Abstract
The current management of intrauterine growth restriction (IUGR) being empirical and aimed at selecting a safe time for delivery. Acknowledging the beneficial effects of l-arginine on endothelial vasculature the present study was designed to evaluate efficacy of l-arginine on bioavailability of nitric oxide (NO) with respect to fetal outcome. With l-arginine supplementation, mean NO levels were significantly increased and a significant mild reduction in systolic/end-diastolic velocity ratio (S/D ratio) was observed on doppler blood flow study, also neonatal outcome improved and incidences of complications were lowered. A deficiency in NO may play an important role in the causation of asymmetric fetal growth restriction. l-Arginine can be used to increase maternal NO levels, enhancing birth weight and decreasing neonatal morbidity. The ideal candidate for arginine therapy according to our study would be IUGR cases with S/D ratio less than 4.96 ± 0.49 and NO levels below 33 μmol/L with minimum of 3 weeks duration of arginine supplementation.
Keywords: IUGR, NO, l-Arginine, Fetal outcome, Birth weight
Introduction
Intrauterine growth restriction (IUGR) is one of the major complications of pregnancy affecting 3–10 % of all gestations [1], It is associated with significant increase in morbidity and mortality in perinatal period and infancy. Out of 22 million small for gestational age infants in the world 21 million belongs to developing countries and India’s share is substantial, around 7–10 million constituting 30 % of live births [2]. There is progressive recognition that adverse consequences of growth deprivation in utero extend beyond early years into later life [3, 4].
Fetal growth restriction can be divided into two types, symmetrical when the fetus is small but well proportioned and asymmetrical, when the fetus’s abdominal growth is restricted. Most of these are linked to placental insufficiency [5]. The human fetoplacental circulation exhibits a low vascular resistance and lacks autonomic innervations. Thus circulating and locally released vasoactive molecules are likely to be involved in control of fetoplacental hemodynamics [6]. The release of local vasoactive molecules such as nitric oxide (NO), from endothelium maintains appropriate placental blood flow, fetal nutrition and oxygenation.
Nitric oxide (NO) is constitutively produced in human vein umbilical cells and platelets from conversion of l-arginine to l-citrulline by endothelial NO synthase [7]. It causes cyclic guanosine monophosphate (cGMP) mediated vascular relaxation and inhibits platelet adhesion [8].
To date, many therapeutic approaches have been used to improve fetal condition. None of the approaches have been of value in a consistent manner. The current management being empirical and primarily aimed at selecting a safe time for delivery [4, 9].
l-Arginine, a nutritionally essential amino acid for the fetus [10], is a precursor for synthesis of NO [11]. Consequently, it may play a critical role in fetal nutrition and oxygenation resulting in improvement of IUGR pregnancy, enhancing birth weight and decreasing neonatal morbidity and mortality [12].
Acknowledging the beneficial effects of l-arginine on endothelial vasculature the present study was designed to evaluate the role of l-arginine in women with IUGR and its correlation with neonatal outcome.
Materials and Methods
Study Design
This hospital based randomised prospective study included 120 antenatal women in the age group of 20–30 years, selected from antenatal clinics and wards of a tertiary care hospital in New Delhi, India. The women were divided into two groups. Group I consisted of 60 pregnant women with fetal growth appropriate for gestational age (AGA). Group II consisted of 60 pregnant women with clinically and sonographically diagnosed asymmetrical IUGR. IUGR was defined as an estimated fetal weight below 10th percentile for gestational age.
Group II was divided into 2 subgroups. Group II A: women receiving l-arginine therapy (study group), Group II B: women not receiving l-arginine therapy (control group). Group IIA were supplemented with oral l-arginine 3 g once daily for 21 days and IIB with placebo. Group assignments were blinded to the patients, doctors and nursing personnel. Group II A and II B were matched for age, parity and period of gestation.
Women with symmetrical IUGR, period of gestation less than 28 wks, toxoplasmosis, rubella, cytomegalovirus, herpes, syphilis and others (TORCH infections), major medical illness like diabetes mellitus, chronic renal failure, heart disease, severe anemia were excluded. Pregnant women with pre-eclampsia, transverse lie, fetal malformation, smoking and history of allergy to food products were also excluded.
Women in both the groups were primigravidae and the gestational age during sampling was between 30 and 40 weeks. All patients were monitored according to standard procedure. Fetal wellbeing was assessed (clinically and sonographically) before and after the therapy in both study and control group. The decision about the time and mode of delivery in each case depended on the conditions of the mother and fetus.
The protocol for the use of l-arginine was approved by Institutional Ethical Committee. A detailed informed consent was taken from all the participants in simple language at the time of enrolment. As far as fetal well-being was observed spontaneous delivery was recommended. In case of fetal distress a caesarean section was performed. After delivery, neonatal outcome was recorded in terms of mode of delivery, birth weight, neonatal complications, appearance, pulse, grimace, activity and respiration (apgar score), resuscitative measures and neonatal intensive care unit(NICU) admission in both groups of IUGR.
Colour Doppler Blood Flow Studies
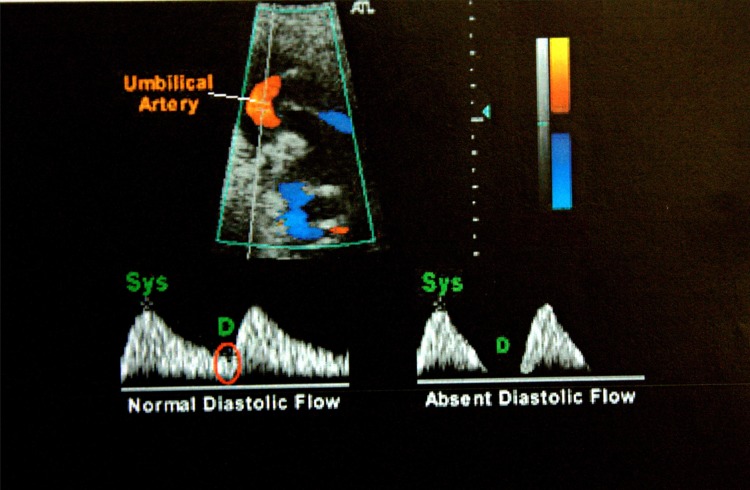
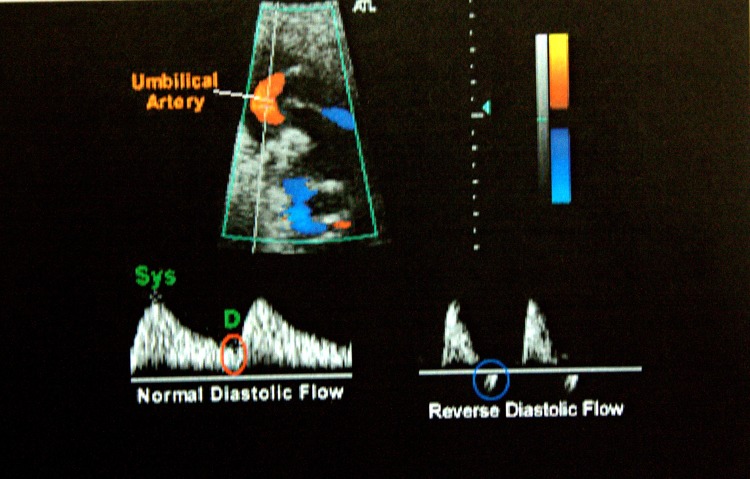
Colour Doppler is an extension of pulsed doppler, in that a colour sign is assigned to the direction of flow. By convention red flows towards the probe and blue flows away from it. The doppler machine used for this study was ATL-HDI5000, SIEMENS. After confirming the asymmetric IUGR sonographically, umbilical artery doppler blood flow peak systolic/end-diastolic velocity ratio (S/D ratio) was done to document abnormality in uteroplacental flow. This vessel normally has forward flow throughout the cardiac cycle and the amount of flow during diastole increases as gestation advances. S/D ratio normally decreases as pregnancy progresses. A high S/D ratio denotes resistance to blood flow to fetus (Fig. 1). In extreme cases of growth restriction end diastolic flow was absent or reversed (Fig. 2). The umbilical artery peak S/D ratio was considered abnormal if it was above the 95th percentile for the gestational age.
Fig. 1.
Umbilical artery flow velocimetry showing normal and absent diastolic flow
Fig. 2.
Umbilical artery flow velocimetry showing normal and reverse end diastolic flow
Sample Collection
Three milliliters of venous blood was collected in plain vials and serum was extracted by centrifugation for estimation of nitric oxide before and after 3 weeks of l-arginine supplementation. Samples were stored at −20 °C until batch was analyzed.
Estimation of Nitric Oxide
Nitric oxide is difficult to measure. In an oxygenated solution, it decomposes to form nitrite and nitrate. Nitrite, a stable end product was estimated as an index of NO using Greiss reaction by method of Mathew et al. [13].
Griess reaction involves the formation of a chromophore during the reaction of nitrite with sulfanilamide and heterocyclic amine of N-(1-napthyl) ethlenediamine (Griess reagent) under conditions of low pH to form a magenta coloured compound, which is measured sphectrophotometrically. This procedure was supplemented with deproteinization and reduction of nitrates to nitrites in the presence of nicotinamide adenine dinucleotide phosphate (NADPH) sensitive reductase, for measurement of NO levels in serum. Deproteinization of samples was done with methanol, which does not influence the sensitivity of detection of NO metabolites.
Statistical Analysis
All data are expressed as mean ± standard deviation (SD) and statistical analysis was conducted using SPSS statistical package, version 16.0 (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered as significant. The student t test was used for normally distributed data.
Results
The characteristics like mean maternal age, parity, gestational age and basal metabolic index (BMI) (Table 1) of the two groups of patients (IUGR with l-arginine vs IUGR with routine therapy) were similar upon randomization. Previous obstetrics history of abortion, IUGR, stillbirth, intrauterine death (IUD) was comparable in both the groups of IUGR.
Table 1.
Characteristics of groups
| Characteristics | Group I (Mean ± SD) | Group II A (Mean ± SD) | Group II B (Mean ± SD) | P value |
|---|---|---|---|---|
| Age | 25.4 ± 3.6 | 25.47 ± 4.3 | 25.17 ± 4.3 | >0.05 |
| Parity | 2.20 ± 1.27 | 24 ± 1.27 | 2.03 ± 1.03 | >0.05 |
| POG | 33.28 ± 1.91 | 33.13 ± 2.19 | 33.0 ± 2.3 | >0.05 |
| BMI | 20.8 ± 3.72 | 20.6 ± 4.74 | >0.05 |
Our study showed significantly lower levels of serum NO levels in Group I (IUGR group) compared to Group II (AGA group), supporting reduced NO availability in IUGR pregnancies. Before treatment maternal serum NO level in Group II A and II B was 26.13 ± 4.64 and 25.37 ± 4.6 µmol/L respectively which was significantly lower than group I (42.48 ± 5.03 µmol/L) (Table 2).
Table 2.
Comparison of serum NO (µMol/L) level between AGA and IUGR group
| AGA (group I) (Mean ± SD) | IUGR (group II) (Mean ± SD) | P value | |
|---|---|---|---|
| 42.48 ± 5.03 | Group II A | 29.70 ± 6.2 | <0.05 |
| Group II B | 25.2 ± 5.71 | <0.05 | |
After treatment with l-arginine, mean serum NO levels (29.0 ± 6.2 µmol/L) in group IIA was significantly higher than that in II B at termination of pregnancy. The mean NO level in group II B at the initiation of study was 25.37 ± 4.6 µmol/L which remain unchanged (25.2 ± 5.71 µmol/L) at termination of pregnancy (Table 3).
Table 3.
Comparison of serum NO levels between study and control group
| Total NO level (µMol/L) (Mean ± SD) | |||||
|---|---|---|---|---|---|
| Mean NO level (group II A) n = 30 | Mean NO level (group II B) n = 30 | ||||
| Pre-treatment | Post treatment | P value | Pre-treatment | Post treatment | P value |
| 26.13 ± 4.64 | 29.70 ± 6.2 | 0.00 | 25.37 ± 4.68 | 25.2 ± 5.71 | >0.05 |
A significant mild reduction in S/D ratio was observed in cases with arginine therapy. In comparison, there was an increase in S/D ratio, absence or reversal of end diastolic blood flow after routine therapy (Figs. 1, 2). The mean pretreatment umbilical artery S/D ratio in group II A was 4.96 ± 0.49 and the mean post treatment S/D ratio was 4.67 ± 0.63, this difference was observed to be statistically significant (0.001). The mean initial umbilical artery S/D ratio in group II B at enrolment was 4.5 ± 0.40 and at termination was 4.82 ± 0.5, the difference was found to be insignificant (Table 4).
Table 4.
Comparison of umblical artery S/D ratio between study and control group
| Mean umblical artery S/D ratio(Mean ± SD) | |||||
|---|---|---|---|---|---|
| Group II A | Group II B | ||||
| Pre-treatment | Post treatment | P value | Pre-treatment | Post treatment | P value |
| 4.96 ± 0.49 | 4.67 ± 0.63 | 0.001 | 4.50 ± 0.40 | 4.82 ± 0.52 | >0.05 |
Percentage of live birth was found to be more in arginine therapy group (88.66 % case in group II A vs 80.0 % in group II B). 13.33 and 20 % of the cases in group II A and II B had intrauterine death respectively. The mean birth weight of the neonates in group II A was 1.90 ± 0.3 kg compared to 1.77 ± 0.53 kg in II B (P > 0.05). Gestational age at delivery was found to be more in arginine therapy group (35.68 ± 2.80 in group II A vs 34.0 ± 3.50 in group II B). There was a higher incidence of lower section cesaerean section (LSCS) in group II B (22.05 % case in group II A vs 38.0 % in group II B). The difference between the Caesarean section rates in the two groups was found to be statistically significant (P < 0.05). Percentage of vaginal delivery was found to be higher in Group II A pregnant females (80 % case in group II A vs 70 % in group II B) (Table 5).
Table 5.
Neonatal characteristics in study and control groups
| Neonatal characteristics | Group II A | Group II B | P value | ||
|---|---|---|---|---|---|
| N = 30 | % | N = 30 | % | ||
| Live births | 26 | 86.66 | 24 | 80 | >0.05 |
| IUD | 4 | 13.33 | 6 | 20 | >0.05 |
| Mean birth weight (kg) | 30 | 1.90 ± 0.38 | 30 | 1.77 ± 0.53 | >0.05 |
| Gestational age @ delivery | 30 | 35.68 ± 2.80 | 30 | 34.0 ± 3.50 | >0.05 |
| LSCS rate | 6 | 22.05 | 9 | 38.0 | <0.05 |
| Vaginal delivery | 24 | 80 | 21 | 70 | >0.05 |
Postnatal assessment showed that apgar score at 1st and 5th minute was higher in the l-arginine group. However, the difference between the apgar scores in neonates of both the groups was statistically insignificant. 46.15 % of neonates in group II A and 58.33 % in group II B had respiratory distress after birth. The frequency of hypoglycemia in group II A and II B were 23.0 and 37.0 % respectively. Hypothermia was seen in 15.3 % of neonates of group II A and 2 % of neonates of group II B. 7.69 and 16.66 % of neonates in group II A and II B respectively were found to have elevated serum bilirubin levels. The incidence of ventricular haemorrhage necrotizing enterocolitis and exchange transfusion was 3.84 % in neonates of group II A compared to 12.5 % in group II B thus suggesting a lower incidence of these complications in Arginine therapy group (Table 6).
Table 6.
Neonatal outcome in study and control group
| Neonatal outcome | Characteristics | Group II A | Group II B | P Value | ||
|---|---|---|---|---|---|---|
| N = 26 | % | N = 24 | % | |||
| At 1 min | ||||||
| APGAR SCORE | <5 | 5 | 19.23 | 6 | 25 | >0.05 |
| 5-7 | 15 | 57.69 | 14 | 5.30 | >0.05 | |
| >7 | 6 | 23.07 | 4 | 16.6 | >0.05 | |
| At 5 min | ||||||
| <5 | 0 | 0 | 2 | 8.33 | >0.05 | |
| 5-7 | 10 | 3.46 | 12 | 50 | >0.05 | |
| >7 | 16 | 61.53 | 10 | 41.60 | >0.05 | |
| Complications | Respiratory distress | 12 | 46.15 | 14 | 58.33 | >0.05 |
| Hypoglycemia | 6 | 23.0 | 9 | 3.50 | >0.05 | |
| Hypothermia | 4 | 15.38 | 6 | 25 | >0.05 | |
| Hyperbilirubinemia | 2 | 7.69 | 4 | 16.66 | >0.05 | |
| VHa, NECb, ETc | 1 | 3.40 | 3 | 12.50 | >0.05 | |
| Resuscitations | Routine | 22 | 84.61 | 17 | 70.83 | >0.05 |
| Bag and mask ventilation | 5 | 19.23 | 14 | 58.33 | >0.05 | |
| Endotracheal intubation | 2 | 7.69 | 3 | 12.5 | >0.05 | |
| PPVd | 4 | 15.38 | 4 | 16.66 | >0.05 | |
| NICUe admission | 8 | 30.76 | 8 | 33.33 | >0.05 | |
| Duration of stay (days) | 9.75 ± 6.0 | 11.30 ± 8.50 | >0.05 | |||
| Neonatal mortality | 4 | 15.3 | 3 | 12.50 | >0.05 | |
aVentricular haemorrhage
bNecrotizing enterocolitis
cExchange transfusion
dPositive pressure ventilation
eNeonatal intensive care unit
In control group a larger number of neonates required resuscitation and ventilation as compared to neonates in l-arginine group. 30.76 and 33.33 % of the neonates of group IIA and II B required NICU admission and had a hospital stay of 9.75 ± 6.0 and 11.38 ± 8.5 days respectively, difference being statistically insignificant. Lower incidence of neonatal death was reported in cases with IUGR treated with arginine (15.3 % of group II A and 12.5 % of II B) (Table 6).
Discussion
Uteroplacental hemodynamics is important in pathophysiology of intra uterine growth retardation. Vascular tone is an essential target of the paracrine and endocrine regulations during pregnancy. NO synthesized in placental vasculature plays a crucial role in maintaining a low fetoplacental perfusion. Studies have shown varying levels of serum NO in third trimester of pregnancy [14, 15]. In our study, as the pregnancy advanced there was a significant increase in serum NO levels in AGA cases. No such increase was seen in IUGR cases when they received routine therapy alone. However a small but significant increase was observed in cases receiving arginine, in consistence with the findings of Neri et al. [16].
The rise in maternal plasma l-arginine concentration increases the net uptake of uteroplacental and fetal arginine which in turn increases insulin secretion and NO production, thus playing a significant role in fetal growth [17]. l-arginine is an insulinotropic amino acid. Vasodilator actions of insulin are mediated by activation of endothelial nitric-oxide synthase (eNOS) and subsequent production of NO. Phosphatidylinositol 3-kinase and protein kinase B (Akt) play important roles in insulin-signaling pathways leading to production of NO in vascular endothelium [18]. It also increases growth hormone levels [16] thereby promoting fetal growth. l-arginine, by increasing NO synthesis, also causes vasodilatation and inhibition of platelet aggregation thereby improving the fetomaternal circulation [16]. In our study, as the period of gestation advanced there was an increase in maternal serum NO levels in subjects receiving arginine (group II A) showing small but significant increase in NO levels compare to routine therapy group (group II B).
Influence of arginine therapy on doppler indices in the umbilical artery was found to be favorable. In the study group the post treatment umbilical artery S/D ratio showed a statistically significant decrease. These results were comparable to a study [19] where a significant decrease of non placental side resistance was seen in IUGR cases receiving arginine. The effect was specific for pregnancies complicated by IUGR associated with unilateral increased resistance in uteroplacental perfusion. In another study [20], it was reported that an intravenous infusion of a NO donor induced a relaxation of uterine artery in second trimester patients with abnormal uterine flow velocity waveforms.
In the study group umbilical artery S/D ratio was found to be decreased which was found to be significant. In the control group when arginine was not given, S/D ratio was found to be increased or end diastolic flow became absent/reversed at the termination of pregnancy (Figs. 1, 2). Four cases had absent end-diastolic flow and nine cases had reverse end diastolic flow when umbilical artery S/D ratio ranged between 5.01–6.0, irrespective of period of gestation (Figs. 1, 2).
A significant mild reduction in S/D ratio was observed with an initial S/D ratio of 4–5 in cases with arginine therapy at all period of gestation. With an initial S/D ratio of 5–6, reduction was observed in cases with arginine therapy in early third trimester only. No reduction was observed in cases with an initial ratio of >6, and in late third trimester despite arginine therapy.
In the present study we found that oral administration of l-arginine was found to improve pregnancy outcome. The mean birth weight of newborns, percentage of live birth, percentage of vaginal delivery and gestational age at delivery was found to be higher in l-arginine group (II A) compared to routine therapy (II B) group, though the difference was not found to be significant. In regards to our results of pregnancy outcome, similar effects of l-arginine has been found by Xiao group [21].
There was a higher incidence of IUD and LSCS in Routine therapy group (II B) compared to arginine (II A) therapy group. The difference between the caesarean section rates in the two groups was statistically significant (P < 0.05). The indication of LSCS in most of the cases was fetal distress followed by non reactive non stress test (NST).
Postnatal assessment showed that apgar score at 1st and 5th min was higher in the l-arginine group. However, the difference between the apgar scores in neonates of both the groups was statistically insignificant. The incidence of complications after birth like intracranial haemorrhage, respiratory distress syndrome, hypoglycaemia, hypothermia, hyperbilirubinemia, ventricular haemorrhage, necrotizing enterocolitis and exchange transfusion were found to be lower in arginine therapy group. Percentage of neonates delivered to routine therapy group mothers requiring resuscitation in terms of routine resuscitation, bag and mask ventilation, endotracheal intubation and positive pressure ventilation was more compared to neonates delivered to mothers who received arginine therapy. The admission of neonates to NICU was also found to be lower in the l-arginine therapy group. Duration of stay at hospital and neonatal mortality was lower in arginine therapy group compared to routine therapy group though the difference was not found to be significant. Similar results were presented by Lampariello et al. [22]. However, in another study Winer et al. [23] reported no significance difference among the group supplemented with arginine compare to control group.
Thus, conclusions drawn from this randomized prospective study are that after oral administration of arginine, when Umbilical Artery S/D ratio decreased and Serum NO Levels increased, there was prolongation of pregnancy leading to increase in gestational age of the fetus thereby facilitating delivery of a baby with higher birth weight, good apgar score and a better perinatal outcome. However when S/D ratio increased, and Serum NO levels did not increase significantly, it was associated with a poor neonatal outcome with increased complications requiring resuscitation, prolonged NICU stay and perinatal mortality.
Hence, during antenatal care all pregnant women and high risk cases should be screened for early detection of IUGR. Besides routine fetal surveillance in IUGR umbilical artery S/D ratio evaluation by doppler blood flow velocitometry is helpful in detecting increased resistance and monitoring of a compromised fetus. These IUGR cases should be given oral l-arginine, a nitric oxide donor, to reduce resistance of feto-placental circulation. As no reduction was observed in cases with an initial ratio of >6, and in late third trimester despite arginine therapy, the ideal candidate for arginine therapy according to our study would be IUGR cases with S/D ratio less than 6 and minimum of 3 weeks duration of arginine administration.
References
- 1.Divon MY, Hsu HW. Maternal and fetal blood flow velocity waveforms in intrauterine growth retardation. Clin Obstet Gynecol. 1992;35:156–171. doi: 10.1097/00003081-199203000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Biswas R, Dasgupta A, Sinha RN, Chauduhri RN. An epidemiological study of low birth weight newborns in the district of Puruliya, West Bengal. Indian J Public Health. 2008;52(2):65–71. [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA. The consequences of being born small: an adaptive perspective. Horm Res. 2006;65:5–14. doi: 10.1159/000091500. [DOI] [PubMed] [Google Scholar]
- 4.Mari G, Hanif F. Intrauterine growth restriction: how to manage and when to deliver. Clin Obstet Gynecol. 2007;50:497–509. doi: 10.1097/GRF.0b013e31804c96a9. [DOI] [PubMed] [Google Scholar]
- 5.Sankaran S, Kyle PM. Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):765–777. doi: 10.1016/j.bpobgyn.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Kusinski LC, Stanley JL, Dilworth MR, Hirt CJ, Andersson IJ, Renshall LJ, et al. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am J Physiol Regul Integr Comp Physiol. 2012;303(1):86–93. doi: 10.1152/ajpregu.00600.2011. [DOI] [PubMed] [Google Scholar]
- 7.Lyall F, Greer IA, Young A, Myatt L. Nitric oxide concentrations are increased in fetoplacental circulation in intrauterine growth restriction. Placenta. 1996;17:165–168. doi: 10.1016/S0143-4004(96)80009-9. [DOI] [PubMed] [Google Scholar]
- 8.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obs Gynecol. 1999;180:499–506. doi: 10.1016/S0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 9.Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496. doi: 10.1016/S0029-7844(01)01780-X. [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Bazer FW, Davis TA, Kim SW, Li P, MarcRhoads J, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review intrauterine growth retardation: implications for the animal sciences. J Anim Sci. 2006;84:2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- 13.Grisham MB, Johnson GG, Lancaster JR., Jr Quantitation of nitrite and nitrate in extra-cellular fluids. Methods Enzymol. 1996;268:237–246. doi: 10.1016/s0076-6879(96)68026-4. [DOI] [PubMed] [Google Scholar]
- 14.Xu K, Dong M, Zhou J. Changes of maternal and umbilical serum nitric oxide in patients with the intrauterine growth retardation. Zhonghua Fu Chan Ke Za Zhi. 2000;35(12):715–716. [PubMed] [Google Scholar]
- 15.Schiessl B, Strasburger C, Bidlingmaier M, Mylonas I, Jeschke U, Kainer F, et al. Plasma and urine concentrations of nitrite/nitrate and cyclic GMP in intrauterine growth restricted and preeclamptic pregnancies. Arch Gynecol Obstet. 2006;274:150–154. doi: 10.1007/s00404-006-0149-8. [DOI] [PubMed] [Google Scholar]
- 16.Neri I, Mazza V, Galassi MC, Volpe A, Facchinetti F. Effects of L-arginine on utero-placental circulation in growth retarded fetuses. Acta Obstet Gynecol Scand. 1996;75:208–212. doi: 10.3109/00016349609047088. [DOI] [PubMed] [Google Scholar]
- 17.Thureen PJ, Baron KA, Fennessey PV, Hay WW., Jr Ovine placental and fetal arginine and metabolism at normal and increased maternal plasma arginine concentrations. Peads Res. 2002;51:464–471. doi: 10.1203/00006450-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser1179. J Biol Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 19.Lakhkar BN, Rajagopal KN, Gaurishankar PT. Doppler prediction of adverse perinatal outcome in PIH and IUGR. Indian J Radiol Imag. 2006;16(1):109–116. doi: 10.4103/0971-3026.29064. [DOI] [Google Scholar]
- 20.Roy A, Mukherjee S, Bhattacharyya SK, Banerjee P, Das B, Patra KK. Perinatal outcome in pregnancies with intra-uterine growth restriction by using umbilical and middle cerebral artery colour Doppler. J Indian Med Assoc. 2012;110(3):154–157. [PubMed] [Google Scholar]
- 21.Xiao XM, Li LP. L-arginine treatment for asymmetric fetal growth restriction. Int J Gynecol Obstet. 2005;88(1):15–18. doi: 10.1016/j.ijgo.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Lampariello C, De Blasio A, Merenda A, Graziano E, Michalopoulou A, Bruno P. Use of L-arginine in intrauterine growth retardation (IUGR): authors’ experience. Minerva Ginecol. 1997;49:577–581. [PubMed] [Google Scholar]
- 23.Winer N, Branger B, Azria E, Tsatsaris V, Philippe HJ, Rozé JC, et al. L-arginine treatment for severe vascular fetal intrauterine growth restriction: a randomized double-bind controlled trial. Clin Nutr. 2009;28(3):243–248. doi: 10.1016/j.clnu.2009.03.007. [DOI] [PubMed] [Google Scholar]