Abstract
The intrinsic excitability of spinal motoneurons is mediated in part by the presence of persistent inward currents (PICs), which amplify synaptic input and promote self-sustained firing. Studies using animal models have shown that PICs are greater in extensor motoneurons over flexor motoneurons, but this difference has not yet been demonstrated in humans. The primary objective of this study was to determine whether a similar difference exists in humans by recording from motor units in biceps and triceps brachii during isometric contractions. We compared firing rate profiles of pairs of motor units, in which the firing rate of the lower-threshold “control” unit was used as an indicator of common drive to the higher-threshold “test” unit. The estimated contribution of the PIC was calculated as the difference in firing rate of the control unit at recruitment versus derecruitment of the test unit, a value known as the delta-F (ΔF). We found that ΔF values were significantly higher in triceps brachii (5.4 ± 0.9 imp/s) compared with biceps brachii (3.0 ± 1.4 imp/s; P < 0.001). This difference was still present even after controlling for saturation in firing rate of the control unit, rate modulation of the control unit, and differences in recruitment time between test and control units, which are known to contribute to ΔF variability. We conclude that human elbow flexor and extensor motor units exhibit differences in intrinsic excitability, contributing to different neural motor control strategies between muscle groups.
Keywords: elbow extensors, elbow flexors, intrinsic excitability, motoneurons, neuromodulation
early studies of motoneuron behavior suggested that alpha-motoneurons fire linearly in proportion to synaptic input and are therefore passive transducers of descending motor commands. However, recent studies have shown that this is not the case (reviewed in Binder et al. 1993; Heckman et al. 2008). Alpha-motoneurons, in fact, possess complex membrane properties capable of nonlinear integration of synaptic inputs. Descending serotonergic and noradrenergic projections from brain stem nuclei are the most powerful neuromodulators of these active membrane properties (Hounsgaard et al. 1988; Lee and Heckman 1999). Monoamines from these projections increase intrinsic motoneuron excitability by depolarizing the resting potential and hyperpolarizing the voltage threshold for spike activation, as well as decreasing the duration of the postspike hyperpolarization (Fedirchuk and Dai 2004; Takahashi and Berger 1990; White and Fung 1989). They also increase motoneuron excitability through the facilitation of persistent inward sodium and calcium currents (PICs), which produce a prolonged depolarization in the cell known as a plateau potential (Bennett et al. 1998; Collins et al. 2002; Hounsgaard et al. 1984). The subsequent increase in neuronal excitability caused by the plateau potential is capable of amplifying synaptic input as much as fivefold (Lee and Heckman 2000) and allows the motoneuron to manifest unique firing properties. One of these properties includes a counterclockwise hysteresis in the frequency-current relationship, in which the amount of current required to keep a motoneuron firing is significantly lower than the amount of current required to recruit the neuron initially (Lee and Heckman 1998a, 1998b).
PICs provide a motoneuron-specific method of prolonged excitation without requiring additional descending drive from supraspinal structures. It has therefore been hypothesized that PICs are most useful for antigravity muscles or those otherwise involved in posture, which must be activated for extended periods of time (Hounsgaard et al. 1988; Kiehn and Eken 1997). Previous electrophysiological studies in the ventral spinal cord of the decerebrate cat and the neonatal rat show that PICs are larger in the extensor pools than in the flexor pools of the limbs (Cotel et al. 2009; Hounsgaard et al. 1988). However, this difference has not been shown yet in humans.
Because we are unable to record directly from individual motoneurons in humans, the magnitude of PICs must be measured indirectly by studying PIC-specific motor unit firing behaviors. The challenge of this approach is in determining whether changes in the firing behavior of a motor unit are due to its intrinsic excitability or due to changes in descending drive. Kiehn and Eken (1997) used paired-motor unit recordings to show that during slow isometric contractions a low-threshold motor unit can be used as an indicator of descending drive to the motoneuron pool. Therefore, changes in the firing behavior of higher-threshold motor units that are not also reflected in the low-threshold unit can be attributed to the intrinsic behavior of those motor units. Gorassini and colleagues (Gorassini et al. 1998, 2002) furthered this approach during ramp contractions. They recorded from pairs of motor units and measured the difference in firing rate at recruitment and derecruitment of the higher-threshold unit (named the test unit) in terms of the firing rate of the lower-threshold unit (named the control unit), producing a value known as the delta-F (ΔF). After specific constraints on the selection of motor unit pairs are employed, the ΔF is an estimation of the magnitude of the PIC in the test unit. The ΔF calculation has been validated in animal models (Bennett et al. 2001), and it has shown appropriate scaling when monoaminergic drive has been artificially increased with amphetamine in humans (Udina et al. 2010).
The purpose of this study was to investigate PICs in flexor and extensor muscles of the upper limb in humans by comparing ΔF values in an elbow flexor (biceps brachii) and an elbow extensor (triceps brachii) muscle. The animal models discussed above suggest that the triceps, as an extensor muscle, will have a higher ΔF than the biceps, as a flexor muscle. However, because humans have bipedal rather than quadrupedal locomotion, the relative lack of postural demand on the muscles of the upper limb in maintaining antigravity trunk support may lessen the need for the difference in intrinsic excitability observed in animals.
METHODS
Ten healthy adults (2 women, 8 men, aged 66.9 ± 6.2 yr) participated in the study. They were asked to abstain from caffeine for 12 h before the experiment to remove any possible effects of caffeine on PICs (Walton et al. 2002, 2003). All procedures were performed in accordance with the Declaration of Helsinki and were approved by the Institutional Review Board at Northwestern University. All subjects gave written informed consent prior to participation in the study.
Experimental arrangement.
Participants were seated in a Biodex chair (Biodex Medical Systems, Shirley, NY) with their dominant arm fixed in 75° shoulder abduction, 45° shoulder flexion (horizontal adduction from the frontal plane), 90° elbow flexion, 15° pronation, and a neutral wrist and finger posture. The participant's shoulder and waist were secured to the chair with straps to minimize auxiliary movements of the trunk. The forearm and hand were encased in a fiberglass cast and coupled via a weight-bearing ring-mount interface to a 6 degree-of-freedom load cell (model 45E15A, JR3, Woodland, CA).
Experimental procedures.
Participants were asked to generate maximum voluntary torque (MVT) for both elbow flexion and elbow extension. MVT was calculated as the average of three maximum torque values within 10% of one another without the last repetition being the greatest. Visual elbow flexion/extension torque feedback was given through a computer monitor in front of the apparatus, and participants were given vigorous verbal encouragement through the duration of the MVT measurements.
For experimental trials used to calculate ΔF, participants completed slow trapezoid contractions in elbow flexion and in elbow extension, each to a target on the computer screen representing 10–15% MVT. Each trial lasted for 40 s and began with 5 s of baseline measurements while the subject was relaxed. Participants were then instructed to flex or extend at the elbow slowly and smoothly such that they reached the target within 10 s, to hold their position at the target for 5 s, and to slowly relax the muscle over another 10 s. The trial then ended with a final 10 s of data collection. A sample trial is shown in Fig. 1A. Subjects were explicitly instructed to relax the muscle being used during the relaxation phase. To ensure proper pacing of the movement, the experimenter counted down the seconds out loud through each trial.
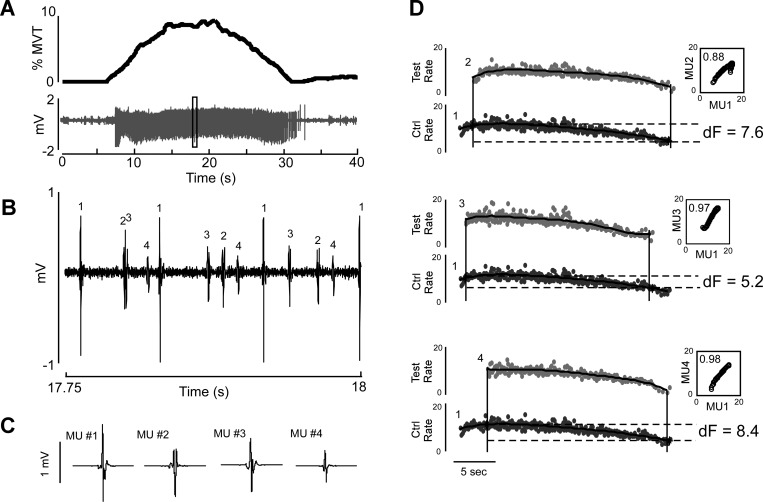
Fig. 1.
Decomposition of intramuscular EMG from triceps brachii during a single elbow extension trial. The participant made an isometric elbow extension up to 10% of maximum voluntary torque (MVT) (A, top). The intramuscular EMG signal (A, bottom) was then decomposed into its constituent motor units (example within the black box enlarged in B and high-pass filtered at 500 Hz; individual motor unit templates shown in C). ΔF values were then calculated for all possible pairwise unit comparisons (D) by fitting 5th-order polynomials to the instantaneous firing rates of the test and control units and taking the difference in firing rate of the polynomial for the lower-threshold control unit (shown here as MU1, black traces) at recruitment and derecruitment of the polynomial for the higher-threshold test unit (shown as MUs 2, 3, and 4, gray traces). Rate-rate plots were generated by plotting the firing rate of the control unit vs. the firing rate of the test unit (D, insets). Only pairs with a coefficient of determination ≥0.7 were included.
The participants completed two or three practice trials before data collection to familiarize themselves with the task. Between trials, participants were given a 1-min break, and they were asked to produce brief contractions back and forth with both agonist and antagonist, to ensure muscle quiescence before the start of the next trial (McPherson et al. 2008). Trials were visually inspected for quality and were discarded if the target was reached too quickly or too slowly, if the ascending and descending phases of the contraction were not equal to and opposite from one another, or if there were sudden increases or decreases in the torque profile during the ascending or descending arms (Udina et al. 2010). Fifty-eight of 77 (75%) collected trials were used for analysis based on the quality of the torque trace as well as clarity of decomposition.
Data collection and analysis.
Orthogonal forces and torques generated at the forearm-load cell interface in the x, y, and z planes were recorded via the load cell and converted into elbow flexion and extension torques with custom MATLAB software employing a Jacobian-based algorithm (The MathWorks, Natick, MA). Torque measurements were digitized at a sampling rate of 1,024 Hz and smoothed with an acausal moving average filter with a 250-ms window.
Intramuscular EMG in the long head of biceps brachii and the lateral head of the triceps brachii were recorded with custom bipolar fine-wire steel electrodes with 1-mm recording surfaces (221-28SS-730, Jari Electrode Supply, Gilroy, CA). Each bipolar unit had barb lengths for the two wires of 1 mm and 2.5 mm. Two electrodes were inserted into each muscle. The signals from each electrode were band-pass filtered (300-10,000 Hz) and amplified (×1-10k) (DAM50 Bio-Amplifier, World Precision Instruments, Sarasota, FL) before digitization at 10,240 Hz (OT Bioelettronica USB2, Turin, Italy). Because torque and intramuscular EMG signals had to be collected on separate computers, a brief 0- to 5-V voltage pulse was generated at the beginning of each trial and recorded by both computers as a reference point for off-line synchronization.
Intramuscular EMG recordings were collected with OTBiolab software (Fig. 1, B and C) (version 1.7.4735.19, OT Bioelettronica) and were decomposed into motor unit spike times with EMGlab software (McGill et al. 2005). Spike times were then converted into instantaneous firing rates by calculating the reciprocal of the interspike interval.
To estimate the influence of PICs in biceps versus triceps, ΔF values were calculated for every possible pair of motor units in a given trial (Fig. 1D) for which the control unit was of a lower threshold and fired during both recruitment and derecruitment of the test unit. In roughly 10% of motor unit pairs per trial the test unit continued to fire after the derecruitment of the control unit, and these pairs were not included. The instantaneous firing rates of both motor units were smoothed by fitting fifth-order polynomials (Gorassini et al. 2002), and the ΔF value was calculated on the polynomials by taking the difference between the values of the control unit at recruitment and derecruitment of the test unit.
The ΔF method relies on four assumptions: 1) that the PIC is activated before or at recruitment in an all-or-nothing manner; 2) that the firing rate of the control unit varies smoothly in proportion to synaptic input so that it can therefore be used as an indicator of that input; 3) that the processing of synaptic input is similar between the test and control units; and 4) that the test and control units share a similar common drive (Gorassini et al. 1998, 2004). To evaluate common drive, the polynomials of the test and control units were plotted against one another in a rate-rate plot. Linear regression was used to calculate the slope of the rate-rate plot as well as the coefficient of determination (r2). Pairs of motor units were included in further analysis only if they had an r2 ≥ 0.7, indicating that ≥70% of the rate modulation of the test unit could be accounted for by the control unit (Udina et al. 2010; Vandenberk and Kalmar 2014).
Since the ΔF is calculated as the difference in firing rate of the test unit relative to the control unit, the maximum ΔF value a pair of motor units can produce is limited by the rate modulation of the control unit. Therefore, it is possible for a control unit with minimal rate modulation to produce a ΔF value that underestimates the true influence of PICs within the test unit. The rate modulation of the control unit was calculated as the difference between the maximum and minimum of the control unit polynomial, only during the time frame in which the test unit is firing (Stephenson and Maluf 2011). To account for the possibility of control unit saturation causing an underestimation of the ΔF, pairs of motor units in which the rate modulation of the control unit fell within 0.5 imp/s of the calculated ΔF for that pair were removed from analysis.
It has been shown that there is a relationship between ΔF and the difference in recruitment time between motor unit pairs, in which a difference in recruitment time less than ∼2 s between test and control units yields a smaller or even negative ΔF value (Stephenson and Maluf 2011). This 2-s limit has been attributed to the length of time required for the PIC to activate (Bennett et al. 1998, 2001), as the recruitment of a test unit before full activation of the control unit PIC compromises the accuracy of the control unit as an indicator of synaptic drive. To control for this, we also calculated difference in recruitment time as the difference in time between the first spikes of the test and control units.
Statistical analysis.
Values are presented as means ± SD. The mean ΔF was calculated first for each muscle in each participant, and then the mean ΔF for each across participants was calculated. Normality of the data was evaluated with the Shapiro-Wilk test. Unpaired Student's t-tests were used to compare ΔF values across muscles and the mean firing rates of the control and test units (Udina et al. 2010). A one-factor ANCOVA was conducted to determine statistically significant differences between ΔF values for biceps and triceps, controlling for recruitment time difference as a covariate. A similar ANCOVA was conducted to control for rate modulation. Statistical significance was set at P = 0.05.
RESULTS
To estimate the relative contribution of PICs in the modulation of elbow flexor and extensor motor units, we recorded the discharge rates of pairs of motor units from the long head of biceps brachii and the lateral head of triceps brachii in 10 healthy participants during isometric trapezoid contractions. Each participant had two or three usable trials for each muscle group, and motor unit yield ranged from 2 to 12 per trial (average motor unit yield: 6.3 ± 2.4 for biceps, 5.7 ± 2.4 for triceps). We then evaluated the data set more vigorously by controlling for two factors: 1) saturation of rate modulation of the control unit and 2) difference in recruitment time between the test and control units. Overall, we found that there were greater ΔF values in triceps motor units than in biceps motor units.
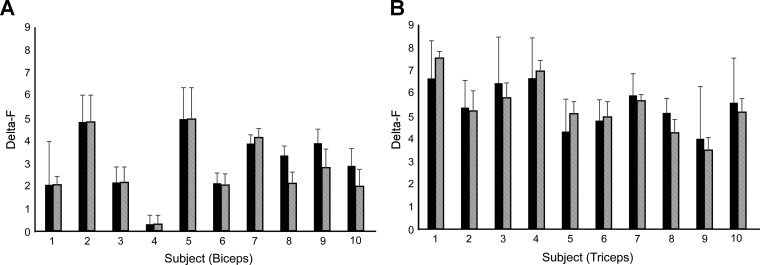
One hundred fifty-two unit pairs in biceps and 328 unit pairs in triceps were used to calculate ΔF values. Of these, 112 unit pairs in biceps and 208 unit pairs in triceps had an r2 ≥ 0.7. The group mean ΔF value for biceps (3.0 ± 1.4) was comparable to those seen in other human studies (Mottram et al. 2009) and significantly lower than that of triceps (5.4 ± 0.9, P < 0.001). The mean ΔF per participant is shown in Fig. 2, A and B, for biceps and triceps, respectively.
Fig. 2.
Average ΔF for each subject in biceps (A) and triceps (B). Black bars denote ΔF with motor unit pairs showing a coefficient of determination >0.7; hatched bars denote ΔF with the same criteria after removal of unit pairs that show possible saturation of the control unit. There were no significant changes in average ΔF when controlling for saturation in biceps (P = 0.6) or triceps (P = 0.9) compared with the first criterion. Error bars denote SE.
Influence of control unit rate modulation on ΔF.
Twenty-three percent of biceps pairs and 46% of triceps pairs fit the criterion of rate modulation saturation and were discarded. The resulting data pool consisted of 86 motor unit pairs from biceps and 111 motor unit pairs from triceps, yielding an average ΔF of 2.7 ± 1.5 and 5.4 ± 1.2 for biceps and triceps, respectively. As with the full data set, the difference in ΔF group means between biceps and triceps was significantly different (P < 0.001). Figure 2 shows the mean ΔF per subject after removal of pairs in which the control unit fit the saturation criterion.
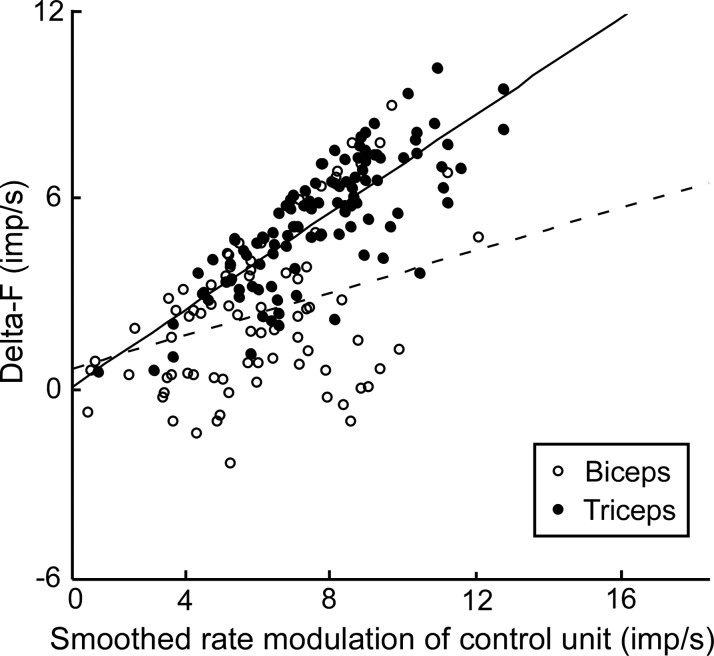
After removal of motor unit pairs exhibiting saturation in rate modulation, smoothed rate modulation of the remaining control units was plotted against ΔF for each muscle, with each data point representing a motor unit pair across all participants (Fig. 3). There was a positive relationship between ΔF and smoothed rate modulation of the control unit for data from both biceps and triceps; however, the slope of this relationship was significantly higher for triceps than for biceps (biceps: y = 0.22x + 0.49, triceps: y = 0.56x − 0.11, P < 0.001).
Fig. 3.
ΔF vs. smoothed rate modulation of the control unit in biceps (○, dashed line) and triceps (●, solid line). Each point represents a single unit pair irrespective of subject. An ANCOVA showed a significant main effect of muscle (P < 0.001) and rate modulation (P < 0.001) on ΔF with a significant interaction (P < 0.001).
Influence of recruitment time difference on ΔF.
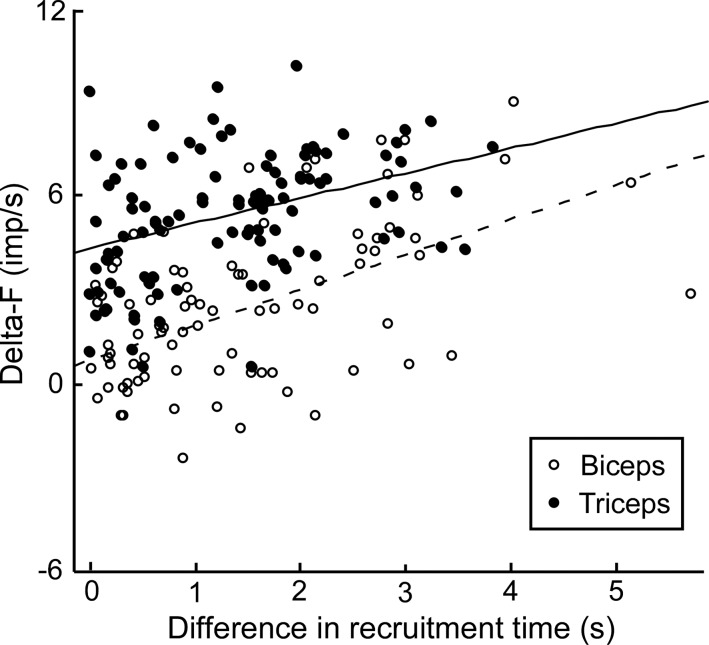
ΔF values for biceps and triceps are shown as a function of recruitment time difference in Fig. 4. There was a significant effect of muscle on ΔF when controlling for recruitment time difference (P < 0.001). There was also a significant effect of recruitment time difference (P < 0.001), but the interaction between muscle and recruitment time difference was not significant (P = 0.23).
Fig. 4.
ANCOVA examining the relationship between ΔF and difference in recruitment time between test and control units in biceps (○, dashed line) and triceps (●, solid line). Each point represents a single unit pair irrespective of subject. A similar analysis using mean ΔF and recruitment time differences for each subject still yields a significant main effect of muscle (P < 0.001) but a nonsignificant main effect of recruitment time difference (P = 0.13) with no significant interaction.
DISCUSSION
Here we provide evidence that, in humans, there is a greater contribution of intrinsic properties to the excitability of extensor motor units than flexor motor units in the upper limb, demonstrated by a larger ΔF value in the triceps brachii compared with biceps brachii. These data are consistent with previous studies demonstrating higher neuronal excitability in the extensors than the flexors of the decerebrate cat (Hounsgaard et al. 1988) as well as the neonatal rat preparation (Cotel et al. 2009).
The interpretation and limitations of the ΔF method have been subject to scrutiny by several groups, who concluded that the ΔF value is subject to a high degree of variability, in part from mechanisms other than PICs. Factors that can increase the variability of ΔF include the choice of control unit, the difference in recruitment time between test and control units, the maximum rate modulation of the control unit, the possible effects of spike frequency adaptation, and the presence and timing of secondary range firing (Powers et al. 2008; Revill and Fuglevand 2011; Stephenson and Maluf 2011; Vandenberk and Kalmar 2014).
Effects of rate modulation of control unit on ΔF.
The positive correlation between ΔF values and the amount of rate modulation in the control unit has been presented previously (Stephenson and Maluf 2011; Udina et al. 2010). Stephenson and Maluf (2011) speculated that this correlation reflects graded synaptic action of the PIC, as opposed to an “all or none” mechanism. Another interpretation is that the ΔF is restricted by the maximum rate modulation of the control unit, raising the possibility that units with limited rate modulation are providing underestimations of the true magnitude of the PIC, particularly if the firing rate of the control unit saturates. Rate modulation of the control unit has been calculated in two ways: as an absolute range of the smoothed firing rate for the entire time the control unit is recruited (Udina et al. 2010) and as the range of the smoothed firing rate limited specifically to the time frame in which the test unit is firing (Stephenson and Maluf 2011). We chose to use the latter, although calculating rate modulation with either method showed that rate modulation in triceps was greater than that in biceps.
When motor unit pairs in which the maximum rate modulation during test unit firing fell within 0.5 imp/s of the ΔF value were eliminated, a significant difference in ΔF between biceps and triceps remained. Furthermore, the ΔF values within either muscle group were not significantly different when comparing values from the full data set and values with saturated control units removed. In fact, the group mean values were virtually identical. Therefore, these data suggest that it is unlikely that saturation in rate modulation substantially impacts ΔF values.
The positive relationship between smoothed rate modulation of the control unit and ΔF of the motor unit pairs not exhibiting saturation in rate modulation was similar to that seen in Stephenson and Maluf (2011). Interestingly, the slope of the ΔF/control rate modulation relationship is significantly greater in triceps than in biceps, suggesting that a given change in synaptic input results in a greater increase in ΔF in triceps than in biceps. The possible contribution of saturation of the control unit having been removed, this difference in slope may support the hypothesis of greater PICs in triceps than in biceps, since greater PIC activation for a given synaptic input would result in a steeper ΔF/rate modulation slope. Given evidence that PICs may be activated in a gradual manner with increases in synaptic input (Elbasiouny et al. 2006), the difference in slopes suggests that the dynamics by which synaptic input activates the PIC may differ between muscles.
Effects of recruitment time difference on ΔF.
To assume linearity in changes in firing rate between test and control units, the PIC of the control unit must be fully activated before the test unit is recruited. If the test unit is recruited before the control unit PIC has activated, the ΔF of the test unit will be an underestimation of its true value. In humans, prior studies have shown that ΔF decreases if the difference in recruitment time is <2 s (Gorassini et al. 2002; Stephenson and Maluf 2011). In the cat, small and negative ΔF values were also observed as recruitment time difference decreased, but relatively large ΔF values were also observed, resulting in an increase in variability but no change in the mean ΔF (Powers et al. 2008). Previous studies have attempted to control for this variable by using motor unit pairs that have a recruitment time difference >1–2 s (Mottram et al. 2009; Udina et al. 2010). However, in vitro recordings have shown that motoneurons may activate PICs within a smaller time frame (Li et al. 2004). By only conducting analysis on motor unit pairs with a recruitment time difference >2 s, subsets of valid motor unit pairs in which the control unit exhibits early PIC activation may be inadvertently excluded (Li et al. 2004).
The present results demonstrating a significant main effect of recruitment time difference on ΔF are in agreement with previous literature (Stephenson and Maluf 2011). There was also a significant main effect of muscle, controlling for differences in recruitment time, reflecting the greater values of ΔF in the triceps than the biceps. However, there was no significant muscle × recruitment time difference interaction, suggesting that the effects of muscle and recruitment time difference contribute independently to the ΔF.
Functional implications.
Because we cannot explain higher ΔF values in triceps through differential effects in recruitment time difference, recruitment thresholds, or saturation in firing rate of the control unit, these data support the hypothesis that extensor motor units are subject to different motor control principles than flexor units. There are several intrinsic mechanisms that could produce a difference in ΔF between extensors and flexors. We speculate that these might include differences in the number of monoaminergic receptors, in the density of L-type CaV1.3 channels that produce the PIC, or in the number of monoaminergic boutons onto extensor versus flexor motoneurons. Any of these could facilitate PIC activation and/or increase the amplitude of the PIC, leading to higher ΔF values and differences in the slope of the ΔF/control rate modulation relationship. Recent studies have shown that the densities of serotonergic and noradrenergic axon terminals in the neck muscles of the cat are 2.3-fold and 1.4-fold greater in extensor motoneurons compared with flexors, suggesting facilitated PICs by an increase in monoaminergic effects (Maratta et al. 2015). Simulation studies have suggested that spike-frequency adaptation may also contribute to ΔF values, especially during trapezoid ramps like those used in the present study (Revill and Fuglevand 2011), but visual inspection of units indicates very little difference in changes in firing rate between biceps and triceps during the plateau portion of the contraction. Furthermore, data from both computer simulations and human investigations indicate that a 5-s plateau would only contribute <0.5 imp/s to a ΔF value (Revill and Fuglevand 2011; Vandenberk and Kalmar 2014), which is not enough to fully explain the difference we have observed between muscles. It is possible that other aspects of intrinsic motoneuron properties, such as the prolongation of spike afterhyperpolarization (AHP) seen in motor units in the cat (Wienecke et al. 2009), may contribute to differences in ΔF between extensors and flexors, but the contributions of changes in AHP to ΔF in humans have not been clarified.
Anatomically, joint angle is an important contributor to ΔF values because of the sensitivity of the PIC to reciprocal inhibition (Hyngstrom et al. 2007). According to analysis of elbow flexor and extensor muscle fascicle excursions at different elbow flexion angles by Murray et al. (2000), having the elbow flexed at 90° results in the long head of the biceps being moderately stretched compared with the lateral head of the triceps. It is possible that having the biceps relatively more stretched than the triceps could contribute to the difference in the ΔF values observed between these muscles. However, it has been shown in the cat that stretching a muscle by changing the joint angle increases the amplitude of the PIC compared with when the muscle is neutral or shortened. Progressive sensory ablation provided strong evidence for the role of reciprocal Ia inhibitory control of the PIC (Hyngstrom et al. 2007). Provided that such reciprocal inhibition exists in the upper limb (Katz et al. 1991), a joint angle where the biceps is stretched should not only increase the biceps PIC but would also inhibit the triceps. If this is the case, our values of ΔF in the biceps may be overestimated and our values of ΔF in the triceps may be underestimated compared with a joint angle where both muscles are equivalently stretched or slackened. As such, we do not believe that the difference in ΔF between biceps and triceps occurs as a result of joint angle.
While differences in intrinsic excitability of flexor and extensor muscles have also been observed in the hindlimbs of animal models (Cotel et al. 2009; Hounsgaard et al. 1988), our results may also be partly explained by factors specific to the muscles of the upper limb. For example, differences in fiber composition between muscles may lead to differences in ΔF, because slow motor units tend to exhibit long-lasting PICs (Lee and Heckman 1998a, 1998b). Autopsy studies, however, suggest that the ratio of slow-twitch to fast-twitch fibers is similar in biceps versus triceps (Elder et al. 1982). Differences in afferent input may also contribute to differences in ΔF. While biceps and triceps have been shown to inhibit each other via Ia reciprocal inhibition (Katz et al. 1991), biceps receives additional group I inhibitory afferents from brachioradialis (Barry et al. 2008; Naito et al. 1996) and pronator teres (Naito et al. 1998). However, considering that both brachioradialis and pronator teres are antagonistic to biceps on the pronation/supination axis and not the flexion/extension axis, the extent to which these muscles contribute to ΔF during elbow flexion is not clear (Buchanan et al. 1986). We also cannot say for certain whether the observed difference in ΔF also applies to the lower limb in humans. Gorassini and colleagues (Gorassini et al. 2002) compared ΔF values in 12 unit pairs in tibialis anterior versus 4 unit pairs in soleus and did not find a significant difference between muscles.
Although ΔF values observed in biceps are similar to those seen in previous studies (Mottram et al. 2009), there is noticeable variability in ΔF values between individual subjects. One source of ΔF variability in biceps may be from the compartmentalization of function within the muscle. Since biceps brachii acts as both a flexor and a supinator, motor units can be recruited in response to flexion torque, supination torque, or a combination of both flexion and supination (ter Haar Romeny et al. 1982, 1984; van Zuylen et al. 1988). Similarly, motor units in triceps brachii have been shown to be recruited in response to pronation/supination as well as extension, even though triceps does not biomechanically contribute to the former (van Zuylen et al. 1988). It is not known how the multifunctionality of a muscle might contribute to its ΔF values during single degree-of-freedom movements. While we tried to be consistent in recording from the lateral and medial long head of biceps corresponding to the location of units that respond to flexion or a combination of flexion and supination (ter Haar Romeny et al. 1982; van Zuylen et al. 1988), it is possible that sampling biases toward units that are also responsive to supination may contribute to the variability of ΔF between participants. Other sources of between-subject variability can involve differences in muscle activation strategies including the relative contributions of synergist muscles and a muscle's angular range of activation, which have been shown to vary between individuals (Buchanan et al. 1986). Considering that we did not control for the fitness level of participants, this may also contribute to subject variability due to the neuromuscular changes that occur with exercise, even in an aged population (Grabiner and Enoka 1995). However, the relationship between exercise and changes in the intrinsic excitability of motoneurons via PIC-mediated mechanisms has not been thoroughly studied.
An important question is whether our observations within a single head are generalizable to the entire muscle. The short and long heads of biceps have previously been considered as a single entity (Buchanan et al. 1989; Cavallari and Katz 1989) and have been shown to receive similar reflex inputs (Barry et al. 2008; Naito et al. 1996; Riley et al. 2008). In a neutral forearm position, the two heads show similar recruitment thresholds and discharge rates. However, when the forearm is supinated, the two heads of biceps show significant differences in recruitment threshold as well as discharge rate (Harwood et al. 2010). With regard to functional compartmentalization, the long head has been shown to contain motor units responsive to flexion only, while motor units in the short head tend to respond to a combination of flexion and supination (van Zuylen et al. 1988). In addition, motor unit discharge variability is greater in the short head of biceps, suggestive of differences in synaptic noise (Harwood et al. 2010). Triceps, by comparison, has fewer studies examining interhead differences, although van Groeningen and Erkelens (1994) did show that recruitment thresholds of motor units are not significantly different across the three heads of triceps, and EMG activation between triceps heads covaries relatively closely with increasing elbow extension (Buchanan et al. 1986). Because serotonergic projections are highly diffuse (Björkland and Skagerberg 1982), it is unlikely that descending monoaminergic drive differs within a muscle, but a comparison of ΔF between muscle heads and across different aspects of muscle functionality will be an important extension of the present work.
The age of our participants introduces some additional caveats in the interpretation of results. Older adults show significantly lower recruitment thresholds and more variable firing rates compared with younger subjects in the upper limb (Harwood et al. 2010; Laidlaw et al. 2000; Tracy et al. 2005), which may be a consequence of motor unit remodeling following a decrease of motor unit number with age (Roos et al. 1997). More importantly, animal models show a decrease in monoaminergic drive to the spinal cord with aging (Ko et al. 1997). However, because the participants in the present study are from an older population, the results are directly relevant to the field of rehabilitation science, in which many individuals with movement disorders are older adults.
As such, the present results provide a foundation for exploring alterations in the activation of flexor versus extensor muscles in individuals with neurological disease or injury. For example, augmented PICs have been implicated in spasticity following spinal cord injury (Gorassini et al. 2004; Li et al. 2004; Murray et al. 2010), and postmortem studies of individuals with Parkinson's disease show degeneration of monoaminergic projections to the spinal cord (Braak et al. 2003; Scatton et al. 1986). Interestingly, individuals with Parkinson's disease exhibit a greater extensor strength deficit in the upper limb (Corcos et al. 1996; Robichaud et al. 2004), but it is unknown whether a loss of monoamines plays a role. Increased understanding of alterations in PIC behavior in flexors versus extensors in these populations may be significant for improving pharmacological and/or rehabilitation strategies for the upper limb.
The present work is a necessary extension of the results seen in animal studies and has interesting implications for the behavior of flexors versus extensors in the upper limb in neurologically intact humans. Postural requirements of shoulder and elbow muscles in humans are much less demanding than those of limb muscles in quadrupeds, because these muscles typically provide postural support for the arm and hand as they interact with the environment rather than providing postural support to the torso to keep the body upright. In such a role, flexors and extensors of the elbow work together to support different arm postures, whereas in the quadruped role the extensors are the clearly dominant muscles for support of the torso. Despite the difference in muscle function between these two cases, however, our results suggest that the pattern of increased PICs seen in quadruped hindlimb extensors compared with flexors is a neural strategy that has been conserved in humans.
Given the multifunctionality of the upper limb and the complex relationships between the muscles that comprise it, further studies are required to explore the contributions of functional anatomy and joint angle to ΔF in humans. Nonetheless, the conclusion that biceps and triceps brachii motoneurons have different levels of intrinsic excitability, and therefore different mechanisms for neural activation, provides important insight for how these muscles behave and interact with one another.
GRANTS
This work was supported by National Institutes of Health Grant NS-085331 and Training Grants T32 HD-057845 and T32 EB-009406, the Craig H. Neilsen Foundation 260215, and the Northwestern Memorial Foundation Parkinson's Disease and Movement Disorders Advisory Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.W., C.K.T., and L.C.M. conception and design of research; J.M.W. and C.K.T. performed experiments; J.M.W. analyzed data; J.M.W., C.K.T., and C.J.H. interpreted results of experiments; J.M.W. prepared figures; J.M.W. drafted manuscript; J.M.W., C.K.T., L.C.M., and C.J.H. edited and revised manuscript; J.M.W., C.K.T., L.C.M., and C.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jules Dewald for use of laboratory space, Dr. Monica Gorassini for technical assistance, and Dr. Randy Powers for his comments on the manuscript.
REFERENCES
- Barry BK, Riley ZA, Pascoe MA, Enoka RM. A spinal pathway between synergists can modulate activity in human elbow flexor muscles. Exp Brain Res 190: 347–359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. How different afferent inputs control motoneuron discharge and the output of the motoneuron pool. Curr Opin Neurobiol 3: 1028–1034, 1993. [DOI] [PubMed] [Google Scholar]
- Björklund A, Skagerberg G. Descending monoaminergic projections to the spinal cord. In: Brain Stem Control of Spinal Mechanisms, edited by Sjolund B, Björklund A. Amsterdam: Elsevier Biomedical, 1982, p. 55–88. [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197–211, 2003. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Almdale DP, Lewis JL, Rymer WZ. Characteristics of synergic relations during isometric contractions of human elbow muscles. J Neurophysiol 56: 1225–1241, 1986. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Rovai GP, Rymer WZ. Strategies for muscle activation during isometric torque generation at the human elbow. J Neurophysiol 62: 1201–1212, 1989. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Exp Brain Res 78: 465–478, 1989. [DOI] [PubMed] [Google Scholar]
- Collins DF, Gorassini M, Bennett D, Burke D, Gandevia SC. Recent evidence for plateau potentials in human motoneurones. Adv Exp Med Biol 508: 227–235, 2002. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson's disease: relationship to rate of force generation and clinical status. Ann Neurol 39: 79–88, 1996. [DOI] [PubMed] [Google Scholar]
- Cotel F, Antri M, Barthe JY, Orsal D. Identified ankle extensor and flexor motoneurons display different firing profiles in the neonatal rat. J Neurosci 29: 2748–2753, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs. J Physiol 570: 355–374, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GC, Bradbury K, Roberts R. Variability of fiber type distributions within human muscles. J Appl Physiol Respir Environ Exercise Physiol 53: 1473–1480, 1982. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol 557: 355–361, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett 247: 13–16, 1998. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Enoka RM. Change in movement capabilities with aging. Exerc Sport Sci Rev 23: 65–104, 1995. [PubMed] [Google Scholar]
- Harwood B, Edwards DL, Jakobi JM. Age independent and position-dependent alterations in motor unit activity of the biceps brachii. Eur J Appl Physiol 110: 27–38, 2010. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holobar A, Zazula D. Correlation-based decomposition of surface electromyograms at low contraction forces. Med Biol Eng Comput 42: 487–495, 2004. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res 55: 391–394, 1984. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405: 345–367, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10: 363–369, 2007. [DOI] [PubMed] [Google Scholar]
- Katz R, Penicaud A, Rossi A. Reciprocal Ia inhibition between elbow flexors and extensors in the human. J Physiol 437: 269–286, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78: 3061–3068, 1997. [DOI] [PubMed] [Google Scholar]
- Ko ML, King MA, Gordon TL, Crisp T. The effects of aging on spinal neurochemistry in the rat. Brain Res Bull 42: 95–98, 1997. [DOI] [PubMed] [Google Scholar]
- Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 23: 600–612, 2000. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998a. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998b. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol 81: 2164–2174, 1999. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004. [DOI] [PubMed] [Google Scholar]
- Maratta R, Fenrich KK, Zhao E, Neuber-Hess MS, Rose PK. Distribution and density of contacts from noradrenergic and serotonergic boutons on the dendrites of neck flexor motoneurons in the adult cat. J Comp Neurol (February 25, 2015). doi: 10.1002/cne.23765. [DOI] [PubMed] [Google Scholar]
- McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods 149: 121–133, 2005. [DOI] [PubMed] [Google Scholar]
- McPherson JG, Ellis MD, Heckman CJ, Dewald JP. Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophysiol 100: 3236–3243, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol 40: 1432–1443, 1977. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol 102: 2026–2038, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray WM, Buchanan TS, Delp SL. The isometric functional capacity of muscles that cross the elbow. J Biomech 33: 943–952, 2000. [DOI] [PubMed] [Google Scholar]
- Naito A, Shindo M, Miyasaka T, Sun YJ, Momoi H, Chishima M. Inhibitory projections from pronator teres to biceps brachii motoneurones in human. Exp Brain Res 121: 99–102, 1998. [DOI] [PubMed] [Google Scholar]
- Naito A, Shindo M, Miyasaka T, Sun YJ, Morita H. Inhibitory projection from brachioradialis to biceps brachii motoneurones in human. Exp Brain Res 111: 483–486, 1996. [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol 100: 292–303, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill AL, Fuglevand AJ. Effects of persistent inward currents, accommodation, and adaptation on motor unit behavior: a simulation study. J Neurophysiol 106: 1467–1479, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley ZA, Baudry S, Enoka RM. Reflex inhibition in human biceps brachii decreases with practice of a fatiguing contraction. J Neurophysiol 100: 2843–2851, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud JA, Pfann KD, Comella CL, Brandabur M, Corcos DM. Greater impairment of extension movements as compared to flexion movements in Parkinson's disease. Exp Brain Res 156: 240–254, 2004. [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve 20: 679–690, 1997. [DOI] [PubMed] [Google Scholar]
- Scatton B, Dennis T, L'Heureux R, Monfort JC, Duyckaerts C, Javoy-Agid F. Degeneration of noradrenergic and serotonergic but not dopaminergic neurones in the lumbar spinal cord of parkinsonian patients. Brain Res 380: 181–185, 1986. [DOI] [PubMed] [Google Scholar]
- Stephenson JL, Maluf KS. Dependence of the paired motor unit analysis on motor unit discharge characteristics in the human tibialis anterior muscle. J Neurosci Methods 198: 84–92, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol 423: 63–76, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar Romeny BM, Denier van der Gon JJ, Gielen CC. Changes in recruitment order of motor units in the human biceps muscle. Exp Neurol 78: 360–368, 1982. [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny BM, van der Gon JJ, and Gielen CC. Relation between location of a motor unit in the human biceps brachii and its critical firing levels for different tasks. Exp Neurol 85: 631–650, 1984. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Maluf KS, Stephenson JL, Hunter SK, Enoka RM. Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle Nerve 32: 533–540, 2005. [DOI] [PubMed] [Google Scholar]
- Udina E, D'Amico J, Bergquist AJ, Gorassini MA. Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor-unit activity. J Neurophysiol 103: 1295–1303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groeningen CJ, Erkelens CJ. Task-dependent differences between mono- and bi-articular heads of the triceps brachii muscle. Exp Brain Res 100: 345–352, 1994. [DOI] [PubMed] [Google Scholar]
- van Zuylen EJ, Gielen CC, Denier van der Gon JJ. Coordination and inhomogeneous activation of human arm muscles during isometric torques. J Neurophysiol 60: 1523–1548, 1988. [DOI] [PubMed] [Google Scholar]
- Vandenberk MS, Kalmar JM. An evaluation of paired motor unit estimates of persistent inward current in human motoneurons. J Neurophysiol 111: 1877–1884, 2014. [DOI] [PubMed] [Google Scholar]
- Walton C, Kalmar JM, Cafarelli E. Effect of caffeine on self-sustained firing in human motor units. J Physiol 545: 671–679, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton C, Kalmar J, Cafarelli E. Caffeine increases spinal excitability in humans. Muscle Nerve 28: 359–364, 2003. [DOI] [PubMed] [Google Scholar]
- White SR, Fung SJ. Serotonin depolarizes cat spinal motoneurons in situ and decreases motoneuron afterhyperpolarizing potentials. Brain Res 502: 205–213, 1989. [DOI] [PubMed] [Google Scholar]
- Wienecke J, Zhang M, Hultborn H. A prolongation of the postspike afterhyperpolarization following spike trains can partly explain the lower firing rates at derecruitment than those at recruitment. J Neurophysiol 102: 3698–3710, 2009. [DOI] [PubMed] [Google Scholar]