Abstract
OBJECTIVE
To evaluate the efficacy of first-trimester markers—pregnancy-associated plasma protein A (PAPPA), free human chorionic gonadotropin β (fhCGβ), alpha-fetoprotein (AFP), placental growth factor (PlGF), and soluble tumor necrosis factor receptor-1 (sTNFR1) together with maternal characteristics (MC) for prediction of early-onset preeclampsia (EOPE).
METHODS
During 2005–2010, the abovementioned biomarkers were analyzed with logistic regression analysis in 64 EOPE and 752 control subjects to determine whether these biomarkers separately and in combination with MC would predict development of EOPE.
RESULTS
PAPPA, fhCGβ, and PlGF levels were lower, whereas AFP and sTNFR1 levels were higher in mothers with EOPE compared to controls. The combination of all markers with MC (age, weight, and smoking status) detected 48% of the mothers with EOPE, with a 10% false-positive rate (FPR).
CONCLUSIONS
First-trimester maternal serum levels of PAPPA, fhCGβ, AFP, PlGF, and sTNFR1, together with MC, are predictive of development of subsequent EOPE. These markers, along with MC, form a suitable panel for predicting EOPE.
Keywords: pregnancy-associated plasma protein A (PAPPA), free β-subunit of human chorionic gonadotropin (fhCGβ), alpha-fetoprotein (AFP), placental growth factor (PlGF), soluble tumor necrosis factor receptor-1 (sTNFR1), early-onset preeclampsia
Introduction
Preeclampsia complicates 3–8% of pregnancies in Western countries, and it is a significant cause of maternal and fetal mortality and morbidity, causing 10–15% of maternal deaths. There is growing evidence that preeclampsia may be a marker for long-term health risks.1 Incidence ranges from 3 to 7% for nulliparas and 1 to 3% for multiparas.2 Early-onset preeclampsia (EOPE) is usually defined as preeclampsia that develops before 34 weeks of gestation, whereas late-onset preeclampsia develops at or after 34 weeks of gestation. In this study, preeclampsia was defined as severe if blood pressure was >160 mmHg systolic or 110 mmHg diastolic. Cases with coexisting HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome were also included in the severe classification.2
Preeclampsia is considered to be an angiogenic disorder originating in very early pregnancy.3,4 Coordinated vascularization of the placenta is essential for a proper placental development, and it involves processes of maternal immune recognition and placental angiogenesis.5–7 Impaired placental trophoblastic invasion is associated with hypoxia and a release of proinflammatory agents and subsequent endothelial damage.8,9
Although the pathophysiology of the disorder is incompletely understood, familial clustering is apparent. According to Cnattingius et al, genetic factors account for more than half of the incidence of preeclampsia, maternal genes contribute more than fetal genes, and a couple effect can occur because of the interaction between genes of the mother and father.10
At present three confirmed genetic susceptibility loci for preeclampsia have been described. These include PEE2 on chromosome 2p25, PEE3 on chromosome 9p13,11 and PEE4, caused by a mutation in the STOX1 gene on chromosome 10q22.12
Pregnancy-associated plasma protein A (PAPPA)
Placental and fetal growth is promoted by the presence in the placenta of PAPPA,13 which is a placental glycoprotein cleaving insulin-like growth factor binding protein-4 (IGFBP4) and positively regulating insulin-like growth factors (IGFs).14 Previously, altered placental activity of IGFs in very early pregnancy, mediated by PAPPA, has been shown to be associated with pregnancy loss, hypertension, preeclampsia, preterm delivery, fetal growth restriction, and fetal death.15–18
Free human chorionic gonadotropin (fhCGβ)
Human chorionic gonadotropin (CG) is a glycoprotein hormone produced by trophoblastic cells of the placenta beginning 10–12 days after conception. First-trimester maintenance of the pregnancy requires the production of CG in the corpus luteum of the ovary, as the placenta takes over the production of progesterone. CG consists of a noncovalent dimer of alpha and beta subunits. The beta subunits define the endocrine function of the dimer.19 Maternal serum beta subunit is the current marker of first-trimester Down syndrome screening. Furthermore, hCG might be involved in the development of preeclampsia, since Zygmunt et al suggested that hCG promotes angiogenesis. The authors’ data indicated a novel function for hCG in uterine adaptation to early pregnancy, as well as in tumor development, underlining the importance of hCG as an unrecognized angiogenic factor.20
Alpha-fetoprotein (AFP)
AFP, produced by the yolk sac and fetal liver, is a major plasma protein in the fetus.21 AFP is probably the fetal counterpart of serum albumin, based on the similarity in the physical properties of AFP and albumin and the fact that their presence is inversely related. During pregnancy, maternal serum AFP has long been recognized as a marker for congenital anomalies of the fetus, eg, congenital nephrosis and spina bifida.22,23 Pregnancies with unexplained midtrimester elevation in maternal serum hCG and/or maternal serum AFP are at increased risk of complications, resulting from placental insufficiency.24
Placental growth factor (PlGF)
PlGF is a homodimering glycoprotein that belongs to the vascular endothelial growth factor (VEGF) subfamily. It is a potent angiogenic factor.25 PIGF stimulates angiogenesis in heart and limb ischemia.26 It is expressed in the villous syncytiotrophoblast27 and in the media of larger stem vessels in the human placenta.28 PIGF, together with VEGF, regulates the development of the placental vasculature, and the result depends on intraplacental oxygen pressure.29 In preeclampsia, placental expression of PlGF is downregulated, whereas its expression is increased in intrauterine growth restriction (IUGR), reflecting possible differences in intraplacental oxygenation.29,30 Changes in expression or function of PIGF, as well as some other angiogenic factors, may interrupt the function of the uteroplacental unit, and thus contribute to many adverse obstetric outcomes.31
Soluble tumor necrosis factor receptor-1 (sTNFR1)
Cytokines have been suggested as possessing the capacity to damage endothelial cells, leading to placental malfunction.6,32 Tumor necrosis factor receptor-1 (TNFR1) is a cytokine receptor that binds tumor necrosis factor alpha (TNFα), and thus activates the signaling pathways controlling inflammatory, immune, and stress responses as well as host defense and apoptosis.33 Cleavage of TNFα and TNFRs from the cell surface by disintegrin and metalloproteinase-17 results in soluble forms. Recent studies have underlined the importance of these soluble forms. Individual differences in sTNFR1 are stable over time and may therefore be useful in the evaluation of harmful effects, although the regulation mechanisms of sTNFR1 are still unclear.34
Previous studies have shown that numerous factors are involved in placental development, and their altered expression and dysfunction may play a role in the development of preeclampsia.35–38 These factors include maternal characteristics (MC) such as weight and smoking, biophysical characteristics such as mean arterial pressure (MAP) and uterine artery pulsatility index (Ut API), and a combination of biochemical markers, eg, PAPPA, fhCGβ, unconjugated estriol (uE3), AFP, PlGF, TNFR1, inhibin A, activin A, endostatin, soluble endoglin (sEng), soluble fms-like tyrosine kinase-1 (sFlt-1), histidinerich glycoprotein (HRG), a disintegrin and metalloprotease-12 (ADAM12), and PP13.
The most significant maternal and fetal complications are related to EOPE (<34 weeks of gestation), the incidence of which has been estimated to be 0.4%.39 Recently, mini-aspirin started prior to 16 weeks of gestation has been shown to be effective in preventing preeclampsia.40 Therefore, in the present study, we wanted to investigate whether maternal serum PAPPA, fhCGβ, AFP, PlGF, and sTNFR1 with MC could be used to identify patients who will subsequently develop EOPE.
Materials and Methods
Between 2005 and 2010, first-trimester serum samples were collected for combined first-trimester trisomy screening at 9 + 0−13 + 6 gestational weeks at Oulu University Hospital, Oulu, Finland. The study complied with the principles of the Declaration of Helsinki. After obtaining approvals from the Institutional Ethics Committee and the National Institute for Health and Welfare, pregnancies with EOPE (n = 64) were identified from this cohort. Women with singleton pregnancies and no major structural or chromosomal anomalies, who developed preeclamptic symptoms prior to 34 + 0 gestational weeks and delivered a live-born or a stillborn child after 24 + 0 gestational weeks, were included. The criteria of the American College of Obstetricians and Gynecologists for preeclampsia were used in this study.41 For the control group, 752 serum samples with a normal pregnancy outcome and full-term delivery were randomly picked from those collected during the same time period. The women screened in North Finland are 98.5% Caucasians. MC, including maternal age, weight, smoking status during pregnancy (smoker/non-smoker), and gestational age (GA) at the time of sample collection and at delivery in both groups, were obtained from patient records as well as the onset and severity of preeclampsia in the study group.
Maternal serum PAPPA and fhCGβ were measured immediately after the sampling, which was conducted in early pregnancy as part of the clinical routine and for combined trisomy screening. The measurements were performed in singlicate with an AutoDELFIA® automatic immunoassay system running AutoDELFIA® PAPPA and AutoDELFIA® fhCGβ time-resolved fluoroimmunoassay kits (PerkinElmer). These methods typically have within-run coefficient of variation (CV) of <2.5% and <3.5% and total CV of <4.5% and <4.5%, respectively. The leftover samples were frozen at −80°C for subsequent biochemical analysis for 3.3 (2.3–4.3) years in the EOPE group and 4.9 (4.8–5.0) years in the control group (P < 0.05).
AutoDELFIA® automatic immunoassay system running AutoDELFIA® AFP and PlGF kits (PerkinElmer) was used to determine the AFP and PlGF levels of the thawed maternal serum samples in singlicate. These methods typically have within-run CV of <1.5% and <6.5% and total CV of <2.5% and <10%, respectively. The AutoDELFIA® system was also used to run prototype AutoDELFIA® sTNFR1 time-resolved fluoroimmunoassay kits (in development by PerkinElmer) to determine the sTNFR1 levels in the samples in singlicate. During this study, the prototype sTNFR1 assay had a total CV of 3.1% for the quality control samples used.
The statistical analysis was performed with Minitab 16 (Minitab Inc.), Analyse-it 2.3 (Analyse-it Software, Ltd.), and TIBCO Spotfire S-plus 8.1 (TIBCO Software Inc.) statistical software packages. Comparisons of the demographic characteristics between the groups were done using the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. The measured PAPPA, fhCGβ, AFP, PlGF, and sTNFR1 concentrations of the control group were log10 transformed to make their distributions normal, and multiple regression analysis was used to test whether MC (GA, maternal weight, smoking status) had an effect on the measured biomarker levels. These median concentration models were refined by using stepwise selection of terms based on analysis of variance (ANOVA).
For the case–control analysis, all the measured biomarker concentrations were converted to multiples of median (MoM) by dividing them by the expected median concentrations derived from the median concentration models. The comparisons between the groups were done using Wilcoxon rank-sum tests on the MoM scale. Logistic regression was used to develop models for predicting the risk of EOPE based on MC (maternal age, weight, and smoking status), PAPPA log10 MoM, fhCGβ log10 MoM, AFP log10 MoM, PlGF log10 MoM, and sTNFR1 log10 MoM. The EOPE prediction performance of the models was then assessed.
First, the risk scores of the control and EOPE samples as predicted by the various models were calculated. Then, based on the known outcomes, receiver operating characteristic (ROC) curves were drawn and EOPE detection rates corresponding to different false-positive rates (FPRs) were determined.
Results
The demographic characteristics of the studied groups are shown in Table 1. Maternal age did not differ statistically between the groups, and the proportion of women 35 years or older was similar in both the groups. Serum storage time before retrospective measurements was shorter, maternal weight was higher, GA at sampling was lower, and there were fewer smokers in the EOPE group as compared to the control group. The EOPE was severe in 85% of the cases.
Table 1.
Demographic characteristics of the study group with EOPE and the control group with normal pregnancy outcome. Values are given as medians (interquartile ranges) or n (% of group). Wilcoxon rank-sum test for continuous variables or Fisher’s exact test for categorical variables was used to compare the EOPE group and the control group.
| CONTROL (n = 737) | EARLY-ONSET PREECLAMPSIA (n = 62) | |
|---|---|---|
| Serum sample storage time at −80°C before retrospective measurements (years) | 4.9 (4.8–5.0) | 3.3 (2.3–4.3)* |
| Maternal age (years) | 29.5 (25.7–33.9) | 29.0 (25.8–33.0) |
| Maternal weight (kg) | 67 (60–77) | 74 (65–85)* |
| Mother smoked during pregnancy | 101 (14%) | 3 (5%)* |
| Gestational age at sampling (day) | 80 (74–85) | 77 (71–83)* |
| Onset of preeclamptic symptoms (week) | 31 (29–33) | |
| Severe preeclampsia | 53 (85%) | |
| Gestational age at delivery (weeks) | >37 | 32 (30–35) |
Note:
P-value <0.05 vs. control.
Multiple regression analysis showed that expected log10 PAPPA, log10 fhCGβ, log10 AFP, log10 PlGF, and log10 sTNFR1 concentrations during gestational weeks 8–13 in the control group were affected by GA, maternal weight, and smoking status in a different way in the case of each analyte. The final refined median concentration models for each analyte are shown in Table 2.
Table 2.
The final refined expected concentration models for each biomarker generated with multiple regression analysis.
| RESPONSE | TERMS | |||
|---|---|---|---|---|
| INTERCEPT | GA | GA2 | WEIGHT | |
| log10 PAPP-A conc. | −2.5473 | +0.12475 | −0.00058686 | −0.0077841 |
| log10 fhCGβ conc. | +3.0596 | −0.0096339 | * | −0.012536 |
| log10 AFP conc. | −0.077889 | +0.018708 | * | −0.011558 |
| log10 PlGF conc | +0.94464 | +0.0037711 | +0.000043530 | +0.0014427 |
| log10 sTNFR1 conc. | +0.79321 | −0.017206 | +0.00010503 | −0.00032380 |
| WEIGHT2 | IF SMOKER | FIT | ||
| R2 | p | |||
| log10 PAPP-A conc. | * | −0.10442 | 0.475 | <0.0001 |
| log10 fhCGβ conc. | +0.000050660 | * | 0.111 | <0.0001 |
| log10 AFP conc. | +0.000060308 | +0.057296 | 0.332 | <0.0001 |
| log10 PlGF conc. | −0.000015176 | +0.12482 | 0.366 | <0.0001 |
| log10 sTNFR1 conc. | +0.000013494 | * | 0.254 | <0.0001 |
Notes: The models describe how the biomarker concentrations in the samples during gestational weeks 8–13 are affected by GA, maternal weight (Weight), and the smoking status of the mother (Smoker). The table lists the coefficients for each term in the model (* = term not used in the model) and also the R2 and p-values of the multiple regression fit.
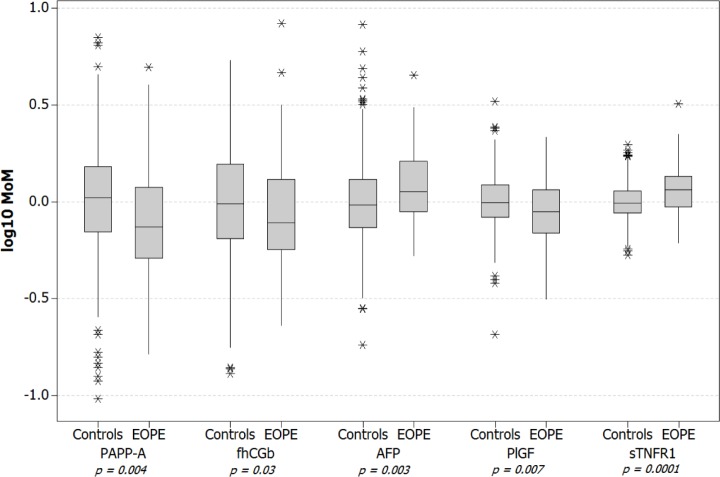
Maternal serum biomarker concentration results were converted to MoMs by dividing each result by the expected concentration calculated for each sample using the median concentration models presented in Table 2. The biomarker results on both concentration and MoM scales are summarized in Table 3 and Figure 1, respectively. The results showed that in early pregnancy, women with subsequent EOPE had lower maternal serum levels of PAPPA (0.74 vs. 1.05 MoMs, P = 0.004), fhCGβ (0.78 vs. 0.97 MoMs, P = 0.03), and PlGF (0.89 vs. 0.99 MoMs, P = 0.007), whereas the EOPE group had higher levels of AFP (1.13 vs. 0.97 MoMs, P = 0.003) and sTNFR1 (1.15 vs. 0.99 MoMs, P = 0.0001) compared to those with a normal pregnancy outcome.
Table 3.
Summary of the PAPP-A, fhCGβ, AFP, PlGF, and sTNFR1 results at 8–13 gestational weeks in the study groups (described in Table 1). Median results (interquartile range) are shown for each biomarker, scale, and studied group.
| BIOMARKER | UNIT | MEDIAN RESULT (INTERQUARTILE RANGE) | |
|---|---|---|---|
| CONTROL (n = 737) | EARLY ONSET PREECLAMPSIA (n = 62) | ||
| PAPP-A | mU/L | 1230 (650–2130) | 650 (493–1440) |
| MoM | 1.05 (0.701–1.52) | 0.741** (0.512–1.19) | |
| fhCGβ | ng/mL | 46.0 (30.0–76.5) | 37.5 (23.8–66.8) |
| MoM | 0.974 (0.648–1.57) | 0.782* (0.568–1.31) | |
| AFP | U/mL | 7.85 (5.59–11.6) | 8.45 (6.15–11.27) |
| MoM | 0.965 (0.736–1.31) | 1.132** (0.888–1.62) | |
| PlGF | pg/mL | 35.9 (27.9–46.0) | 28.9 (20.0–39.0) |
| MoM | 0.994 (0.830–1.22) | 0.889* (0.687–1.16) | |
| sTNFR1 | ng/mL | 1.38 (1.18–1.62) | 1.57 (1.39–1.80) |
| MoM | 0.986 (0.873–1.14) | 1.15*** (0.940–1.35) | |
Notes: Wilcoxon rank-sum test on MoM scale, cases vs. controls:
P-value <0.05,
P-value <0.005, and
P-value <0.0005.
Figure 1.
First-trimester biomarker levels in pregnancies with subsequent EOPE and in the control pregnancies with a normal outcome.
Six different risk prediction models for EOPE were developed with logistic regression. The models had the following general formula: EOPE risk = 1/(1 + e−(Terms)). The terms and coefficients of the risk models are shown in Table 4.
Table 4.
The refined risk prediction models for EOPE developed with logistic regression.
| TERM | COEFFICIENT | |||||
|---|---|---|---|---|---|---|
| MODEL 1 | MODEL 2 | MODEL 3 | MODEL 4 | MODEL 5 | MODEL 6 | |
| Intercept | 0.41127 | 0.28915 | 0.19145 | −0.023350 | 0.05199 | −0.055762 |
| Age | −0.030713 | −0.029929 | −0.028477 | −0.022789 | −0.032847 | −0.028769 |
| 1/Weight | −1.3109 | −1.2866 | −1.2974 | −1.3126 | −1.4467 | −1.4857 |
| If smoker | −1.1564 | −1.1435 | −1.1615 | −1.1177 | −1.2892 | −1.4312 |
| a | * | −1.0077 | −0.43395 | −0.51840 | 0.084412 | 0.016534 |
| b | * | −0.56651 | −0.55287 | −0.49845 | −2.1102 | −2.0219 |
| c | * | * | −2.6762 | −1.9844 | −4.7405 | −4.2068 |
| d | * | * | * | 1.4547 | * | 0.64703 |
| e | * | * | * | * | 10.025 | 9.5883 |
| ab | * | 0.12386 | 0.27990 | 0.45154 | 0.47314 | 0.57672 |
| ac | * | * | 2.1575 | 2.5684 | 2.7419 | 3.1240 |
| bc | * | * | −3.0772 | −1.7972 | −8.3745 | −7.8537 |
| ad | * | * | * | 0.59192 | * | 0.56955 |
| bd | * | * | * | 0.74704 | * | −0.46573 |
| cd | * | * | * | −4.9472 | * | −4.9112 |
| ae | * | * | * | * | −4.5906 | −5.7087 |
| be | * | * | * | * | 13.957 | 15.0399 |
| ce | * | * | * | * | 9.4703 | 11.528 |
| de | * | * | * | * | * | 10.563 |
Notes: The models describe how the biomarker measurement results of the samples during gestational weeks 8–13, together with MC, affect the risk for an EOPE outcome. The table lists the coefficients for each term in the model (* = term not used in the model).
Abbreviations: a, PAPP-A log MoM; b, fhCGβ log MoM; c, PlGF log MoM; d, AFP log MoM; e, sTNFR1 log MoM.
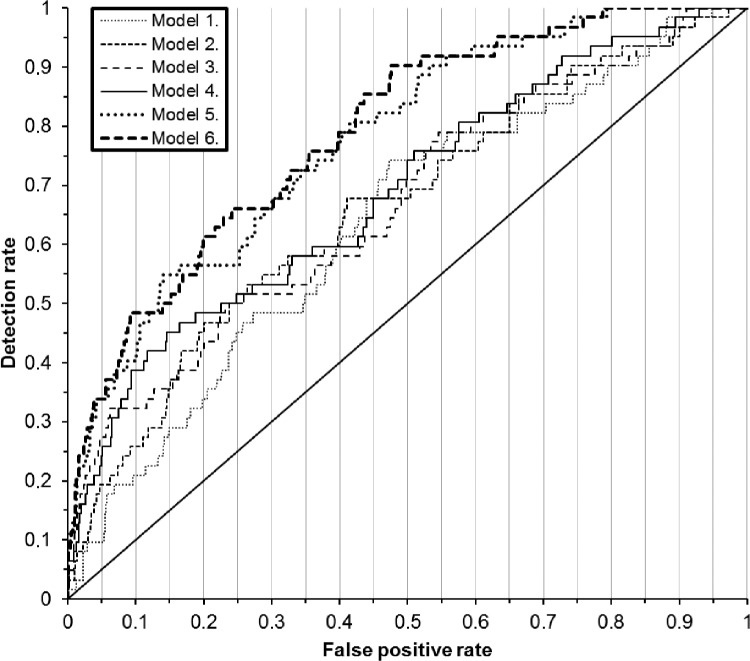
Risk scores were calculated for the groups with different risk model formulae. Figure 2 shows the ROC curves, and Table 5 summarizes the detection rates corresponding to different FPRs as determined from the risk scores and known pregnancy outcomes of the samples. The best model for the prediction of EOPE in the first trimester included MC with PAPPA, fhCGβ, AFP, PlGF, and sTNFR1. With a 10% FPR, the detection rate of EOPE was 48% and the corresponding ROC curve (area under the curve (AUC)) was 0.79 (0.73–0.85). Assuming a 0.38% incidence of EOPE, the positive predictive value (PPV) using MC and all the markers was 2.5% and the negative predictive value (NPV) was 99.74%.
Figure 2.
ROC curves of the prediction of subsequent EOPE during gestational weeks 8–13 by different risk models. See Tables 4 and 5 for the explanation of the risk models.
Table 5.
Prediction of subsequent EOPE during gestational weeks 8–13 by using MC and first-trimester maternal serum biomarker levels as risk predictors. For each risk model, the modeled detection rates are shown with different FPRs together with ROC curve AUC.
| RISK MODEL | LOGISTIC REGRESSION MODELED DETECTION RATE AND ROC AUC (95% CONFIDENCE INTERVAL) | |||
|---|---|---|---|---|
| FPR 5% | FPR 10% | FPR 15% | AUC | |
| Model 1. (MC) | 10% (4–20%) | 21% (12–33%) | 29% (18–42%) | 0.64 (0.57–0.71) |
| Model 2. (MC + PAPP-A + fhCGβ) | 19% (10–31%) | 26% (16–38%) | 35% (24–49%) | 0.66 (0.59–0.74) |
| Model 3. (MC + PAPP-A + fhCGβ + PlGF) | 27% (17–40%) | 32% (21–45%) | 35% (24–49%) | 0.67 (0.59–0.74) |
| Model 4. (MC + PAPP-A + fhCGβ + AFP + PlGF) | 23% (13–35%) | 39% (27–52%) | 45% (32–58%) | 0.69 (0.62–0.76) |
| Model 5. (MC + PAPP-A + fhCGβ + PlGF + sTNFR1) | 34% (22–47%) | 40% (28–54%) | 55% (42–68%) | 0.78 (0.72–0.84) |
| Model 6. (MC + PAPP-A + fhCGβ + AFP + PlGF + sTNFR1) | 34% (22–47%) | 48% (35–61%) | 50% (37–63%) | 0.79 (0.73–0.85) |
Discussion
The present study showed that mothers with subsequent EOPE had lower PAPPA, fβhCG, and PlGF levels and higher AFP and sTNFR1 concentrations in the first trimester than mothers with a normal pregnancy outcome. The combination of these biomarkers with MC detected 48% of women with subsequent EOPE in a low-risk population, with 10% FPR. These findings may reflect the unresolved etiology of preeclampsia, relating to impaired trophoblast invasion into maternal myometrium and apoptosis.42
Preeclampsia, which is a multiorgan disease, is defined according to the onset of hypertension and proteinuria after 20 gestational weeks, but its pathogenesis lies in early pregnancy. Therefore, it is clinically challenging to detect this disorder early enough to develop treatment strategies for prevention or cure. Currently, there is no other cure than delivery and removal of the placenta for preeclampsia, but from a clinical point of view, the detection of early-onset severe preeclampsia would allow timely initiation of prophylactic therapy and appropriate antenatal surveillance to prevent the most severe adverse outcomes. It would be essential to find a screening method for low-risk first pregnancies, as the incidence of preeclampsia is the highest among primigravidas.2 The simplest test for preeclampsia would only require one healthcare visit, and therefore, various biomarker panels with or without MAP and UtA PI have been investigated eagerly.38,43–48 The best EOPE detection rate, 96% with 10% fixed FPR, has been achieved using Ut API, MAP, PAPPA, and PlGF with maternal markers.43 In some studies, uterine artery pulsatility, even though being statistically significant, did not increase the detection rate.49 It is difficult to compare some of the previous results with our study, as information concerning whether the tests were performed in a low-or a high-risk population is lacking. Because of strong clustering and the impact of genes in preeclampsia, maternal factors and medical findings in early pregnancy are likely to have a significant impact, even though from a practical point of view, a simple blood sample would be clinically more feasible.
We selected PAPPA and fhCGβ for our biomarker panels as they are currently available in most early pregnancies because of high participation in integrated trisomy screening, and they are reported to be associated with elevated risk of preeclampsia.50–52 PlGf and sTNFR1 were chosen for their strong association with angiogenesis, and PlGF shows an excellent predictive value in the second trimester in the detection of subsequent preeclampsia.53
The results in our study are comparable with previous studies. However, there are also conflicting reports. According to a North American study on a nulliparous low-risk population, MC were significantly associated with preeclampsia, while several biomarkers (ADAM12, PP13, PlGF, sFlit, and endoglin) had no clinical utility in prediction of preeclampsia in the first trimester.54 The authors concluded that univariable analysis of maternal age, race, marital status, years of education, source of medical payment, prenatal caregiver, body mass index (BMI), and systolic blood pressure at enrollment revealed significant association with preeclampsia.
In this study, the predictive value of each biomarker separately was low, which is not surprising since the levels of these biomarkers in normal pregnancies have shown to peak later in the second trimester, except in the case of fβhCG.55 In our study, the addition of biomarker levels to MC increased ROC AUC values, indicating that their assessments increased the predictive value of the diagnostic panel for preeclampsia (Table 5). The most significant increase was detected when TNFRs1 was added to the panel, while the addition of AFP to the panel only increased the ROC AUC value from a mean value of 0.78 to 0.79 (Table 5).
We are fully aware of the difference in the storage time of the samples between the groups. However, Law et al showed no difference between PlGF samples analyzed freshly or stored for a period of more than three years. In addition, stable storage conditions in the freezer have shown no effect on PAPPA and fβhCG concentrations.56,57 A mean of three-day earlier sampling time in the EOPE group may have affected our results. The peak secretion of all the studied biomarkers except hCG is later than in the first trimester.55,58 Thus, later sampling in the EOPE group would most probably only reinforce our findings. Maternal obesity and smoking are known risk factors for preeclampsia, and these factors as well as the difference in the sampling time were taken into account in the statistical analyses and should not therefore confound the results. We regret that information concerning maternal medical history, and blood pressure values were not available for this study.
Footnotes
ACADEMIC EDITOR: Zeev Blumenfeld, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Teemu Korpimaki is a senior chemist and Heikki Kouru a senior statistician working for PerkinElmer, Turku, Finland. Oulu University Hospital purchases biochemical products from PerkinElmer for early pregnancy trisomy screening. Other authors have no potential conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: AY, JM, and MR. Analyzed the data: KM, TK, and HK. Wrote the first draft of the manuscript: AY. Contributed to the writing of the manuscript: TK, HK, and MR. Agreed with manuscript results and conclusions: AY, KM, TK, HK, JM, and MR. Jointly developed the structure and arguments for the paper: TK and HK. Made critical revisions and approved the final version: KM, TK, HK, and MR. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Smith GN, Pudwell J, Walker M, Wen SW. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J Obstet Gynaecel Can. 2012;34(9):830–835. doi: 10.1016/S1701-2163(16)35381-6. [DOI] [PubMed] [Google Scholar]
- 2.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayobi JM. Preeclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–474. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 4.Pijnenborg R, Anthony J, Davey DA, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98(7):648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 5.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266(5190):1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 7.Staff AC, Dechend R, Redman CW. Review: preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: two new hypotheses. Placenta. 2012;34:s73–s78. doi: 10.1016/j.placenta.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JM, Redman CW. Preeclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341(8858):1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 9.Redman CW. Current topic: preeclampsia and the placenta. Placenta. 1991;12(4):301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 10.Cnattingius S, Reilly M, Pawitan Y, Lichtenstein P. Maternal and fetal genetic factors account for most of familial aggregation of preeclampsia: a population-based Swedish cohort study. Am J Med Genet A. 2004;130A(4):365–371. doi: 10.1002/ajmg.a.30257. [DOI] [PubMed] [Google Scholar]
- 11.Laivuori H, Lahermo P, Ollikainen V, et al. Susceptibility loci for preeclampsia on chromosomes 2p25 and 9p13 in Finnish families. Am J Hum Genet. 2003;72:168–177. doi: 10.1086/345311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk M, Mulders J, Poutsma A, et al. Maternal segregation of the Dutch preeclampsia locus at 10q22 with a new member of the winged helix gene family. Nat Genet. 2005;37:514–519. doi: 10.1038/ng1541. [DOI] [PubMed] [Google Scholar]
- 13.Peterson SE, Simhan HN. First-trimester pregnancy-associated plasma protein A and subsequent abnormalities of fetal growth. Am J Obstet Gynecol. 2008;198:43–45. doi: 10.1016/j.ajog.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence JB, Oxvig C, Overgaard MT, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci USA. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87:1762–1767. doi: 10.1210/jcem.87.4.8430. [DOI] [PubMed] [Google Scholar]
- 16.Pihl K, Larsen T, Krebs L, Christiansen M. First trimester maternal serum PAPP-A, beta-hCG and ADAM12 in prediction of small-for-gestational-age fetuses. Prenat Diagn. 2008;28:1131–1135. doi: 10.1002/pd.2141. [DOI] [PubMed] [Google Scholar]
- 17.Barrett SL, Bower C, Hadlow NC. Use of the combined first-trimester screen result and low PAPP-A to predict risk of adverse fetal outcomes. Prenat Diagn. 2008;28:28–35. doi: 10.1002/pd.1898. [DOI] [PubMed] [Google Scholar]
- 18.Krantz D, Goetzl L, Simpson JL, et al. First Trimester Maternal Serum Biochemistry and Fetal Nuchal Translucency Screening (BUN) Study Group Association of extreme first-trimester free human chorionic gonadotropin-beta, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am J Obstet Gynecol. 2004;191:1452–1458. doi: 10.1016/j.ajog.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 19.Talmadge K, Boorstein WR, Fiddes JC. The human genome contains seven genes for the beta-subunit of chorionic gonadotropin but only one gene for the beta-subunit of luteinizing hormone. DNA. 1983;2:281–289. doi: 10.1089/dna.1983.2.281. [DOI] [PubMed] [Google Scholar]
- 20.Zygmunt M, Herr F, Keller-Schoenwetter S, et al. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]
- 21.Ingram RS, Scott RW, Tilghman SM. Alpha-fetoprotein and albumin genes are in tandem in the mouse genome. Proc Natl Acad Sci USA. 1981;78:4694–4698. doi: 10.1073/pnas.78.8.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wald NJ, Brock DJ, Bonnar J. Prenatal diagnosis of spina bifida and anencephaly by maternal serum-alpha-fetoprotein measurement. A controlled study. Lancet. 1974;1:765–767. doi: 10.1016/s0140-6736(74)92838-4. [DOI] [PubMed] [Google Scholar]
- 23.Ryynänen M, Seppälä M, Kuusela P, et al. Antenatal screening for congenital nephrosis in Finland by maternal serum alpha-fetoprotein. Br J Obstet Gynaecol. 1983;90(5):437–442. doi: 10.1111/j.1471-0528.1983.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 24.Heinonen S, Ryynänen M, Kirkinen P, Saarikoski S. Elevated midtrimester maternal serum hCG in chromosomally normal pregnancies is associated with preeclampsia and velamentous umbilical cord insertion. Am J Perinatol. 1996;13(7):437–441. doi: 10.1055/s-2007-994384. [DOI] [PubMed] [Google Scholar]
- 25.Athanassiades A, Lala PK. Role of placenta growth factor (PIGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta. 1998;19(7):465–473. doi: 10.1016/s0143-4004(98)91039-6. [DOI] [PubMed] [Google Scholar]
- 26.Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PIGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Fit1. Nat Med. 2002;8(8):831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 27.Vuorela P, Hatva E, Lymboussaki A, et al. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod. 1997;56(2):489–494. doi: 10.1095/biolreprod56.2.489. [DOI] [PubMed] [Google Scholar]
- 28.Khaliq A, Li XF, Shams M, et al. Localisation of placenta growth factor (PIGF) in human term placenta. Growth Factors. 1996;13(3–4):243–250. doi: 10.3109/08977199609003225. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed A, Kilby MD. Hypoxia or hyperoxia in placental insufficiency? Lancet. 1997;350(9081):826–827. doi: 10.1016/S0140-6736(05)62027-2. [DOI] [PubMed] [Google Scholar]
- 30.Khaliq A, Dunk C, Jiang J, et al. Hypoxia down-regulates placenta growth factor, whereas fetal growth restriction up-regulates placenta growth factor expression: molecular evidence for “placental hyperoxia” in intrauterine growth restriction. Lab Invest. 1999;79(2):151–170. [PubMed] [Google Scholar]
- 31.Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997;18(8):657–665. doi: 10.1016/s0143-4004(97)90007-2. [DOI] [PubMed] [Google Scholar]
- 32.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359–1375. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 33.Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR. Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol. 2012;36(3):261–270. doi: 10.1159/000342333. [DOI] [PubMed] [Google Scholar]
- 34.Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76(3):262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- 35.Metcalfe A, Langlois S, Macfarlane J, Vallance H, Joseph KS. Prediction of obstetrical risk using maternal serum markers and clinical risk factors. Prenat Diagn. 2014;34(2):172–179. doi: 10.1002/pd.4281. [DOI] [PubMed] [Google Scholar]
- 36.Ay E, Kavak ZN, Elter K, Gokaslan H, Pekin T. Screening for preeclampsia by using maternal serum inhibin A, activin A, human chorionic gonadotropin, unconjugated estriol, and alpha-fetoprotein levels and uterine artery Doppler in the second trimester of pregnancy. Aust N Z J Obstet Gynaecol. 2005;45(4):283–288. doi: 10.1111/j.1479-828X.2005.00412.x. [DOI] [PubMed] [Google Scholar]
- 37.Thissier-Levy S, Boucoiran I, Luo ZC, et al. Endostatin levels and the risk of subsequent preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2013;170(2):396–400. doi: 10.1016/j.ejogrb.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 38.Myers JE, Kenny LC, McCowan LM, et al. Angiogenic factors combined with clinical risk factors to predict preterm preeclampsia in nulliparous women: a predictive test accuracy study. Br J Obstet Gynaecol. 2013;120(10):1215–1223. doi: 10.1111/1471-0528.12195. [DOI] [PubMed] [Google Scholar]
- 39.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early-versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544–545. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Roberge S, Giguère Y, Villa P, et al. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Am J Perinatol. 2012;29(7):551–556. doi: 10.1055/s-0032-1310527. [DOI] [PubMed] [Google Scholar]
- 41.ACOG Committee on Practice Bulletins—Obstetrics ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 42.Kadyrov M, Kingdom JC, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol. 2006;194(2):557–563. doi: 10.1016/j.ajog.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 43.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33(1):8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 44.Audibert F, Boucoiran I, An N, et al. Screening for preeclampsia using first-trimester serum markers and uterine artery Doppler in nulliparous women. Am J Obstet Gynecol. 2010;203(4):383.e1–383.e8. doi: 10.1016/j.ajog.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Di Lorenzo G, Ceccarello M, Cecotti V, et al. First trimester maternal serum PIGF, free β-hCG, PAPP-A, PP-13, uterine artery Doppler and maternal history for the prediction of preeclampsia. Placenta. 2012;33(6):495–501. doi: 10.1016/j.placenta.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Bolin M, Wikström AK, Wiberg-Itzel E, et al. Prediction of preeclampsia by combining serum histidinerich glycoprotein and uterine artery Doppler. Am J Hypertens. 2012;25(12):1305–1310. doi: 10.1038/ajh.2012.112. [DOI] [PubMed] [Google Scholar]
- 47.Park FJ, Leung CH, Poon LC, Williams PF, Rothwell SJ, Hyett JA. Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. Aust N Z J Obstet Gynaecol. 2013;53(6):532–539. doi: 10.1111/ajo.12126. [DOI] [PubMed] [Google Scholar]
- 48.Kuc S, Koster MP, Franx A, Schielen PC, Visser GH. Maternal characteristics, mean arterial pressure and serum markers in early prediction of preeclampsia. PLoS One. 2013;8(5):e63546. doi: 10.1371/journal.pone.0063546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myatt L, Clifton RG, Roberts JM, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU) The utility of uterine artery Doppler velocimetry in prediction of preeclampsia in a low-risk population. Obstet Gynecol. 2012;120(4):815–822. doi: 10.1097/AOG.0b013e31826af7fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer K, Cowans NJ, Nicolaides KH. Low levels of maternal serum PAPP-A in the first trimester and the risk of preeclampsia. Prenat Diagn. 2008;28(1):7–10. doi: 10.1002/pd.1890. [DOI] [PubMed] [Google Scholar]
- 51.Keikkala E, Vuorela P, Laivuori H, Romppanen J, Heinonen S, Stenman UH. First trimester hyperglycosylated human chorionic gonadotropin in serum—a marker of early-onset preeclampsia. Placenta. 2013;34(11):1059–1065. doi: 10.1016/j.placenta.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Karahasanovic A, Sørensen S, Nilas L. First trimester pregnancy-associated plasma protein A and human chorionic gonadotropin-beta in early and late preeclampsia. Clin Chem Lab Med. 2013;1:1–5. doi: 10.1515/cclm-2013-0338. [DOI] [PubMed] [Google Scholar]
- 53.Chappell LC, Duckworth S, Seed PT, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128(19):2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 54.Myatt L, Clifton RG, Roberts JM, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119(6):1234–1242. doi: 10.1097/AOG.0b013e3182571669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21(1):9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Law LW, Sahota DS, Chan LW, Chen M, Lau TK, Leung TY. Effect of long-term storage on placental growth factor and fms-like tyrosine kinase 1 measurements in samples from pregnant women. J Matern Fetal Neonatal Med. 2010;23(12):1475–1480. doi: 10.3109/14767051003678242. [DOI] [PubMed] [Google Scholar]
- 57.Cruz J, Cruz G, Minekawa R, Maiz N, Nicolaides KH. Effect of temperature on free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A concentration. Ultrasound Obstet Gynecol. 2010;36(2):141–146. doi: 10.1002/uog.7688. [DOI] [PubMed] [Google Scholar]
- 58.Bischof P, DuBerg S, Herrmann W, Sizonenko PC. Pregnancy-associated plasma protein-A (PAPP-A) and hCG in early pregnancy. Br J Obstet Gynaecol. 1981;88(10):973–975. doi: 10.1111/j.1471-0528.1981.tb01683.x. [DOI] [PubMed] [Google Scholar]