonged bout of limb ischemia rather than employing brief repeated cycles of ischemia followed by reperfusion. The cardio- and neuroprotective literature indicates that in virtually every species tested 5 min per bout of RLIC is optimal (Ishida et al. 1997) and that the systemic effects of RLIC are dependent on the alternating pattern of occlusion and reperfusion (Hausenloy and Yellon 2009; Ishida et al. 1997; Zhao 2009).
One possibility is that the pain induced by RLIC causes a release of factors that could have enhanced motor learning, given that pain is associated with a host of functional, anatomical, and chemical changes in the nervous system (Siddall 2013). However, we did not find any relationships between average pain rating and motor learning in our data (data not reported). Pain processing is highly complex, and while it can lead to neuroplasticity, the changes are found primarily in the somatosensory cortex (Flor et al. 1997). Because performance on our stability platform balance task does not require the use of the upper extremity that received the sometimes painful stimulus, it is unlikely that the changes in balancing performance were a consequence of pain-induced sensory reorganization.
The data presented in this report also align well with findings from an emerging area of research investigating the potential of intermittent respiratory hypoxia, achieved by reducing the oxygen content of inhaled air, to induce plasticity. These studies demonstrate that exposing an animal to a transient state of respiratory hypoxia induces plasticity that manifests as a strengthening of spared pathways to motoneurons and improved motor recovery after neurological injury (Baker and Mitchell 2000; Fuller et al. 2003; Golder and Mitchell 2005; Ling et al. 2001; Lovett-Barr et al. 2012; Turner and Mitchell 1997). Specifically, intermittent respiratory hypoxia improves both phrenic (Dale-Nagle et al. 2010; Fuller et al. 2003; Lovett-Barr et al. 2012) and forelimb (Lovett-Barr et al. 2012) motor function in rodent models of chronic cervical spinal cord injury. The cascade of molecular and epigenetic changes induced by intermittent respiratory hypoxia is complex, and it remains unclear whether there are shared mechanisms between those involved with plasticity after intermittent respiratory hypoxia and those activated by RLIC.
RLIC did not increase cognitive learning or retention.
Given that the mechanisms by which RLIC leads to augmented plasticity in our study and to neuroprotective phenotypes are thought to be humoral, we anticipated enhanced learning to occur in both motor and cognitive domains. The most likely explanation for our finding is that the associative recognition task, which many participants reported to be extremely challenging, offered only a narrow dynamic range in which participants could improve. Overall performance on the associative recognition task was poor, and RT did not change over the course of training. The task was likely too difficult and therefore was not a good test of the hypothesis. An alternative explanation for our findings is that we did not detect an improvement in cognitive learning because of the specificity of the associative recognition task. While the motor task engaged many systems of the brain including the vestibular system, visual system, motor system, somatosensory system, and cognitive centers, the cognitive task that we used was largely hippocampally dependent (Bunge et al. 2004; Duzel et al. 2003; Yonelinas et al. 2001). Tasks that require multiple brain regions or networks may generate a larger learning “signal” that would be more easily detected at the behavioral level. A nondeclarative, nonmotor task, such as a serial RT task, may be more analogous to the motor learning task that showed an effect of RLIC and could be used in subsequent studies. A task with a wider range should be used in future probes of cognitive learning.
RLIC did not cause an increase in muscle activation or muscle strength.
The fact that we did not find changes, and more specifically large changes, within or across sessions at the neuromuscular level secondary to RLIC suggests that the observed motor performance gains were not a consequence of improved muscle activation or muscle force generation but rather due to learning of the complex relationship between stabilizing the balance board and controlling upright posture. In contrast to our findings, intermittent respiratory hypoxia appears to have a facilitative effect on the neuromuscular system in persons with incomplete spinal cord injury, with increased lumbosacral motor output (43 ± 17% to 83 ± 23%) and maximal voluntary plantar flexion torque (82 ± 33%) occurring within a session (Trumbower et al. 2012) and augmented walking speed and endurance occurring across five sessions (Hayes et al. 2014). Such a discrepancy may reflect differences between some or all of the specific mechanisms underlying plasticity following RLIC relative to those activated by intermittent respiratory hypoxia.
RLIC did not cause an increase in serum BDNF.
Given that neurotrophic growth factors such as BDNF have key roles in many forms of neuroplasticity (Schinder and Poo 2000; Thoenen 1995, 2000), and that BDNF is a key contributor to neuroplasticity resulting from intermittent respiratory hypoxia (Baker-Herman et al. 2004; Baker-Herman and Mitchell 2002; Wilkerson and Mitchell 2009), we hypothesized that increased BDNF production by hippocampal (Conner et al. 1997; Hall et al. 2000) and corticospinal (Giehl et al. 1998) neurons could be a mechanism by which RLIC enhances learning. Our serum BDNF data were highly variable and showed no consistent changes secondary to RLIC or training. One possible explanation for our negative BDNF result is that we measured serum BDNF, while studies in animal models measured BDNF content directly in the respiratory and nonrespiratory motor nuclei in the cervical spinal cord (Baker-Herman et al. 2004; Lovett-Barr et al. 2012; Satriotomo et al. 2012). Another probable explanation for our findings is that serum BDNF is highly variable and is influenced by a multitude of factors including age (Lommatzsch et al. 2005; Katoh-Semba et al. 2007), sex (Lommatzsch et al. 2005), exercise (Ferris et al. 2007; Tang et al. 2008), sleep (Cirelli et al. 2006; Hairston et al. 2004), menstrual cycle (Cubeddu et al. 2011; Lommatzsch et al. 2005), and genetics (Egan et al. 2003; Kleim et al. 2006). We tried to minimize the external factors through our exclusion criteria and by maintaining consistency regarding the time of day that the blood was drawn, but many of these factors were beyond our control. Moreover, BDNF, if it does play a role in the observed phenotype, is likely only one of many factors involved in this mechanism. Future studies investigating the phenomenon of RLIC to promote motor learning could benefit from “bench-to-bedside” experiments in animal models in which more spatially and temporally dependent measures of BDNF and other potential mediators of plasticity can be measured.
Ideally there would already be an established serum biomarker of plasticity that we could use to assess the learning response to RLIC in humans. Unfortunately, at the present time there are no serum markers of neuroplasticity that accurately reflect physiological events underlying neuroplasticity (Corbett et al. 2014; Cramer et al. 2011; Jickling and Sharp 2011; Krakauer et al. 2012; Milot and Cramer 2008; Pearson-Fuhrhop et al. 2009). Knowledge of mechanisms gained from preclinical cardio- and neuroprotection experiments suggests that a threshold for tissue ischemia needs to be crossed to produce the desired effect and that, once the threshold is crossed, a cascade of adaptive signaling events ensues. Therefore, it might be wiser at this point in time to explore serum biomarkers of ischemic conditioning (Gidday 2006; Koch et al. 2014). Because serum markers of ischemic conditioning suffer from the same challenges as those of neuroplasticity, future studies may consider screening a panel of potential serum markers from each of the major signaling pathways known to be altered by conditioning (Koch et al. 2014). This approach would permit simultaneous examination of changes in serum markers and behavior.
Conclusions and significance.
The evidence presented here suggests that RLIC can enhance motor learning and retention in young adults. The effects of age, genetics, and comorbidities on responses induced by RLIC are not yet understood (Hausenloy and Yellon 2011). Future research is needed to explore the effects of each of these potential confounders and identify the characteristics of people who will be most responsive to the intervention. Additionally, before RLIC is introduced in a clinical population, optimal conditioning, training, and RLIC parameters need to be established that maximize learning effects while minimizing adverse responses (Brooks and Andrews 2013). Eventually, RLIC could represent a safe (Bilgin-Freiert et al. 2012; Gonzalez et al. 2014), inexpensive, and clinically feasible way to enhance motor learning and improve rehabilitation outcomes in individuals recovering from neurological injury or other motor impairment.
GRANTS
This research was funded by the Foundation for Physical Therapy and the Mr. and Mrs. Spencer T. Olin Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.M.C.-A., J.M.G., J.-M.L., and C.E.L. conception and design of research; K.M.C.-A. performed experiments; K.M.C.-A., J.-M.L., T.H., and C.E.L. analyzed data; K.M.C.-A., J.M.G., J.-M.L., T.H., and C.E.L. interpreted results of experiments; K.M.C.-A. prepared figures; K.M.C.-A. drafted manuscript; K.M.C.-A., J.M.G., J.-M.L., T.H., and C.E.L. edited and revised manuscript; K.M.C.-A., J.M.G., J.-M.L., T.H., and C.E.L. approved final version of manuscript.
REFERENCES
- Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, Gaunt ME. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 116: 98–105, 2007. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R. The English Lexicon Project. Behav Res Methods 39: 445–459, 2007. [DOI] [PubMed] [Google Scholar]
- Bilgin-Freiert A, Dusick JR, Stein NR, Etchepare M, Vespa P, Gonzalez NR. Muscle microdialysis to confirm sublethal ischemia in the induction of remote ischemic preconditioning. Transl Stroke Res 3: 266–272, 2012. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation 96: 1641–1646, 1997. [DOI] [PubMed] [Google Scholar]
- Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375: 727–734, 2010. [DOI] [PubMed] [Google Scholar]
- Brand D, Ratan RR. Epigenetics and the environment: in search of the “toleroasome” vital to execution of ischemic preconditioning. Transl Stroke Res 4: 56–62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain 116: 511–525, 1993. [DOI] [PubMed] [Google Scholar]
- Brevoord D, Kranke P, Kuijpers M, Weber N, Hollmann M, Preckel B. Remote ischemic conditioning to protect against ischemia-reperfusion injury: a systematic review and meta-analysis. PLoS One 7: e42179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks MJ, Andrews DT. Molecular mechanisms of ischemic conditioning: translation into patient outcomes. Future Cardiol 9: 549–568, 2013. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cogn 56: 141–152, 2004. [DOI] [PubMed] [Google Scholar]
- Cherry KM, Lenze EJ, Lang CE. Combining d-cycloserine with motor training does not result in improved general motor learning in neurologically intact people or in people with stroke. J Neurophysiol 111: 2516–2524, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47: 2277–2282, 2006. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Faraguna U, Tononi G. Changes in brain gene expression after long-term sleep deprivation. J Neurochem 98: 1632–1645, 2006. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17: 2295–2313, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M, Bilgin-Freiert A, Ellingson B, Dusick JR, Liebeskind D, Saver J, Gonzalez NR. Peripheral vascular disease as remote ischemic preconditioning, for acute stroke. Clin Neurol Neurosurg 115: 2124–2129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Nguemeni C, Gomez-Smith M. How can you mend a broken brain? Neurorestorative approaches to stroke recovery. Cerebrovasc Dis 38: 233–239, 2014. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D, Chen WG, Cohen LG, deCharms C, Duffy CJ, Eden GF, Fetz EE, Filart R, Freund M, Grant SJ, Haber S, Kalivas PW, Kolb B, Kramer AF, Lynch M, Mayberg HS, McQuillen PS, Nitkin R, Pascual-Leone A, Reuter-Lorenz P, Schiff N, Sharma A, Shekim L, Stryker M, Sullivan EV, Vinogradov S. Harnessing neuroplasticity for clinical applications. Brain 134: 1591–1609, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu A, Bucci F, Giannini A, Russo M, Daino D, Russo N, Merlini S, Pluchino N, Valentino V, Casarosa E, Luisi S, Genazzani AR. Brain-derived neurotrophic factor plasma variation during the different phases of the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology 36: 523–530, 2011. [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci 3: 1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann NY Acad Sci 1198: 252–259, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO. Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis 8: 123–129, 1999. [DOI] [PubMed] [Google Scholar]
- Ding Q, Ying Z, Gomez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience 192: 773–780, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 8: 398–412, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 24: 843–851, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci 23: 9439–9444, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann E, Lessmann V, Brigadski T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 76C: 610–627, 2014. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269, 2003. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol 96: 1486–1495, 2004. [DOI] [PubMed] [Google Scholar]
- Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 39: 728–734, 2007. [DOI] [PubMed] [Google Scholar]
- Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 224: 5–8, 1997. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23: 2993–3000, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94: 2193–2200, 1996. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7: 437–448, 2006. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Schutte A, Mestres P, Yan Q. The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism. J Neurosci 18: 7351–7360, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 25: 2925–2932, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez NR, Connolly M, Dusick JR, Bhakta H, Vespa P. Phase I clinical trial for the feasibility and safety of remote ischemic conditioning for aneurysmal subarachnoid hemorrhage. Neurosurgery 75: 590–598, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston IS, Peyron C, Denning DP, Ruby NF, Flores J, Sapolsky RM, Heller HC, O'Hara BF. Sleep deprivation effects on growth factor expression in neonatal rats: a potential role for BDNF in the mediation of delta power. J Neurophysiol 91: 1586–1595, 2004. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci 3: 533–535, 2000. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Balnave R, Adams R. Grip strength testing reliability. J Hand Ther 7: 163–170, 1994. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 370: 575–579, 2007. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 79: 377–386, 2008. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis 204: 334–341, 2009. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol 8: 619–629, 2011. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82: 104–113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Hoda MN, Bhatia K. Remote limb perconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke? Stroke 44: 1191–1197, 2013. [DOI] [PubMed] [Google Scholar]
- Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Hansen TM, von Weitzel-Mudersbach P, Simonsen CZ, Damgaard D, Gottrup H, Svendsen K, Rasmussen PV, Ribe LR, Mikkelsen IK, Nagenthiraja K, Cho TH, Redington AN, Botker HE, Ostergaard L, Mouridsen K, Andersen G. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 45: 159–167, 2014. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 14: 1363–1368, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock 8: 86–94, 1997. [DOI] [PubMed] [Google Scholar]
- Janda DH, Geiringer SR, Hankin FM, Barry DT. Objective evaluation of grip strength. J Occup Environ Med 29: 569–571, 1987. [PubMed] [Google Scholar]
- Jennings RB, Murry CE, Reimer KA. Preconditioning myocardium with ischemia. Cardiovasc Drugs Ther 5: 933–938, 1991. [DOI] [PubMed] [Google Scholar]
- Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurotherapeutics 8: 349–360, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Wakako R, Komori T, Shigemi H, Miyazaki N, Ito H, Kumagai T, Tsuzuki M, Shigemi K, Yoshida F, Nakayama A. Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. Int J Dev Neurosci 25: 367–372, 2007. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106: 2881–2883, 2002. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet 374: 1557–1565, 2009. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci 9: 735–737, 2006. [DOI] [PubMed] [Google Scholar]
- Koch S. Preconditioning the human brain: practical considerations for proving cerebral protection. Transl Stroke Res 1: 161–169, 2010. [DOI] [PubMed] [Google Scholar]
- Koch S. Moving towards preconditioning for neurological disorders: are we ready for clinical trials? Transl Stroke Res 4: 15–18, 2013. [DOI] [PubMed] [Google Scholar]
- Koch S, Della-Morte D, Dave KR, Sacco RL, Perez-Pinzon MA. Biomarkers for ischemic preconditioning: finding the responders. J Cereb Blood Flow Metab 34: 933–941, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Gonzalez N. Preconditioning the human brain: proving the principle in subarachnoid hemorrhage. Stroke 44: 1748–1753, 2013. [DOI] [PubMed] [Google Scholar]
- Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke 42: 1387–1391, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a KATP channel-dependent mechanism. Transplantation 79: 1691–1695, 2005. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair 26: 923–931, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res 72: 1293–1299, 1993. [DOI] [PubMed] [Google Scholar]
- Laskey WK. Brief repetitive balloon occlusions enhance reperfusion during percutaneous coronary intervention for acute myocardial infarction: a pilot study. Catheter Cardiovasc Interv 65: 361–367, 2005. [DOI] [PubMed] [Google Scholar]
- Levy LM, Ziemann U, Chen R, Cohen LG. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol 52: 755–761, 2002. [DOI] [PubMed] [Google Scholar]
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 105: 651–655, 2010. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XQ, Sheng R, Qin ZH. The neuroprotective mechanism of brain ischemic preconditioning. Acta Pharmacol Sin 30: 1071–1080, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging 26: 115–123, 2005. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg 9: 222–226, 1984. [DOI] [PubMed] [Google Scholar]
- McClanahan TB, Nao BS, Wolke LJ, Martin BJ, Mertz TE, Gallagher KP. Brief renal occlusion and reperfusion reduces myocardial infarct size in rabbits (Abstract). FASEB J 7: A118, 1993. [Google Scholar]
- McNevin NH, Shea CH, Wulf G. Increasing the distance of an external focus of attention enhances learning. Psychol Res 67: 22–29, 2003. [DOI] [PubMed] [Google Scholar]
- McNulty PA, Macefield VG, Taylor JL, Hallett M. Cortically evoked neural volleys to the human hand are increased during ischaemic block of the forearm. J Physiol 538: 279–288, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, Li G, Ren C, Luo Y, Ling F, Jia J, Hua Y, Wang X, Ding Y, Lo EH, Ji X. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 79: 1853–1861, 2012. [DOI] [PubMed] [Google Scholar]
- Milot MH, Cramer SC. Biomarkers of recovery after stroke. Curr Opin Neurol 21: 654–659, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90: 2466–2475, 2001. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol Heart Circ Physiol 273: H1707–H1712, 1997. [DOI] [PubMed] [Google Scholar]
- Patel HH, Moore J, Hsu AK, Gross GJ. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol 34: 1317–1323, 2002. [DOI] [PubMed] [Google Scholar]
- Pearson-Fuhrhop KM, Kleim JA, Cramer SC. Brain plasticity and genetic factors. Top Stroke Rehabil 16: 282–299, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 275: H1542–H1547, 1998. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87: 893–899, 1993. [DOI] [PubMed] [Google Scholar]
- Reaz M, Hussain M, Mohd-Yasin F. Techniques of EMG signal analysis: detection, processing, classification and applications. Biol Proced Online 8: 11–35, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 151: 1099–1103, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 237: 103–115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P, Newman MA, Shehatha JS, Redington AN, Konstantinov IE. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg 25: 127–134, 2010. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Lang CE. Using dual tasks to test immediate transfer of training between naturalistic movements: a proof-of-principle study. J Mot Behav 44: 313–327, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci 23: 639–645, 2000. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci 117: 191–200, 2009. [DOI] [PubMed] [Google Scholar]
- Siddall PJ. Neuroplasticity and pain: what does it all mean? Med J Aust 198: 177–178, 2013. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117: 34–50, 1988. [DOI] [PubMed] [Google Scholar]
- Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation 112: 2143–2148, 2005. [DOI] [PubMed] [Google Scholar]
- Stapels M, Piper C, Yang T, Li M, Stowell C, Xiong ZG, Saugstad J, Simon RP, Geromanos S, Langridge J, Lan JQ, Zhou A. Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance. Sci Signal 3: 111, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, Gao Y. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol 114: 58–83, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell C, Wang L, Arbogast B, Lan JQ, Cioffi GA, Burgoyne CF, Zhou A. Retinal proteomic changes under different ischemic conditions—implication of an epigenetic regulatory mechanism. Int J Physiol Pathophysiol Pharmacol 2: 148–160, 2010. [PMC free article] [PubMed] [Google Scholar]
- Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett 431: 62–65, 2008. [DOI] [PubMed] [Google Scholar]
- Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury—a review. J Surg Res 150: 304–330, 2008. [DOI] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, Ragert P. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 30: 11670–11677, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science 270: 593–598, 1995. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res 128: 183–191, 2000. [DOI] [PubMed] [Google Scholar]
- Thompson JW, Narayanan SV, Koronowski KB, Morris-Blanco K, Dave KR, Perez-Pinzon MA. Signaling pathways leading to ischemic mitochondrial neuroprotection. J Bioenerg Biomembr 47: 101–110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraman A, Yildirim NU. The falling risk and physical fitness in older people. Arch Gerontol Geriatr 51: 222–226, 2010. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26: 163–172, 2012. [DOI] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol 499: 543–550, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinten-Johansen J, Shi W. The science and clinical translation of remote postconditioning. J Cardiovasc Med (Hagerstown) 14: 206–213, 2013. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain 125: 1402–1413, 2002. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 217: 116–123, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Gerraty R, Satterthwaite TD, Loughead J, Campellone T, Elliott MA, Turetsky BI, Gur RC, Gur RE. Striatal intrinsic reinforcement signals during recognition memory: relationship to response bias and dysregulation in schizophrenia. Front Behav Neurosci 5: 81, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G, Weigelt M, Poulter D, McNevin N. Attentional focus on suprapostural tasks affects balance learning. Q J Exp Psychol A 56: 1191–1211, 2003. [DOI] [PubMed] [Google Scholar]
- Yang Q, Wang Y, Feng J, Cao J, Chen B. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: the potential roles played by microglia. Neuropsychiatr Dis Treat 9: 1077–1086, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett 479: 161–165, 2010. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NE, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: an fMRI study. Neuroreport 12: 359–363, 2001. [DOI] [PubMed] [Google Scholar]
- Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab 29: 873–885, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Hurdles to clear before clinical translation of ischemic postconditioning against stroke. Transl Stroke Res 4: 63–70, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H579–H588, 2003. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci 18: 1115–1123, 1998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci 18: 7000–7007, 1998b. [DOI] [PMC free article] [PubMed] [Google Scholar]