Abstract
Noninvasive brain-computer-interfaces (BCI) coupled with prosthetic devices were recently introduced in the rehabilitation of chronic stroke and other disorders of the motor system. These BCI systems and motor rehabilitation in general involve several motor tasks for training. This study investigates the neurophysiological bases of an EEG-oscillation-driven BCI combined with a neuroprosthetic device to define the specific oscillatory signature of the BCI task. Controlling movements of a hand robotic orthosis with motor imagery of the same movement generates sensorimotor rhythm oscillation changes and involves three elements of tasks also used in stroke motor rehabilitation: passive and active movement, motor imagery, and motor intention. We recorded EEG while nine healthy participants performed five different motor tasks consisting of closing and opening of the hand as follows: 1) motor imagery without any external feedback and without overt hand movement, 2) motor imagery that moves the orthosis proportional to the produced brain oscillation change with online proprioceptive and visual feedback of the hand moving through a neuroprosthetic device (BCI condition), 3) passive and 4) active movement of the hand with feedback (seeing and feeling the hand moving), and 5) rest. During the BCI condition, participants received contingent online feedback of the decrease of power of the sensorimotor rhythm, which induced orthosis movement and therefore proprioceptive and visual information from the moving hand. We analyzed brain activity during the five conditions using time-frequency domain bootstrap-based statistical comparisons and Morlet transforms. Activity during rest was used as a reference. Significant contralateral and ipsilateral event-related desynchronization of sensorimotor rhythm was present during all motor tasks, largest in contralateral-postcentral, medio-central, and ipsilateral-precentral areas identifying the ipsilateral precentral cortex as an integral part of motor regulation. Changes in task-specific frequency power compared with rest were similar between motor tasks, and only significant differences in the time course and some narrow specific frequency bands were observed between motor tasks. We identified EEG features representing active and passive proprioception (with and without muscle contraction) and active intention and passive involvement (with and without voluntary effort) differentiating brain oscillations during motor tasks that could substantially support the design of novel motor BCI-based rehabilitation therapies. The BCI task induced significantly different brain activity compared with the other motor tasks, indicating neural processes unique to the use of body actuators control in a BCI context.
Keywords: neuroprostheses, motor action, EEG, proprioceptive feedback
in an aging society, motor rehabilitation is becoming increasingly important, and one of the main focuses of the field is the use of neuroimaging techniques to understand the effects of the different treatments. It has been demonstrated that changes in neural networks correlate with improvements in motor function (Fritsch et al. 2010; Koralek et al. 2012; Sehm et al. 2010) and that some strategies exciting the motor neural networks in the absence of movements through motor imagery determine motor improvements in patients who have undergone stroke (Sharma et al. 2006). However, a better understanding of the relevant neurophysiological processes involved during motor rehabilitation is needed to improve treatment design, optimize efficacy, and reduce intervention time. The use of brain-computer-interfaces (BCI) in motor rehabilitation could help individuals without residual movements to control a rehabilitation robotic device and at the same time serve as rehabilitation assessment tool (Ramos-Murguialday et al. 2013). During BCI intervention, one can track changes in brain activity during treatment and the patients' involvement during training (tracking volitional features in EEG or other neural activities) and correlate these with functional and neuronal changes to predict or assess recovery. However, different motor components produce very similar brain activity, and as such the decoding of those different components is a complex problem. Although, motor tasks and motor task components involved in motor rehabilitation (active and passive movement and motor imagery) have been extensively studied, these three motor tasks elements (active and passive movement and motor imagery) were never studied in the same participants within the same experimental session, minimizing the experimental variability.
Active (self-paced) limb movements have been mainly described to be accompanied by three different types of event-related desynchronization and synchronization (ERD/ERS) patterns at the scalp EEG: 1) contralateral-dominant sensorimotor rhythm (SMR) in the alpha and beta ERD before movement (premovement ERD) (Pfurtscheller and Neuper 2006); 2) bilateral symmetrical alpha and beta ERD during execution of movement; and 3) contralateral-dominant beta-rebound (beta-ERS) within the first second after movement offset (Pfurtscheller and Lopes da Silva 1999). It has been shown that the topography of the alpha changes is quite different for the low alpha band (8–10 Hz) and the high alpha band (10–12 Hz), suggesting that there may be two different SMR alpha rhythms contributing to the ERD during active movement (Pfurtscheller and Berghold 1989). The lower SMR alpha ERD is more prominent at parietal electrodes, and the topography of the higher SMR alpha ERD is more similar to that of the SMR beta ERD (range 17–30 Hz), i.e., beginning in contralateral central regions becoming bilateral with the movement and extending to frontal and parietal regions (Alegre et al. 2004). Movement-related slow cortical potentials during active movement have been very well studied and divided into two main components: 1) the slow cortical potentials occurring during intention or anticipation of an upcoming movement, which is also called the Bereitschaftspotential for self-paced movements (Barrett et al. 1986); and 2) the motor potential occurring at the time of the execution (Deecke et al. 1969).
Several studies demonstrated that, after movement onset, synchronization (ERS) in the ipsilateral and in surrounding, not required, contralateral areas appears, whereas, in the corresponding contralateral region, the desynchronization continues until the end of the motor sequence (ERD) (Alegre et al. 2004). This activation pattern has been associated with the concept of focal ERD surrounded by ERS, whereby the less involved ipsilateral motor area neurons and the surrounding contralateral regions are deactivated to increase the gain of the required active regions (Cassim et al. 2001; Hummel et al. 2002; Müller-Putz et al. 2007; Pfurtscheller and Neuper 2006), specific for the upper alpha frequency range. Furthermore, after movement offset, a positive slow cortical potential appears, which may reflect the inhibition of the motor cortex after termination of the intended movement, as well as afferent sensory projections to other motor-related areas acting as proprioceptive feedback (Birbaumer et al. 1990; Lee et al. 1986; Müller et al. 2003; Müller-Putz et al. 2007).
On the other hand, during a passive movement, voluntary planning and preparation are absent, yet the afferent somatosensory components (skin mechanoreceptors, muscle spindles, and joint receptors) produce SMR variations similar to those observed during active movements (Alegre et al. 2002; Keinrath et al. 2006) with the absence of any preparatory ERD/ERS. In a PET study, Mima and colleagues (1999) compared active and passive movements. During passive movements activity was observed in the contralateral primary and secondary somatosensory cortex, whereas the classical motor network, including the primary motor cortex, the supplementary motor area, the premotor cortex, the cerebellum, and the basal ganglia, was active during volitional movements. They concluded that, during passive movements, there is a lack of sensorimotor attention (i.e., attention toward afferent information of movements), necessary to regulate movement control, which is present during active movements. Several groups observed similar activation patterns, using EEG and electrocorticography data, between executed and passively performed movements (Cho et al. 2011; Lee et al. 1986; Lotze et al. 2003; Müller-Putz et al. 2007). However, other studies have shown that primary motor cortex neurons receive and process sensory afferent inputs from muscle spindles, without generating any active movements and thus are activated during passive movement (Naito 2004). However, most of these studies did not control for involuntary ipsilateral and contralateral upper-limb electromyogram (EMG) activation, which could indicate that unseen micromovements may have affected the observed EEG activation (Ramos-Murguialday et al. 2010).
Motor imagery has been proposed as a tool for motor rehabilitation in combination with BCI (Birbaumer et al. 2008) and has been defined as rehearsal of movement without a joint overt action (Grezes and Decety 2001). The cognitive process of motor imagery does engage supraspinal structures but usually lacks any spinal motor neuron activation. However, as of yet, no description satisfactorily differentiates between the various forms of movement imagination, as the neural response clearly differs depending on the perspective of the imagination (first person “me” or third person “others”) and the strategy used (motor vs. sensory for example). On the other hand, and most relevant for active brain control of rehabilitation robots, it has been proven that kinesthetic motor imagery, described as a “first person process” elicits very similar brain activity patterns to that obtained during active movement execution (Neuper et al. 2005). Neurophysiologically, the neural system used for the execution of a movement has been presented as equivalent to the system activated during the kinesthetic imagination of the same movements (Lotze et al. 1999) although the activation is stronger during active movements (Grezes and Decety 2001; Jeannerod and Frak 1999; Pfurtscheller and Neuper 1997). Furthermore, the observation of movements was found to have an impact on the activity in sensorimotor areas, reflecting processes of motor preparation and motor programming and activating the premotor cortex, the supplementary motor area (SMA), and the precentral cortex (Grezes et al. 1998; Halder et al. 2011), indicating the importance of volitional initiation for a visuo-motor task.
When addressing the relevant brain activation components in relation to active and passive movements, motor imagery follows the same neurophysiological patterns as active movements (Ehrsson et al. 2002; Gerardin et al. 2000), except for the overt execution of the movement, no proprioception, no spinal activations, etc. It is important to notice that imagery of unilateral right hand movement can be accompanied by a contralateral beta ERD and an ipsilateral beta ERS (Pfurtscheller and Neuper 1994, 1997) similar to what has been observed for active movement. However, it is still unclear whether the primary motor cortex (M1), despite being predominantly responsible for the motor command, is necessarily engaged during the imagination of a movement. Most studies postulate the S1, the SMA, the cerebellum, and the premotor and precentral cortex to be active during motor imagery (Sharma et al. 2006). Whereas some studies suggest an apparent but weaker involvement of M1 (Grezes and Decety 2001; Jackson et al. 2003; Kasess et al. 2008; Lacourse et al. 2005), others dispute its role, arguing that motor imagery is primarily related to the planning phase of a movement rather than the final execution (Dechent et al. 2004; de Vries and Mulder 2007). Thus it might be that the M1 is not necessary for an accurate movement representation in the brain but is merely coactivated during imagery. However, it has to be stated that most of these studies lacked appropriate controls for involuntary ipsilateral and contralateral upper-limb EMG activation, which as previously mentioned could have affected the observed EEG activation (Ramos-Murguialday et al. 2010). This involuntary upper-limb EMG activation may be caused by a plethora of reasons: 1) undesired movement of the hand during imagery of the same movement to cause EEG activity attributable to movement instead of only imagery; 2) muscle activity during resting when subjects were asked to remain relaxed; 3) bilateral muscle activity during active movement resulting in more bilateral EEG activity when subjects were asked to move one hand and relax the other one.
Regarding the timing of a motor task, one has to differentiate between the preparatory and the execution phases. During unilateral hand movement, the preparatory phase is associated with a contralateral alpha and central beta event-related desynchronization (ERD) that appears 1–2 s before simple movement onset and becomes bilaterally symmetrical in the execution phase (Derambure et al. 1999; Stancak and Pfurtscheller 1996a, 1996b). Conversely, in the case of motor imagery of the same unilateral hand movement, the contralateral ERD is present during the whole imagery process, in a manner similar to the preparation of a self-paced hand movement (Pfurtscheller and Neuper 1997; Pfurtscheller et al. 2006). These observations indicate that similar (but not identical) neural structures in sensorimotor areas become activated in the course of preparation of a motor action and during the mental simulation of the same action without its execution (Jeannerod and Decety 1995). Generally mu and/or beta components display a somatotopically organized activation (ERD) pattern, with both the premovement ERD and the imagination-related ERD appearing to reflect similar types of brain states in premotor and motor areas responsible for movement preparation, regardless of whether an action will be performed or imagined (Neuper and Pfurtscheller 1999). However, sensorimotor rhythms such as mu and central beta can be modified by executed, passive, imagined, or observed movement and other cognitive tasks (Alegre et al. 2002; Derambure et al. 1999; Jasper and Penfield 1949; Leocani et al. 1997; Muthukumaraswamy and Johnson 2004; Neuper and Pfurtscheller 1999; Pfurtscheller and Neuper 1997; Pineda 2005; Salmelin and Hari 1994; Weiller et al. 1996).
When addressing the relevant brain states during the use of robots for neurorehabilitation, passive motor trainings have not been shown to result in gains in motor function because active participation and volition seem necessary to produce significant motor function improvement (Hogan et al. 2006; Hu et al. 2009; Lotze et al. 2003). The control of a robot or prosthetic device and the feedback contingency are of vital importance to enable neuro-motor rehabilitation; as in an active motor rehabilitation treatment the feedback plays an important role in coupling intention and action (Ramos-Murguialday et al. 2013).
The critical elements of feedback have been distinguished according to three aspects, including feedback modality (visual, auditory, haptic, etc.), the timing of feedback (continuous or binary), and the transmission of the feedback (contingent or noncontingent). The expression “continuous” refers to whether the feedback is presented throughout the entire trial; alternatively, in a binary fashion, the feedback is presented once at the end of each trial. Because a therapist cannot accurately control all the aspects of feedback, robots have been introduced as tools to improve repetition, precision, and control during motor rehabilitation. Robots can be used as tracking systems (no friction and only track limb movements and forces) and for assisting (compensating gravity, guided paths, etc.) and resisting (increasing friction, perturbing trajectories, etc.). To control a robot during these three types of rehabilitation scenarios, a classifier (based on force, movement, EMG, EEG, etc.) to detect action intention and to operate the robot joints is necessary. When utilizing the commonly used linear classifier to control a robot, a threshold has to be defined based on force, velocity, and muscle or brain activity, and feedback is provided each time the activation level crosses that activity threshold. This could happen either at the end of the trial (binary) or during the ongoing trial (continuous). The distinction between contingent and noncontingent defines the amount of association in time between the response (force, velocity, and muscle or brain activity) and the feedback signal.
In this study, we investigated the difference in brain activity during several commonly studied motor rehabilitation tasks, including motor imagery and passive and active movements. During these tasks, we analyzed EEG frequency changes over time. Furthermore, we added an extra task linking brain oscillations (related to the imagination of hand opening and closing) with hand movements using a robotic hand orthosis (haptic BCI). The aim of our work was to analyze the cortical energy changes in the theta, alpha, beta, and low gamma bands during four motor tasks compared with a resting condition. Three of the motor tasks were related to motor rehabilitation (motor imagery, active, and passive movement) and were compared with the BCI task, in which the three elements of the tasks are mixed to link brain activity attributable to motor imagery of hand opening/closing and the exact same movements via a BCI controlled robotic hand orthosis. Furthermore, we intended to study the relationship of these EEG oscillation changes with the preparation, planning, and execution of each of the motor tasks and hypothesized that 1) each motor task will present individual EEG signatures (EEG power at locations L1, L2…Ln during time periods t1, t2…tn), i.e., characteristic EEG power at different locations during different time periods compared with the other motor tasks (especially against rest that was used as reference); 2) EEG activity during brain control of the orthotic device (BCI condition) would resemble neural activation generated during active movement.
METHODS
Experimental Procedure
Nine healthy volunteers (age range 26.6 ± 4 yr; all subjects were right-handed) were recruited for the experiments. Participants were sitting in an upright position wearing a 128-channel EEG cap. The experimental protocol was approved by the ethics committee of the University of Tubingen, Medical Faculty, with all participants providing written, informed consent before participating in this study. The predominant hand (right) of the participant was fixed to a hand orthosis (Fig. 1C), and participants were presented randomly with five different auditory cues, indicating the motor tasks to perform, as follows.
Fig. 1.
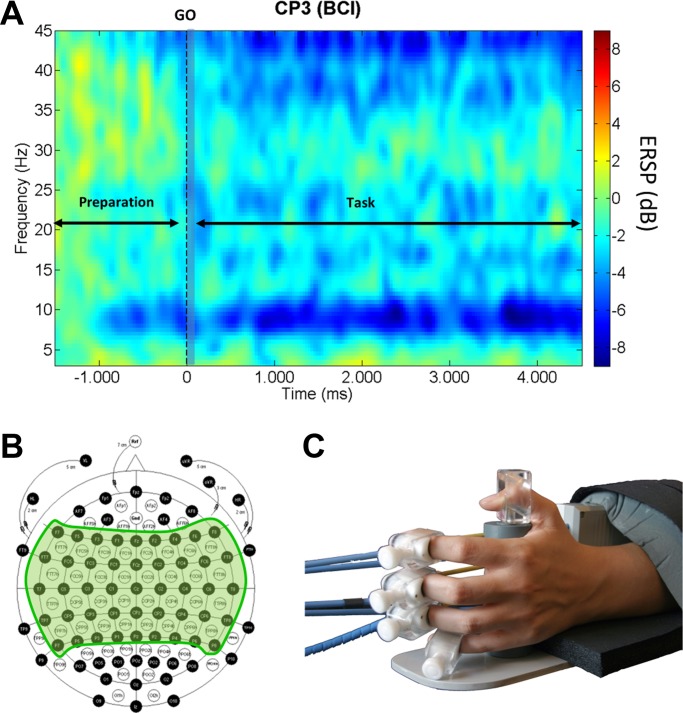
Experimental protocol. A: channel CP3 time-frequency event-related spectral perturbation (ERSP) during the brain-computer-interface (BCI) task. We divided our analysis in 3 time periods: preparation (−1,500 to 0 ms), onset (0 to 200 ms; shaded in light gray), and during task (200 to 4,500 ms). B: EEG 128-channel cap used for the recordings. Shaded in green, 61 electrodes used during the experiments over the motor areas. C: close look at the orthosis with the fingers attached. Bowden cables pull and push participants' fingers (open and close the hand) depending on the task to execute.
Motor imagery without direct control.
Motor imagery without direct control (MI) of the orthosis was performed; i.e., the participant imagined moving and imagined feeling the hand moving without moving it and with no movement of the orthosis.
Motor imagery with direct control.
Motor imagery with direct control (BCI task) of the orthosis was performed; i.e., the brain Rolandic rhythm oscillations (SMR) during imagining of hand opening and closing drove the movements of the orthosis attached to the hand.
Passive movements.
Passive movements of the orthosis were performed; i.e., the hand attached to the orthosis was opened and closed passively without any concurrent mental task.
Active movement.
Active movement was performed; i.e., the participant was required to open and close the hand attached to the orthosis, the orthosis following the movements.
Rest condition.
The final stage was rest.
The participants' SMR oscillations were directly linked with the orthosis movements during the BCI task only. Consequently, during a BCI task trial, SMR decrease in power (desynchronization) was “rewarded” with orthosis movement, and, vice versa, an increase in SMR power (synchronization) was “penalized” with the restriction of orthosis movement.
The orthosis range of motion was adjusted for each participant before starting the experiment. Two seconds after hearing the corresponding “task to perform” auditory cue, a “GO” cue was presented, and the participant performed the appropriate motor task for 5 s before movement was terminated by an auditory “end” cue at the end of the task period. All the spoken auditory cues were previously recorded and normalized in pitch, volume, and length to avoid cue differences. All participants had no prior BCI experience and completed five experimental sessions; each session was undertaken on a different day and consisted of completing 10 runs of 25 trials, each resulting in 50 trials per condition per session. An EEG screening was performed the day before the first training session and was used as a calibration session to identify the features, i.e., electrodes and frequency bins presenting higher r2 values when comparing MI and rest, which were to be used by the BCI classifier. More information about the BCI calibration and functioning can be found in Ramos-Murguialday et al. (2012).
Data Acquisition
EEG data were acquired using a BrainAmp 128-channel amplifier from Brainproducts (Munich, Germany). An EasyCap 128-channel EEG cap (modified 10–20 system) (Herrsching, Germany) was used for EEG data acquisition, referenced to the nasion and grounded anteriorly to Fz. Only 61 EEG channels over the motor areas on both hemispheres were used, focusing on premotor, motor, and postcentral areas (Fig. 1B). Additionally, we measured horizontal electrooculogram on both eyes and vertical electrooculogram on the right eye for artifact correction and EMG on both upper arms and forearms for the detection of unwanted muscle contractions (e.g., muscle contraction during rest or MI). Data were sampled at 500 Hz and transmitted to a PC for both storage and real-time signal processing, using the BCI2000 platform (www.bci2000.org). EMG data were acquired using four bipolar Ag/AgCl electrodes from Myotronics-Noromed (Tukwila, WA) placed on antagonistic muscle pairs, one close to the external epicondyle on the extensor digitorum (forearm extensor), one on the flexor carpi radialis (forearm flexor), one on the external head of the biceps (upper arm flexor), and the last one placed on the external head of the triceps (upper arm extensor). The EEG and EMG electrode impedance was always kept under 5 and 20, kOhm respectively.
Signal Processing
We performed a time-frequency analysis using a 1.142-s sliding window with an overlap of 26 ms. EEG data were first filtered using a band-pass filter (2–45 Hz) and segmented from 2 s before to 5 s after the GO cue; the baseline from −1.5 to −1 s before the GO cue was removed from each segment to remove offsets, and the event-related spectrum perturbation was then calculated using Morlet transforms (Daubechies 1996). The Morlet transforms used three cycles at lowest frequencies and 23.04 at highest and a time window of 1,114 ms and 30 ms overlap. A 200-ms time window from −1.5 to −1.3 s before the GO cue was used as baseline for the event-related spectra perturbation analysis (normalization). Eighty-five linear-spaced frequencies from 3 Hz to 45 Hz and 200 time points were generated.
To test for significant differences in brain activation within subjects and between motor tasks, we used nonparametric statistics (bootstrapping), not assuming a particular distribution. We performed bootstrap analyses to compare brain activity (time frequency) during the motor tasks. Comparisons were performed always in a pairwise manner, i.e., comparing two motor tasks and adopting the comparison rest vs. motor task (BCI, MI, and passive and active movement) as reference for our analyses of each motor task (Table. 1).
Table 1.
Changes in time and frequency recorded from EEG electrodes placed over the motor strip during four motor tasks and rest in three time periods
| Motor Task |
Active Movement |
Passive Movement |
Imagined Movement |
BCI Condition |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Period | Prior | Onset | During | Prior | Onset | During | Prior | Onset | During | Prior | Onset | During | |
| ERD (Power decrease) | |||||||||||||
| Contralateral | 3–6 Hz | – | – | – | – | – | X | – | X* | X | x | – | X |
| 6–14 Hz | – | – | XXXX | x | X | XXXX | – | XX | XXXX | – | – | XXXX | |
| 15–30 Hz | – | – | X | x | XX | XXX | – | XX | XX | XX | XX | XX | |
| 30–45 Hz | x | – | – | X | X | x | X | X | x | X | X | x | |
| Ipsilateral | 3–6 Hz | – | – | – | – | – | X | – | – | X | x* | – | X |
| 6–14 Hz | – | – | XXXX | – | X | XXXX | – | X | XXXX | –* | X | XXXX | |
| 15–30 Hz | – | – | X | – | X | XXX | – | X | XX | X | X | XX | |
| 30–45 Hz | x | – | – | – | X | x | X | X | x | – | X | x | |
| ERS (Power Increase) | |||||||||||||
| Contralateral | 3–6 Hz | XX | – | X | – | – | – | X | – | – | – | – | X |
| 6–14 Hz | XXXX | – | – | – | – | – | XX | – | – | x | – | – | |
| 15–30 Hz | XX | – | x | – | – | – | X | – | – | – | – | – | |
| 30–45 Hz | X | – | XXXX | X | – | – | X | – | – | x | – | – | |
| Ipsilateral | 3–6 Hz | XX* | – | X | – | – | – | X | – | – | – | – | X |
| 6–14 Hz | XXXX | – | – | – | – | – | XX | – | – | – | – | – | |
| 15–30 Hz | X | – | x | – | – | – | X | – | – | x | – | – | |
| 30–45 Hz | X | – | XXXX | X | – | – | X | – | – | x | – | – | |
Significant power change during motor tasks compared with rest. Significant decrease and increase in power before (preparation), at onset (planning), and during (execution) the 4 different motor tasks [active movement, passive movement, motor imagery (MI) and MI with proprioceptive feedback (brain-computer interface, BCI)]. X stands for significant power change intensity in power and time (i.e., difference in number of pixels in the time-frequency plot showing significant power changes). The number and size of the Xs represent the difference in power in each time period for each task (being x little and XXXX great significant power changes). Cells in underlined font indicate significant changes during BCI condition only, and cells in bold font indicate EEG activity changes related to the BCI condition and active movements only when compared with rest.
Unique task-specific significant changes in power, present during a specific time period (prior, onset, during) in that motor task when compared with rest only (i.e., no significant power changes when comparing during that period of time to any of the other 3 tasks to rest individually). Prior, onset, and during stand for the time periods with respect to the “GO” cue from −1,500 to 0 ms (preparation for a task), 0 to 200 ms (task initiation in response to an imperative cue), and 200 to 4,500 ms, respectively (during task execution), as described in Fig. legend 1. ERD/ERS, event-related desynchronization and synchronization.
When comparing task 1 with task 2, we subtracted in a trial-by-trial basis task 1 from task 2 time-frequency spectra, and then we averaged these differences and created a time-frequency spectra representing the mean spectral difference between tasks. Motor tasks were also compared between them to detail analysis.
For the spectral difference between task time-frequency plot (DIFF) generation, a baseline removal (power normalization) was performed using a common power baseline calculated averaging both task baseline means. This common baseline was then subtracted from each task time-frequency plot before computing the mean spectral difference time-frequency plot. (Fig. 2C).
Fig. 2.
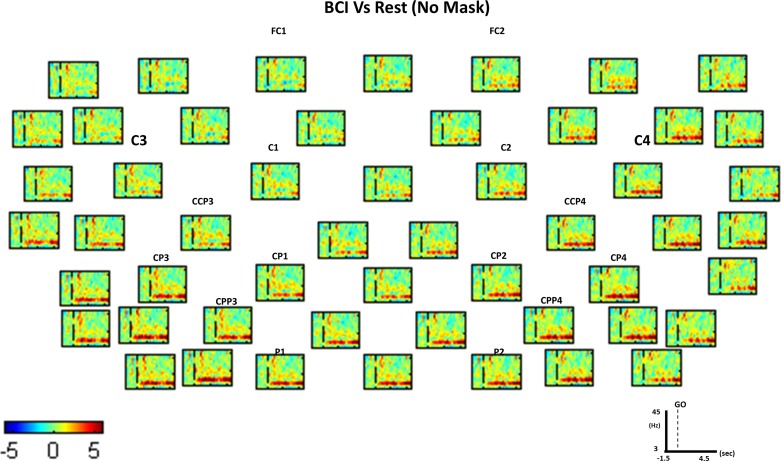
Significance mask. A: time-frequency plots for the active movement task (baseline removed using bootstrap analysis). B: time-frequency plots for the active movement task (baseline removed using bootstrap analysis). C: difference between active movement and rest (rest-active power values) tasks using a common baseline. D: significance mask obtained during the bootstrap analysis comparing active movement and rest tasks. Everything not in green is statistically significant (P < 0.01). The vertical dashed line indicates the time in which the “GO” cue was presented. Red color indicates desynchronization (DS) (power decrease in dB), and blue shows synchronization (S) (power increase in dB) during BCI compared with EEG activity during the rest condition, as expressed in the color bar.
Spectral differences between tasks not resulting as significant using the EEGLAB toolbox (http://sccn.ucsd.edu/eeglab/) were masked and plotted green (Fig. 2). The null hypothesis distribution used to determine significance thresholds was estimated by accumulating surrogate data from 200 bootstrap replications, shuffling the single-trial spectral DIFF estimates using a two-tailed bootstrap significance probability level (Burgess and Gruzelier 2008; Efron and Tibshirani 1994). The t1 and t99 percentiles obtained for every frequency and time bin (every “pixel” of the time-frequency plot) from the spectral DIFF were calculated and averaged from all 200 bootstraps. This allowed us to determine at each time and frequency bin the power values determining significance (i.e., with P = 0.01). No averaging of the values of the percentiles was performed in the frequency dimension because at different frequencies we have different power values (higher at lower frequencies), and this would produce false positives in low frequencies and false negatives in high frequencies. Averaging of the percentiles in time was performed to see significant changes with respect to all changes in time during the entire 7 s of the task, including preparation, onset, and task execution, to compute the bootstrap significance level (i.e., mask).
It is very important to note that, in this study, we considered significant differences in the time-frequency spectra between classes, only clusters (group of neighboring pixels in time or frequency dimension) of more than 18 pixels presenting the same sign significant difference in power with a minimum size in the x-axis (0.5 Hz/pixel) and y-axis (33 ms/pixel) of 3 pixels. We consider that the percentiles averaging over time and the cluster analysis compensate for the lack of a multiple-comparison correction to an acceptable extent without losing information related to correlation and noise.
Our main goal of the study was to describe spectral differences between motor tasks and describe features that might serve as differentiating factors between conditions. If we use hypothesis testing and correct for multiple comparisons, we would ignore and lose class power (i.e., correlation and noise information are lost). Thus some of the features alone would not result in significant differences, but, if used together, some features might become significant and help a classifier differentiate between classes (i.e., predictability based on recording). To demonstrate this effect, we used a nonparametric framework and determined the Monte-Carlo estimates from the permutation distribution to compare spectra correcting for multiple comparisons. Permutation statistics were computed for each sample in the time-frequency space as implemented in the Fieldtrip open-source toolbox (Oostenveld et al. 2011). We used 1,000 random permutations on a dependent-sample t-test and an adjusted α-level of P < 0.025 per tail. Although these sample-wise statistics produce a massive number of comparisons [i.e., the product of the number of channels (61) by times (200) by frequencies (85)], the cluster-based correction method was demonstrated to treat the multiplicity problem effectively without losing sensitivity for spectral modulations (Maris and Oostenveld 2007) (Fig. 9).
Fig. 9.
Spectral time-frequency measures statistical comparison. Top: topographical distribution of time-frequency plots for the difference in power between the BCI and the resting class. A: time-frequency spectral perturbation data. B: time-frequency spectral perturbations shown in A but using a significance mask calculated using bootstrapping techniques and corrected for multiple comparisons. Everything not in green is statistically significant. C and D: enlarged channel CP3 time-frequency spectra without and with significance mask, respectively. The vertical dashed line indicates the time in which the GO cue was presented. ERSP is measured in dB difference during BCI task compared with EEG activity during the rest condition. The areas contoured by a black line represent significant activity in spectral changes before multiple-comparison correction.
The EMG data were filtered using a high-pass filter at 10 Hz, bipolarized, and rectified. The waveform length of the signal (Farry et al. 1996), which provided us information about amplitude and frequency of the EMG signal, was extracted for every motor task and rest. After extracting the waveform length of the signal, the EMG data at rest were scanned for artifacts. Any rest trial with EMG activity exceeding 3 SDs at any point in time, at any electrode, within the analyzed time window (1.5 to 4 s with respect to the GO cue) was removed, and the remaining “considered artifact-free” trials were used to calculate an EMG activity threshold; this threshold was set at 3 SD from the mean of activity at rest. Any EMG activity exceeding this threshold (from −2 to 5 s with respect to the GO cue) for a continuous period of more than 25 ms was considered a muscle contraction. Trials presenting muscle activity at any electrode during the resting task or on the resting hand during any motor task and absence of activity during active opening and closing of the hand were excluded from the EEG analysis. A minimum number of 15 trials per person was required to be included in the analysis, which resulted in 193, 182, 234, 203, and 155 trials for MI, BCI, passive movement, active movement, and rest, respectively.
The time-frequency change analysis was divided in three time windows (Fig. 1A) as follows: 1) preparation period comprising the 1.5 s before the GO cue presentation starting 0.5 s after the instruction period in which the participants were presented with the task to be performed; 2) time immediately after presentation of the GO cue comprising the 200 ms after the cue; 3) time interval during the task 5 s that the trial lasted. We subdivided time windows in four frequency ranges: theta (3–6 Hz), alpha (6–14 Hz), beta (15–30 Hz), and gamma (30–45 Hz).
RESULTS
Preparation Period, with Instructions Before the GO Cue Presentation
During this time, the participants heard an auditory cue instructing them which motor task should be performed and had 2 s to prepare themselves before the imperative GO cue, indicating the beginning of the motor task action.
Theta (3–6 Hz).
During the last 200 ms before the GO cue, a significant increase in power was observed in all electrodes, which was larger at contralateral premotor electrodes compared with MI and rest (5–8 Hz) (Fig. 3; electrode C1). Contrastingly, when BCI was compared with rest, a significant decrease of power (5–7 Hz) occurred 1.5 to 1.2 s before cue onset in the contralateral and precentral ipsilateral electrodes (Fig. 4; electrode C1). Furthermore when we compared preparation for active movement with rest, significant theta (3–5 Hz) increase in power was observed from 700 to 350 ms before the GO cue in all electrodes; however, this increase was relatively constant and stronger in parieto-central electrodes.
Fig. 3.
Motor imagery (MI) vs. rest spectral power. Top: bootstrap analysis (grand average) comparing MI task and rest of 52 electrodes from the 61 used (for clarity, 9 electrodes were excluded from the figure). Everything not in green is statistically significant (P < 0.01). The vertical dashed line indicates the time in which the GO cue was presented. Red color indicates DS (power decrease in dB), and blue shows S (power increase in dB) during MI compared with EEG activity during the rest condition, as expressed in the color bar. Bottom: enlarged views of different channels of time-frequency power difference between these 2 classes.
Fig. 4.
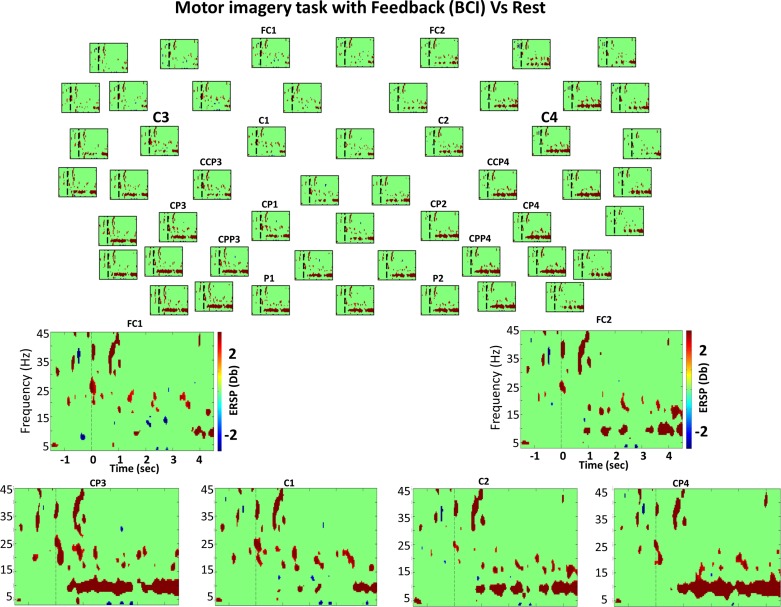
Bootstrap analysis (grand average) comparing MI with feedback (BCI task) and rest. Top: bootstrap analysis (grand average) comparing the BCI task and rest of 52 electrodes from the 61 used (for clarity, 9 electrodes were excluded from the figure). Everything not in green is statistically significant (P < 0.01). The vertical dashed line indicates the time in which the GO cue was presented. Red color indicates DS (power decrease in dB), and blue shows S (power increase in dB) during BCI compared with EEG activity during the rest condition, as expressed in the color bar. Bottom: enlarged views of different channels of time-frequency power difference between these 2 classes.
When we compared preparation for active movement with preparation for MI, BCI, and passive movement, we observed significantly higher power in theta frequencies during preparation for active movement in all electrodes (from 500 to 300 ms before the GO cue compared with MI and the last 300 ms compared with BCI and passive movement preparation before the GO cue) (Fig. 6). No significant difference was observed in the theta band before passive movement.
Fig. 6.
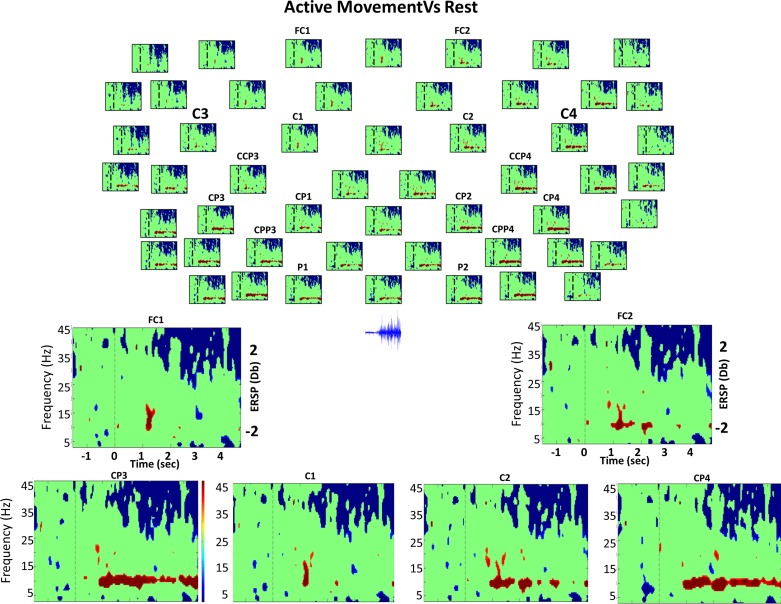
Bootstrap analysis (grand average) comparing active movement and rest. Top: bootstrap analysis (grand average) comparing the active movement task and rest of 52 electrodes from the 61 used (for clarity, 9 electrodes were excluded from the figure). Everything not in green is statistically significant (P < 0.01). The vertical dashed line indicates the time in which the GO cue was presented. Red color indicates DS (power decrease in dB), and blue shows S (power increase in dB) during active compared with EEG activity during the rest condition, as expressed in the color bar. Bottom: enlarged views of different channels of time-frequency power difference between these 2 classes. Below EEG electrodes in the topographical view, raw electromyogram (EMG) activity during active movement task. We can observe the significant activity in gamma and mu rhythm to correspond to the presence of muscle recruitment.
Alpha (6–14 Hz).
Significant increase in power was observed for the last 250 and 800 ms before the GO cue in all (mainly parietal) electrodes preparing for MI and active movement tasks, respectively (MI: 8–12 Hz; active: 6–10 Hz). The significant increase in power in the alpha band during the preparation for active movement started earlier and was much stronger than during the preparation for MI, eliciting significant differences continuously until the GO cue presentation (Fig. 6). A 200-ms decrease in power was observed in all electrodes from 1,500 to 1,300 ms before the GO cue when we compared preparing for passive movement and preparing to rest (Fig. 5). This effect seems to be a prolongation of a baseline effect. Interestingly, no significant difference in power was observed when we compared the BCI task with the passive movement. Preparation for the BCI task evokes significant power increase in the alpha rhythm frequency band compared with preparation to rest in fronto-central contralateral electrodes only (Fig. 4; electrode FC1), implying that expecting brain control of proprioceptive activation without muscle contraction (as in the BCI task) does not elicit a significant alpha power increase, as during the preparation for an active movement or for a motor imagery of that same movement in all electrodes.
Fig. 5.
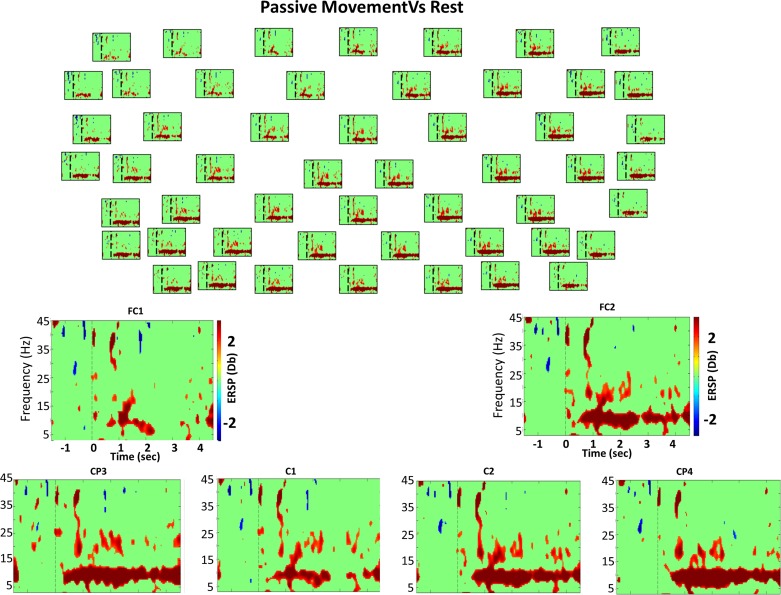
Bootstrap analysis (grand average) comparing passive movement and rest. Top: bootstrap analysis (grand average) comparing the passive movement task and rest of 52 electrodes from the 61 used (for clarity, 9 electrodes were excluded from the figure). Everything not in green is statistically significant (P < 0.01). The vertical dashed line indicates the time in which the GO cue was presented. Red color indicates DS (power decrease in dB), and blue shows S (power increase in dB) during passive compared with EEG activity during the rest condition, as expressed in the color bar. Bottom: enlarged views of different channels of time-frequency power difference between these 2 classes.
Beta (15–30 Hz).
During the preparation for BCI and MI tasks compared with preparation to rest, we observed from 500 to 200 ms before the GO a significant increase in power in the 16–18-Hz band in the precentral electrodes and in ipsilateral central electrodes, respectively (possibly related to attention) (Klimesch 2012). During preparation for BCI, passive movement, and active movement, we found significant power changes during the last 500 ms before the beginning of the task. Significant decreases in power during preparation for BCI and passive movement from 18 to 22 Hz [BCI: all electrodes (mainly contralateral); passive: contralateral electrodes] were observed. Significant increases in power during preparation for active movement in all electrodes, what was more pronounced in postcentral electrodes bilaterally (15–17 Hz and 21–23 Hz), were found.
Nevertheless, when we compared preparation for passive movements and BCI, two significant periods of significantly lower power preparing for BCI from −700 to −500 and the last 100 ms extending to 300 ms after the GO cue were found at 24–30 Hz and 18–22 Hz, respectively. Although preparation for MI just showed a significant power increase right before the cue in low beta (16–18 Hz), preparation for active movement and BCI showed a significant increase in power from 16–24 Hz compared with preparation for passive movement. These results indicate two beta band components during task preparation: 1) the contralateral 18–23-Hz activity (decrease in power) might comprise preparation for passive proprioceptive (without muscle contraction) activation (present before BCI and passive movement only); 2) the ipsilateral precentral 15–18-Hz increase in power might correspond to attention to a task or preparation to execute or imagine movement maintaining hand posture (present only before BCI, MI, and active movements), in line with the findings of Hatsopoulos and Donoghue (2009).
Gamma (30–45 Hz).
During preparation for all motor tasks, 30–45-Hz significant changes in power were found compared with preparing to rest in all electrodes.
Onset or First 200 ms After the GO Cue Presentation
Theta and alpha (3–14 Hz).
These two frequency bands presented similar activity, and therefore the significant changes are reported in the same section. No significant differences were found when we compared the rest task onset with the BCI and active movement tasks onsets. The MI task showed a significant decrease in power (8–12 Hz) in all electrodes (Fig. 3) at the onset. However, this decrease in power was largest in the contralateral electrodes, predominantly in the postcentral areas and in ipsilateral precentral electrodes; interestingly, for the passive movement task, a significantly high alpha rhythm (12–14 Hz) power decrease was found in the medial-precentral electrodes (Cz, Fz, F1, F2, Fcz, Fc1, and Fc2).
Beta (15–30 Hz).
All motor tasks but active movement showed significantly lower power in all electrodes in this frequency band compared with rest at onset. For the MI and BCI task onsets, the significant decrease in power was broadbanded (18–28 Hz) predominantly over contralateral regions and highest in postcentral areas. Contrastingly, during passive and active movement task onset, the significant decrease in power was in a narrow band (24 to 27 Hz) mainly over ipsilateral-postcentral regions for active movement and across all electrodes for passive movement. The largest differences were observed in contra-postcentral electrodes (Fig. 5; electrode CP3).
Interestingly, when we compared BCI with passive and active movement task onsets, significantly larger contralateral power decreases (18–22 Hz) were seen during the BCI task onset. These decreases were only over the contralateral precentral electrodes when BCI was compared with passive onset.
Gamma (30–45 Hz).
Only a 100-ms period of significant power decrease (35–42 Hz) was observed in all electrodes during the first 200 ms after the GO cue presentation during MI, BCI, and passive movement onset, which was absent during active movement onset.
During the Task, Excluding the First 200 ms
Theta (3–6 Hz).
During MI, BCI, and passive movement tasks, a significant power decrease was found in all electrodes from 750 to 900 ms after the cue compared with rest. However, for the active movement task, a significant power increase was found during the same interval (700 to 1,000 ms) across all electrodes (Fig. 6). During the BCI and active movement tasks, segments of frequency clusters presenting significant theta power increases (3–6 Hz) were observed after the first 1,800 ms until the end of the trial, again across all electrodes. Furthermore, in the active movement task, the power increase was larger than for the BCI tasks and more pronounced in contralateral areas. However, during the BCI task, the power increase was significantly larger compared with that of the MI task. During passive movement, two segments of frequency clusters presenting a significant power decrease, between 1.2–1.5 and 3.6–3.8 s, were found (3–7 Hz) in all electrodes. These time points are exactly the times in which the orthosis changes from opening to closing (i.e., orthosis stops for 150 ms to change from opening to closing, changing the sign of the velocity because maximum open or closed position was reached). In this frequency band (theta; 3–6 Hz), BCI presents similar brain activity to that during active movement.
Alpha (6–14 Hz).
This is the frequency band in which a significant decrease in power was more prominent for all the motor tasks compared with rest. During execution of all motor tasks, alpha rhythm was the frequency range that presented more significant power changes compared with rest. The same spatial distribution was found in all tasks, with significant decreases in power observed over all electrodes, but was generally more pronounced and continuous in ipsilateral electrodes for precentral electrodes and contralateral for postcentral electrodes. Thus identifying the ipsilateral precentral cortex as an integral element of motor regulation was expected. We will refer to this significant decrease in power spatial distribution as a “significance diagonal” from contra-postcentral to ipsi-precentral electrodes in motor areas.
During MI and BCI tasks, the significant difference in power occurred in the 7–10-Hz frequency band (Figs. 3 and 4). During the passive movement task, this power difference occurred in the 6–13-Hz frequency band (Fig. 5). During the active movement task, it occurred in the 8–11-Hz frequency band (Fig. 6).
The MI task alone was the only motor task showing clearly significant power decreases from 200 ms before the onset until the end of the trial (Fig. 3; electrode CP3), but only for the contralateral postcentral electrodes. After the initial 300 ms, significant decreases in power could be observed. The significant decrease in the ipsilateral electrodes displayed the earliest response, particularly in parietal electrodes before appearing later in the medial and finally in the precentral electrodes (posterior to anterior).
During MI across the medio-precentral electrodes, there were two periods presenting a significant decrease in power, one at the beginning of the trial for 350 ms and the second starting 1.5 s after the GO cue and lasting until the end of the trial (Fig. 3; electrode C2).
During the BCI task, the significant decrease in power started early in contralateral postcentral electrodes 300 ms after the GO cue and then continued until 2,100 ms after the beginning of the trial, and, after 350 ms of no significant change in power, it decreased significantly again until the end of the trial (Fig. 4). The significant decrease in power was observed for ipsilateral-postcentral electrodes throughout the entire trial.
During passive movement tasks, we observed the beginning of significant spectral changes at around 200 ms in the contralateral hemisphere compared with rest, similar to what we observed when BCI was compared with rest (Fig. 5; electrode CP3). In contrast, in the ipsilateral side, the significant spectral changes started 150 ms earlier than during BCI task, at 200 ms in the postcentral areas, and were delayed toward precentral areas (Fig. 5; electrode CP4).
The significant decrease in power during passive movements was present until the end of the trial in medial-ipsilateral electrodes.
Active movement was the task with the longest latency to the first significant decrease in power of the alpha rhythm compared with rest, starting 800 ms after the GO cue until the end of the trial in postcentral areas only. This late onset of significant decrease in power could be explained by a longer latency to the first movement onset for all users, as demonstrated by the EMG activity presented in Fig. 3B. In the active movement case, the significant decrease in power was continuously present toward the end of the trial only in the significance diagonal (contra-postcentral, ipsi-postcentral, and fronto-ipsilateral), and its latency was the same in the postcentral medio-lateral electrodes, as opposed to Takahashi et al. (2011).
We found more (more time-frequency bins and/or larger difference in power) reduced power (7–10 Hz) during MI than during BCI for the first 500 ms compared with rest mainly in contralateral precentral electrodes. However, we observed more power decrease during BCI than during MI compared with rest for the last 2.5 s of the trial in temporo-medial and medio-postcentral ipsilateral areas. Passive movement presented higher power than BCI during the first 2.5 s (6–13 Hz) in ipsilateral central and medio-central and precentral.
During the last second of passive movement and MI task, we observed smaller ERD (8–12 Hz) in postcentral areas (mainly ipsilateral) compared with the BCI task. On the other hand, during BCI, we observed periods of lower power (8–10 Hz) than during active movement, mainly in contralateral-postcentral electrodes and in ipsilateral-postcentral electrodes during the last 1.5 s (Fig. 3).
In summary, these data suggest that, during BCI, the significant power decrease in ipsilateral-medio-postcentral electrodes at frequencies between 8 and 10 Hz during the last second of the trial might be a unique effect of the haptic BCI task compared with the other motor tasks and rest.
Beta (15–30 Hz).
Contrary to most of the previous results commented on in the literature (Alegre et al. 2004; Derambere et al. 1999; Pfurtscheller and Lopes da Silva 1999; Pfurtscheller and Neuper 1994, 1997; Stanack and Pfurtscheller 1996a, 1996b). Only limited and very short periods of significant power changes were found during the active movement task in low beta (18–24 Hz) compared with rest (Fig. 6; electrode C2). However, significant decreases in power during active movement were present mainly in medio-precentral and medio-postcentral electrodes (SMA), which started before movement initiation and continued during movement, mainly in the first 1.5 s after muscle contraction started, in line with the previous work (Ohara et al. 2000, Pfurtscheller et al. 2003). A short 200-ms period of significant power increase was observed for all electrodes during active movement in the 14–16-Hz range, concurrently with the first significant increase in power in low gamma, high beta frequency range.
During MI and BCI tasks, a significant decrease in power compared with rest was found for the first 450 ms (MI: 14–20 Hz; BCI: 20–24 Hz) mainly across the significance diagonal, whereas, during the passive movement task, just the frequency range from 20 to 24 Hz showed a significant power decrease, and, during the MI and BCI tasks compared with rest, segments of frequency clusters presenting significant changes in power were found throughout the trial. Interestingly, we observed a significantly high beta decrease in power (20–24 Hz) only for the BCI task, in a manner similar to that observed during the passive movement task compared with rest.
During passive movements, intermittently significant decreases in power were found in the 16–24-Hz frequency range, becoming more continuous from 1 to 3.5 s after the GO cue (Fig. 5; electrode CP3) compared with rest. The decrease in power in the beta range during passive movements was larger than the decrease during the MI task in ipsilateral centro-postcentral electrodes during the entire trial and smaller in contralateral electrodes during the first 300 ms.
When we compared MI and BCI, we found significantly larger power decreases in the beta band (18–22 Hz) for the BCI task. This decrease occurred toward the end of the trial and was localized mainly to the ipsilateral-postcentral electrodes. In contrast, if BCI and passive movement were compared, we again found significantly larger decreases in power (18–22 Hz) during the BCI task; however, this was localized in contralateral medio-postcentral electrodes and occurred during the first 500 ms of the trial. These results reflect the influence of proprioception in the ipsilateral beta (20–24 Hz) decrease in power and of the motor volition in the first milliseconds after the GO cue in the contralateral beta (20–24 Hz) decrease in power. Furthermore, these results indicate either operant regulation or proprioceptive feedback and are both key factors in maintaining desynchronization in the 20–24-Hz beta frequency range, as opposed to the main role of the MI in the significant beta desynchronization reported previously (Pfurtscheller and Neuper 1997), which we found to happen in the 14–20-Hz range. Furthermore, significant beta band decreases in power were larger and more continuous during MI than during active movement tasks.
Compared with rest, the passive movement produced more significant changes in beta frequency range than the active movement, MI, and BCI tasks. Significant changes in the 20–24-Hz frequency band were observed during BCI and passive movement tasks only compared with rest. All this suggests the implication of these rhythms (20–24 Hz) in proprioception without active muscle contraction; however, during the BCI task, the significant power changes were less continuous.
Gamma (30–45 Hz).
During MI, BCI, and passive movement, a 200-ms period of significant decrease in power (30–45 Hz) was present in all electrodes 800 ms after the GO cue compared with rest. During active movement, a significant increase in power (38–45 Hz) from 200 to 400 ms after the GO cue was observed. This significant power increase continued in contralateral postcentral electrodes, spreading to a broader frequency band (26–45 Hz) starting earlier in contralateral-postcentral electrodes, and was sustained until the end of the trial. The significant gamma increase in power in the 26–45-Hz frequency range started concurrently with significant decreases in power of the slow oscillations (4–10 Hz) in the contralateral hemisphere (Fig. 6), in a manner consistent with previous research (Pfurtscheller and Neuper 1994; Pfurtscheller et al. 1993, 2003). Generally significant increase in power (26–45 Hz) started earlier in postcentral contralateral electrodes compared with central and precentral electrodes, with the onset latency of the significant increase in power increasing together with the distance to postcentral electrodes; thus the more frontal the location of the electrode, the larger the onset latency. However, in the ipsilateral hemisphere, no delay between postcentral and precentral areas was observed.
DISCUSSION
We described changes in time and frequency recorded from EEG electrodes placed over the motor strip during four motor tasks and rest and summarized our findings in three time periods (preparation, onset, and during task) and some standard frequency bands (Table 1). Significant power change is represented by X values, calculated as the number of pixels presenting significant power changes when we compared that motor task to rest during that time period. The number and size of the Xs represent the difference in power in each time period for each task, with x as little and XXXX as great significant power changes. We calculated the maximum and minimum X value for each time period over all the tasks and segmented it in eight equally large Pow ranges (x, xx, xxx, xxxx, X, XX, XXX, XXXX).
The presence/absence of X values does not necessarily mean identical activity in tasks presenting/not presenting X values in the same time period (e.g., if X is present before BCI task and passive movement, it does not necessarily mean that before both, tasks result in identical power changes). The X values could result from significant activity changes during different time periods or slightly different frequency bins when task-related power changes were compared with resting-related power changes. To statistically compare between motor tasks power changes, additional analyses were carried out (Fig. 7).
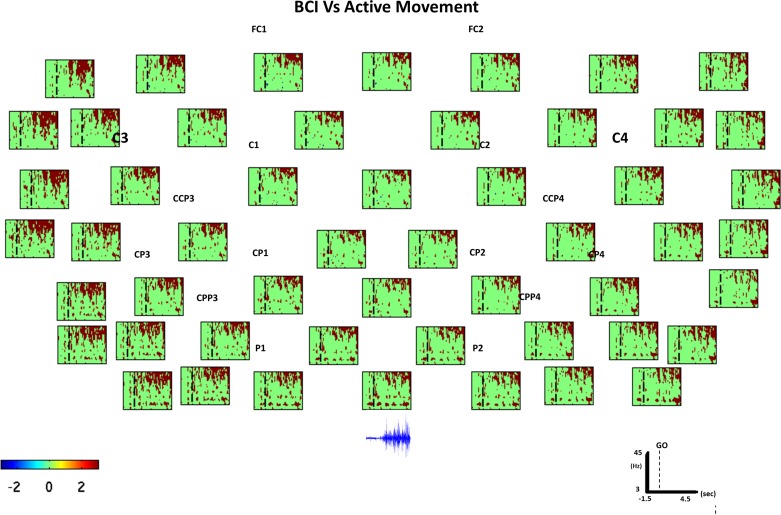
Fig. 7.
Active movement vs. BCI spectral power comparison. Bootstrap analysis (grand average) comparing MI with feedback (BCI) task and active movement. Everything not in green in statistically significant (P < 0.01). The vertical dashed line indicates the time in which the GO cue was presented to the participants. Red color indicates DS (power decrease in dB), and blue shows S (power increase in dB) during BCI compared with EEG activity during the active movement condition, as expressed in the color bar. Below the last channel line (parietal electrodes), we plotted raw EMG activity acquired on top of the extensor digitorum muscle during the active movement condition. The EMG amplitude increase corresponds with significantly higher gamma power in the low gamma region during active movement compared with the BCI condition.
The aim of our work was to analyze the cortical energy changes in the theta, alpha, beta, and low gamma bands preparing for, at onset, and during the execution of four motor tasks compared with a resting condition. In most of the previous literature investigating spectral changes of motor tasks, time-frequency power changes have been analyzed using a baseline to normalize and to observe spectral changes with respect to that predefined baseline. Intertrial intervals or instruction periods are commonly used as baseline periods. In this work, although we did not correct for multiple comparisons, we used statistical analyses to describe relevant spectral changes between motor tasks, using a resting class as reference. Hypothesis testing that corrects for multiple comparisons ignores and lacks correlation and noise information, which indeed would mask features that combined may help a classifier to differentiate between classes (i.e., predictability based on recording) (Fig. 9). Our results could therefore be taken as a study toward identifying the features that might help classify different motor tasks using EEG spectral changes.
The control for involuntary muscle contractions was performed because EMG activity has been observed in forearm extensor muscles during contralateral forearm activity and during mental activity, without finger movements being required. Similarly, Whitham et al. (2007) observed EMG activity in paralyzed subjects with a single limb excluded from paralysis, and as such it has been suggested that EMG activation during mental activity may be related to evolutionarily important mechanisms for preparedness to defend oneself or react; namely mental activity may constitutively activate protective programs of readiness for action (Whitham et al. 2007).
Preparation Period, with Instruction Period Before the GO Cue Presentation
We observed that preparing for BCI does not resemble preparing for active movement or MI EEG activity. We found that preparation for BCI (hand movements without muscle contractions) presented significantly lower preparatory theta and alpha power compared with MI and active movement preparation (presenting significant increase in power) compared with preparation for rest. This might be explained by participants shifting their attention more toward the proprioception than toward the actual MI during this preparatory phase. It has to be noted that the comparisons here were made against preparing to rest, which indeed presented power changes during preparation very similar to all the motor tasks (Fig. 2B).
In our data, we demonstrated how preparing to move induced a significant power increase in SMA and centro-parietal electrodes. In recordings with intracranial EEG, it has been observed that movement-associated ERD is preceded by a weak ERS in a nonmovement condition at around 7–12 Hz, compared with a slightly stronger and focal early ERS in two electrode contacts from the left postcentral Rolandic region in a movement condition (Klopp et al. 2001). Klopp and colleagues suggested that the early ERS could reflect either functional inhibition, in our case movement inhibition or movement preparation, whereas the late peri-Rolandic ERD represents a state of transient functional activation. Klimesch et al. (2007) demonstrated that alpha ERS is elicited in situations where people withhold or control the execution of a response and is obtained over sites that probably exert top-down control, for instance corticospinal control of motorneurons. Thus they proposed that alpha ERS reflects a top-down inhibitory control process and concluded that alpha ERS plays an active role for the inhibitory control and timing of cortical processing, whereas ERD reflects the gradual release of inhibition, associated with the emergence of complex spreading activation processes. Therefore, a 7–12-Hz ERS should be present during the preparation phase of all motor tasks. However, compared with preparation to rest, we found mainly significant ERS during preparation for MI (centro-parietal electrodes), preparation for active movement (SMA and centro-parietal electrodes), and preparation for BCI (minimally in contralateral fronto-central electrodes). These results follow the observations of Desmurget et al. (2009) that planning or intention of motor actions takes place in parietal cortex, whereas actual movements are produced by activation in the premotor cortex, although movement proprioception (feeling the movement) is induced through activating parietal areas.
We did not observe the decrease in the mu and/or beta band power described in previous work (Derambure et al. 1999; Pfurstcheller and Neuper 1997; Pfurtscheller et al. 2006; Stanack and Pfurtscheller 1996a, 1996b), probably because of our comparison to preparing for rest instead of comparing it to resting. This can be seen in Fig. 2A, where a clear ERD before the GO cue can be observed. However, the goal of our study was to compare the different tasks that can occur during a rehabilitation training, and therefore comparing it to the task rest allowed us to subtract changes attributable to the protocol (mainly cue presentation and timing).
During preparation for all motor tasks (MI, BCI, passive movement, and active movement), we found significant changes in power in the beta frequency band in contralateral postcentral electrodes at two clearly differentiated narrow frequency bands. This suggests several beta band components during task preparation: 1) 18–23-Hz power decrease in contralateral electrodes might comprise preparation for proprioception of the movement without active muscle contraction (present before BCI and passive movement only); 2) 18–23-Hz power increase might correspond to preparation for proprioception of the movement with active muscle contraction (present before active movement only in all electrodes); and 3) 15–18-Hz power increase in medio-postcentral electrodes (present before MI, BCI, and active movement only) could correspond to preparation for a volitional motor action maintaining hand posture (Hatsopoulos and Donoghue 2009).
Onset or First 200 ms After the GO Cue Presentation
The contralateral decrease in power in the alpha frequency range at MI onset continued until the end of the task, whereas, in all the other motor tasks, there was no significant alpha power change at onset. This might be a result of neural processes involving motor afferents. On the other hand, in the beta frequency range, all motor tasks but active movement presented significant power decrease at task onset. All these significant power decreases in beta oscillations (22–26 Hz) were observed during passive movement, MI, and BCI, suggesting a simultaneous involvement of passive proprioception-related neural networks during kinesthetic MI onset.
During the Task, Excluding the First 200 ms
During the execution of all the tasks, we observed a significant alpha power decrease spatial distribution compared with rest, significance diagonal. Contra-postcentral, medio-central, and ipsi-precentral electrodes in motor areas comprise this spatial distribution of significant alpha power changes, in line with previous work (Babiloni et al. 1999; Crone et al. 1998; Pfurtscheller and Berghold 1989; Pfurtscheller et al. 2000; Toro et al. 1994). However, the ipsilateral activation has not been investigated further.
During MI, BCI, and passive movement tasks compared with rest, ERD starts to be significant in parietal areas earlier during the execution of the task compared with central areas and in central areas compared with fronto-central areas. Significant decrease in power appearance occurred at the same time in contralateral electrodes. This might be interpreted as a propagation effect, referring only to the onset of significant alpha ERD when MI, BCI, and passive movement tasks are compared with the rest task.
In Fig. 8, we can see that the alpha ERD is continuous during the BCI task after the onset. However, these alpha power changes are not marked as significant continuously. Nevertheless, this effect needs further investigation and analysis to prove its significant appearance.
Fig. 8.
BCI vs. rest spectral power comparison without significance mask. Time-frequency topographical plot of the difference between active movement and rest (rest-active power values) tasks using a common baseline. The common baseline (−1.5 to −1.3 s) has been subtracted from each task time frequency before subtraction between tasks was done. Everything not in green in statistically significant (P < 0.01) compared with the common baseline. The vertical dashed line indicates the time in which the GO cue was presented to the participants. Red color indicates DS (power decrease in dB), and blue shows S (power increase in dB) during BCI compared with EEG activity during rest, as expressed in the color bar.
Topographical analysis of the phase of ongoing oscillations between recording sites has led to the detection of “traveling” waves, best documented for alpha frequencies moving in a task-dependent manner, e.g., from anterior to posterior sites (Takahashi et al. 2011). The fact that travel speed is in the range of neural transmission (Schack et al. 2003) indicates that spreading direction of alpha reflects communication between brain areas. This suggests that the observed significant ipsilateral alpha wave decrease in power, starting in posterior sites earlier and in anterior sites later during passive movements, MI, and BCI task, might indicate sensory information propagating from parietal to motor and premotor areas and not motor intention or planning because the delay toward frontal electrode latency of significant alpha power changes is not present during active movement, a conclusion again supporting previous findings (Schwartz 1994, 2004). During a BCI task, we could expect a similar effect to the one observed during active movement because the movements of the hand with the orthosis were controlled by motor-related oscillations on the contralateral hemisphere as in the active movement task; however, this was not the case, probably because of delay between efferent (brain command) and afferent (BCI-guided orthosis movement) activity. The fact that, during motor imagery (MI and BCI) and passive movement, the brain does not use precise somatosensory feedback could delay the appearance of significant alpha power decrease in ipsilateral brain areas via uncrossed afferent fibers arriving directly to precentral central areas and postcentral areas. During active movements, the ipsilateral cortex is activated at the same time presumably with movement-related sensory feedback.
The significant decrease of ipsilateral hemispheric alpha rhythm (6–10 Hz) activation during MI might be due to different events, such as active inhibition of movements, reception of an efference copy from the contralateral hemisphere, or inhibitory inputs. Regarding the sustained ipsilateral significant decrease in alpha (8–10 Hz) power during active movements, this might be due to a combination of interhemispheric flow (efference copy), active suppression of mirror movements, and an afferent somatosensory input projecting also to the ipsilateral hemisphere via uncrossed fiber tracts (Korvenoja et al. 1995).
It has been demonstrated that alpha ERD during passive movements disappeared if local nerve block was administered and proprioception of the upper limb was blocked (Chou-Ching et al. 2003), indicating that this frequency is directly related to movement proprioceptive input. Therefore, we should not see significant alpha decrease in power during MI; however, we do indeed observe a significant decrease in power in the alpha band during MI, suggesting that either alpha comprises two components and one is related to motor volition or there is an afferent recall during kinesthetic MI.
It has been proposed that ERS should be produced for planning of action and ERD during sensory processing (Klimesch 2012). The mu rhythm is expected to desynchronize over the respective areas of the homunculus, reflecting the downstream modulation of motor neurons (Pineda 2005). However, areas (such as the SMA) that are involved in planning of movements should exhibit an increase in power. Indeed with people observing another person doing a complex motor task, Muthukumaraswamy and Johnson (2004) and Muthukumaraswamy et al. (2004) found ERD over sensory-motor areas but ERS over the SMA. However, we observed alpha band ERS during preparation for active movement, BCI, and MI in SMA but not during the execution of these motor tasks. This effect could be due to the need for planning before the task starts, whereas, once the task has started, no more planning is needed (too simple task) and only motor correction responding to sensory activity takes place.
Neuper et al. (2008) demonstrated that abstract feedback (e.g., a thermometer on a screen) from brain oscillations during a BCI task produces similar mu ERD/ERS compared with watching a video of a hand moving. Because our participants watched the hand being moved by the orthosis during the active and passive movements and BCI conditions, the alpha ERD during the task we observed can be a combination of “mirror movement” and proprioceptively produced activity.
It has been suggested that different dynamics in the temporal pattern of oscillatory change in alpha and beta bands indicate that parallel processes may be occurring in the neuronal network configurations involved in generating these rhythms during movement, with those generating beta rhythms having more of a motor component and those generating alpha rhythms having more of a somatosensory component (Jurkiewicz et al. 2006). In line with these results, Patino et al. (2006) showed that, with chronic deafferentation, there is a larger ERD during active movement performance in the alpha rhythm only, and they speculated an effort-related effect because it should be more difficult to perform a movement without sensory feedback, whereas the beta dynamics remained unaffected after chronic deafferentation and in transient ischemia (Schnitzler et al. 1997). Although in these studies it was not possible to distinguish whether the stronger alpha power decrease was due to increased effort or to the removal of the expected sensory input, i.e., larger prediction error requiring increased neural processing, it was shown that the removal of afferent feedback does not significantly influence the strength of beta dynamics and at least does not weaken the alpha rhythm dynamics during active movements. However, in the work of Schnitzler et al. (1997), a complete alpha range was used in the analysis, ignoring the different subcomponents within alpha frequency range; therefore it could have affected the motor component of the alpha rhythm only. Our data show a larger significant beta decrease in power during passive movement, MI, and BCI than during active movement, suggesting a more inhibitory activity modulated by sensory input in the beta frequency range, i.e., the participant feels or imagines feeling the hand moving and inhibits muscle contraction.
However, results from human electroencephalographic studies suggest that an increase (and not a decrease) in power in the beta range is associated with inhibition of the excitatory state of the motor cortex (Gilbertson et al. 2005). There is also clinical evidence regarding the origin of this inhibition in patients with frontal lobe damage that exhibit “unwilled” automatic movements (Archibald et al. 2001). These clinical studies suggest that the prefrontal, anterior cingulate, and supplementary motor cortexes may contribute the necessary inhibition to prevent triggering of movement commands generated in activated motor and premotor cortical areas. Following these studies, we should have observed beta ERD in these areas during active movement. However, during active movement, only sparse significant decreases in power were observed in the low-beta frequency range mainly at medio-central and ipsilateral electrodes, as opposed to the majority of the literature results (Alegre et al. 2004; Derambere et al. 1999; Pfurtscheller and Lopes da Silva 1999; Pfurtscheller and Neuper 1994, 1997; Stanack and Pfurtscheller 1996a, 1996b). In our study, we used an extra resting task with the same protocol (warning cue with instructions “Rest,” imperative cue “GO,” and “end cue”) and compared it time point by time point and frequency band by frequency band as opposed to the baseline used in those studies (normally an average from each frequency band during some seconds before the task), and this could have influenced the statistical comparison (Fig. 9).
We observed several spectral components only related to 1) volition (i.e., all volitional tasks, including BCI, MI, and active movement, showing the same activation); 2) passive proprioception only (i.e., without muscle contraction, including BCI and passive movement, showing the same activation); and 3) proprioception (i.e., all proprioceptive tasks, including BCI, passive movement, and active movement, showing the same activation) (Table 2).
Table 2.
Significant power change related to proprioception or volition only
| Time |
Prior |
Onset |
During |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency Band | Electrode | Contralateral | Medial | Ipsilateral | Contralateral | Medial | Ipsilateral | Contralateral | Medial | Ipsilateral | |
| Alpha | 6–10 Hz | Front. | V(ERS) | – | – | – | – | – | – | – | |
| Centr. | – | – | – | – | – | – | – | – | – | ||
| PostCentr. | – | – | – | – | – | – | – | – | – | ||
| 10–14 Hz | Front. | – | – | – | – | – | – | – | – | – | |
| Centr. | – | – | – | – | – | – | – | – | – | ||
| PostCentr. | – | – | – | – | – | – | – | – | – | ||
| Beta | 15–18 Hz | Front. | – | V(ERS) | V(ERS) | – | – | – | – | ||
| Centr. | – | – | – | – | – | – | – | – | – | ||
| PostCentr. | – | – | – | – | – | – | – | – | |||
| 19–24 Hz | Front. | PP(ERD) | – | – | – | – | – | PP(ERD) | PP(ERD) | PP(ERD) | |
| Centr. | PP(ERD) | – | – | – | – | – | PP(ERD) | PP(ERD) | PP(ERD) | ||
| PostCentr. | PP(ERD) | – | – | – | – | – | PP(ERD) | PP(ERD) | PP(ERD) | ||
Significant decrease (ERD) and increase (ERS) in power before (preparation), at onset (planning), and during (execution) for the 4 different motor tasks (active movement, passive movement, MI, and MI with proprioceptive feedback, i.e., BCI) when compared with rest allowed us to identify significant changes in frequency at different electrodes related to: 1) volition (V), i.e., all volitional tasks (BCI, MI, and active movement) showing the same activation, 2) passive proprioception only (PP), i.e., without muscle contraction (BCI and passive movement); and 3) proprioception (P), i.e., all proprioceptive tasks (BCI, passive movement, and active movement).
The frequency range from 20 to 24 Hz appears to be related to proprioception without muscle contraction because only passive movement and BCI tasks showed significant activity in this band at the time orthosis movement started; therefore, we decided to subdivide the frequency ranges used in Table 1 to analyze these effects more in detail. Furthermore, the BCI condition presented significantly higher activity in this band compared with passive movement, implying that movement imagery linked to passive movements increased the activity in this frequency band in line with previous BCI control results (Ramos-Murguialday et al. 2012). This could be explained following the results observed by Kayser et al. (2009), in which they found that feedback interactions during multisensory processing are associated with beta band activity. However, our results regarding the 20–24-Hz band, indicate that this somatosensory processing is related to proprioception without muscle contraction because it was absent during active movement.
Beta band activity has been also identified as related to “keeping online” the mapping between somatosensory inputs and motor actions required by the context. Indeed, beta oscillations have been related to top-down attention and sensorimotor decision making (Siegel et al. 2012). Thus, because the active movement task was a simple opening and closing of the hand, this could have been the reason for the absence of more beta activity desynchronization during active movement, and therefore we suggest that beta ERD is related to proprioceptive information processing and/or the inhibition of muscle contractions.
Summarizing, we observed significant alpha and beta power differences before, at onset, and during the motor tasks that related to passive proprioception or volition only (Table 2); before the task, we observed volition-only-related ERS (6–10 Hz) in contralateral frontal electrodes and passive proprioceptive-related ERD (19–24 Hz) in contralateral electrodes. At task onset, we did not observe any specific volition-only- or proprioception-only-related ERD or ERS. During the task, we observed passive proprioception-only-related ERD 19–24 Hz in all electrodes.
In addition to the main investigated motor-related frequency bands using EEG (alpha and beta), in subdural recordings, contralateral dominant gamma clusters (gamma ERS) have been observed during the execution phase. Our strict filtering of trials containing EMG activity during tasks might have made the detection of gamma activity more reliable. It is important to notice that induced gamma and beta oscillations embedded in desynchronized alpha band activity have been already observed previously (Pfurtscheller and Neuper 1994; Pfurtscheller et al. 1993). We found significant EEG gamma power changes (24–45 Hz) during the task to be concurrent with muscle contraction and cooccurring with slow-oscillation (theta and alpha) significant changes in power (Fig. 6). Although we have not statistically proven the correlation between theta and gamma activity, recent results using intracranial EEG reported delta and gamma band to contribute significantly more strongly than other frequency bands when decoding muscle activity (Shin et al. 2012) and end-point trajectories (Nakanishi et al. 2013). Furthermore, it has been shown that theta oscillations modulated power at gamma frequency, such that the gamma oscillations cooccur with the incoming sensory information, i.e., during active sensing (Siegel et al. 2012). Therefore, we expected to observe cooccurring gamma and theta oscillations during active movement and BCI task; however, this was not the case during BCI.
We concluded that we did not observe slow oscillations cooccurring with gamma oscillations during the BCI task because 1) the BCI system delay linking afferent and efferent activity was not optimal, as naturally happening during active movement, and the slow oscillations could not modulate power at gamma frequency; and 2) significant gamma changes in power are related to muscle contraction only.
Another limitation of the study is to what extent the feedback in the BCI task affects the time-frequency modulations. We cannot discern which of the differences in time-frequency activity between MI and BCI were due to proprioception or to the feedback (visual and proprioceptive) of the BCI, which would strengthen the ERD control and therefore SMR activity, as demonstrated previously (Buccino et al. 2001; Lapenta et al. 2013; Ramos-Murguialday et al. 2012).
During passive movement and active movement, the proprioception is continuous; during BCI (reflected in our results in the theta band during the task), the control of the orthosis will vary depending on the SMR modulation. Therefore, we cannot conclude that, if there is not a modulation at the same time during BCI and passive or active movement, then there is not a common signature between BCI and these two classes (passive and active movement).
Relevant for SMR-based BCI control of orthoses is the fact that theta (3–6 Hz) and alpha (8–10 Hz) frequency band power changes during MI, BCI, and active movement do not show significant differences. This means that classification of EEG changes attributable to active movement or MI could be differentiated from passive movement-generated desynchronization when using a combination of theta and alpha frequency band features from medio-postcentral electrodes on the contralateral hemisphere. Furthermore, our findings showed that active movement could be easily decoded using low gamma band activity after adequate limb and head EMG artifact filtering. We found theta power increase during the trial to be the feature differentiating BCI and active movement from MI and passive movement (significantly higher power during BCI and MI compared with passive movement and MI), and it could be related to voluntary hand movement generation.
Conclusion
We analyzed the cortical energy changes in the alpha, beta, and low gamma bands before, at onset, and during four motor tasks, from which three of the four motor tasks were related to motor rehabilitation (MI and active and passive movement) and were compared with the other motor task (BCI), in which the three of them were mixed. During the BCI task, we linked brain activity attributable to MI of hand opening/closing and the exact same movement via a BCI-controlled robotic hand orthosis. We demonstrated that spectral power changes in alpha and beta SMR frequency ranges before and during each of the motor tasks (motor imagery, BCI, and active and passive movements) presented low and high components for each band, separating the involvement of these low and high components of alpha and beta band in proprioception and volitional motor commands.
Furthermore, we demonstrated that each motor task presented EEG signatures, which were significantly different from the other motor tasks, except for passive movement. The BCI task incorporated all EEG signatures from passive movement while presenting some EEG signatures shared only with active movement and others related only to the BCI task. On the other hand, and somehow opposite to our goal when closing the loop (BCI), we observed that EEG activity during brain control of the orthotic device in the BCI task did not resemble neural activation generated before (ERS in theta and alpha bands) and during (ERS in beta and low gamma bands) active movement. This was probably due to the absence of muscle contraction during BCI (low gamma ERS), the presence of proprioceptive feedback false positives/negatives, the delay caused by MI classification not being 100% correct, and because signal processing presents a larger delay compared with natural cortico-muscular activations. However, as mentioned above, we found specific significant power changes at frequencies and time windows present during BCI task and active movement only. We observed EEG signatures present during the BCI task only, indicating that the BCI task involves particular neural oscillations not present during MI, passive movement, or active movement tasks. These findings raise the question of the existence of a different brain state when brain-controlling (BCI) afferent pathways (haptic feedback) bypass subcortical, spinal, and peripheral effectors.
In this work, we pinpointed EEG signatures related to motor tasks and motor task components involved in motor rehabilitation, which could be used toward the design and development of an EEG-based user-involvement assessment tool for motor rehabilitation interventions. Such a tool could provide the therapist with information regarding the involvement of the patient during the rehabilitation motor training by being able to classify whether the patient is not trying to move and only passive components are present, or whether any volition component is present and the patient is trying to move, but movement is not possible. These features differentiating brain oscillations during motor tasks involved in motor rehabilitation could contribute substantially in the development of more advanced motor rehabilitation therapies like brain-controlled and shared-controlled (EEG-EMG) orthotics. It is important to notice that significant power changes were found before the beginning of the trial in all frequency ranges, suggesting that the prediction of motor intention is very plausible, and therefore so is the development of faster EEG-based BCI control.
The proprioceptive feedback broadened the significant changes in frequency power mainly in alpha and beta bands and therefore increased BCI performance, biasing it as the power at these frequency bands was used as inputs for the linear classifier. We know from previous work (Ramos-Murguialday et al. 2012, 2013) that healthy participants and patients can learn to control the proprioceptive BCI, but an adequate selection of features could optimize learning by avoiding afferent bias. For this purpose, we suggest following the work of Siegel et al. (2012) in using spectral fingerprints to distinguish between similar EEG activity in different behavioral levels.
GRANTS
This work was funded by the Deutsche Forschungsgemeinschaft (DFG) project KU 1453-1 and Koselleck to N. Birbaumer (BI 195/58-1), the Werner Reichardt Center of Integrative Neuroscience (CIN) (2011-18 and 2008-6), the Baden Wuerttemberg Stiftung (ROB-1), the Bundes Ministerium für Bildung und Forschung (BMBF Förderzeichen 01GQ0831), the European Union Information and Communication Technologies Framework Programme 7 (ICT-FP7) (HUMOUR 231724; WAY 288551), the European Union COST action (TD1006), the Indian-European collaborative research and technological development projects (INDIGO-DTB2-051), the VolkswagenStiftung, and the National Natural Science Foundation of China (NSFC 31450110072).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.R.-M. and N.B. conception and design of research; A.R.-M. performed experiments; A.R.-M. analyzed data; A.R.-M. and N.B. interpreted results of experiments; A.R.-M. prepared figures; A.R.-M. and N.B. drafted manuscript; A.R.-M. and N.B. edited and revised manuscript; A.R.-M. and N.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Sebastian Halder, Juergen Mellinger, and Juergen Dax for hard work and dedication toward development of the BCI-orthosis platform. The authors thank Eva Maria Hammer and Silvia Kofler for assistance with the EEG experiments.
REFERENCES
- Alegre M, Labarga A, Gurtubay IG, Iriarte J, Malanda A, Artieda J. Beta electroencephalograph changes during passive movements: Sensory afferences contribute to beta event-related desynchronization in humans. Neurosci Lett 331: 29–32, 2002. [DOI] [PubMed] [Google Scholar]
- Alegre M, Gurtubay IG, Labarga A, Iriarte J, Malanda A, Artieda J. Alpha and beta oscillatory activity during a sequence of two movements. Clin Neurophysiol 115: 124–130, 2004. [DOI] [PubMed] [Google Scholar]
- Archibald SJ, Mateer CA, Kerns KA. Utilization behavior: clinical manifestations, and neurological mechanisms. Neuropsychol Rev 11: 117–130, 2001. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Cincotti F, Rossini PM, Neuper C, Pfurtscheller G, Babiloni F. Human movement-related potentials vs desynchronization of EEG alpha rhythm: a high-resolution EEG study. Neuroimage 10: 658–65, 1999. [DOI] [PubMed] [Google Scholar]
- Barrett G, Shibasaki H, Neshige R. Cortical potentials preceding voluntary movement: evidence for three periods of preparation in man. Electroencephalogr Clin Neurophysiol 63: 327–339, 1986. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Elbert T, Canavan AGM, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev 70: 1–40, 1990. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kübler A, Perelmouter J, Taub, Flor EH. A spelling device for the paralysed. Nature 398: 297–298, 1999. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Ramos Murguialday A, Cohen L. Brain-computer-interface (BCI) in paralysis. Curr Opin Neurol 21: 634–638, 2008. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci 13: 400–404, 2001. [PubMed] [Google Scholar]
- Burgess A, Gruzelier J. Methodological advances in the analysis of event-related desynchronization data: reliability and robust analysis. In: Handbook of Electroencephalography and Clinical Neurophysiology, revised series, Vol 6, edited by Pfurtscheller G, Lopes da Silva FH. Amsterdam, Netherlands: Elsevier Science, 2008, p. 139–158. [Google Scholar]
- Cassim F, Monaca C, Szurhaj W, Bourriez JL, Defebvre L, Derambure P, Guieu JD. Does post-movement beta synchronisation reflect an idling motor cortex? Neuroreport 12: 3859–3863, 2001. [DOI] [PubMed] [Google Scholar]
- Cho W, Vidaurre C, Hoffmann U, Birbaumer N, Ramos-Murguialday A. Afferent and efferent activity control in the design of brain computer interfaces for motor rehabilitation. Eng Med Biol Soc Proc Annu Int Conf IEEE 2011: 7310–7315, 2011. [DOI] [PubMed] [Google Scholar]
- Chou-Ching K, Ming-Shaung L, Chih-Hsu H. Influence of passive thumb movements on mu wave. J Med Biol Eng 23: 37–44, 2003. [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson WT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain 121: 2271–2299, 1998. [DOI] [PubMed] [Google Scholar]
- Daubechies I. Where do wavelets come from?—A personal point of view. Proc IEEE 84: 510–513, 1996. [Google Scholar]
- Dechent P, Merboldt KD, Frahm J. Is the primary motor cortex involved in motor imagery? Cogn Brain Res 19: 138–144, 2004. [DOI] [PubMed] [Google Scholar]
- Deecke L, Scheid P, Kornhuber HH. Distribution of readiness potential, pre-motion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Exp Brain Res 7: 158–168, 1969. [DOI] [PubMed] [Google Scholar]
- Derambure P, Defebvre L, Bourriez JL, Cassim F, Guieu JD. Event-related desynchronization and synchronization. Reactivity of electrocortical rhythms in relation to the planning and execution of voluntary movement. Neurophysiol Clin 29: 53–70, 1999. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science 324: 811, 2009. [DOI] [PubMed] [Google Scholar]
- de Vries S, Mulder T. Motor imagery and stroke rehabilitation: a critical discussion. J Rehabil Med 39: 5–13, 2007. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: CRC, 1994. [Google Scholar]
- Ehrsson HH, Kuhtz-Buschbeck JP, Forssberg H. Brain regions controlling nonsynergistic versus synergistic movement of the digits: a functional magnetic resonance imaging study. J Neurosci 22: 5074–5080, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farry KA, Walker ID, Baraniuk RG. Myoelectric teleoperation of a complex robotic hand. IEEE Trans Robot Autom 12: 775–788, 1996. [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66: 198–204, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardin E, Sirigu A, Lehe'ricy S, Poline JB, Gaymard B, Marsault C, Agid Y, Le Bihan D. Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex 10: 1093–1104, 2000. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci 25: 7771–7779, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Costes N, Decety J. Top down effect of the strategy to imitate on the brain areas engaged in perception of biological motion: a PET investigation. Cogn Neuropsychol 15: 553–582, 1998. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp 12: 1–19, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder S, Agorastos D, Veit R, Hammer EM, Lee S, Varkuti B, Bogdan M, Rosenstiel W, Birbaumer N, Kubler A. Neural mechanisms of brain-computer interface control. Neuroimage 55: 1779–1790, 2011. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos NG, Donoghue JP. The science of neural interface systems. Annu Rev Neurosci 32: 249–66, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan N, Krebs HI, Rohrer B, Palazzolo JJ, Dipietro L, Fasoli SE, Stein J, Hughes R, Frontera WR, Lynch D, Volpe BT. Motions or muscles? Some behavioral factors underlying robotic assistance of motor recovery. J Rehabil Res Dev 43: 605–618, 2006. [DOI] [PubMed] [Google Scholar]
- Hummel F, Andres F, Altenmüller E, Dichgans J, Gerloff C. Inhibitory control of acquired motor programmes in the human brain. Brain 125: 404–420, 2002. [DOI] [PubMed] [Google Scholar]
- Hu XL, Tong KY, Song R, Zheng XJ, Leung WW. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabil Neural Repair 23: 837–846, 2009. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Lafleur MF, Malouin F, Richards CL, Doyon J. Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. Neuroimage 20: 1171–1180, 2003. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Penfield W. Electrocorticograms in man: effect of the voluntary movement upon the electrical activity of the precentral gyrus. Arch Psychiatr Z Neurol 183: 163–174, 1949. [Google Scholar]
- Jeannerod M, Decety J. Mental motor imagery: a window into the representational stages of action. Curr Opin Neurobiol 5: 727–732, 1995. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Frak V. Mental imaging of motor activity in humans. Curr Opin Neurobiol 9: 735–739, 1999. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. Neuroimage 32: 1281–1289, 2006. [DOI] [PubMed] [Google Scholar]
- Kasess CH, Windischberger C, Cunnington R, Lanzenberger R, Pezawas L, Moser E. The suppressive influence of SMA on M1 in Motor imagery revealed by fMRI and dynamic causal modeling. Neuroimage 40: 828–837, 2008. [DOI] [PubMed] [Google Scholar]
- Kayser C, Logothetis NK. Directed interactions between auditory and superior temporal cortices and their role in sensory integration. Front Integr Neurosci 4: 3–7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinrath C, Wriessnegger S, Mueller-Putz GR, Pfurtscheller G. Post-movement beta synchronization after kinaesthetic illusion, active and passive movements. Int Psychophysiol 62: 321–327, 2006. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16: 606–617, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res Rev 53: 63–88, 2007. [DOI] [PubMed] [Google Scholar]
- Klopp J, Marinkovic K, Clarke J, Chauvel P, Nenov V, Halgren E. Timing and localization of movement-related spectral changes in the human peri-Rolandic cortex: intracranial recordings. Neuroimage 14: 391–405, 2001. [DOI] [PubMed] [Google Scholar]
- Koralek AC, Jin X, Long JD, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature 483: 331–335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvenoja A, Wikstrom H, Huttunen J, Virtanan J, Laine P, Aronen HJ, Seppalainen AM, Ilmoniemi RJ. Activation of ipsilateral primary sensorimotor cortex by median nerve stimulation. Neuroreport 6: 2589–2593, 1995. [DOI] [PubMed] [Google Scholar]
- Lacourse MG, Orr EL, Cramer SC, Cohen MJ. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage 27: 505–519, 2005. [DOI] [PubMed] [Google Scholar]
- Lapenta OM, Minati L, Fregni F, Boggio PS. Je pense donc je fais: transcranial direct current stimulation modulates brain oscillations associated with motor imagery and movement observation. Front Hum Neurosci 7: 256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BI, Lüders H, Lesser RP, Dinner DS, Morris HH. Cortical potentials related to voluntary and passive finger movements recorded from subdural electrodes in humans. Annu Neurol 20: 32–37, 1986. [DOI] [PubMed] [Google Scholar]
- Leocani L, Toro C, Manganotti P, Zhuang P, Hallett M. Eventrelated coherence and event-related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self-paced movements. Electroencephalogr Clin Neurophysiol 104: 199–206, 1997. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hülsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci 11: 491–501, 1999. [DOI] [PubMed] [Google Scholar]
- Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain 126: 866–872, 2003. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007. [DOI] [PubMed] [Google Scholar]
- Mima T, Sadato N, Yazawa S, Hanakawa T, Fukuyama H, Yonekura Y, Shibasaki H. Brain structures related to active and passive finger movements in man. Brain 122: 1989–1997, 1999. [DOI] [PubMed] [Google Scholar]
- Müller GR, Neuper C, Keinrath C, Gerner HJ, Pfurtscheller G. Event-related beta EEG changes during wrist movements induced by functional electrical stimulation of forearm muscles in man. Neurosci Lett 340: 143–147, 2003. [DOI] [PubMed] [Google Scholar]
- Müller-Putz GR, Zimmermann D, Graimann B, Nestinger K, Korisek G, Pfurtscheller G. Event-related beta EEG-changes during passive and attempted foot movements in paraplegic patients. Brain Res 1137: 84–91, 2007. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW. Changes in Rolandic mu rhythm during observation of a precision grip. Psychophysiology 41: 152–156, 2004. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Cogn Brain Res 19: 195–201, 2004. [DOI] [PubMed] [Google Scholar]
- Naito E. Sensing limb movements in the motor cortex: how humans sense limb movement. Neuroscientist 10: 73–82, 2004. [DOI] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH. Kinesthetic illusion of wrist movement activates motor-related areas. Neuroreport 12: 3805–3809, 2001. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Yanagisawa T, Shin D, Fukuma R, Chen C, Kambara H, Yoshimura N, Hirata M, Yoshimine T, Koike Y. Prediction of three-dimensional arm trajectories based on ECoG signals recorded from human sensorimotor cortex. PLoS One 8: e72085, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Motor imagery and ERD. In: Event-Related Desynchronization and Related Oscillatory Phenomena of the Brain. Handbook of Electroencephalography and Clinical Neurophysiology, vol. 6, edited by Pfurtscheller G and Lopes da Silva FH. Amsterdam, Netherlands: Elsevier, 1999, p. 303–325. [Google Scholar]
- Neuper C, Scherer R, Reiner M, Pfurtscheller G. Imagery of motor actions: Differential effects of kinaesthetic and visual-motor mode of imagery in single-trial EEG. Cogn Brain Res 25: 668–677, 2005. [DOI] [PubMed] [Google Scholar]
- Neuper C, Scherer R, Wriessnegger S, Pfurtscheller G. Moor imagery and action observation: odulation of sensorimotor brain rhythms during mental control of a brain computer interface. Clin Neurophysiol 120: 239–247, 2008. [DOI] [PubMed] [Google Scholar]
- Ohara S, Ikeda A, Kunieda T, Yazawa S, Baba K, Nagamine T, Taki W, Hashimoto N, Mihara T, Shibasaki H. Movement-related change of electrocorticographic activity in human supplementary motor area proper. Brain 123: 1203–1215, 2000. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino L, Chakarov V, Schulte-Mönting J, Hepp-Reymond MC, Kristeva R. Oscillatory cortical activity during a motor task in a deafferented patient. Neurosci Lett 401: 214–218, 2006. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol 72: 250–258, 1989. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Event-related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci Lett 174: 93–96, 1994. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neurosci Lett 239: 65–68, 1997. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Future prospects of ERD/ERS in the context of brain-computer interface (BCI) developments. Prog Brain Res 159: 433–437, 2006. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Kalcher J. 40-Hz oscillation during motor behavior in man. Neurosci Lett 164: 179–182, 1993. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related desynchronization. In: Handbook of Electroencephalography and Clinical Neurophysiology, vol. 6, edited by Pfurtscheller G and Lopes da Silva FH. Amsterdam, Netherlands: Elsevier, 1999, p. 51–66. [Google Scholar]
- Pfurtscheller G, Neuper C, Krausz G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin Neurophysiol 111: 1873–1879, 2000. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Woertz M, Supp G, Lopes da Silva FH. Early onset of post-movement beta synchronization in the supplementary motor area (SMA) during self-paced finger movement in man. Neurosci Lett 339: 111–114, 2003. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlögl A, Lopes da Silva FH. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage 31: 153–159, 2006. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res Brain Res Rev 50: 57–68, 2005. [DOI] [PubMed] [Google Scholar]
- Ramos-Murguialday A, Soares E, Birbaumer N. Upper limb EMG artefact rejection in motor sensitive BCIs. Eng Med Biol Soc Proc 2010 Annu Int Conf IEEE 2010: 1–6, 2010. [Google Scholar]
- Ramos-Murguialday A, Schürholz M, Caggiano V, Wilgruber M, Caria A, Hammer EM, Halder S, Birbaumer N. Proprioceptive feedback and brain computer interface (BCI) based neuroprostheses. PLoS One 7: e47048, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Murguialday A, Broetz D, Rea M, Laeer L, Yilmaz O, Brasil FL, Liberati G, Curado MR, Garcia-Cossio E, Vyziotis A, Cho W, Agostini M, Soares E, Soekadar S, Caria A, Cohen LG, Bibaumer N. Brain-machine-interface in chronic stroke rehabilitation: a controlled study. Ann Neurol 74: 100–108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 60: 537–550, 1994. [DOI] [PubMed] [Google Scholar]
- Schack B, Weiss S, Rappelsberger P. Cerebral information transfer during word processing: where and when does it occur and how fast is it? Hum Brain Mapp 19: 18–36, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, Salenius S, Salmelin R, Jousmäki V, Hari R. Involvement of primary motor cortex in motor imagery: a neuromagnetic study. Neuroimage 6: 201–208, 1997. [DOI] [PubMed] [Google Scholar]
- Schwartz AB. Distributed motor processing in cerebral cortex. Curr Opin Neurobiol 4: 840–846, 1994. [DOI] [PubMed] [Google Scholar]
- Scwartz AB. Cortical neural prosthetics. Annu Rev Neurosci 27: 487–507, 2004. [DOI] [PubMed] [Google Scholar]
- Sehm B, Perez MA, Xu B, Hidler J, Cohen LG. Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex 20: 34–45, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: A backdoor to the motor system after stroke? Stroke 37: 1941–1952, 2006. [DOI] [PubMed] [Google Scholar]
- Shin D, Watanabe H, Kambara H, Nambu A, Isa T, Nishimura Y, Koike Y. Prediction of muscle activities from electrocorticograms in primary motor cortex of primates. PLoS One 7: e47992, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev 13:121:131, 2012. [DOI] [PubMed] [Google Scholar]
- Stancak A Jr, Pfurtscheller G. The effects of handedness and type of movement on the contralateral preponderance of mu-rhythm desynchronisation. Clin Neurophysiol 99: 174–182, 1996a. [DOI] [PubMed] [Google Scholar]
- Stancak A Jr, Pfurtscheller G. Event-related desynchronisation of central beta rhythms during brisk and slow self-paced finger movements of dominant and nondominant hand. Brain Res Cogn Brain Res 4: 171–183, 1996b. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Saleh M, Penn RD, Hatsopoulos NG. Propagating waves in human motor cortex. Front Hum Neurosci 5: 1–8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro C, Deuschl G, Thatcher R, Sato S, Kufta C, Hallett M. Event-related desynchronization and movement-related cortical potentials on the ECoG and EEG. Electroencephalogr Clin Neurophysiol 93: 380–389, 1994. [DOI] [PubMed] [Google Scholar]
- Weiller C, Juptner M, Fellows S, Rijntjes M, Leonhardt G, Kiebel S, Muller S, Diener HC, Thilmann AF. Brain representation of active and passive movements. Neuroimage 4: 105–110, 1996. [DOI] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Broberg M, Wallace A, DeLosAngeles D, Lillie P, Hardy A, Fronsko R, Pulbrook A, Willoughby JO. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin Neurophysiol 118: 1877–1888, 2007. [DOI] [PubMed] [Google Scholar]