Abstract
European starlings (Sturnus vulgaris) exhibit seasonal changes in singing and in the volumes of the neural substrate. Increases in song nuclei volume are mediated at least in part by increases in day length, which is also associated with increases in plasma testosterone (T), reproductive activity, and singing behavior in males. The correlations between photoperiod (i.e. daylength), T, reproductive state and singing hamper our ability to disentangle causal relationships. We investigated how photoperiodic-induced variation in reproductive state modulates the effects of T on singing behavior and song nuclei volumes in adult female starlings. Female Starlings do not naturally produce measureable levels of circulating T but nevertheless respond to exogenous T, which induces male-like singing. We manipulated photoperiod by placing birds in a photosensitive or photorefractory state and then treated them with T-filled or empty silastic implants. We recorded morning singing behavior for three weeks, after which we assessed reproductive condition and measured song nuclei volumes. We found that T-treated photosensitive birds sang significantly more than all other groups including T-treated photorefractory birds. All T-treated birds had larger song nuclei volumes than with blank-treated birds (despite photorefractory T-treated birds not increasing song-rate). There was no effect of photoperiod on the song nuclei volumes of T-treated birds. These data show that the behavioral effects of exogenous T can be modulated by reproductive state in adult female songbirds. Furthermore, these data are consistent with other observations that increases in singing rate in response to T are not necessarily due to the direct effects of T on song nuclei volume.
Keywords: female songbird, testosterone, song, neuroplasticity, photoperiodism, birdsong, HVC, enkephalin
INTRODUCTION
A complex web of endogenous and exogenous cues controls singing in temperate-zone songbirds. External cues such as photoperiod and the social milieu can interact with a bird’s hormonal and physiological state (including the state of neural control systems) to determine the rate and quality of singing (Tramontin and Brenowitz, 2000; Catchpole and Slater, 2008; Kroodsma, 2005). For example, increases in photoperiod are known to induce increases in plasma testosterone (T), singing behavior, and the volumes of song nuclei in male European starlings (Bernard and Ball, 1995; Riters, et al, 2000; Dawson, et al, 2001; Stevenson, et al, 2010).
The administration of exogenous T can induce an increase in singing and the volumes of song nuclei in male and some female songbirds independent of photoperiodic cues, indicating that the effects of photoperiod on song behavior and underlying brain plasticity may be mediated by T (Nottebohm, 1980; Hausberger, et al, 1995; Harding, 2004; see Schlinger and Brenowitz, 2002 for a review). There is evidence that reproductive state (e.g. breeding, non-breeding, or pre-breeding) can modulate T-induced behavioral and neural changes in mammals and birds (Campbell, et al, 1978; Ellis and Turek, 1983; Bernard, et al, 1997; Smith, et al, 1997). Data collected from male starlings suggest that a pre-breeding ‘photosensitive’ state increases sensitivity to exogenous T on song nuclei volumes, resulting in larger volumes of the Song nucleus HVC in photosensitive compared with non-breeding ‘photorefractory’ birds treated with similar doses of T (Bernard and Ball, 1997). This is not true of all temperate-zone songbirds; photoperiod (i.e. reproductive state) does not alter the effects of exogenous T in male song sparrows (Melospiza melodia; Nowicki and Ball, 1989; Ball and Nowicki, 1990). The interrelation of photoperiod, steroids, song nuclei plasticity, and song behavior are not well understood.
European starlings are an excellent model system to study the modulatory role of photoperiod on T-induced singing behavior and song quality. Starlings, as in a number of other songbird species, display absolute photorefractoriness after exposure to long day lengths for an extended period of time (weeks to months of exposure to “long days” depending on species; Burger, 1947; Nicholls, et al, 1988; Dawson et al., 2001). This state of photorefractoriness is characterized by regression of the gonads to a prepubescent-like state, as well as an inability to respond to long day lengths including photoperiods of constant daylight (Burger, 1947; Dawson, et al, 1985; Falk and Gwinner, 1988; MacDougall-Shackleton, et al, 2009). When males are exposed to short day lengths for an extended period of time, the birds become photosensitive (i.e., responsive) to the stimulating effects of long day lengths (Nicholls, et al, 1988; Dawson, et al, 2001).
Photoperiod is also involved in the control of the reproductive cycle in female starlings. This regulation does not involve changes in concentrations of T circulating in plasma (Dawson and Goldsmith, 1983; Dawson, 1984, 1997; Stevenson, et al, 2012). In addition, natural singing behavior in female starlings is partially regulated by photoperiod (Pradhan, et al, 2008). Female starlings tend to sing in the non-breeding season when day lengths are short and significantly reduce song output in the breeding season when day lengths increase and male conspecifics compete for mates (Pavlova et al, 2005; Pavlova et al, 2007a, 2007b).
However, adult female starlings respond to exogenous administration of T by singing male-like songs (Hausberger, et al, 1995; De Ridder, et al, 2002). These findings suggest that the activational effects of T largely cause sex differences in song behavior in adult starlings rather than organizational effects during ontogeny (Arnold, et al, 1996; Wade and Arnold, 2004). Adult female starlings treated with T provide a useful model system to investigate the activational properties of T independent of prior exposure to male-like concentrations of circulating T.
We used female starlings to study how the effects of T on singing behavior, song quality, and song system morphology are modulated by photoperiodic (i.e. reproductive) state. It has been assumed that T-induced changes in females are modulated by the same mechanisms as in males (Madison, et al, 2014), but this has not been tested. Based on the hypothesis that reproductive state can modulate the effectiveness of T on brain and behavior, we predicted that photosensitive females would be more responsive to the effects of T and exhibit increased song behavior compared to photorefractory females treated with T and sham-treated controls. Finally, we predicted that the volumes of song nuclei would be larger in T-treated photosensitive female starlings compared with photorefractory females treated with T and sham-treated controls.
METHODS
Animals and Photoperiodic Treatments
Twenty-eight wild-caught adult female European starlings were used in this experiment. All birds were captured using a drop down V-trap in early March 2007 and late February 2013. Upon arrival in the laboratory, birds were group-housed and maintained on a natural photoperiod (8L:16D; lights on at 1200 hr EDT, lights off at 2000 hr). Shortly after arrival all birds were laparotomized and the gonads examined in order to confirm sex and assess reproductive condition. All birds were housed in groups on 8L:16D for 6 to 7 months before the start of the experiment to maintain a photosensitive state. Animal husbandry of the starlings was in accordance with guidelines published by the National Research Council (2010). All experimental procedures were approved by the Johns Hopkins University Animal Care and Use Committee and adhered to standards of the Society for Neuroscience.
The twenty-eight female starlings were randomly assigned to one of two photoperiodic conditions: 1) long-day photorefractory or 2) short-day photosensitive. Fourteen birds were transferred to group housing on a long-day photoperiod (16L:8D; lights on 0700 hr EDT, lights off 2300 hr EDT) and fourteen birds remained in group housing on a short-day photoperiod (8L:16D; lights on at 1200 hr EDT, lights off at 2000 hr EDT) to maintain a photosensitive state.
T-Implantation
Previous data have shown that housing starlings on long-days for a minimum of eight to ten weeks can induce photorefractoriness, as determined by gonad size, molt and beak score (Dawson and Goldsmith, 1983). After 10 weeks, both the photosensitive and the photorefractory groups received either a single 10mm length Silastic capsule (1.47mm i.d., 1.96mm o.d.) containing crystalline T (nphotosensitive = 7; nphotorefractory = 7) or a 10mm length empty Silastic capsule (1.47mm i.d., 1.96mm o.d.) blank implant (nphotosensitive = 7; nphotorefractory = 7). Implants were inserted through a small incision (approx. 2–3mm) over the left flank subcutaneously. Immediately following implantation, birds were transferred to individual sound attenuated chambers. Photoperiod was held constant for each individual bird. Birds were implanted for a total of 3 weeks and during that time behavioral measurements were taken daily.
Behavioral Measurements
Bird vocalizations were recorded daily using an electret microphone (Radioshack Model 33-3013) and digitized using a custom software package with 16-bit resolution and 44.1kHz sampling rate. Over the 3-week experimental manipulation, song samples were automatically recorded for a 2-hour period starting when the lights were turned on in the morning. Recordings were high pass filtered with a cutoff frequency of 900 Hz.
Songs were operationally defined as periods of at least 5 seconds of singing with no more than 3 seconds of silence (see Bernard, Eens, and Ball, 1996). The total number of songs per recording per day was tallied. The daily count was averaged across the week of treatment (i.e. week 1, week 2 and week 3). In addition, birds that sang complete song bouts were identified for further behavioral analysis. Complete song bouts were defined as songs with at least three of the four phrase-types characteristic of male starling song. These songs were then quantified for a motif analysis to provide the relative size of the bird’s vocal repertoire.
As previously stated, only complete song bouts (i.e. songs where at least three of the four phrase types characteristic of starling song were present) were used for the motif analysis. Not all birds sang complete song bouts. As a result only a subset of the birds were included in this analysis (Photosensitive + T, n=5; Photorefractory + T, n=4). One photosensitive control and one photorefractory control were observed singing complete bouts during the 3-week treatment period. However, since there was only 1 animal in each group, we could not include them in the statistical analysis of repertoire and do not present their data, as we cannot make a valid comparison to the T-treated groups.
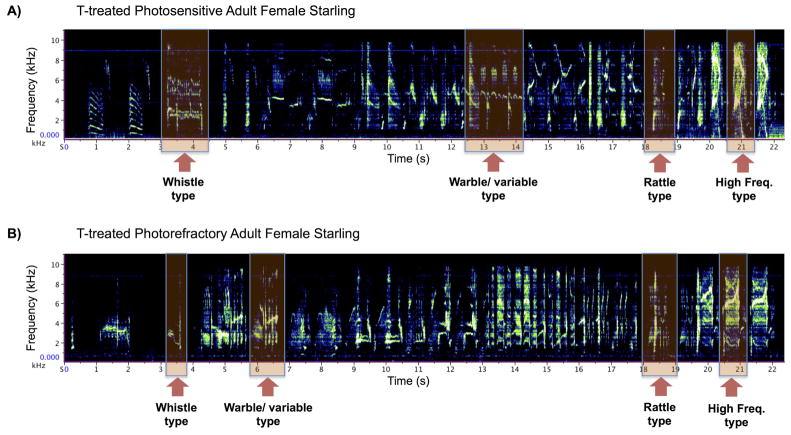
The motif analysis was used to estimate the repertoire size of individual birds. Sound analysis was done using Ravenlite (Cornell Ornithology Lab, Ithaca, NY) and each song (N=1,849) was visualized using oscillograms and spectrograms. Each unique motif/phrase was counted and categorized into one of four phrase types characteristic of starling song (whistle, variable/warble, rattle, and high frequency phrase types; Eens, 1997; see figure 1).
Figure 1.
Sound spectrographs of T-treated adult female starling songs. The x-axis represents time and the y-axis frequency. Amplitude is represented by the brightness of the vocalization (dark = low amplitude, bright = high amplitude). Displayed are songs from two different T-treated females in two different photoperiodic conditions taken from the same day of study, A) a photosensitive T-treated female starling and B) a T-treated photorefractory female starling. As illustrated, starling song is a complex arrangement of harmonically variable vocalizations and though rich in acoustic diversity the various syllable phrases that make up starling song can be characterized into one of four motif or phrase types. The labeled boxes highlight exemplars of the four phrase types; whistle, warble/variable, rattle, and high frequency phrases.
Peripheral Physiology
Beak color was assessed before and after hormonal implantation. In starlings, beak color ranges from yellow to black (Hicks, 1934; Kessel, 1957). Bright yellow beaks indicate that testosterone is present in the blood while black beaks indicate that little or no T is available in the blood (Ball and Wingfield, 1987). The beak is therefore an indicator of hormonal condition. Beak scoring was as follows: 0 - completely black beak; 1 - 2/3 black beak with 1/3 yellow at the base; 2 – 2/3 yellow beak with 1/3 black toward the tip; 3 - completely yellow beak (Wydoski, 1964).
Radioimmunoassay
Blood samples were taken before T implantation and at the end of the experiment via puncture of the alar wing vein with a 25-gauge needle. 300–500 μl of blood was collected into caraway capillary tubes at both sampling times. The blood samples were transferred into tubes and centrifuged at 9000 rpm for 15 minutes. The plasma was removed and stored in vials at −20° prior to T assay. Plasma T concentrations were analyzed in a single run of duplicates (50μl) using a commercially available 125I Coat-A-Count kit for total testosterone (Siemens Medical Solutions Diagnostics, Los Angeles, CA). This kit provides reliable hormone concentrations and has been validated for and previously used in starlings (Stevenson, et al, 2008; Stevenson and Ball, 2009; Cornil, et al, 2009). The antiserum is highly specific for testosterone (i.e. 100pg/ml) and shows negligible cross reactivity with other steroids including dihydrotestosterone (<3.5%); 17β-estradiol (< 0.01%); corticosterone (< 0.01%).
Perfusion & Peptide Immunocytochemistry
At the end of the experiment, birds were deeply anaesthetized with secobarbitol (50mg/ml IM) and perfused transcardially with heparinized 0.1M phosphate buffered saline (PBS) pH 7.5, followed by 4% paraformaldehyde. The brains were extracted, weighed, and placed in 4% paraformaldehyde and left overnight at 4° C. Immediately following that the ovaries and oviducts were dissected out and the wet weight was measured and recorded. The following morning, the brains were transferred into a sucrose solution (30% sucrose in 0.1 M PBS) and left overnight at 4° C. The brains were frozen with dry ice for two min. and then stored in a freezer (−70°C) until sectioning. Brains were sectioned coronally (40 μm thick) using a cryostat.
Every third section was mounted onto gelatin-coated slides and Nissl-stained with thionin. Adjacent sections were processed with immunocytochemical techniques for enkephalin (ENK). The boundaries of the forebrain nucleus lateral magnocellular nucleus of the anterior nidopallium (LMAN) and medial magnocellular nucleus of the anterior nidopallium (MMAN) cannot be reliably discerned in Nissl-stained material but can be distinguished in ENK positive material (Ball et al, 1988; Bottjer and Alexander, 1995; Stevenson and Ball, 2010). The boundaries of HVC are clearly defined by both Nissl and ENK immunoreactive fiber staining (Stevenson and Ball, 2010).
The brains were processed in random order such that the time from tissue collection to processing was similar across groups. Brain sections were washed in 0.1 M PBS twice, once in 0.5% H2O2 for 15 min., then washed three times in 0.1 M PBS and left overnight in normal goat serum (20% solution in 0.3% PBS/T [Triton X]) at 4°C. Sections were incubated in primary antibody (1:2000 for ENK) for 24 hrs. In the morning the sections were washed three times with 0.1% PBS/T, then incubated in biotinylated secondary antibody (goat anti rabbit IgG, 1:250) for 1 hr, washed three times in 0.1% PBS/T, incubated in avidin biotin horseradish-peroxidase complex (Vectastain ABC, Elite Kit 1:200) for 1 hr, and then washed again three times in 0.1% PBS/T. The sections were then incubated in biotinylated tyramine (1:150 in PBS/T) for 1 hr, washed three times in 0.1% PBS/T, incubated in streptavadin horseradish peroxidase (1:200 in PBS/T) for 1 hr, and then washed another three times in 0.1% PBS/T. The antibodies were visualized by incubating the sections with diaminobenzidine (Sigma Fast DAB) for 6 minutes. Finally, sections were washed three times with 0.1 M PBS and mounted onto gelatin coated microscope slides. Sections were then serially dehydrated in ethanol and then placed in xylene for 5 min. The slides were then coverslipped using Permount (Fisher).
Song Nuclei Volume Reconstruction
Digital images of brain sections containing the regions of interest were captured using a microscope with a CCD camera connected to a computer. Digital images of the brain images were analyzed and the peptidergic-defined boundaries for each nucleus were traced using Openlab (Improvision® a Perkin-Elmer company). The regions of interests were Area X, the lateral magnocellular nucleus of the anterior nidopallium (LMAN), HVC (used as its proper name), and the robust nucleus of the arcopallium (RA). The volume of each region of interest was reconstructed; the areas of sections containing the region of interest were summed with the sampling interval (120 μm) using the formula for a truncated cone (developed by Smith et al., 1995). This method has been used previously in European starlings (Bernard and Ball, 1995; Bentley et al., 1999; Bernard and Ball, 1997). For each bird, both the left and right hemispheres were measured and the average between hemispheres was calculated. Volumes of each nucleus were measured and normalized to the post-perfusion brain weight of the individual, which was weighed immediately after brain extraction, just prior to post-fixation in 4% paraformaldehyde. After the raw value of the nuclei volume was calculated. The raw value was normalized by dividing the nuclei volume by the total brain weight in grams. Prior to normalization, the mean volumes of the song nuclei were well within the previously reported range(s) for untreated adult female starlings (Ball et al, 1994; Bernard et al, 1993).
Statistical Analysis
The open source programming language R (version 3.1.0) was used for statistical analyses; we used the statistical, nlme, and ggplot2 packages (R Core Team, 2013; Pinheiro, et al, 2014; Wickham, 2009). We analyzed the song rate data using a mixed-design analysis of variance (ANOVA). For the repeated measure song rate (mean daily song count by week) and interaction variables (week by photoperiod, week by T-treatment, and week by photoperiod by T-treatment), p-values for effects were corrected using a Greenhouse-Geisser correction for non-sphericity. Since the variables photoperiod and T-treatment are binary, post-hoc tests were not necessary for these main effects. Post-hoc pairwise comparisons were made for the main effect of the repeated measures variable, as well as all significant interaction effects. All pairwise comparisons were corrected for multiple comparisons using Westfall’s procedure (Westfall, 1997; Bretz, et al, 2010). All possible pairwise combinations for the main and interaction effects were tested.
For the physiological measures (i.e. T-RIA, beak score, ovary and oviduct weight) and the brain volume data, a MANOVA was used to test for significant effects of treatment and interaction effects. For statistically significant interaction effects in the MANOVA, post-hoc pairwise comparisons were made and were corrected for multiple comparisons using Westfall’s procedure (Westfall, 1997; Bretz, et al, 2010). All possible pairwise combinations for significant interaction effects were tested.
The number of song samples used for motif analysis varied dramatically between individuals and groups, which resulted in a multimodal distribution of songs per sampling period. Further, not all birds sang complete bouts during the course of treatment, leaving unequal sizes for final motif analyses. This non-normal distribution of singing and unequal group sizes made parametric statistical analysis inappropriate. We used non-parametric statistics (Wilcoxon rank sum test) to test for effects of treatment on the number of unique phrases because equal sample size and normal distribution are not required for assessment of independent observations. All statistically significant differences reported were evaluated with respect to α = 0.05. For statistically significant main and interaction effects, generalized eta squared values are reported. For statistically significant pairwise comparisons of the main and interaction effects, Cohen’s d values are reported.
RESULTS
Effect of T on song rate and syllable repertoire
The latency to sing varied widely by individual and by treatment. Indeed, some birds did not sing over the course of treatment; those birds were included in the analysis of the data unless otherwise noted. There was a significant main effect of photoperiod (F(1,24) = 4.39, p < 0.05, η2 = 0.12) on the overall rate of singing (mean number of song bouts per recording period); photosensitive birds sang more than photorefractory. Likewise, there was a significant main effect of T-treatment (F(1,24) = 5.31, p < 0.05, η2 = 0.14) on the overall rate of singing; T-treated birds sang more than blank-treated birds. Though there was a trend in the interaction between photoperiod and T-treatment, the interaction effect was not statistically significant (F(1,24) = 3.72, p = 0.07).
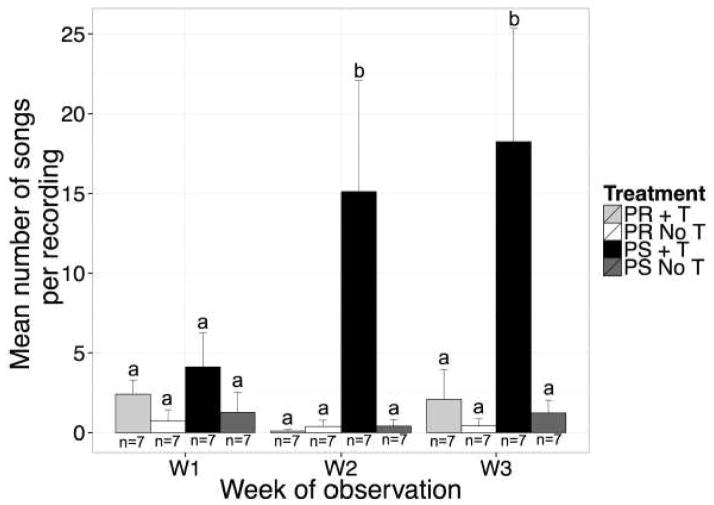
Interestingly, there was a significant main effect of the repeated measures variable week of observation (F(2,48) = 4.20, p < 0.05, η2 =0.09). Likewise, there were significant interaction effects with this variable; namely, there was a significant interaction between week and photoperiod (F(2,48) = 5.87, p < 0.05, η2 = 0.13), week and T-treatment (F(2,48) = 4.84, p < 0.05, η2 = 0.11), and a three way interaction of week, photoperiod, and treatment (F(2,48) = 6.03, p < 0.05, η2 = 0.13; Figure 2).
Figure 2.
Bar graph illustrating the mean number of songs produced per 2 hour recording session. Black bars represent T-treated photosensitive birds. Dark gray bars represent photosensitive control birds. Light gray bars represent T-treated photorefractory birds. White bars represent photorefractory controls. In general T was much more effective in inducing high rates of singing in photosensitive than in photorefractory female starlings. See results section for more detail.
The main effect of week was further investigated. Pairwise post-hoc comparisons showed that birds sang more during week 3 of treatment compared to week 1 (z = 2.354, p < 0.05, d = 0.44). However, there was no difference between week 3 and week 2 of treatment (z = 1.04, p = 0.30) or week 2 and week 1 of treatment (z = 1.31, p = 0.19). The two-way interaction between week and photoperiod was further investigated. Pairwise post-hoc comparisons showed that during week 1 and week 2, the effect of photoperiod was not statistically significant; however, the effect of photoperiod during week 2 approached a statistical trend (zweek1 = 0.81, p = 0.42; zweek2 = 1.92, p = 0.05). Further, during week 3 the effect of photoperiod was significant; photosensitive birds sang more than photorefractory birds (z = 1.98, p < 0.05, d = 0.37). The two-way interaction between week and T-treatment yielded a similar pattern. Pairwise post-hoc comparisons showed that during week 1 and week 2, the effect of T-treatment was not statistically significant and only approached a statistical trend (z = 1.69, p = 0.09; z = 1.83, p = 0.07). Conversely, during week 3, T-treated birds sang more than blank-treated control birds (z = 2.22, p < 0.05, d = 0.42).
Furthermore, the three-way interaction of week of observation, photoperiod and T-treatment yielded a specific pattern of behavior in post-hoc comparisons. During weeks 2 and 3, differences in the interaction between photoperiod and T-treatment were significant. Specifically, photosensitive T-treated birds sang more than photorefractory T-treated (zweek2 = 3.05, p < 0.05, d = 0.82; zweek3 = 3.08, p < 0.01, d = 0.82), photosensitive blank-treated birds (zweek2 = 2.99, p < 0.05, d = 0.80; zweek3 = 3.25, p < 0.01, d = 0.87), and photorefractory blank-treated birds (zweek2 = 2.99, p < 0.05, d = 0.80; zweek3 = 3.403, p < 0.01, d = 0.91). Conversely, photorefractory T-treated birds did not differ from photorefractory blank-treated birds (zweek2 = 0.06, p = 1.00; zweek3 = 0.32, p = 0.95) or photosensitive blank-treated birds (zweek2 = 0.06, p = 1.00; zweek3 = 0.17, p = 0.95) during week 1. Furthermore, photosensitive blank-treated birds did not differ from photorefractory blank-treated birds (zweek2 = 0.00, p = 1.00; zweek3 = 0.15, p = 0.95).
However, during week 1, differences in the interaction between photoperiod and T-treatment were not significant, specifically, photosensitive T-treated birds did not differ from photorefractory T-treated (z = 0.88, p = 0.61), photosensitive blank-treated birds (z = 1.47, p = 0.31), and photorefractory blank-treated birds (z = 1.75, p = 0.30) during week 1. Likewise, photorefractory T-treated birds did not differ from photorefractory blank-treated birds (z = 0.87, p = 0.66) or photosensitive blank-treated birds (z = 0.59, p = 0.66) during week 1. Finally, photosensitive blank-treated birds did not differ from photorefractory blank-treated birds (z = 0.28, p = 0.78) during week 1.
Starling song is composed of a highly variable number of syllables and syllable/phrase types arranged into either long or short song bouts. Long song is complex, male-typical, and is composed of a diverse array of syllable/phrase types. We measured the relative repertoire size of birds that sang complete song bouts to further assess the quality/complexity of songs in birds that sang complete song bouts. Photorefractory T-treated singers tended to have a larger vocal repertoire compared with photosensitive T-treated singers (total number of unique phrases; Wilcoxon rank sum test, W = 18.5, p < 0.05, d = 1.67; Figure 3A). However, they did not actually differ in the number of specific phrase types including whistle phrases (W = 16, p = 0.17), variable/warble phrases (W = 7, p = 0.52), rattle phrases (W = 15, p = 0.25), and high frequency phrases (W = 14, p = 0.33; Figure 3B).
Figure 3.
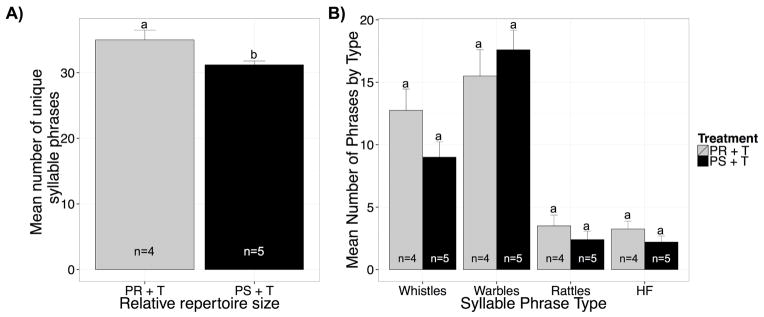
Bar graphs illustrating the analysis of song repertoire in the photosensitive and photorefractory T-treated female starlings. A) Differences in the relative repertoire size in the T-treated photosensitive (black bars) vs the photorefractory females (gray bars). Too few blank-treated females sang to perform this analysis in the control birds. B) Mean number of phrases of each of the four types defined in figure 1 in the T-treated photosensitive (black bars) vs the photorefractory females (gray bars).
Circulating steroids and reproductive physiology
At the end of the experiment blood was collected and the concentration of testosterone (T) was measured. We found that there was a significant effect of T-treatment on the amount of T measured in plasma; T-treated birds had more T than blank-treated (F(1,24) = 39.06, P < 0.001, η2 = 0.59; Figure 4A). There was no effect of photoperiod on the amount of T measured in plasma (F(1,24) = 1.80, p = 0.19). Likewise, there was no effect of the interaction between photoperiod and T-treatment (F(1,24) = 1.80, p = 0.19)
Figure 4.
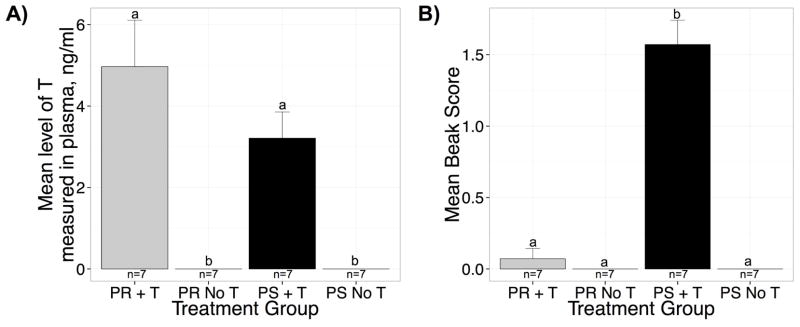
Bar graphs illustrating different measures of the effectiveness of T-treatment. A) Concentrations of T in the blood based on measurements via RIA on samples collected 3 weeks after implantation just prior to brain collection. T-treated birds had significantly higher T than blank-treated birds. T was not different between T-treated photosensitive (black bars) and T-treated photorefractory (gray bars) females. B) Bar graphs illustrating the mean beak score for females in all the groups. The higher the score the higher the percentage of the beak that is yellow in color rather than black. The beak becomes fully yellow eventually if T is present in the blood. Photosensitive (black bars) T-treated birds had beaks that had a much higher percentage covered in yellow than photorefractory T-treated birds or the blank-treated control groups. See text for more details.
Exogenous T in male starlings is known to change the color of the beak from black to yellow (Goldsmith and Nicholls, 1984). We measured the beak scores of all birds both pre- and post-treatment. All of the birds started with black, low-score beaks. At the end of the study, there was a significant main effect of photoperiod on beak scores (F(1,24) = 66.15, p < 0.001, η2 = 0.28; Figure 4B). In addition, there was a significant main effect of T-treatment on beak scores (F(1,24) = 79.35, p < 0.001, η2 = 0.34). Likewise, there was a significant interaction effect between photoperiod and T-treatment (F(1,24) = 66.15, p < 0.001, η2 = 0.28). Photosensitive T-treated females had higher beak scores than T-treated photorefractory (z = 11.50, p < 0.001, d = 3.07), photorefractory controls (z = 12.05, p < 0.001, d = 3.22), and photosensitive controls (z = 12.05, p < 0.001, d = 3.22). Photorefractory T-treated females were not different from photorefractory controls (z = −0.55, p = 0.85) or photosensitive controls (z = −0.55, p = 0.85) and the controls were not different from one another (z = 0.00, p = 0.99).
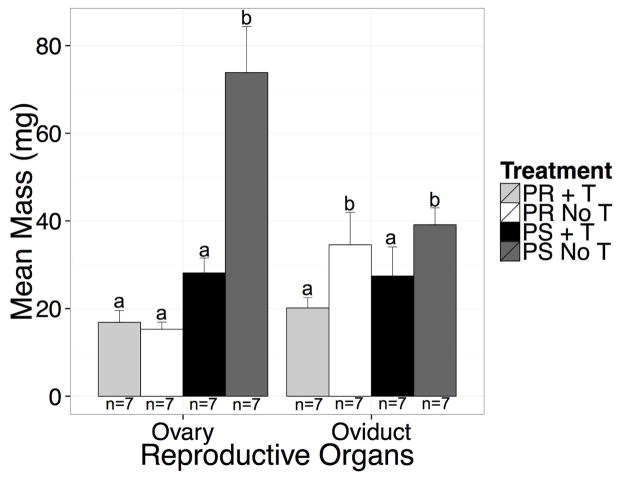
Ovaries and oviducts were extracted after transcardial perfusion and weighed (Figure 5). We found that there was a significant main effect of photoperiod on the wet weight of the ovary; photosensitive birds had larger ovaries than photorefractory birds (F(1,24) = 36.93, p < 0.001, η2 = 0.40). There was a significant main effect of T-treatment; T-treated birds had smaller ovaries than blank-treated birds (F(1,24) = 14.75, p < 0.001, η2 = 0.16). In addition, there was a significant interaction effect of photoperiod and T-treatment (F(1,24) = 16.92, p < 0.001, η2 = 0.18). Photosensitive controls had the heaviest ovaries compared with T-treated photosensitive females (z = 5.62, p < 0.001, d = 1.50), T-treated photorefractory females (z = 7.01, p < 0.001, d = 1.87), and photorefractory controls (z = 7.21, p < 0.001, d = 1.93). Photorefractory control females were not different from photorefractory T-treated birds (z = −0.19, p = 0.85) or photosensitive T-treated birds (z = 1.58, p = 0.25) and the T-treated birds were not different from one another (z = 1.39, p = 0.25). There was a significant main effect of T-treatment on the weight of the oviduct; T-treated birds had smaller oviducts compared with blank-treated birds (F(1,24) = 5.73, p < 0.05, η2 = 0.19). There was no effect of photoperiod on oviduct weight (F(1,24) = 1.18, p = 0.29). Likewise, there was no effect of the interaction of photoperiod and T-treatment (F(1,24) = 0.06, p = 0.80).
Figure 5.
Bar graph illustrating the mean ovary and oviduct mass for all the experimental groups. Photosensitive females not treated with T (dark gray bars) had the largest ovary mass.
Effect of T on volumes of Song nuclei
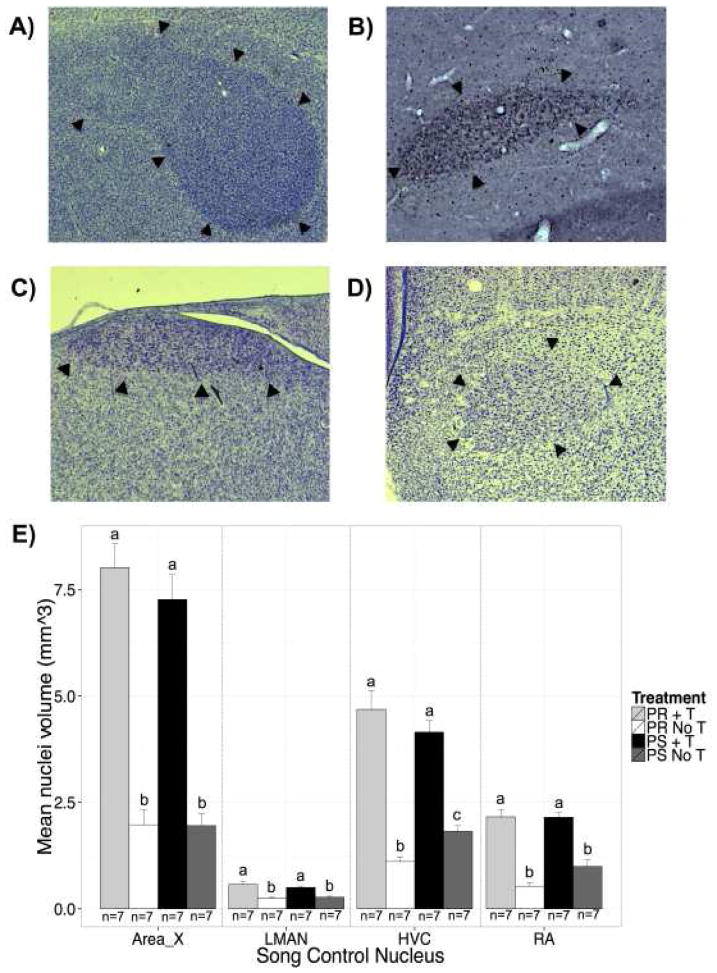
We measured the volumes of the song nuclei Area X, LMAN, HVC, and RA, and found a significant main effect of T-treatment (Area X, F(1,24) = 152.83, p < 0.001, η2 = 0.86; LMAN, F(1,24) = 44.84, p < 0.001, η2 = 0.64; HVC, F(1,24) = 117.38, p < 0.001, η2 = 0.80; RA, F(1,24) = 115.79, p < 0.001, η2 = 0.79; Figure 6). T-treated birds had larger song nuclei volumes. There was no effect of photoperiod on the volumes of these brain regions (Area X, F(1,24) = 0.71, p = 0.41; LMAN, F(1,24) = 0.29, p = 0.60; HVC, F(1,24) = 0.10, p = 0.75; RA, F(1,24) = 3.03, p = 0.09). Furthermore, the interaction effect of photoperiod and T-treatment was not significant for the volumes of Area X (F(1,24) = 0.62, p = 0.43), LMAN (F(1,24) = 1.37, p = 0.25), and RA (F(1,24) = 3.31, p = 0.08) though RA volumes approached a trend. However, there was a significant interaction effect of photoperiod and T-treatment for the volume of nucleus HVC (F(1,24) = 5.20, p < 0.05, η2 = 0.04).
Figure 6.
Photomicrographs of the regions of interest and bar graphs illustrating the mean volume of 4 key forebrain song nuclei in the females in all 4 experimental groups. Representative photomicrographs of the staining quality show the regions of interest for volume reconstruction, namely, song nuclei A) Area X, B) LMAN, C) HVC, and D) RA. E) Bar graphs show that T-treatment resulted in a marked increase in the volume of all four nuclei in both the photosensitive and photorefractory birds compared with blank-treated birds. There were no significant differences in the mean volume of the song nuclei between the T-treated photorefractory and the T-treated photosensitive birds. In the case of HVC and RA nucleus volume was larger in the photosensitive blank-treated control birds than in the photorefractory blank-treated birds. Black bars represent T-treated photosensitive birds. Dark gray bars represent photosensitive control birds. Light gray bars represent T-treated photorefractory birds. White bars represent photorefractory control birds. Abbreviations: HVC used as its proper name; LMAN, lateral magnocellular nucleus of the anterior nidopallium; RA, robust nucleus of the arcopallium.
T-treated photosensitive females had significantly larger song nuclei volumes than photorefractory controls (HVC, z = 9.35, p < 0.001, d = 2.50) and photosensitive controls (HVC, z = −6.52, p < 0.001, d = 1.74). T-treated photorefractory females had significantly larger HVC volumes than photorefractory controls (z = −10.49, p < 0.001, d = 2.80) and photosensitive controls (z = −7.66, p < 0.001, d = 2.05). Photosensitive and photorefractory T-treated birds did not differ in HVC volumes (z = −1.13, p = 0.26). However, photosensitive controls had larger volumes of HVC compared to photorefractory controls (z = 2.83, p < 0.01, d = 0.76).
DISCUSSION
We exposed wild-caught adult female European starlings to two photoperiodic treatment regimens that resulted in the birds being in a photosensitive (i.e., pre-breeding or early breeding state) or photorefractory (i.e., non-breeding state) physiological state. We then implanted birds from both groups with silastic capsules filled with T or with blank blank implants. We found that T-treated photosensitive birds sang significantly more than either the T-treated photorefractory birds or blank treated controls. However, during week 1 of study, T-treated photosensitive females sang only slightly more than the other groups, and this difference was not significant. T-treated photorefractory singers were found to have a small but statistically significant increase in relative song repertoire size compared with T-treated photosensitive singers. There was no effect of photoperiod on the repertoire size of any individual category.
Plasma T was higher three weeks post-implantation in the T-treated birds compared with the controls. Furthermore, there was no statistical difference between T-treated photosensitive and T-treated photorefractory birds in plasma T, indicating that the T-filled capsules were equally effective in those groups. Beak color in starlings is a sensitive indicator of prolonged exposure to circulating androgens (or lack thereof; Ball and Wingfield, 1987) and we found a significant effect of treatment on beak color scores: T-treated photosensitive females had significantly higher beak scores than photorefractory females and blank-treated birds. However, some photorefractory T-treated birds did appear to be at the beginning phase of responding to elevated T with a change in beak color at the end of the study. It is possible that the beaks of female starlings in a photorefractory state take longer to respond to T than those in a photosensitive state.
Interestingly, we found a significant effect of treatment on measures of reproductive physiology. The ovaries of blank-treated control photosensitive females tended to be heavier than all other treatment conditions. Likewise, there was a significant effect of T-treatment on the weight of the oviduct; T-treated birds had smaller oviducts. This finding is not surprising, as it is well known that exogenous T can reduce the size of gonads and inhibit ovary function in females, perhaps via negative feedback mechanisms (e.g., Barraclough, 1961). There was no effect of photoperiod on the weight of the oviduct. This is most likely due to a floor effect because no bird was in full breeding condition and the oviduct was regressed to its smallest nonfunctioning state; birds were only in non-breeding and pre-breeding states.
We found that there was a significant effect of T-treatment on the volumes of song nuclei, including Area X, LMAN, HVC, and RA. T-treated birds had larger song nuclei volumes compared with blank-treated birds. Further, there was no effect of photoperiod on song nuclei volume in the T-treated birds, suggesting an equivalent effect of exogenous T on this measure regardless of reproductive state.
Intriguingly, though there was no difference in the volumes of Area X and LMAN in the blank-treated birds, photosensitive controls had larger HVC (and possibly RA) volumes compared with photorefractory controls. This finding is consistent with a growing literature based on studies of male songbirds indicating that song nuclei can start growing prior to gonads increasing in size and sex steroid hormone concentrations reaching their maxima (e.g., Tramontin et al., 2000; Caro et al., 2005; Hurley et al., 2008). The song nuclei HVC and RA in photosensitive female starlings seem to be able to grow, albeit with a relatively small effect size, even when the birds are on short days.
Overall, our findings are consistent with the notion that refractory states attenuate birds’ ability to respond to sex steroid hormones. As has been suggested for a variety of photoperiodic species, being in a ‘post’-breeding (i.e. non-breeding) state is not only associated with a decrease in gonadal size and secretion, but also with a changes in the signaling properties of target tissues thus affecting the ability to respond to T (Campbell, et al, 1978; Ellis and Turek, 1983). Most notably, being in a ‘post’-breeding state decreases T’s ability to increase singing rate.
Although we did not observe a significant effect of photoperiod on brain region volumes for the T-treated birds, we did observe significant effects of photoperiod on song behavior for T-treated photosensitive and photorefractory birds (e.g. song rate). Importantly, the Song system does not regulate all aspects of song behavior (Alward, et al, 2014; Ball, et al, 2008). Recent data have demonstrated that the rate or motivation to sing in response to steroids is controlled by the preoptic area, (POA) and when T is directly implanted into the POA the rate of singing increases similarly to that of systemic treatments (Alward, et al, 2014). Other evidence from male starlings indicates that the rate of singing is regulated by the POA (Riters and Ball, 1999; Ball, et al, 2002). It may well be that in females song rate is controlled in a similar manner, and thus the POA of photosensitive females is more sensitive to the actions of T thereby increasing their rate of singing. If this is the case it is not surprising that T might have a differential effect on song rate, as a function of reproductive state, despite T retaining its effectiveness in inducing increases in the volume of the song nuclei.
The volumetric changes observed in the song system in response to T-treatment, though not directly related to song rate, may nevertheless directly affect syllable stereotypy. Recent data from canaries shows that over a three-week treatment period, T-treated adult female photosensitive canaries show similar patterns of growth in the song system, and over this treatment period there is a significant linear increase in the stereotypy of individual syllable iterations (Madison, et al, 2014). It is likely that we observed the same phenomenon here: T-treated photosensitive female starlings sang with greater frequency and also experienced the general T-induced growth in volume. However, we cannot definitively say that photorefractory T-treated females are also increasing syllable stereotypy; given the low rate of singing, it is not likely that enough song is being produced to properly detect or measure the stereotopy of individual syllables.
As previously mentioned, T-treated photorefractory birds tending to have slightly larger overall repertoires compared to T-treated photosensitive birds. However, upon closer inspection the number of unique phrases in each of the four respective categories did not differ as an effect of photoperiod. Since the song nuclei responded to T in similar manner (i.e. similar volume changes) in photosensitive and photorefractory birds, the quality of songs produced should have been the same overall. We did not find that.
One possible explanation of the song repertoire data is that T-treated photorefractory females were in a different state of neuronal excitability and that they had either not engaged sensorimotor learning (or were in a much earlier stage of sensorimotor learning). Castrated and intact male starlings sing at similar rates and have songs of similar lengths throughout the year; however, intact males have a larger syllable repertoire size (Van Hout, et al, 2009). Exogenous administration of T in castrated male starlings can induce a turnover in song repertoire, with the addition and deletion of particular syllable phrase types and overall increase in the repertoire size (Van Hout, et al, 2009, 2012).
Exogenous T is also thought to recapitulate sensorimotor learning in adult female songbirds that sing in response to treatment resulting in a masculinization of song (Hausberger, et al, 1995). Sensorimotor learning is the dynamic modification of the acoustic features and arrangement of syllables/phrases that requires an active and intact song system. Photorefractoriness may inhibit T-induced state changes in neuronal excitability, essentially inhibiting the recapitulation of sensorimotor learning. This potentially means that the phrases incorporated into song may include vocal errors that are so different from other iterations that they could be mistakenly identified as unique.
T could be changing the state of the neurons in the Song system by changing the electrophysiological properties of the cells. This putative state change would result in a change in the probability of a neuron firing. Evidence from other songbird species supports this notion. In particular, there are data suggesting that T (and putatively its metabolites) play a role in the modulation of the electrophysiological properties of the song system across season (Park, et al, 2005; Meitzen, et al, 2007, 2009).
In Gambel’s white-crowned sparrows, slice preparations of nucleus RA from males in a breeding condition show a more than two-fold increase in spontaneous firing activity relative to slices prepared from males in a non-breeding condition (Park, et al, 2005). Further, this seasonal modulation of spontaneous firing activity is reliant on both estrogenic and androgenic signaling (Park, et al, 2005). A similar finding was shown in male song sparrows; male birds captured during the spring had a more than three-fold increase in spontaneous RA firing activity relative to males captured in the fall (Meitzen, et al, 2007).
Likewise, it was found that the RA-projecting neurons in the HVC of male Gambel’s white-crowned sparrows in a breeding condition (i.e. long days plus T) had increases in the membrane time constant, capacitance, and evoked and spontaneous firing rate(s) relative to non-breeding controls (i.e. short days no T; Meitzen, et al, 2009). Relatedly, in castrated male zebra finches, relative to intact controls, there was a suppression of spontaneous and evoked firing rates, membrane time constants, and membrane capacitance of RA-projecting neurons (Wong, et al, 2014).
Further, in adult female canaries it was found that exogenous administration of T increased the number of neuronal soma-somatic gap junctions in HVC relative to controls (Gahr and Garcia-Segura, 1996). Furthermore, androgens have been shown to hasten the developmental transition of N-methyl-D-aspartate– excitatory postsynaptic currents from slow to fast in the song nuclei of male zebra finches; this finding is not observed in non-Song areas (White, et al, 1999).
Across seasons, males of a variety of species including starlings add new syllables to their repertoire and ostensibly experience a recapitulation of at least some aspects of sensorimotor learning (Nottebohm and Nottebohm, 1978; Samson, 1978; Bernard, et al, 1996). Thus, the difference in phrase repertoire observed between T-treated photosensitive and photorefractory female starlings may not represent a difference in vocal repertoire/ability but rather a difference in the stage of T-induced vocal development. Taken all together, the effects of reproductive state on T-induced song behavior appear not to be related to differences in song nuclei volume, but to actions in other brain regions (in particular the POA) and to potential differences in the excitability of song system neurons.
However, we cannot exclude the possibility that some of the findings observed are due to estrogenic metabolites of T and not androgenic effects. In light of our current findings, it may be that photorefractoriness suppressed estrogenic facilitation of song behavior. Exogenous T can increase the amount of aromatase activity in the brain, meaning that local synthesis of estrogens may be necessary for specific features of T-induced song behavior (Fusani, et al, 2001, 2003). Though aromatase expression and activity tends to be sexually dimorphic, exogenous T may be up-regulating aromatase expression and activity in the photosensitive T-treated female starlings, thus facilitating the behavioral differences (Schumacher and Balthazart, 1986; Foidart, et al, 1994; Fusani, et al, 2001, 2003; Peterson, et al, 2005).
This hypothesis is supported by data from female canaries, which show that exogenous T administration can increase aromatase activity in the forebrain (Fusani, et al, 2001). Furthermore, blocking aromatase during T-treatment suppresses song masculinization; songs have aberrant duration and a greater proportion of the songs have abnormally slow-repeated syllable phrases (male-typical song has fast repeated syllable phrases; Fusani, et al, 2003). Though we did not test this explicitly, it is likely that in addition to being sensitive to the androgenic actions of T, photosensitive-females were also responsive to the actions of estrogenic metabolites (via T-induced up-regulation of brain aromatase). Conversely, photorefractory females were likely insensitive to estrogenic metabolites, though it is unclear whether photorefractoriness inhibits estrogen synthesis (via suppression of aromatase activity) or inhibits local estrogenic signaling in the brain.
CONCLUSION
We investigated whether T is equally effective in inducing changes in brain and behavior of adult female starlings in two different reproductive states (pre-breeding and ‘post’/non-breeding). The data demonstrate that reproductive state modulates the effects of T. Further, these data show that studies of female song production may shed new light on our understanding of neuroplasticity and neuroendocrine effects on vocal learning. By critically analyzing the changes that occur in response to T we can glean general principles about song behavior and its hormonal regulation. Elevating circulating levels of testosterone to male-like concentrations in females significantly changes song activity. Song becomes more male-like in structure and rates of singing increases. Changes in song behavior can happen independently of volume changes to song nuclei. However, T may not be equally effective as seasonal photoperiodic conditions change.
Manuscript Highlights.
Adult female starlings were put into a photosensitive or photorefractory state.
Birds were then treated with either testosterone (T) or empty control implants.
We studied the modulatory effect of reproductive state on T-induced singing.
T-treated photosensitive birds sang at a higher rate compared to all other groups.
T-treated birds had larger song control nuclei volumes than blank-treated controls.
Acknowledgments
This work was funded by NIH/NINDS RO1 35467 to GFB. Partial support is provided by SSTC PAI P7/17 from Belgian Science Policy Office to GFB. TJS was on an NSERC Predoctoral Fellowship (PGS-D 334570). We would like to thank Adam Troyer for providing a field site to capture starlings.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alward BA, Balthazart J, Ball GF. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proceedings of the National Academy of Sciences. 2013;110(48):19573–19578. doi: 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Wade J, Grisham W, Jacobs EC, Campagnoni AT. Sexual differentiation of the brain in songbirds. Developmental Neuroscience. 1996;18:124–136. doi: 10.1159/000111400. [DOI] [PubMed] [Google Scholar]
- Ball GF, Casto JM, Bernard DJ. Sex differences in the volume of avian song control nuclei: comparative studies and the issue of brain nucleus delineation. Psychoneuroendocrinology. 1994;19(5):485–504. doi: 10.1016/0306-4530(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: Multiple sites of action of sex steroid hormones. Frontiers in Neuroendocrinology. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Ball GF, Riters LV, MacDougall-Shackleton SA, Balthazart J. 2008: Sex differences in brain and behavior and the neuroendocrine control of the motivation to sing. In: Ziegler HP, Marler PR, editors. Neuroscience of Birdsong. Cambridge University Press; Cambridge: 2008. pp. 320–331. [Google Scholar]
- Ball GF, Nowicki S. Assessment of song quality in photorefractory and photosensitive song sparrows. Animal Behaviour. 1990;40:986–987. [Google Scholar]
- Ball GF, Wingfield JC. Changes in plasma levels of luteinizing hormone and sex steroid hormones in relation to multiple-broodedness and nest-site density in male starlings. Physiological Zoology. 1987;60:191–199. [Google Scholar]
- Barraclough C. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Goldsmith AR, Juss TS, Dawson A. The effects of nerve growth factor and anti-nerve growth factor antibody on the neuroendocrine reproductive system in the European starling Sturnus vulgaris. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1997;181:133–141. doi: 10.1007/s003590050100. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Van’t Hof TJ, Ball GF. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4674–4679. doi: 10.1073/pnas.96.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard DJ, Ball GF. Two histological markers reveal a similar photoperiodic difference in the volume of the high vocal center in male European starlings. Journal of Comparative Neurology. 1995;360:726–734. doi: 10.1002/cne.903600415. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Ball GF. Photoperiodic condition modulates the effects of testosterone on song control nuclei volumes in male European starlings. General and Comparative Endocrinology. 1997;105:276–283. doi: 10.1006/gcen.1996.6829. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Casto JM, Ball GF. Sexual dimorphism in the volume of song control nuclei in European starlings: Assessment by a Nissl stain and autoradiography for muscarinic cholinergic receptors. Journal of Comparative Neurology. 1993;334(4):559–570. doi: 10.1002/cne.903340405. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Eens M, Ball GF. Age- and behavior-related variation in volumes of song control nuclei in male European starlings. Journal of Neurobiology. 1996;30:329–339. doi: 10.1002/(SICI)1097-4695(199607)30:3<329::AID-NEU2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Wilson FE, Ball GF. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea) Brain Research. 1997;760:163–169. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- Bretz F, Hothorn T, Westfall P. Multiple comparisons using R. CRC Press; 2010. [Google Scholar]
- Bottjer SW, Alexander G. Localization of met-enkephalin and vasoactive intestinal polypeptide in the brains of male zebra finches. Brain Behavior and Evolution. 1995;45:153–177. doi: 10.1159/000113547. [DOI] [PubMed] [Google Scholar]
- Burger JW. On the relation of day-length to the phases of testicular involution and inactivity of the spermatogenetic cycle of the starling. Journal of Experimental Zoology. 1947;105:259–267. doi: 10.1002/jez.1401050207. [DOI] [PubMed] [Google Scholar]
- Campbell CS, Finkelstein JS, Turek FW. The interaction of photoperiod and testosterone on the development of copulatory behavior in castrated male hamsters. Physiology & Behavior. 1978;21:409–415. doi: 10.1016/0031-9384(78)90101-4. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird song: biological themes and variations. Bird song: biological themes and variations. 1995;i–viii:1–248. [Google Scholar]
- Cornil CA, Stevenson TJ, Ball GF. Are rapid changes in gonadal testosterone release involved in the fast modulation of brain estrogen effects? General and Comparative Endocrinology. 2009;163:298–305. doi: 10.1016/j.ygcen.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. Changes in plasma thyroxine concentrations in male and female starlings (Sturnus vulgaris) during a photo-induced gonadal cycle. General and Comparative Endocrinology. 1984;56:193–197. doi: 10.1016/0016-6480(84)90030-3. [DOI] [PubMed] [Google Scholar]
- Dawson A. Plasma-luteinizing hormone and prolactin during circannual rhythms of gonadal maturation and molt in male and female European starlings. Journal of Biological Rhythms. 1997;12:371–377. doi: 10.1177/074873049701200409. [DOI] [PubMed] [Google Scholar]
- Dawson A. The effects of a single long photoperiod on induction and dissipation of reproductive photorefractoriness in European starlings. General and Comparative Endocrinology. 2001;121:316–324. doi: 10.1006/gcen.2001.7601. [DOI] [PubMed] [Google Scholar]
- Dawson A, Follett BK, Goldsmith AR, Nicholls TJ. Hypothalamic gonadotrophin-releasing hormone and pituitary and plasma FSH and prolactin during photostimulation and photorefractoriness in intact and thyroidectomized starlings (Sturnus vulgaris) Journal of Steroid Biochemistry and Molecular Biology. 1984;20:1538–1538. doi: 10.1677/joe.0.1050071. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith AR. Plasma prolactin and gonadotrophins during gonadal development and the onset of photorefractoriness in male and female starlings (Sturnus vulgaris) on artificial photoperiods. Journal of Endocrinology. 1983;97:253–260. doi: 10.1677/joe.0.0970253. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith AR, Nicholls TJ. Development photorefractoriness intact castrated male starlings (Sturnus vulgaris) exposed different periods long-day lengths. Physiological Zoology. 1985;58:253–261. [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. Journal of Biological Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- De Ridder E, Pinxten R, Mees V, Eens M. Short- and long-term effects of male-like concentrations of testosterone on female European starlings (Sturnus vulgaris) Auk. 2002;119:487–497. [Google Scholar]
- Ding L, Perkel DJ. Dopamine modulates excitability of spiny neurons in the avian basal ganglia. Journal of Neuroscience. 2002;22:5210–5218. doi: 10.1523/JNEUROSCI.22-12-05210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eens M. Understanding the complex song of the European starling: An integrated ethological approach. Advances in the Study of Behavior. 1997;26:355–434. [Google Scholar]
- Ellis GB, Turek FW. Testosterone and photoperiod interact to regulate locomotor activity in male hamsters. Hormones and Behavior. 1983;17:66–75. doi: 10.1016/0018-506x(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Falk H, Gwinner E. Timing of photorefractoriness in the European starling: significance of photoperiod early and late in the reproductive cycle. Biology of Reproduction. 1988;39:1004–1008. doi: 10.1095/biolreprod39.5.1004. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Baum FR, Campbell CS. Entrainment of the female hamster to reversed photoperiod: role of the pineal. Physiology & Behavior. 1978;21:105–111. doi: 10.1016/0031-9384(78)90283-4. [DOI] [PubMed] [Google Scholar]
- Foidart A, De Clerck A, Harada N, Balthazart J. Aromatase-immunoreactive cells in the quail brain: effects of testosterone and sex dimorphism. Physiology & behavior. 1994;55(3):453–464. doi: 10.1016/0031-9384(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Fusani L, Hutchison JB, Gahr M. Testosterone regulates the activity and expression of aromatase in the canary neostriatum. Journal of neurobiology. 2001;49(1):1–8. doi: 10.1002/neu.1061. [DOI] [PubMed] [Google Scholar]
- Fusani L, Metzdorf R, Hutchison JB, Gahr M. Aromatase inhibition affects testosterone-induced masculinization of song and the neural song system in female canaries. Journal of neurobiology. 2003;54(2):370–379. doi: 10.1002/neu.10141. [DOI] [PubMed] [Google Scholar]
- Gahr M, GarciaSegura LM. Testosterone-dependent increase of gap-junctions in HVC neurons of adult female canaries. Brain Research. 1996;712:69–73. doi: 10.1016/0006-8993(95)01448-9. [DOI] [PubMed] [Google Scholar]
- Goldsmith AR, Nicholls TJ. Recovery of photosensitivity in photorefractory starlings is not prevented by testosterone treatment. General and Comparative Endocrinology. 1984;56:210–217. doi: 10.1016/0016-6480(84)90033-9. [DOI] [PubMed] [Google Scholar]
- Harding CF. Hormonal modulation of singing - Hormonal modulation of the songbird brain and singing behavior. In: Zeigler HPMP, editor. Behavioral Neurobiology of Birdsong. 2004. pp. 524–539. [DOI] [PubMed] [Google Scholar]
- Hausberger M, Henry L, Richard MA. Testosterone-induced Singing in Female European Starlings (Sturnus vulgaris) Ethology. 1995;99:193–208. [Google Scholar]
- Hicks LE. Individual and Sexual Variations in the European Starling. Bird-Banding. 1934;5:103–118. [Google Scholar]
- Kessel B. A Study of the Breeding Biology of the European Starling (Sturnus vulgaris L.) in North America. American Midland Naturalist. 1957;58:257–331. [Google Scholar]
- Kroodsma DE. The singing life of birds: the art and science of listening to birdsong. Houghton Mifflin; Boston: 2005. [Google Scholar]
- MacDougall-Shackleton SA, Stevenson TJ, Watts HE, Pereyra ME, Hahn TP. The evolution of photoperiod response systems and seasonal GnRH plasticity in birds. Integrative and Comparative Biology. 2009;49:580–589. doi: 10.1093/icb/icp048. [DOI] [PubMed] [Google Scholar]
- Madison FN*, Rouse ML*, Balthazart J, Ball GF. Reversing song behavior phenotype: Testosterone driven induction of singing and measures of song quality in adult male and female canaries (Serinus canaria) General and Comparative Endocrinology. 2014 Sep 26; doi: 10.1016/j.ygcen.2014.09.008. pii: S0016-6480(14)00365-7. [Epub ahead of print] Shared first authorship. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Perkel DJ, Brenowitz EA. Seasonal changes in intrinsic electrophysiological activity of song control neurons in wild song sparrows. Journal of Comparative Physiology A. 2007;193(6):677–683. doi: 10.1007/s00359-007-0222-1. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Weaver AL, Brenowitz EA, Perkel DJ. Plastic and Stable Electrophysiological Properties of Adult Avian Forebrain Song-Control Neurons across Changing Breeding Conditions. Journal of Neuroscience. 2009;29:6558–6567. doi: 10.1523/JNEUROSCI.5571-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; 2011. [PubMed] [Google Scholar]
- Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiological Reviews. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Research. 1980;189:429–436. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME. Relationship between song repertoire and age in canary, Serinus-canarius. Zeitschrift Fur Tierpsychologie. 1978;46:298–305. [Google Scholar]
- Nowicki S, Ball GF. Testosterone induction of song in photosensitive and photorefractory male sparrows. Hormones and Behavior. 1989;23:514–525. doi: 10.1016/0018-506x(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Park KH, Meitzen J, Moore IT, Brenowitz EA, Perkel DJ. Seasonal-like plasticity of spontaneous firing rate in a songbird pre-motor nucleus. Journal of neurobiology. 2005;64(2):181–191. doi: 10.1002/neu.20145. [DOI] [PubMed] [Google Scholar]
- Pavlova D, Pinxten R, Eens M. Female song in European Starlings: Sex differences, complexity, and composition. Condor. 2005;107:559–569. [Google Scholar]
- Pavlova DZ, Pinxten R, Darras VM, Eens M. Effects of nestboxes and males on female song activity in the European starling: an experimental study. Behaviour. 2007a;144:1255–1271. [Google Scholar]
- Pavlova DZ, Pinxten R, Eens M. Seasonal singing patterns and individual consistency in song activity in female European starlings (Sturnus vulgaris) Behaviour. 2007b;144:663–680. [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Royal Society B: Biological Sciences. 2005;272(1576):2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-118. 2014 http://CRAN.R-project.org/package=nlme.
- Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of DHEA metabolism in the male and female songbird brain. Journal of neurochemistry. 2008;104(1):244–253. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder E, Pinxten R, Mees V, Eens M. Short- and long-term effects of male-like concentrations of testosterone on female European starlings (Sturnus vulgaris) Auk. 2002;119:487–497. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org/ [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Hormones and Behavior. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Hormones and Behavior. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Samson FB. Vocalizations of Cassin’s Finch in Northern Utah. The Condor. 1978;80:203–210. [Google Scholar]
- Schlinger BA, Brenowitz EA. Neural and hormonal control of birdsong. Hormones, brain and behavior. 2002;2:799–839. [Google Scholar]
- Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain research. 1986;370(2):285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–U74. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC. Seasonal changes in the size of the avian song control nucleus HVC defined by multiple histological markers. Journal of Comparative Neurology. 1997;381:253–261. doi: 10.1002/(sici)1096-9861(19970512)381:3<253::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC, Baptista LF. Seasonal changes in song nuclei and song behavior in Gambel’s white-crowned sparrows. Journal of neurobiology. 1995;28:114–125. doi: 10.1002/neu.480280110. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Ball GF. Anatomical Localization of the Effects of Reproductive State, Castration, and Social Milieu on Cells Immunoreactive for Gonadotropin-Releasing Hormone-I in Male European Starlings (Sturnus vulgaris) Journal of Comparative Neurology. 2009;517:146–155. doi: 10.1002/cne.22159. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Ball GF. Photoperiodic Differences in a Forebrain Nucleus Involved in Vocal Plasticity: Enkephalin Immunoreactivity Reveals Volumetric Variation in Song Nucleus IMAN but Not NIf in Male European Starlings (Sturnus vulgaris) Developmental Neurobiology. 2010;70:751–763. doi: 10.1002/dneu.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Bentley GE, Ubuka T, Arckens L, Hampson E, MacDougall-Shackleton SA. Effects of social cues on GnRH-I, GnRH-II, and reproductive physiology in female house sparrows (Passer domesticus) General and Comparative Endocrinology. 2008;156:385–394. doi: 10.1016/j.ygcen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Hahn TP, Ball GF. Variation in Gonadotrophin-Releasing Hormone-1 Gene Expression in the Preoptic Area Predicts Transitions in Seasonal Reproductive State. Journal of Neuroendocrinology. 2012;24:267–274. doi: 10.1111/j.1365-2826.2011.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: How a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends in Neurosciences. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Turek FW, Loseeolson SH, Ellis GB. Pinealectomy and lesions of the suprachiasmatic nucleus affect the castration response in hamsters exposed to short photoperiods. Neuroendocrinology. 1983;36:335–339. doi: 10.1159/000123477. [DOI] [PubMed] [Google Scholar]
- Van Hout AJM, Eens M, Balthazart J, Pinxten R. Complex modulation of singing behavior by testosterone in an open-ended learner, the European Starling. Hormones and behavior. 2009;56(5):564–573. doi: 10.1016/j.yhbeh.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Van Hout AJM, Pinxten R, Darras VM, Eens M. Testosterone increases repertoire size in an open-ended learner: An experimental study using adult male European starlings (Sturnus vulgaris) Hormones and behavior. 2012;62(5):563–568. doi: 10.1016/j.yhbeh.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Behavioral Neurobiology of Birdsong. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Wang S, Liao C, Li F, Liu S, Meng W, Li D. Castration modulates singing patterns and electrophysiological properties of RA projection neurons in adult male zebra finches. PeerJ. 2014;2:e352. doi: 10.7717/peerj.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall PH. Multiple testing of general contrasts using logical constraints and correlations. Journal of the American Statistical Association. 1997;92(437):299–306. [Google Scholar]
- White SA, Livingston FS, Mooney R. Androgens modulate NMDA receptor-mediated EPSCs in the zebra finch song system. Journal of Neurophysiology. 1999;82:2221–2234. doi: 10.1152/jn.1999.82.5.2221. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- Wydoski RS. Seasonal changes in the color of Starling bills. The Auk. 1964;81:542–550. [Google Scholar]
- Zakon HH. The effects of steroid hormones on electrical activity of excitable cells. Trends in Neurosciences. 1998;21:202–207. doi: 10.1016/s0166-2236(97)01209-5. [DOI] [PubMed] [Google Scholar]