Abstract
Background
There is little data regarding use of mineralocorticoid antagonists (MRAs) for patients reduced LV ejection fraction (LVEF) following acute myocardial infarction (MI). We determined the frequency and temporal trends of MRA use in these patients.
Methods
We performed a retrospective review of all cases of acute MI between June 1, 2010 and April 1, 2012. Patients were considered eligible for MRA therapy if they were admitted with acute MI with LVEF ≤ 40 % and had heart failure symptoms or a history of diabetes.
Results
Of 3910 cases of acute MI, 332 patients were considered eligible for MRA therapy. MRA therapy was prescribed for 92/332 (28 %) eligible patients, while 66 of 1142 (6 %) of ineligible patients were so treated. Over the study period, usage in eligible and ineligible patients rose significantly (22 to 30 %, p = 0.08 and 4 to 7 %, p = 0.04 respectively).
Conclusions
Prescription of MRAs for eligible patients occurred in a minority of patients, and demonstrated a modest increase over time. In patients without an indication for MRAs, a similar trend was observed. Further study is required to better understand barriers to appropriate use of MRAs in this patient population.
Keywords: Heart failure, Myocardial infarction, Mineralocorticoid receptor antagonists
Background
Pharmacologic interventions such as angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor antagonists (ARBs) and beta blockers are known to benefit patients with heart failure (HF) and reduced left ventricular ejection fraction (LVEF) complicating acute myocardial infarction (MI). In 2003, following publication of the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), mineralocorticoid receptor antagonist (MRA) therapy was included in this list [1].
The use of MRA therapy in patients with HF and reduced LVEF is not new. Following the publication of the Randomized Aldactone Evaluation Study (RALES) trial, use of spironolactone was considered standard therapy for patients with moderate to severe HF and LVEF < 35 % [2]. However, registries have shown a low uptake in MRA therapy for patients with post-MI and chronic HF [3,4]. Following the RALES trial, spironolactone use increased from 3 to only 24 % of patients meeting enrollment criteria [3]. Interestingly, MRA use increased from 3 to 18 % in patients not meeting RALES enrollment criteria, with a concurrent increase in hospitalizations for hyperkalemia [3,5].
Evidence for MRA therapy in patients with reduced LVEF has been accumulating over time. Recent studies such as Eplerenone in Patients with Systolic Heart Failure and mild symptoms (EMPHASIS-HF) study showed a significant reduction in mortality with the addition of eplerenone to standard HF therapy in patients with mild to moderate symptoms and reduced LVEF [6]. Consequently, the Canadian Cardiovascular Society (CCS) recommended an MRA be considered for patients already on standard HF therapy who meet these enrolment criteria [7].
MRAs are thought to be underutilized, and at our centre we anecdotally noted a low rate of MRA prescriptions for post-MI patients with reduced LVEF. In the Canadian Heart Failure network, outpatient MRA therapy utilization approaches 38 % [8]. According to CIHI data, Calgary area hospitals report a mortality rate below the national median for MI [9]. This represented an opportunity to describe the rate of MRA prescription in a large cohort of post-MI patients who were presumably well treated following supportive publications for MRA use. In addition, temporal changes in prescription of MRAs could be assessed. We hypothesized that appropriate utilization of MRAs would be low with an increase in use over time.
Methods
We completed a retrospective review of patients discharged alive with a diagnosis of acute MI (ICD-10 code I-21) from three Calgary metropolitan area hospitals between June 1, 2010 and April 1, 2012. We excluded patients who underwent hospital transfer and patients who did not have an assessment of LV function.
From this group of patients we collected the following clinical information: LVEF, type of MI (ST elevation (STEMI) or non-ST elevation MI (NSTEMI)), length of hospitalization, admission blood pressure, admission heart rate, and admitting service. We obtained past medical history of: HF, previous MI, hypertension, diabetes, dyslipidemia, and smoking. In addition the following laboratory information was collected: discharge serum creatinine, peak potassium and peak troponin. Glomerular filtration rate (GFR) was estimated using the MDRD equation [10]. Use of MRA (either spironolactone or eplerenone), beta-blockers, ACE-inhibitors and ARBs were assessed based on discharge summaries and electronic prescription records. LVEF was recorded in order of preference based on the 2D biplanar Simpson’s model, subjective estimated LVEF and subjective global impression.
Patients were considered eligible for MRA therapy if there was documented LVEF ≤ 40 % and symptoms of HF or a history of diabetes. Patients were considered ineligible if there was documented: allergy or intolerance to MRA therapy, a serum potassium level > 5.0 meq/L, estimated GFR <30 mL/min/1.73 m2, or documented patient refusal. These criteria were based on current ACC guidelines [11]. A standardized data extraction tool was used to minimize bias during the data collection process.
We explored temporal change by comparing prescribing rates of MRAs before (period A) and after (period B) the date of publication of EMPHASIS-HF trial (January 6, 2011) for both eligible and ineligible patients. This date was chosen to investigate the effects of a landmark trial publication on medication use. All data were reported using descriptive statistics with means and standard deviations for continuous variables and simple percentages for categorical variables. Fisher exact tests were used to analyze differences in proportions according to categorical stratifications. Linear regression was used to determine a trend in overall use with the least squares method after dividing usage rates by quarters, centered around the EMPHASIS-HF publication date. We performed a logistic regression analysis using the least squares method to determine factors associated with MRA prescription, with significance denoted by p < 0.05. Analyses were conducted using Stata version 13 (Stata Corp., College Station, Texas). Ethics approval was obtained from the University of Calgary Conjoint Health Research Ethics Board.
Results
We identified 3910 patients discharged alive with a diagnosis of acute MI, of whom 1474 had documented reduced LVEF. Five hundred ninety-nine patients had documented LVEF ≤ 40 %, of which 332 met our eligibility criteria for MRA therapy without exclusions. Patient characteristics are outlined in Table 1. Study flow sheet outlining patient inclusion is shown in Fig. 1. Overall, 92 of 332 eligible patients (28 %) were prescribed MRA therapy. This percentage was numerically lower during period A (24/108, 22 %) versus those discharged in period B (68/224 or 30 %, p = 0.07, see Fig. 2), however this was not statistically significant. If all prescriptions filled within 6 months of discharge were included, MRAs were prescribed in 33/108 (31 %) patients for period A and 79/224 (35 %) for period B (p = 0.23 between periods).
Table 1.
Population characteristics for patients with documented systolic dysfunction
| Eligible (n = 332) | Ineligible (n = 1142) | ||
|---|---|---|---|
| Demographics | Male (%) | 250 (75.3 %) | 842 (73.7 %) |
| Age (years) | 67.6 +/− 12.8 | 64.7+/− 13.2 | |
| Length of stay (days) | 15 +/− 21 | 9 +/− 13 | |
| Medical history | Hypertension | 199 (59.9 %) | 624 (54.6 %) |
| Dyslipidemia | 132 (39.8 %) | 407 (35.6 %) | |
| Diabetes | 187 (56.3 %) | 272 (23.8 %) | |
| Smoking | 107 (32.2 %) | 399 (34.9 %) | |
| Myocardial infarction | 117 (35.2 %) | 257 (22.5 %) | |
| Heart failure | 86 (25.9 %) | 77 (6.7 %) | |
| Clinical data | Systolic blood pressure (mmHg) | 114 +/−18 | 119 +/− 23 |
| Heart rate (beats/min) | 78 +/− 15 | 72 +/− 14 | |
| Ejection fraction | 32.7 +/− 7.1 | 45.6+/− 6.9 | |
| STEMI | 141 (42.5 %) | 637 (55.8 %) | |
| Laboratory data | Peak troponin T (μg/L) | 4.0 +/− 5.9 | 3.7+/− 4.9 |
| Peak potassium (mmol/L) | 4.5 +/− 0.4 | 4.5 +/− 0.5 | |
| Estimated GFR (mL/min/1.73 m2) | 81 +/− 36 | 77 +/− 30 |
EF ejection fraction, μg/L micrograms per liter, μmol/L micromole per liter, mmHg millimeters mercury, mmol/L millimoles per liter, STEMI ST elevation myocardial infarction. All numerical values shown +/− standard deviation
Fig. 1.
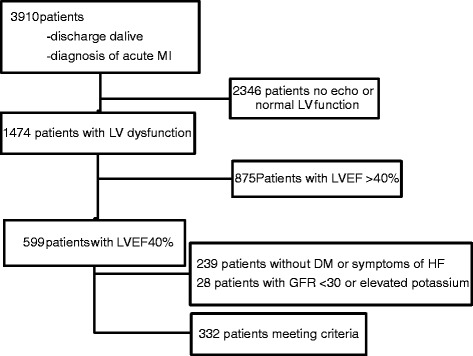
Study flow sheet outlining patient inclusion and exclusion. DM diabetes mellitus, GFR glomerular filtration rate, HF heart failure, LV left ventricle, LVEF left ventricular ejection fraction
Fig. 2.
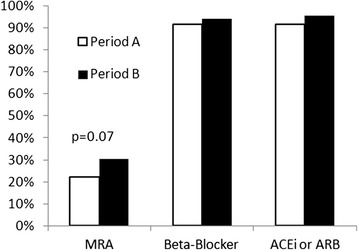
Prescriptions of MRA, beta-blockers, and ACE-inhibitors or ARBs in patients meeting criteria for MRA usage between study periods. MRA mineralocorticoid receptor antagonist, ACE-i angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker
We identified 1142 patients with systolic dysfunction who did not meet our criteria. In these patients, MRAs were prescribed in 16/401 (4 %) patients during period A and 50/741 (7 %) during period B (p = 0.04 between periods, see Fig. 3).
Fig. 3.
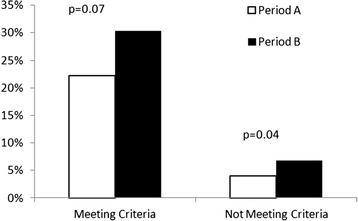
Use of MRAs in patients meeting and not meeting our criteria between study periods
When considering only patients admitted to a cardiology service, 32 % were prescribed MRAs, with 16/71 patients (23 %) given during period A and 54/148 (36 %) for period B (p = 0.03). For patients not meeting our criteria the corresponding proportions were 14/323 (4 %) and 40/585 (7 %, p = 0.08). Prescribing rates between periods were not analyzed for other admitting services due to low patient numbers. Cumulative prescribing rates for eligible patients were; cardiovascular surgery 7/43 (16 %), family practice 7/33 (21 %), and internal medicine 6/18 (33 %). For ineligible patients, the rates of MRA prescription were: cardiovascular surgery 4/96 (4 %) family practice 4/58 (7 %) and internal medicine 3/36 (8 %). There were no significant differences in prescribing rates between admitting services.
The proportion of eligible patients prescribed MRAs by quarter are displayed in Fig. 4. However the coefficient of determination (R2) was only 0.036 (p = 0.02). For comparison purposes, we also collected the prescription rates for other therapies with longstanding indications for patients with acute MI (see Fig. 1). Beta-blockers were prescribed at similar rates across periods (99/108, 92 % vs. 211/224, 94 %). There were similar findings for ACE-inhibitors and ARBs.
Fig. 4.
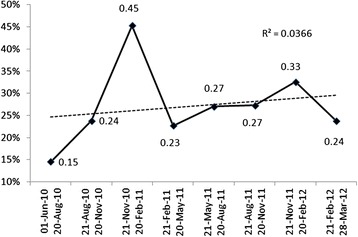
Proportion of patients using MRAs by quarter with overall trend in use
We performed a logistic regression analysis to identify factors associated with MRA prescriptions in both eligible and ineligible patients. We assessed the following possible associated factors: age, gender, length of hospitalization, history of HF, hypertension, diabetes, smoking, dyslipidemia, and previous MI, systolic blood pressure, heart rate, type of MI, EF, estimated GFR, peak troponin, and potassium. The results of this analysis are outlined in Table 2. In patients eligible for MRA therapy, lower EF, history of smoking, and history of dyslipidemia were associated with higher rates of MRA prescription (all p < 0.05). In patients who were considered ineligible for MRA therapy, lower EF and history of HF were associated with higher rates of MRA prescription (all p < 0.05).
Table 2.
Logistic regression analysis to identify factors associated with MRA prescription
| Eligible | Ineligible | ||||
|---|---|---|---|---|---|
| OR (95 % CI) | Adjusted p-value | OR (95 % CI) | Adjusted p-value | ||
| Demographics | Age | 1.01 (0.98–1.03) | 0.69 | 1.00 (0.98–1.02) | 0.91 |
| Female | 0.97 (0.51–1.83) | 0.92 | 2.22 (1.27–3.88) | 0.01 | |
| Length of stay | 1.01 (0.99–1.02) | 0.33 | 1.01 (0.99–1.03) | 0.17 | |
| Medical history | Heart failure | 1.66 (0.83–3.32) | 0.15 | 2.38 (0.97–5.85) | 0.06 |
| Hypertension | 0.99 (0.56–1.75) | 0.97 | 1.24 (0.70–2.17) | 0.46 | |
| Dyslipidemia | 0.47 (0.26–0.85) | 0.01 | 0.73 (0.41–1.29) | 0.40 | |
| Diabetes | 1.06 (0.61–1.83) | 0.84 | 1.33 (0.69–2.56) | 0.28 | |
| Smoking | 1.84 (1.03–3.27) | 0.04 | 1.39 (0.81–2.39) | 0.23 | |
| MI | 0.99 (0.50–1.95) | 0.98 | 1.05 (0.54–2.03) | 0.89 | |
| Clinical data | SBP | 0.99 (0.97–1.00) | 0.16 | 1.00 (0.99–1.01) | 0.58 |
| Heart rate | 1.01 (0.99–1.03) | 0.17 | 0.99 (0.97–1.01) | 0.40 | |
| LVEF | 0.93 (0.90–0.97) | 0.00 | 0.93 (0.90–0.96) | 0.00 | |
| STEMI | 1.44 (0.74–2.80) | 0.28 | 1.62 (0.85–3.10) | 0.15 | |
| Laboratory data | Troponin T | 1.02 (0.97–1.07) | 0.39 | 1.05 (1.00–1.09) | 0.05 |
| Potassium | 0.50 (0.23–1.08) | 0.08 | 1.01 (0.56–1.79) | 0.99 | |
| Estimated GFR | 1.00 (0.99–1.01) | 0.87 | 1.00 (0.99–1.01) | 0.74 |
Analysis of factors associated with increased rates of MRA prescription. CI, confidence interval; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; μg/L, micrograms per liter; μmol/L, micromole per liter; mmHg, millimeters mercury; mmol/L, millimoles per liter; OR, odds ratio; STEMI, ST elevation myocardial infarction; SBP, systolic blood pressure
Discussion
We had hypothesized that MRA prescription would be suboptimal in eligible patients with reduced LVEF following acute MI. Over time, there was a trend towards an increase in the utilization of MRA therapy for both eligible and ineligible patients, although this was not statistically significant in patients eligible for MRA therapy. Overall, prescribing rates were significantly lower than we found for beta-blockers and ACE-inhibitors or ARB’s. For these agents we found a very high usage rate which did not change over time, as one might expect of an established standard of care. We’ve shown that across three medical centers where overall survival for MI is better than the norm, there is a low rate of MRA usage [9]. Indeed, this level is below that seen in other jurisdictions, such as in Madrid, Spain (50 %), [12] and in many US hospitals [4].
Previous studies have identified suboptimal use of MRA therapy for patients with HF and reduced LVEF, but have not, until recently, reported usage rates of MRAs in post-MI patients with low LVEF [13,14]. While there has been a clear lack of emphasis on MRA usage in eligible patients following acute MI, reports regarding the usage in chronic HF have hypothesized a lack of confidence in diagnosis, concerns regarding medication use in fragile patients, poor awareness of research evidence and individual preference as barriers in HF management [15,16]. Additionally, some physicians may feel that adherence to guidelines does not change clinical outcomes, which may be true specifically for MRA therapy [15]. While there was a trend towards an increase in prescriptions between periods this seemed to reflect an overall upward trend in usage. This suggests a role for ongoing educational efforts such as education at the time of guideline implementation, continuing education, and audit-feedback systems [16].
While we did find an increase in appropriate prescriptions over time, we also found a smaller, but statistically significant increase in use in patients not meeting our criteria for MRA usage. We identified EMPHASIS-HF as a landmark trial in MRA therapy that may have renewed enthusiasm for this class of medications even though it investigated a different patient population then our current study [6]. We did see an increase in patients we deemed ineligible for MRA therapy which may reflect a direct impact from this publication or a more global trend towards increased use. Interestingly, there were similar findings following publication of the RALES trial, suggesting that landmark trials have important effects outside of their studied populations [3]. Therefore, it may be of benefit to specifically outline both inclusion and exclusion criteria in major studies, guidelines and educational efforts to optimize clinical decision making.
Our study had several important limitations. Due to the retrospective nature of our study, only data that was collected for clinical purposes was recorded. This was a single-center experience limiting the generalizability of the results and the small sample size limited our ability to detect a difference in prescribing rates, particularly between admitting services. Our exclusion criteria were more restrictive than would typically be used in clinical practice. This would have lead to a falsely high usage rate, making the low use found in our study more significant. Finally, in the EPHESUS study, patients were randomized to eplerenone therapy at an average of 7.3 days [1]. It’s possible that patients were discharged before MRA therapy could be initiated, however the low rates of prescriptions in follow-up and lack of impact of length of hospitalization on utilization argues that this is not the case.
Conclusions
Despite its limitations, our study highlights some important considerations. Use of MRAs has increased, but they continue to be underutilized. Further efforts to improve the appropriate usage of MRAs are required. Finally, interventions other than publication of landmark trials are likely required to accelerate optimal usage of MRAs in eligible patients.
Funding sources
No industry sourced funding was received, directly or indirectly for the conception, design, initiation, performance, analysis or documentation of this study.
Footnotes
Competing interests
Dr. Howlett has received consultant fees from Pfizer Canada, the manufacturer of eplerenone.
Authors’ contributions
RM was responsible for applying for ethics approval, collecting and interpreting data, and preparation of the manuscript. JH was responsible for conception and implementation of the project as well as preparation of the manuscript. Both authors have read and approved the final manuscript.
References
- 1.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 3.Masoudi FA, Gross CP, Wang Y, Rathore SS, Havranek EP, Foody JAM, et al. Adoption of spironolactone therapy for older patients with heart failure and left ventricular systolic dysfunction in the United States, 1998–2001. Circ. 2005;112:39–47. doi: 10.1161/CIRCULATIONAHA.104.527549. [DOI] [PubMed] [Google Scholar]
- 4.Rassi AN, Cavender MA, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, et al. Temporal trends and predictors in the use of aldosterone antagonists post-acute myocardial infarction. J Am Coll Cardiol. 2013;61:35–40. doi: 10.1016/j.jacc.2012.08.1019. [DOI] [PubMed] [Google Scholar]
- 5.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med. 2004;351:543–51. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 6.Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 7.McKelvie RS, Moe GW, Cheung A, Costigan J, Ducharme A, Estrella-Holder E, et al. The 2011 Canadian Cardiovascular Society Heart Failure Management Guidelines Update: focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can J Cardiol. 2011;2011(27):319–38. doi: 10.1016/j.cjca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Arnold J, Ignaszewski A, Howlett J, LeBlanc MH, Liu P, Guiterrez R, et al. Changes in drug utilization in patients referred to outpatient heart failure clinics in Canada between 1999 and 2007. J Am Coll Cardiol. 2009;53:A166. [Google Scholar]
- 9.Canadian Institutes of for Health Information (CIHI) Canadian hospital reporting project. 2010. [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circ. 2013;2013(127):e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-de-Sa E, Martinez A, Anguita M, Dobarro D, Jimenez-Navarro M. Aldosterone receptor antagonist use after myocardial infarction. Data from the REICIAM registry. Rev Esp Cardiol. 2011;64:981–7. doi: 10.1016/j.recesp.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Patel UD, Hernandez AF, Liang L, Peterson ED, LaBresh KA, Yancy CW, et al. Quality of care and outcomes among patients with heart failure and chronic kidney disease: a get with the guidelines—heart failure program study. Am Heart J. 2008;156:674–81. doi: 10.1016/j.ahj.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TY, Dai D, Hernandez AF, Bhatt DL, Heidenreich PA, Fonarow GC, et al. The importance of consistent, high-quality acute myocardial infarction and heart failure care results from the American Heart Association’s Get with the Guidelines Program. J Am Coll Cardiol. 2011;58:637–44. doi: 10.1016/j.jacc.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Heywood JT, Liu Y, et al. Associations between outpatient heart failure process-of-care measures and mortality clinical perspective. Circ. 2011;123:1601–10. doi: 10.1161/CIRCULATIONAHA.110.989632. [DOI] [PubMed] [Google Scholar]
- 16.Sinuff T, Cook D, Giacomini M, Heyland D, Dodek P. Facilitating clinician adherence to guidelines in the intensive care unit: a multicenter, qualitative study. Crit Care Med. 2007;35:2083–9. doi: 10.1097/01.ccm.0000281446.15342.74. [DOI] [PubMed] [Google Scholar]


