Abstract
Purpose
To review treatments for osteoarthritis of the knee (OAK) received by patients across five European countries, and to obtain patients’ perceptions and willingness to pay for current treatments.
Patients and methods
A prospective, internet-based, double-blind survey of adults with OAK was conducted in France, Germany, Italy, Spain, and the United Kingdom. The questionnaire included questions about diagnosis, treatment history, and perceptions of OAK treatments, followed by a discrete choice-based conjoint exercise to identify preferred attributes of OAK treatments, evaluating 14 sets of four unbranded products.
Results
Two thousand and seventy-three patients with self-reported OAK completed the survey; 17.4% of patients rated their knee pain as drastically affecting their ability to perform normal daily activities, and 39.3% of employed patients reported that they had lost work time because of OAK. The most common treatments were exercise (69.7%), physical therapy (68.2%), and nonprescription oral pain medication (73.9%). Treatments perceived as most effective were: viscosupplement injections (74.1%), narcotics (67.8%), and steroid injection (67.6%). Patient co-pay, duration of pain relief, and type of therapy exhibited the largest impact on patient preference for OAK treatments. The average patient was willing to pay €35 and €64 more in co-pay for steroid and viscosupplement injections, respectively, over the cost of oral over-the-counter painkillers (per treatment course, per knee) (each P<0.05).
Conclusion
OAK is a debilitating condition that affects normal daily activities. In general, treatments most commonly offered to patients are not those perceived as being the most effective. Patients are willing to pay a premium for treatments that they perceive as being more effective and result in longer-lasting pain relief, and those that can be administered with fewer visits to a physician.
Keywords: osteoarthritis, treatment, survey, viscosupplementation
Introduction
Osteoarthritis of the knee (OAK) is the most common form of arthritis and is a debilitating condition that is common in Europe.1,2 The condition is multifactorial, with biochemical, mechanical, and behavioral components.1 Prevalence varies globally; symptomatic OAK in Europe has a reported prevalence of 5.2% in France, 5.4% in Italy, 10.2% in the UK, and 10.2% in Spain.3–6 Age and sex are important risk factors for OAK, with higher prevalence reported in female and elderly populations.7–11 Body mass index is another important risk factor for OAK, with risk increasing exponentially with body mass index.12
OAK has a negative impact upon quality of life, and this impact increases with disease progression.13 Previous studies have reported various detrimental effects of OAK upon daily functionality, including pain, and impairments in physical functioning and physical role.14–16 In addition to simply seeking clinical benefit for an individual chronic condition, a number of factors influence OAK treatment choices for patients, including management of comorbidities, risks and benefits of new treatments, knowledge of their condition, and knowledge of other patients in similar positions.17–20 A recent study showed that treatments such as total knee arthroplasty can lead to dissatisfaction in approximately one-half of patients as a result of ongoing pain, and this dissatisfaction can persist for over a decade after treatment.21 Similarly, a study measuring willingness to pay for pain relief from disability-related pain found that patients were more willing to pay for relief from their pain than for functional improvements; the amount that patients were willing to pay per month was US$1,428.22 Studies such as these demonstrate that it is important to ensure patients with OAK have access to effective treatments that confer maximal effectiveness and satisfaction.23
In the current study, adult patients with self-reported OAK in France, Germany, Italy, Spain, and the UK were invited to take part in an internet-based survey. The study aimed to elucidate how living with OAK affects patient quality of life, current OAK treatment patterns, patient perceptions of treatment effectiveness, and patients’ willingness to pay for specific attributes of these treatments. Treatments ranging from physical therapy to injection (steroid or viscosupplement [VS]) were evaluated. A separate choice-based conjoint analysis was designed to estimate relative willingness to pay for different attributes of each treatment, and determine which of these attributes are considered most important by the patient.
Methods
Survey design
Study sample
A prospective, internet-based, double-blind survey recruited adult participants with self-reported OAK in one or both knees. Previous studies have shown that self-reported physician-level diagnoses of osteoarthritis (OA) are reliable, with a concordance of 86.9% with primary-care records.24,25 Initially, 55,007 participants in France, Germany, Italy, Spain, and the UK were recruited through established consumer market research panels in each country, which ensured that the identity of the study sponsor and the identities of the participants were unknown to one another (ie, double-blinded). The number of respondents was determined according to the number of parameters/attributes used in the conjoint analysis, as well as the number of choice-based questions contained in the survey that could be asked of each respondent before observing a decline in data quality. A sample of 400 patient respondents was deemed sufficient in each country (analytic domain) to provide acceptable standard errors of conjoint part-worth utilities with 14 choice sets per respondent. Study panels were created from opt-ins by patients across a wide variety of channels designed to maximize representativeness of the panels to the general public.
The patient self-reported online survey included questions on patient demographics, diagnosis, treatment history, attitudes, and perceptions of OAK treatments. The survey validation instrument comprised six in-person paper and pencil “pretests”. These pretests were administered to qualifying patients to ensure that the wording of all questions and answer choices was interpreted as intended, and were not included in the analysis. During the six in-person patient pretests, no difficulty in understanding phrasing, question formats, or survey stimuli was reported. Survey questions were coauthored by Reason Research (Philadelphia, PA, USA) and Genzyme Corporation, a Sanofi Biosurgery (Cambridge, UK) company, and underwent compliance review in accordance with Sanofi’s compliance system for market research; questions were reviewed for accuracy of medical terminology and overall clarity of phrasing. All respondents agreed to standard opt-in terms and conditions required for such a study. Furthermore, the study involved simply asking for a history of treatments used and future treatment preferences, rather than administering treatment. Given these factors, the need for further research ethics approval was not considered necessary.
Several treatments were included in the survey: exercise, physical therapy, acupuncture, magnetic pulse therapy, topical creams/liniments/patches, glucosamine/chondroitin sulfate, oral pain medication available without a prescription, cyclooxygenase (COX)-2 inhibitors (oral medications that require a prescription), narcotics (oral medications requiring a prescription), steroid injection, VS injection, arthroscopic knee surgery, and other treatment. The definition of current treatments included treatments that the respondent was receiving at the time of the survey or had received recently (patients may have been receiving more than one type of treatment concurrently). A 14-question conjoint exercise was embedded within the survey. The online survey took 45 minutes to complete, with the results being gathered electronically and tabulated in SPSS v 21 (IBM Corporation, Armonk, NY, USA).
Participants of ≥40 years of age with self-reported OAK in one or both knees were included in the study. Participants were required to be able to select the specialty of their treating physician from an aided list. Participants also had to meet a minimum household income requirement, representing local poverty definitions adjusted for size of household. This restriction was included to avoid the anticipated dilution of the measure of willingness to pay due to economic limitations; it was estimated that unreimbursed patient co-payments could add up to £500/€350 for a single course of treatment on one knee (not including the office visit co-pay). In France, the minimum annual household income required ranged from €9,640 for a single-person household to €14,460 for a household of three or more persons. The corresponding minimums in Germany ranged from €9,910 to €20,820; in Spain from €7,530 to €15,820; in Italy from €7,140 to €19,400; and in the UK from £6,450 to £15,600 (mean exchange rate during data collection [November 2012] was 1.23 GBP/EUR). There were two exclusion criteria: participants who had undergone knee-replacement surgery in one or both knees (or who were planning to undergo surgery in the 12 months following participation in the survey), and participants associated with a market research company, pharmaceutical company, drug manufacturer, advertising agency, newspaper, magazine, TV or radio station, or any other news organization.
Patient responses were gathered online from December 2012 to January 2013 through self-administered online questionnaires in the respondent’s national language. Participation was voluntary, and reasonable compensation was offered in the form of monetary and/or virtual currency, as per standard industry practice. Questionnaires were translated by Reason Research, and verified for accuracy by Sanofi. Prior to analysis, data were examined and cleaned of respondents with suspicious answer patterns. For example, 51 (0.1%) of 55,007 patients who claimed to have all eleven of the medical conditions listed in the comorbidity question were discarded. An additional data-cleaning step was conducted to remove data collected from respondents who completed the survey in too short a time or who displayed no variation in answers to 20 distinct seven-point rating scale questions (“straight-lining”).
Study questionnaire
The questionnaire was divided into four sections: 1) patient demographics and familiarity with OAK treatments; 2) respondent-reported pain rating on a scale of 1–10, and the impact of their OAK according to the Activities of Daily Living Questionnaire;26,27 3) patient-reported rating of effectiveness and satisfaction of current treatments; and 4) choice-based questions. Questions related to employment status, disposable income, and insurance coverage were also included to provide context for co-pay sensitivity analysis derived in the conjoint analysis.
Conjoint model analysis
Model design
A choice-based conjoint model was designed to understand the impact of various attributes on OAK product choice, and to determine which attributes of OAK treatments are most highly valued by patients. The questions were created using experimental design principles of independence and balance of the features (fractional factorial design). By independently varying the features shown to respondents, and observing the responses to the product profiles, the analysis statistically deduced which attribute levels have the greatest impact upon patient choice.
Various product package inserts and proprietary data held by Sanofi were used to design the attributes and levels used in modeling.28 Prevailing co-payments for steroid/cortisone injections and various brands of VS injection treatments were carefully reviewed in each country to identify the most common dosing regimens, benefit outcomes, adverse events, and range of co-pays associated with oral and injection treatments. The range of levels of each efficacy attribute was designed to span the relevant range of clinical outcomes. The range of out-of-pocket co-payments made by patients spanned the economically relevant range for each type of treatment.
The dependent variable was discrete treatment choice (the selection in the bottom row of Table 1), a common practice in conducting surveys;29 the explanatory variables were the product attributes. Conjoint modeling provided rescaled zero-centered part-worths (ie, utilities) for each attribute level, which were then analyzed to understand the drivers of treatment choice. Within each attribute, these estimated utilities were scaled to sum to 0, so that less acceptable attribute levels received lower utilities (including negative and zero values). Utilities were interval-scaled rather than ratio-scaled. Changes in utility were calculated by assessing the impact of product attributes and attribute levels upon patient preferences using Sawtooth Software’s CBC v 6.0 (Orem, UT, USA).
Table 1.
Example of respondent exercise screen: “Which of the following treatments would be your most preferred for your Osteoarthritis of the Knee? Select one”
| Treatment characteristic | Treatment A | Treatment B | Treatment C | Treatment D |
|---|---|---|---|---|
| Type | An injection (or series of injections) of a viscosupplement | Oral medication that requires a prescription from your doctor | Oral pain medications that you can buy without a prescription | An injection of steroid or cortisone |
| Frequency of dosing | 1 injection (in a single office visit) | Oral tablet, 3–4 times per day (as needed) | Oral tablet, 3–4 times per day (as needed) | 1 injection (in a single office visit) |
| Time to wait for pain relief to start | Pain relief may start within 1 week after the first injection, reaching its maximum within 4 weeks | Within 3 hours | Within 3 hours | Within 3 hours |
| Magnitude of pain relief | Reduces pain from a level 10 to 5 | Reduces pain from a level 10 to 5 | Reduces pain from a level 10 to 5 | Reduces pain from a level 10 to 5 |
| Duration of pain relief | Up to 12 months | Up to 12 hours | Up to 8 hours | Up to 3 months |
| Ability to slow progression of the disease | No impact | No impact | No impact | No impact |
| Out-of-pocket cost per knee (not including office visit co-pays) | €100 per course of treatment | €5 per month (€30 for 6 months) | €5 per month (€30 for 6 months) | €0 (no out-of-pocket cost) |
| Your most preferred treatment | ○ | ○ | ○ | ○ |
Note: “○” denotes the button for responders to make their choice.
Fourteen sets of four unbranded products were evaluated during the internet survey, resulting in 14 observations of the dependent variable (product choice) for each patient. Selecting 14 sets of unbranded products maximized the efficiency of the survey design and served to minimize expected standard errors. Similarly, the principle of orthogonality (or independence of attributes) was applied, maximizing design-efficiency and minimizing expected standard errors of part-worths (each under 0.05). All oral and injection products were blinded; product characteristics were shown to patients, including prevailing co-pays within country, but brand names were not. The product attributes evaluated in the analysis were: type of therapy, frequency of dosing, time to wait for pain relief to start, duration of pain relief, magnitude of pain relief, ability to slow disease progression, and co-pay. Co-pay refers to the out-of-pocket cost per knee not including office visit co-pays. Further details on the attributes used (except co-pay) is given in Table 2. The side-effect profiles of the four types of treatments (as presented in Table 3) were shown prior to the conjoint exercise but not explicitly modeled as attributes, given the primary focus of the study on willingness to pay for efficacy.
Table 2.
Attribute levels used in the analysis (except co-pay)
| Treatment characteristic | Oral | Steroid injection | Viscosupplement (hyaluronic acid) injection |
|---|---|---|---|
| Frequency of dosing | • Oral tablet, 1–2 times per day (as needed) • Oral tablet, 3–4 times per day (as needed) |
• 1 injection (in a single office visit)* | • 1 injection (in a single office visit) • 3 injections (in 3 office visits, over 3 subsequent weeks) • 5 injections (in 5 office visits, over 5 subsequent weeks) |
| Time to wait for pain relief to start | • Within 3 hours* | • Within 3 hours • Within 4 hours to 24 hours |
• Pain relief may start within 1 week after the first injection, maximum within 4 weeks • Pain relief may start within 2 weeks, maximum within 4 weeks • Pain relief may start within 3 weeks, maximum within 4 weeks |
| Duration of pain relief | • Up to 8 hours • Up to 12 hours |
• Up to 1 month • Up to 3 months |
• Up to 1 month • Up to 3 months • Up to 6 months • Up to 12 months |
| Magnitude of pain relief | • Reduces pain from a level 10 to 7 • Reduces pain from a level 10 to 5 • Reduces pain from a level 10 to 2 |
||
| Ability to slow disease progression | • No impact* | • No impact • Some impact |
|
| Co-pay† (not including office visit costs)** | • co-pay level 1 to 2† | • Co-pay level 1 to 5† |
Notes:
Fixed levels, not varied in conjoint analysis.
Vary by country.
Co-pay refers to the out-of-pocket cost per knee not including office visit co-pays. The range of out-of-pocket co-payments made by patients spanned the economically relevant range for each type of treatment.
Table 3.
Example of potential side effects assessment screen
| Oral medications that you can buy without a prescription | Oral prescription medication | An injection of steroid or cortisone | An injection (or series of injections) of a viscosupplement |
|---|---|---|---|
| May cause GI complications, and liver damage (with high doses) | May interact with other drugs and cause drowsiness, confusion, skin rash, and abdominal pain. Some medications (NSAIDs) may cause increased risk of heart attack and stroke | May cause pain and swelling, and long-term use may damage cartilage and connective tissue | May include mild and temporary injection-site pain, swelling, heat and/or redness, rash and itching, bruising around the joint, and/or fluid accumulation |
Abbreviations: GI, gastrointestinal; NSAID, nonsteroidal anti-inflammatory drug.
An example of patient choice exercise screen is shown in Table 1. Each patient completed 14 such exercises, selecting their most preferred treatment on each screen (one screen per exercise). The patient was required to select a treatment on each screen before proceeding to the next screen/exercise.
Co-pay attribute levels
Co-pay for oral over-the-counter (OTC) and prescription treatments ranged from €0 to €5 per month (€30 for 6 months) in Euro currency countries and £0 to £5 per month (£30 for 6 months) in the UK. Steroid (cortisone) injections in Euro currency countries ranged from €0 to €50 and in the UK from £0 to £50. VS injections in Euro currency countries ranged from €0 to €350 and in the UK from £0 to £500. Three additional intermediate co-pays were tested for VS injections in each country; for example, €100, €175, and €250 co-pays were tested between €0 to €350 in Germany, Spain, and Italy.
Duration of pain relief attribute levels
In alignment with the language used in marketing OAK products, duration of pain relief was stated as lasting up to a certain amount of time, rather than lasting the full amount of time. A single dose of oral treatments, for example, offers duration of relief of “up to 12 hours”, whereas for injection products the longest duration from a single injection was “up to 12 months”. While framing the duration of relief in more absolute temporal terms (specifically as “exactly 12 hours” or “exactly 12 months”) would have removed the potential variance from patient to patient when making assumptions of the true duration of relief per patient, this would have compromised real-world validity. Priority was therefore placed on mirroring the decisions that patients must make in the real-world situation, which include reacting to the ambiguity of language present in treatment package inserts.
Conjoint analysis plan
Conjoint analysis generated numeric utilities for each attribute level, derived from multivariate regression, representing the relative importance of each product feature on the treatment choice. Utilities therefore provided a measure of derived importance as opposed to stated importance. The impact of various attributes could be made directly by forcing patients to make trade-offs between attribute levels, such as between duration of pain relief and co-pay. After calculation, the utilities of each product attribute level were recombined to represent the net utility (assumed proportional to real-world value) of a hypothetical product.
An attribute-specific design was used, whereby there was a clinically relevant limitation of the combination of attributes applied in the design. In measuring the intrinsic value of the type of therapy, unique features of VS injections, such as ability to slow disease progression, were captured in separate attributes, independent of type of therapy.30,31 Thus, the type of therapy attribute is not a reflection of features unique to one treatment or another.
A hierarchical Bayes logit model specific to each country was estimated using Sawtooth Software CBC/HB v 6.0. Separate models were constructed for each country because the co-pays that were shown to patients reflected structurally different non-reimbursed patient co-pays. A “none of the above” option was not included, as the inexpensive oral OTC product (cost to patient ranging from free to £5/€5 per month) served as the proxy for the no-treatment option.
Statistical analysis
In the survey analysis, population comparisons were performed using a Wilcoxon rank sum test to determine statistical difference between all populations. Individual populations were compared using a chi-square test. In the conjoint model analysis, two-tailed z-tests of proportions and t-tests of means (using SPSS with Bonferroni correction) were conducted to determine statistical significance between utilities (taken at P<0.05).
Results
Baseline characteristics
Survey results from 2,073 participants met the criteria, with 1.4% of the total completed interviews discarded as a result of suspected straight-lining. The baseline characteristics of the study population are shown in Table 4.
Table 4.
Summary of baseline characteristics of the study population
| Survey characteristic | Value |
|---|---|
| Study population location | |
| Germany | 415 |
| Italy | 400 |
| France | 437 |
| Spain | 406 |
| UK | 415 |
| Age (years) | |
| Mean | 57.3 |
| Median | 57.0 |
| Range | 40–86 |
| Sex (%) | |
| Female | 66.3 |
| Extent of OAK (%) | |
| One knee only | 32.5 |
| Both knees | 67.5 |
| Type of doctor managing OA (%) | |
| Specialist physician/surgeon | 45.4 |
| General physician | 43.0 |
| Other | 11.6 |
Note: Sample size (N=2,073).
Abbreviations: OA, osteoarthritis; OAK, osteoarthritis of the knee.
Effect of OAK pain on daily living activities
Responders were asked to rate the severity of pain associated with OAK when taking no medication (Table 5). Responses were classified according to OAK severity (mild, moderate, or severe), defined in terms of the ability to undertake normal activities. OAK was rated as severe by 360 (17.4%) respondents, with pain that “drastically limits normal activities”. A further 949 (45.8%) responders experienced moderate OAK, meaning “pain on a daily basis which limits normal activity”. In total, pain that limited normal activities was experienced by 63.2% of respondents. Across the mild, moderate, and severe OAK ratings combined, 10.1% of respondents rated their daily pain as 8–10 on a scale where 0 was “no pain” and 10 was the “worst pain imaginable”, while 61.0% rated pain at 4–7 on the same scale.
Table 5.
Severity of OAK-associated pain
| Response | Patients (n) | Patients (%) | Intensity of pain (%)*
|
||
|---|---|---|---|---|---|
| 0–3 | 4–7 | 8–10 | |||
| Mild: able to undertake normal activities but with a certain degree of pain | 764 | 36.9 | 50.1 | 47.5 | 2.4 |
| Moderate: experience pain on a daily basis that limits normal activities | 949 | 45.8 | 18.8 | 73.6 | 7.7 |
| Severe: pain drastically limits normal activities and makes routine activities difficult | 360 | 17.4 | 10.6 | 56.4 | 33.1 |
| Total | 2,073 | 100 | 28.9 | 61.0 | 10.1 |
Notes: Respondents were asked: “How would you characterize your level of knee pain when not taking any medications to treat your OA of the knee (OAK)”; with the possible responses of mild, moderate, or severe.
Respondents rated their daily pain on a scale where 0 was “no pain” and 10 was the “worst pain imaginable”.
Abbreviations: OA, osteoarthritis; OAK, osteoarthritis of the knee.
The impact of OAK pain on daily living activities of the patient is reported in Table 6. Pain was experienced constantly by 31.3% of respondents, with the pain affecting daily mood of 43.8% and sleep patterns of 35.9% of respondents. Over one-half of the respondents agreed that OAK pain had caused them to decrease normal activities during the previous 12 months to 18 months.
Table 6.
Impact of OAK-associated pain on quality of life
| Respondent statement | Agree or strongly agree (%)a |
|---|---|
| My knee pain makes me dependent on pain medication to get me through the day | 38.0 |
| My knee pain makes me feel older than my age | 54.9 |
| I experience pain constantly (around the clock) | 31.3 |
| My knee pain is impacting my daily mood | 43.8 |
| My knee pain prevents me from sleeping well through the night | 35.9 |
| My knee pain limits my ability to do what I want to do on a daily basis | 47.6 |
| My knee pain has made me decrease my normal activities over the last 12 months to 18 months | 51.1 |
Notes: Respondents were asked whether they agreed with a series of statements about OAK impact upon daily functioning. Responses were scored on a scale from 1 to 7, where 1 was “do not agree at all” and 7 was “strongly agree”.
Score of 5–7.
Abbreviation: OAK, osteoarthritis of the knee.
Table 7 describes the impact of OAK on the employment of respondents, of which 38.4% were retired and 14.3% were not working. Among patients who worked, 39.3% had missed time from work or had reduced their hours of work because of their OAK.
Table 7.
Current employment status and impact of OA of the knee on respondent’s work schedule
| Employment status | % | Have you missed time from work, or reduced your work schedule due to OA? |
|---|---|---|
| Employed full time | 35.0 | Yes* |
| Employed part time | 12.4 | Yes* |
| Retired | 38.4 | N/A |
| Not working | 14.3 | N/A |
Note:
39.3% of the respondents who were employed missed time from work, or reduced their work schedule due to OA.
Abbreviations: N/A, not applicable; OA, osteoarthritis.
Respondent experiences of current treatments
The survey recorded the treatments for OAK being used by respondents and their experiences of them; these data are shown in Table 8. The most common treatments (received by $50% patients) were: exercise (69.7%); physical therapy (68.2%); topical creams, liniments, and patches (63.5%); and oral pain medication available without a prescription (73.9%). It was notable that the list of most common treatments did not vary according to the severity of OAK, with the sole exception that prescription oral pain medications were taken by ≥50% of patients with severe pain.
Table 8.
Respondent experiences with current OAK treatments
| Treatment | Treatments used (according to OAK severity)#
|
Effectiveness of treatment#
|
Satisfaction with treatment#
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild (%) | Moderate (%) | Severe (%) | All patients (%) | Too soon to tell (%) | Not effective (%) | Moderately effective (%) | Very effective (%) | Not satisfied (%) | Moderately satisfied (%) | Very satisfied (%) | |
| All treatments | 6.6 | 12.5 | 27.9 | 53.0 | 12.4 | 37.2 | 50.4 | ||||
| Exercise | 70.7 | 70.5 | 65.6 | 69.7 | 4.0 | 13.9 | 35.8 | 46.3 | 12.8 | 37.4 | 49.8 |
| Physical therapy | 61.3 | 72.6 | 71.1 | 68.2 | 4.8 | 11.2 | 24.6 | 59.4 | 12.9 | 38.4 | 48.7 |
| Acupuncture | 21.9 | 28.1 | 26.9 | 25.6 | 8.1 | 18.8 | 26.2 | 46.9 | 11.7 | 38.0 | 50.3 |
| Magnetic pulse therapy | 20.7 | 28.5 | 25.3 | 25.0 | 9.1 | 17.9 | 28.7 | 44.3 | 12.7 | 39.9 | 47.4 |
| Topical creams, liniments, patches | 60.1 | 66.4 | 63.3 | 63.5 | 2.3 | 17.7 | 39.8 | 40.2 | 12.2 | 39.7 | 48.1 |
| Glucosamine, chondroitin sulfate | 29.1 | 31.5 | 38.3 | 31.8 | 9.9 | 20.6 | 31.3 | 38.2 | 13.2 | 36.6 | 50.2 |
| Oral pain medication available without a prescription | 72.9 | 77.2 | 67.2 | 73.9 | 3.5 | 12.7 | 36.7 | 47.1 | 13.1 | 38.2 | 48.7 |
| COX-2 inhibitors (oral medications that require a prescription) | 22.6 | 34.0 | 40.6 | 31.0 | 15.7 | 5.1 | 18.7 | 60.4 | 11.7 | 35.5 | 52.8 |
| Narcotics (oral medications that require a prescription) | 26.8 | 42.0 | 53.9 | 38.5 | 7.1 | 5.1 | 19.9 | 67.8 | 12.5 | 38.2 | 49.3 |
| Steroid injection | 22.4 | 39.5 | 48.6 | 34.8 | 10.4 | 5.7 | 16.4 | 67.6 | 10.3 | 36.6 | 53.1 |
| Viscosupplement (hyaluronic acid) injection | 17.5 | 29.6 | 29.4 | 25.1 | 8.8 | 6.7* | 10.4 | 74.1* | 8.6* | 25.3 | 66.0* |
| Arthroscopic knee surgery | 18.2 | 21.3 | 26.7 | 21.1 | 10.8 | 12.1 | 17.2 | 60.0 | 14.7 | 35.2 | 50.1 |
| Other treatment | 10.3 | 11.5 | 12.8 | 11.3 | 8.1 | 9.8 | 15.8 | 66.3 | 15.0 | 36.8 | 48.3 |
Notes: The percentage of patients who received OAK treatments, both current and recent, is given in relation to OAK severity. The effectiveness of the current treatments and respondent satisfaction were measured on a scale of 1–7 (where 1 was “not at all effective/satisfied” and 7 was “extremely effective/satisfied”) and expressed as a percentage of patients with each opinion.
Wilcoxon rank sum test, all groups P<0.0001.
Chi-square P<0.0001 (viscosupplement compared with all treatments).
Abbreviations: COX-2, cyclooxygenase-2; OAK, osteoarthritis of the knee.
Patients were asked to rate the effectiveness (in terms of pain relief) of their current treatments on a scale from 1 (not at all effective) to 7 (extremely effective), as shown in Table 8. Treatments that were perceived as being very effective (score of 5–7) by the majority of patients were: physical therapy (59.4%), COX-2 inhibitors (60.4%), narcotics (67.8%), steroid injection (67.6%), VS injection (74.1%), and arthroscopic knee surgery (60.0%). With the exception of physical therapy, the treatments rated as most effective did not correlate with the treatments most commonly received. The proportion of patients who rated VS injection to be very effective (74.1%) was significantly higher than for all treatments taken together (53.0%, P<0.0001, chi-square test), and the proportion of patients rating VS to be not effective (6.7%) was significantly lower than for all treatments taken together (12.5%, P<0.0001, chi-square test).
Patients were also asked to rate their satisfaction with their current treatment, on a scale from 1 (not at all satisfied) to 7 (extremely satisfied), as shown in Table 8. Treatments with which ≥50% of patients were very satisfied (rating score 5–7) were: acupuncture (50.3%), glucosamine, chondroitin sulfate (50.2%), COX-2 inhibitors (52.8%), steroid injection (53.1%), VS injection (66.0%), and arthroscopic knee surgery (50.1%). None of the treatments with the highest satisfaction ratings were among the treatments most commonly received. The proportion of patients very satisfied with VS (66.0%) was significantly higher than for all treatments taken together (50.4%, P<0.0001, chi-square test), and the proportion of patients not satisfied with VS (8.6%) was significantly lower than for all treatments taken together (12.4%, P<0.0001, chi-square test).
Determinants of treatment choice
Stated preference
Injections into the knee were perceived by patients to be among the most effective treatments and scored highly in terms of patient satisfaction. Steroid injections were received by 34.8% of patients overall and by 48.6% of patients with severe OAK pain. VS injections were received by 25.1% of patients overall and by 29.4% of patients with severe pain. The conjoint analysis aimed to determine which attributes of these treatments are most highly valued.
Derived preference (conjoint analysis)
Figure 1 shows the utilities for the four treatment types tested in the conjoint analysis. Both injection treatments were significantly preferred over both oral treatments, demonstrated by non-overlap of the respective confidence intervals between the oral and injectable treatments. This finding is consistent with the satisfaction ratings returned by patients (Table 8). Further, and also in agreement with satisfaction ratings, VS injection was preferred to steroid injection.
Figure 1.
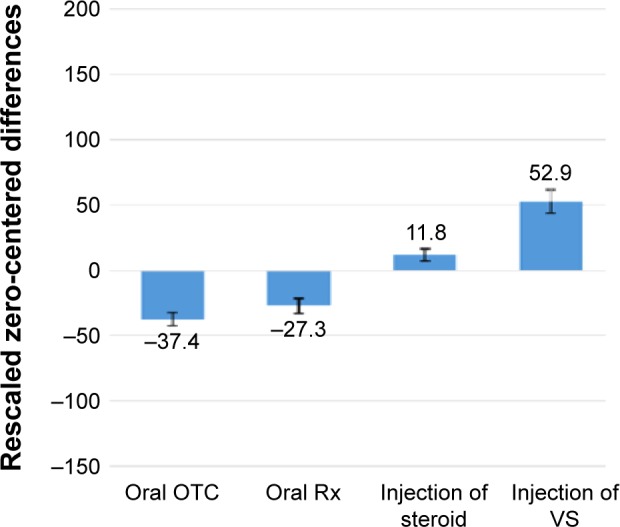
Impact of varying the type of treatment upon utility premium.
Notes: The differences between adjacent utilities indicate the relative importance of moving from one level (type of treatment) to an adjacent level. Error bars indicate 95% confidence interval.
Abbreviations: OTC, over the counter; Rx, medical prescription; VS, viscosupplement.
Product attributes driving willingness to pay
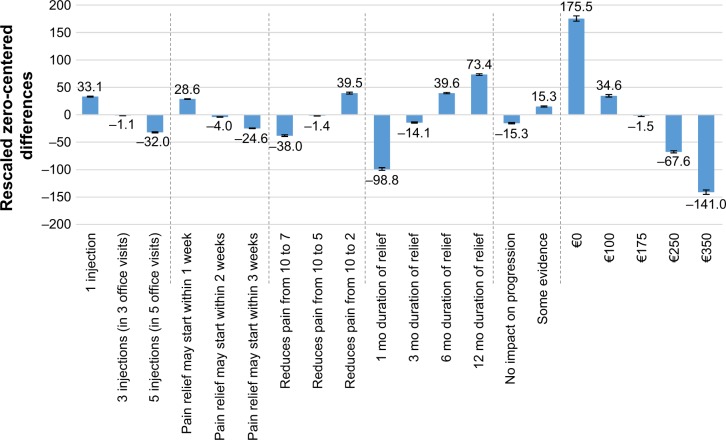
To assess product attributes driving willingness to pay, a utility-to-Euro conversion was performed, using the impact of the co-pay attribute as a measure of utility. Utility/co-pay conversion was independent for each attribute, with conversions used as a measure of premium paid for higher-level benefits within a single attribute. As shown in Figure 2, there was an “improvement” of 140.8 utility points when the co-pay was reduced from €100 (34.6 utility points) to €0 (175.5 utility points). Therefore, a change of 140.8 utility points equates to a monetary patient co-pay value of €100. The utility associated with each of the injection attributes is shown in Figure 2, and the currency conversion can be applied to these utility gains. Of the attributes examined by conjoint analysis, patient co-pay, duration of pain relief, and type of therapy (for example, oral OTC or steroid injection) exhibited the largest impact on patient preference for OAK treatments, as shown in Figure 2. Considering only the type of therapy and co-pay as the sole determinants of patient preference, the average patient would be willing to pay €35 and €64 more for steroid and VS injections, respectively, over the cost of oral OTC painkillers (per treatment course, per knee) (each P<0.05).
Figure 2.
Impact of varying attributes of patient characteristics upon their choice of OAK treatment.
Note: Error bars indicate 95% confidence interval.
Abbreviations: mo, month/s; OAK, osteoarthritis of the knee.
Oral treatment co-pay was modeled separately from injectable co-pay. Relative to a 1-month supply of oral OTC pain relief tablets stating “3 to 4 times a day” dosing, an otherwise identical bottle offering “1 to 2 times a day” dosing was perceived to be worth €7 more. Therefore, it can be considered that equal utility exists for a month of free tablets offering “3 to 4 times a day” dosing and €7 tablets offering “1 to 2 times a day” dosing.
Specific to VS injections, the improvement in utility of 1 month of pain relief (−98.8 utility points) to 6 months of pain relief (39.6 utility points) is 138.4 utility points (P<0.05, t-test). This would have a co-pay value of €98.3. Therefore, a patient would be equally willing to pay for a free VS injection offering 1 month of relief or a €100 co-pay for a VS injection offering 6 months of relief. In this scenario, the incremental 5 months of pain relief is worth roughly €100. By comparison, 12 months of pain relief (167.2 utility points) has a co-pay value of €122.3 over 1 month of pain relief. Similarly, a dosing frequency of one injection had a utility gain of 65.1 points (€46.2 co-pay) over therapy that requires five injections (and five office visits, P<0.05, t-test). Pain relief within a week of commencing treatment had a utility gain 53.2 (€37.8 co-pay) compared with pain relief within 3 weeks (P<0.05, t-test). Pain reduction from a score of 10 to a 2 on the pain scale had a utility gain of 77.5 (€55.0 co-pay) over pain reduction from a 10 to a 7 (P<0.05, t-test).
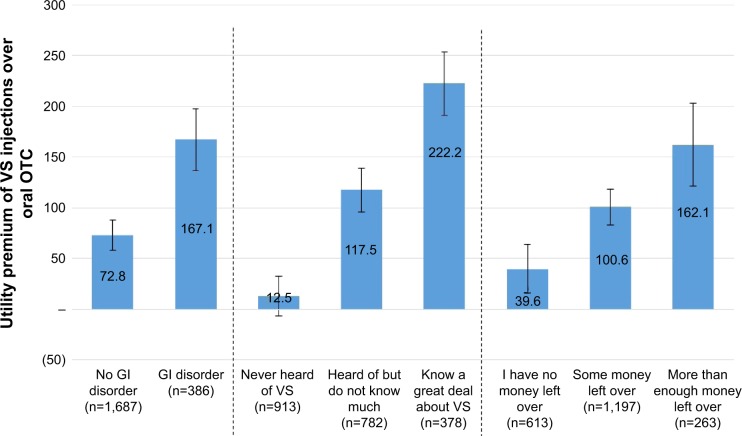
It was possible to assess patient characteristics tied to preferences for specific treatments through the use of individual-level conjoint utilities. Patients stating a preference for higher co-pay injections over less costly oral treatments had a larger utility gain moving from oral treatments to injection treatments than patients who preferred less costly options. Oral OTC treatments and VS injections were the two types of therapy with the largest difference in utilities. Figure 3 shows the patient factors linked to a preference for VS treatment over oral OTC treatments, based on results obtained from the survey.
Figure 3.
Patient factors linked to preference for VS injections over oral OTC.
Note: Error bars indicate 95% confidence interval.
Abbreviations: GI, gastrointestinal; OTC, over the counter; VS, viscosupplement.
As shown in Figure 3, a higher utility premium for VS injections over oral OTC treatments was observed in patients with gastrointestinal (GI) disorders compared with patients without GI disorders. This preference is likely to arise from the GI adverse effects associated with prolonged use of some OTC analgesics.32 Patients more familiar with VS injections or who had higher disposable income also had significantly greater utility premiums for VS injections than patients with little/no knowledge of VS injections and lower disposable income, respectively. Notably, the number of past treatments attempted was significantly correlated with the utility premium for VS injection (Pearson’s r=0.19, P<0.01) (data not shown). However, age, sex, the presence of OAK in both knees, and other comorbidities did not significantly impact the utility premium placed on VS injections.
Discussion
The current study found that the treatments most commonly received by patients with OAK are not generally the same as the treatments that score highest on measures of perceived effectiveness or of patient satisfaction. Using the co-pay system as a means of monetary conversion, the study also provides a measure of the worth of different attributes associated with OAK treatment and the premium that patients are willing to pay for them.
In the stated preference analysis, knee injections were rated as very effective by the largest proportion of patients receiving these treatments and also provided the highest levels of satisfaction. In the derived preference analysis, the treatment type (as well as duration of effect attribute) had the greatest impact upon treatment preference, with injections being significantly preferred to oral medications. However, knee injections were received only by a minority of patients (between 25% and 35%), irrespective of disease severity. A higher preference for injections was associated with patients who had GI disorders, a greater knowledge about treatments, and higher disposable income. As might be expected, duration of pain relief and fewer injections were also significant determinants of treatment choice (P<0.05). The analysis also showed that the out-of-pocket cost of injections, relative to prescription medications and other treatments in some European countries, was a barrier to their use.
Although the study was designed to be a comprehensive study of willingness to pay in European patients, there are some limitations. Firstly, the self-reporting of OAK could lead to a reporting bias, although self-reporting has been shown to compare with clinical records.24,25 Ensuring a representative population of patients with OAK is also important; care was taken to ensure a consistent and representative patient population, with patients sampled from many demographics. Although difficult to align with the entire population of patients with OAK, the mean age of 57.3 years and presence of 66.3% of females in the study can be considered suitably comparable with European epidemiological estimates.7–11 Despite being performed according to a widely used methodology, one possible further limitation of the survey was that participation required a degree of computer literacy and Internet access; this may represent a degree of bias against non-information-technology-literate responders in a predominantly elderly survey population. Finally, the survey validation instrument consisted of six in-person pretests, and could perhaps have been more extensive. A potential limitation associated with the conjoint methodology is that co-pay sensitivity is nonlinear between tested levels, with results linearly interpolated between tested values.
The current study demonstrates that patients are willing to pay a €100 co-pay for treatment with an efficacy increasing from 1 month to 6 months, and a €122.3 co-pay for one with an efficacy increasing from 1 month to 12 months. In addition, patients are willing to pay a premium of €64 co-pay for VS injections over oral OTC painkillers and €35 for steroid injections (per course of treatment, per knee). A previous patient preference study examined the willingness of patients to adopt alternative treatments to prevent progression of OA.23 It was found that while 16.4% of patients tested would reject alternative treatments under all conditions, 59.2% of patients had a strong preference for trying new therapies in all scenarios, in order to prevent OA disease progression. A recent study described a willingness in patients with OA to accept a 400% increase in myocardial infarction risk in order to achieve a reduction in ambulatory pain associated with OA, suggesting the importance of maintaining daily activities and reducing pain to patients with OAK.29
For future analyses, the present study can be generalizable to other countries with a similar treatment algorithm, health care system framework, and ability to pay. From the current study, it is possible to conclude that patients who have GI disorders, have more disposable income, and are more knowledgeable in managing their OAK are more willing to pay for VS injections. The study found that duration of pain relief and fewer injections are also significant determinants of treatment choice among patients with OAK.
Acknowledgments
Janek Hendrich assisted with the drafting of the article and received financial support from Sanofi (Paris, France) via employment with HERON™ Commercialization.
Footnotes
Author contributions
Each of the authors declares they have made substantial contributions to each of the following: the conception and design of the study, acquisition of data, analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be submitted; and agree to be accountable for all aspects of the work.
Disclosure
John Posnett is employed by HERON™ Commercialization. Sanjeev Dixit and Brooks Oppenheimer are employed by Reason Research. Sven Kili was an employee of Sanofi Biosurgery during the time that this project was initiated and developed and during the preparation of this report. Nazanin Mehin is employed by Sanofi. The study was funded by Sanofi. The authors report no other conflicts of interest in this work.
References
- 1.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Kingsbury SR, Gross HJ, Isherwood G, Conaghan PG. Osteoarthritis in Europe: impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology (Oxford) 2014;53:937–947. doi: 10.1093/rheumatology/ket463. [DOI] [PubMed] [Google Scholar]
- 3.Salaffi F, De Angelis R, Grassi W, MArche Pain Prevalence. INvestigation Group (MAPPING) study Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The MAPPING study. Clin Exp Rheumatol. 2005;23:819–828. [PubMed] [Google Scholar]
- 4.Guillemin F, Rat AC, Mazieres B, et al. 3000 Osteoarthritis group Prevalence of symptomatic hip and knee osteoarthritis: a two-phase population-based survey. Osteoarthritis Cartilage. 2011;19:1314–1322. doi: 10.1016/j.joca.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Lopez JC, Laffon A, Blanco FJ, Carmona L, EPISER Study Group Prevalence, risk factors, and impact of knee pain suggesting osteoarthritis in Spain. Clin Exp Rheumatol. 2008;26:324–332. [PubMed] [Google Scholar]
- 6.Wills AK, Black S, Cooper R, et al. Life course body mass index and risk of knee osteoarthritis at the age of 53 years: evidence from the 1946 British birth cohort study. Ann Rheum Dis. 2012;71:655–660. doi: 10.1136/ard.2011.154021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Mannoni A, Briganti MP, Di Bari M, et al. Epidemiological profile of symptomatic osteoarthritis in older adults: a population based study in Dicomano, Italy. Ann Rheum Dis. 2003;62:576–578. doi: 10.1136/ard.62.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacey RJ, Thomas E, Duncan RC, Peat G. Gender difference in symptomatic radiographic knee osteoarthritis in the Knee Clinical Assessment – CAS(K): a prospective study in the general population. BMC Musculoskelet Disord. 2008;9:82. doi: 10.1186/1471-2474-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiem U, Lamsfuß R, Günther S, et al. Prevalence of self-reported pain, joint complaints and knee or hip complaints in adults aged $40 years: a cross-sectional survey in Herne, Germany. PLoS One. 2013;8:e60753. doi: 10.1371/journal.pone.0060753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira D, Peleteiro B, Araújo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011;19:1270–1285. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Zhou ZY, Liu YK, Chen HL, Liu F. Body mass index and knee osteoarthritis risk: a dose-response meta-analysis. Obesity (Silver Spring) 2014;22:2180–2185. doi: 10.1002/oby.20835. [DOI] [PubMed] [Google Scholar]
- 13.Altman RD. Early management of osteoarthritis. Am J Manag Care. 2010;16(Suppl Management):S41–S47. [PubMed] [Google Scholar]
- 14.Salaffi F, Carotti M, Stancati A, Grassi W. Health-related quality of life in older adults with symptomatic hip and knee osteoarthritis: a comparison with matched healthy controls. Aging Clin Exp Res. 2005;17:255–263. doi: 10.1007/BF03324607. [DOI] [PubMed] [Google Scholar]
- 15.Kauppila AM, Kyllonen E, Mikkonen P, et al. Disability in end-stage knee osteoarthritis. Disabil Rehabil. 2009;31:370–380. doi: 10.1080/09638280801976159. [DOI] [PubMed] [Google Scholar]
- 16.Dawson J, Linsell L, Zondervan K, et al. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology (Oxford) 2004;43:497–504. doi: 10.1093/rheumatology/keh086. [DOI] [PubMed] [Google Scholar]
- 17.Cheraghi-Sohi S, Bower P, Kennedy A, et al. Patient priorities in osteoarthritis and comorbid conditions: a secondary analysis of qualitative data. Arthritis Care Res (Hoboken) 2013;65:920–927. doi: 10.1002/acr.21897. [DOI] [PubMed] [Google Scholar]
- 18.Bogardus ST, Jr, Holmboe E, Jekel JF. Perils, pitfalls, and possibilities in talking about medical risk. JAMA. 1999;281:1037–1041. doi: 10.1001/jama.281.11.1037. [DOI] [PubMed] [Google Scholar]
- 19.Hulka BS, Wheat JR. Patterns of utilization. The patient perspective. Med Care. 1985;23:438–460. doi: 10.1097/00005650-198505000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Cher DJ, Miyamoto J, Lenert LA. Incorporating risk attitude into Markov-process decision models: importance for individual decision making. Med Decis Making. 1997;17:340–350. doi: 10.1177/0272989X9701700311. [DOI] [PubMed] [Google Scholar]
- 21.Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8–13 years of followup. Acta Orthop. 2014;85:229–233. doi: 10.3109/17453674.2014.916487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuck A, Adamowicz W, Jacobs P, Ohinmaa A, Dick B, Rashiq S. The willingness to pay for reducing pain and pain-related disability. Value Health. 2009;12:498–506. doi: 10.1111/j.1524-4733.2008.00457.x. [DOI] [PubMed] [Google Scholar]
- 23.Fraenkel L, Suter L, Cunningham CE, Hawker G. Understanding preferences for disease-modifying drugs in osteoarthritis. Arthritis Care Res (Hoboken) 2014;66:1186–1192. doi: 10.1002/acr.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlow JH, Turner AP, Wright CC. Comparison of clinical and self-reported diagnoses for participants on a community-based arthritis self-management programme. Br J Rheumatol. 1998;37:985–987. doi: 10.1093/rheumatology/37.9.985. [DOI] [PubMed] [Google Scholar]
- 25.Rasooly I, Papageorgiou AC, Badley EM. Comparison of clinical and self reported diagnosis for rheumatology outpatients. Ann Rheum Dis. 1995;54:850–852. doi: 10.1136/ard.54.10.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 27.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 28.Vignon E, Valat JP, Rossignol M, et al. Osteoarthritis of the knee and hip and activity: a systematic international review and synthesis (OASIS) Joint Bone Spine. 2006;73:442–455. doi: 10.1016/j.jbspin.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Hauber AB, Arden NK, Mohamed AF, et al. A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthritis Cartilage. 2013;21:289–297. doi: 10.1016/j.joca.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Hall S, Hanna F, et al. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12:195. doi: 10.1186/1471-2474-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pal S, Thuppal S, Reddy KJ, et al. Long-term (1-year) safety and efficacy of a single 6-mL injection of Hylan G-F 20 in Indian patients with symptomatic knee osteoarthritis. Open Rheumatol J. 2014;8:54–68. doi: 10.2174/1874312901408010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balmaceda CM. Clinical trial data in support of changing guidelines in osteoarthritis treatment. J Pain Res. 2014;7:211–218. doi: 10.2147/JPR.S45321. [DOI] [PMC free article] [PubMed] [Google Scholar]