Abstract
Extensive data from animal and human studies indicate a role of vitamin D in erythropoiesis. Iron and vitamin D deficiencies are implicated with adverse health effects in children even if they are asymptomatic. The potential relationship between the two remains poorly understood. A cross-sectional study was performed in the period from 1st May 2012 through 30th April 2013 and subjects were classified into vitamin D deficiency (VDD), vitamin D insufficiency (VDI) and vitamin D sufficiency (VDS) groups according to their 25(OH) D levels. A total of 263 children were included in the analysis. Anaemia was present in 66 % of 25(OH) D deficient subjects compared with 35 % in vitamin D sufficient individuals (p < 0.0001). The association of breast feeding and development of VDD was also significant (p < 0.05). Serum levels of 25(OH) D were found lower in female sex and if the analysis was performed in the winter/spring season. Physicians should therefore assess vitamin D levels in all anaemic children and ensure adequate supplementation to prevent deficiencies.
Keywords: Vitamin D deficiency, Iron deficiency anaemia, Supplementation, Children, Season, Breast feeding
Introduction
India is a vast tropical country and majority of its population reside in areas receiving ample sunlight throughout the year and hence Vitamin D deficiency (VDD) is considered uncommon in India. However data available from published literature claim VDD as very common in India in all the age groups and involving both sexes across the country [1, 2]. Skin complexion, poor sun exposure, vegetarian food habits and lack of vitamin D food fortification programme in the country may explain the high prevalence of VDD in India despite its sunny climate [3]. Vitamin D is a steroid hormone important for serum calcium and phosphorus homeostasis and plays a pivotal role in neuromuscular function and optimal skeletal health. It can be obtained from the diet or made in the skin after exposure to ultraviolet B radiation (UVB) from the sun. Vitamin D is converted to its major circulating form, 25-hydroxyvitamin D by the liver and then to an activated form 1,25-dihydroxyvitamin D, by the kidney. The plasma 25(OH) D levels are quite stable over several days or weeks and typically fluctuate with Vitamin D intake and UVB exposure. VDD leads to rickets in childhood and musculoskeletal disorders like osteomalacia in adolescent and adult populations [4]. There are evidence which suggest a role of hypovitaminosis D in path physiology of many other clinical situations like autoimmune diseases (crohn’s disease, multiple sclerosis, rheumatoid arthritis and type I diabetes mellitus), cardiovascular diseases, infections, cancers, foetal health, and exercise performance [5]. Recent studies have reported VDD as being associated with various types of anaemia like iron deficiency anaemia, anaemia of chronic kidney disease, and anaemia of infection. Iron deficiency anaemia is one of the most widespread forms of anaemia in the world. It has a prevalence of 35–45 % in Indian children. Vitamin D has been demonstrated in bone marrow to affect functioning of bone marrow with levels reaching several hundred-fold higher in bone marrow as compared with plasma [6]. Iron and Vitamin D are important micronutrients which are required for the growth and development of children. Iron deficiency can lead to cognition defects, memory impairment along with impaired immune function, frequent infections and iron deficiency anaemia. Moreover iron is an important co-factor for many enzymes like 1α-hydroxylase required for hydroxylation of 25(OH) D to 1,25(OH)2 D. Despite these important observations there are very few studies which report the association of VDD with anaemia especially in this region where VDD is so rampant. Therefore in this study, we investigated the prevalence and risk factors for VDD and its association with iron deficiency anaemia in children of Northern India.
Materials and Methods
Two hundred and sixty-three children attending the outpatient department of Chacha Nehru Bal Chikitsalya Hospital New Delhi for acute disorders such as gastroenteritis, viral exanthematous fever, otitis media, pneumonia between age of 3 months to 12 years were included in the study from May 2012 till April 2013. Children with systemic illness such as celiac disease, liver, kidney disorders and haematological disorders like thalassemia, G-6PD deficiency, anemia other than iron deficiency anemia were excluded from the study. The study period was set to 1 year to remove any bias which may occur due to seasonal variation. Clinical examination was followed by a blood tests including complete blood count (CBC) and serum levels of ferritin, iron, TIBC, % transferrin saturation, vitamin D, calcium, phosphorus and alkaline phosphatise as adviced by physician. Anaemia was defined as Hb < 11 g/dl as per WHO guidelines with MCV below 70 fl and elevated red cell distribution width (RDW) >15 % as abnormal and suggestive of iron deficiency. Along with this iron deficiency anaemia was identified if subjects met ≥2 of the following criteria: serum ferritin <12 ng/ml, TIBC >450 µg/dl, % transferrin saturation <15 % [7]. Serum ferritin is an acute phase protein with increase in levels during inflammation. In this study if serum ferritin was >12 ng/ml with elevated C-reactive protein (CRP) than MCV, RDW and % transferrin saturation levels were used to diagnose iron deficiency anaemia.
Further patients were grouped as Vitamin D Deficiency (VDD) was defined as serum 25(OH) D levels <30 nmol/L, vitamin D insufficiency (VDI) as 25(OH) D levels between 30 and 75 nmol/L and vitamin D sufficiency (VDS) as >75 nmol/L [8, 9]. Serum 25(OH) D was measured by an ELISA kit from DLD Diagnostika GMBH (Alderhost, Hamburg, Germany). CBC was analysed on MS-9 analyser, serum ferritin was estimated by chemiluminescence immunoassay method on Access-2 Beckman Coulter analyser. Serum levels of iron and TIBC (total iron binding capacity) were analyzed by ferrozene method, calcium by arsenazo method, phosphorus by phosphomolybdate UV method and alkaline phosphatase by p-nitrophenyl phosphate AMP method on Olympus AU-400 analyser. % Transferrin saturation was calculated from iron and TIBC. The study was cleared by Institution Ethical committee.
Statistical Analysis
Data was expressed as mean ± standard deviation values. The data between the groups was analyzed by using student’s t test and 1 way analysis of variance (ANOVA). Pearson correlation test was used to determine the correlation between 25(OH) D and Hb levels. A p value of <0.05 was accepted as significant. A multivariable logistic regression examined the simultaneous influence of several variables on the risk for VDD. All statistics were performed by using SPSS for windows 12.0 software (SPSS Inc., Chicago, IL, USA).
Results
Table 1 shows the study population characteristics like gender, feeding pattern, admission history, vitamin D, alkaline phosphatase status of the children attending the outpatient department. Out of the 263 children enrolled for the study, 29 (11 %) were below 1 year of age whereas 90 (34 %) and 144 (55 %) were between 1–5 and >5 years respectively. Amongst these 148 (56 %) were male and 115 (44 %) were female. The prevalence of breast feeding was 74 % in infants <5 years of age whereas 57 % were bottle fed. More than half the children were hospitalized for some ailment. Our measurement of vitamin D status indicated that 25 subjects (86 %) in <1 year age group, 70 subjects (78 %) in 1–5 years age group and 128 subjects (89 %) >5 years had serum 25(OH) D levels below 75 nmol/L. Only 11 % of the total patients took vitamin-mineral supplementation. 114 patients (43 %) had elevated levels of ALP (p value = 0.025).
Table 1.
Subject population characteristics
| Characteristics | Age <1 year | Age 1–5 years | Age >5 years |
|---|---|---|---|
| Total no | 29 (11) | 90 (34) | 144 (55) |
| Female sex | 07 (25) | 38 (42) | 70 (49) |
| Breast milk only | 09 (31) | 43 (48) | – |
| Breast milk and formula | 15 (52) | 21 (23) | – |
| Formula only | 05 (17) | 27 (30) | – |
| 25OH vitamin D deficiency (levels <30 nmol/L) | 11 (38) | 32 (36) | 44 (31) |
| 25OH vitamin D insufficiency (levels 30–75 nmol/L) | 14 (48) | 38 (42) | 84 (58) |
| 25OH vitamin D sufficiency (levels >75 nmol/L) | 04 (14) | 18 (20) | 15 (10) |
| Elevated ALP | 21 (72) | 49 (54) | 44 (31) |
Values are presented as-number (%)
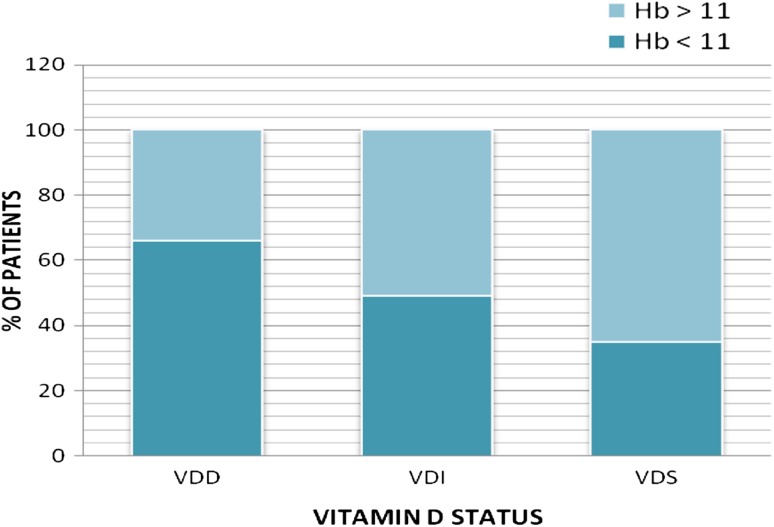
Figure 1 shows the estimation of Hb levels (<11 g/dl & >11 g/dl) according to the vitamin D status (VDD, VDI, VDS) of the patients. The patients with Hb < 11 g/dl were 66 % of total in the VDD group with mean Hb as 8.73 ± 1.97 g/dl, 49 % of total in the VDI group with mean Hb as 9.02 ± 1.66 g/dl and 35 % of total in the VDS group with mean Hb as 9.19 ± 1.42 g/dl (p = 0.556). It shows that number of patients with low Hb levels (<11 g/dl) was more in Vitamin D Deficient group compared to Vitamin D Sufficient group. So, Iron deficiency anaemia was more prevalent in the VDD group. The Pearson’s correlation between 25(OH) D and Hb levels was r = 0.317; p = 0.013. We did not find significant correlation of iron, TIBC, ferritin with vitamin-D. Table 2 shows that out of the 263 children enrolled in the study 86 % had deficient levels of vitamin D (VDD + VDI) and 39 % were girls. Mean age of the children who were VDD was 65.57 ± 46.67 months, whereas in VDI group 79.56 ± 45.14 months and in the VDS group 59.78 ± 47.09 months. Thirty-five (39 %) in the VDD group, 29 (21 %) in the VDI group and 24 (65 %) in VDS group were breast fed. Thus the association between the presence of VDD and breast feeding was statistically significant (p < 0.05). Mean Hb, iron, % transferrin saturation was significantly decreased in the VDD group compared to the VDI and VDS group. TIBC was significantly increased in the VDD group. No significant difference was observed in levels of serum ferritin, calcium, phosphorus and alkaline phosphatise (Table 2).
Fig. 1.
Ratio of iron deficiency anaemia among VDD, VDI and VDS
Table 2.
Comparison of haematological and biochemical profiles according to vitamin D status (in total 263 patients)
| Parameters | Vit D <30 nmol/L (VDD) | Vit D 30–75 nmol/L (VDI) | Vit D >75 nmol/L (VDS) | p value |
|---|---|---|---|---|
| Total no. (%) | 90 (34) | 136 (52) | 37 (14) | <0.05 |
| Mean age (MO) | 65.57 ± 46.67 | 79.56 ± 45.14 | 59.78 ± 47.09 | <0.05 |
| Female sex (%) | 43 (48) | 59 (43) | 13 (35) | >0.05 |
| Breast feeding (%) | 35 (39) | 29 (21) | 24 (65) | <0.05 |
| 25(OH) vit D (nmol/L) | 15.8 ± 8.05 | 47.04 ± 12.11 | 120.31 ± 26.21 | <0.0001 |
| Mean Hb (g/dl) | 9.86 ± 2.60 | 10.59 ± 2.27 | 11.25 ± 1.86 | <0.01 |
| Mean ferritin (ng/ml) | 28.44 ± 25.08 | 33.36 ± 25.73 | 39.30 ± 36.32 | >0.05 |
| Mean iron (µg/dl) | 43.65 ± 27.51 | 63.34 ± 14.57 | 114.18 ± 23.75 | <0.001 |
| Mean TIBC (µg/dl) | 461.65 ± 49.76 | 382.55 ± 52.49 | 353.23 ± 89.45 | <0.001 |
| Mean transferrin saturation (%) | 11.77 ± 11.24 | 19.27 ± 13.85 | 38.20 ± 5.41 | <0.001 |
| Mean Ca (mg/dl) | 8.50 ± 1.56 | 8.29 ± 1.24 | 8.83 ± 1.22 | >0.05 |
| Mean PO4 (mg/dl) | 4.19 ± 1.55 | 4.13 ± 1.13 | 4.29 ± 1.27 | >0.05 |
| Mean ALP (IU/L) | 705.98 ± 513.27 | 561.17 ± 468.41 | 443.02 ± 230.63 | >0.05 |
Values are presented as mean ± SD. p values were obtained using 1-way ANOVA
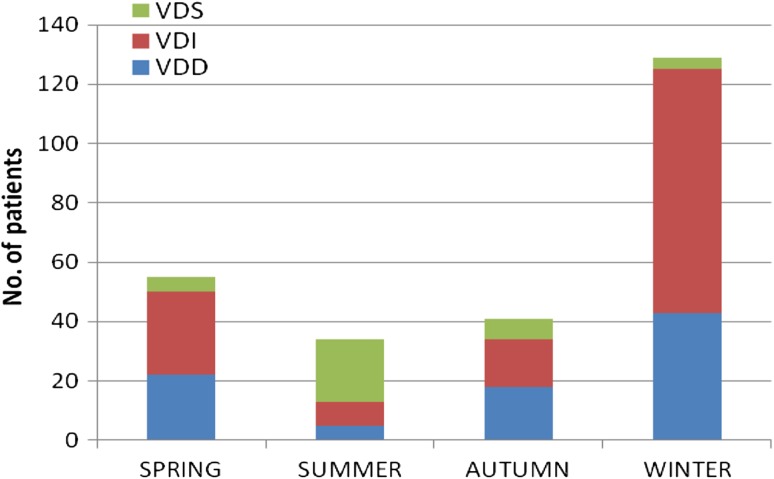
We also analysed the number of patients who were vitamin D deficient, insufficient and sufficient and the levels of vitamin D according to the season (Fig. 2). The number of patients with vitamin D levels <75 nmol/L was 50 (91 %) in spring (March–May), 125 (97 %) in winter (December–February), 13 (38 %) in summer (June–August) and 34 (83 %) in autumn (September–November). The mean level of serum 25(OH) D was significantly lower in spring/winter than in summer/autumn (41.03 ± 40.04 v/s 52.80 ± 29.77 nmol/L, p = 0.0105). The winter season had the highest rate of VDD (49 %). Serum Hb, ferritin, iron and % transferrin saturation were also lower in the winter but the differences were not statistically significant (Table 3).
Fig. 2.
Analysis of subjects according to seasonal variation
Table 3.
ODDS Ratio for development of VDD
| Variable | Odds ratio | 95 % CI |
|---|---|---|
| Female sex | 1.57 | 0.76–3.24 |
| Breast feeding | 0.74 | 0.35–1.57 |
| Serum Hb level | 3.60 | 1.77–7.35* |
| Vitamin D tested in winter/spring | 11.58 | 5.12–26.23* |
* p value <0.001
By multivariable regression analysis the risk factor for development of VDD was significant in children who had anaemia (p < 0.001) and if 25(OH) D levels were tested in winter/spring season (p < 0.001).
Discussion
Vitamin D and iron are important nutrients required for the growth and development of children. Out of the 263 children enrolled in the study 86 % were deficient in vitamin D levels (<75 nmol/L) whereas 15 % had sufficient levels of vitamin D. Amongst these deficient children 89 % were female whereas 69 % were male. A total of 33 % of the infants were breast fed and VDD was observed in 40 % of them. According to Balasubramanian [3], a greater risk of VDD and rickets was observed in infants who were exclusively breast fed without appropriate exposure to sunlight and adequate vitamin D supplementation. Human breast milk contains low levels of vitamin D and all breast fed infants should be supplemented with 400 IU of vitamin D per day regardless of being given formula feeds. However there are very few parents which follow doctor’s advice for vitamin D supplementation as they believe breast milk has all nutrients [1]. In our study only 11 % of the children were taking any type of supplementation despite having lowered vitamin D and iron concentration. A greater prevalence and risk of anaemia was observed in individuals with VDD compared to those with normal 25(OH) D levels. In this study 66 % of the children had Hb < 11 g/dl in the vitamin D deficient group (VDD + VDI) whereas only 39 % of the children had Hb < 11 g/dl in the vitamin D sufficient group. Subjects with VDD also had lowered levels of iron, % transferrin saturation and elevated levels of TIBC in comparison to VDI and VDS group. No difference was observed in mean levels of ferritin in any of the groups. This may be explained by the fact that ferritin is an acute phase protein and increases in inflammation. In a study by Grindulis et al. [10], the prevalence of iron and VDD was assessed in asian toddlers. A significant association between VDD and poor iron status was observed. Two-fifths of the children were anaemic, two-fifths had low plasma levels of vitamin D and one-fifth had both these features. Children with VDD had significantly lower levels of Hb and serum iron. These results are consistent with our findings and suggest more than a simple overlap in between both the deficiencies. Another study showed no differences in levels of Hb and serum iron in vitamin D deficient (VDD + VDI) and VDS groups. However 58 % of the children with iron deficiency anaemia had VDI and 39 % had VDD [11, 12]. In another study conducted in Al-Medina 37.9 % of all rachitic children were anaemic. Furthermore the Hb level was lower in children with active rickets in comparison to healed rickets. According to them vitamin D plays a role in proliferation and differentiation of stem cells in bone marrow and may play a role in red cell proliferation. A deficiency of vitamin D may therefore affect the Hb metabolism and give rise to anaemia [13]. A seasonal comparison of vitamin D levels revealed that the 25(OH) D levels were lower the spring/winter season as compared to the autumn/summer season this was in support of previous studies [14, 15]. No significant difference was observed in levels of calcium, phosphorus and ALP in different seasons. A multivariable logistic analysis indicated seasonal variation as an important factor for development of VDD. This highlights the importance of sunlight and the need for vitamin D supplementation during winter/spring season. However the amount and duration of sunlight exposure required for maintenance of normal levels is unknown. Since many factors like clothing, race, skin colour, sunscreen use, weather, latitude of residence, time of day etc. affect the amount of sunlight exposure. Requirement also varies with age, sex, pregnancy, lactation, presence of co morbidities etc. [16]. Hence physicians should aim at finding the optimal dose and duration for each patient. VDD seems to be associated with anaemia however the potential relationship between the two remains poorly understood. One possible mechanism is that vitamin D directly stimulates erythroid precursors in the bone marrow. It has been reported that hematons (the buffy coat of bone marrow containing erythroid precursors, fibroblast, endothelial cells, lipid laden cells, and macrophages) contain significantly higher concentrations of 25(OH) D and 1,25(OH)2 D levels than bone marrow plasma. High local concentrations of 1,25(OH)2 D in hematopoietic tissues may directly activate erythroid precursor cells in a paracrine fashion though it is only a speculation [17]. Anaemia may also in turn lead to VDD. It is known that iron deficiency impairs fat and vitamin A intestinal absorption. Therefore the absorption of vitamin D may also be impaired. This may contribute to the development of VDD [18]. Iron is also a cofactor for the enzyme 1 α-hydroxylase (CYP27B1) which is responsible for the hydroxylation of 25(OH) D to 1,25(OH)2 D and iron deficiency may therefore inhibit the activation of vitamin D leading to its deficiency [19]. Anaemic patients also tend to be more fatigued and may restrict themselves from going outside and obtaining adequate sun exposure for vitamin D synthesis [20]. Our study showed a greater prevalence of anaemia in children who had VDD, however further studies on larger number of subjects are required before we can substantiate our findings to the whole population.
There were some limitations to our study. The age of our subjects was varied so their feeding habits and dietary intake might have differed. Number of individuals in different age groups was small, so more data will be needed for elaborative comparison. Larger number of patients was of low socioeconomic status, so our findings cannot be generalised to other populations in relation to nutritional status. In summary our study showed increased prevalence of VDD in children with anaemia. Deficiency was also predominant in infants who were breast fed, if sex was female and if analysis was done in winter/spring season.
Conclusion
Physicians should therefore to ensure that vitamin D levels are evaluated in anaemic children and to provide adequate supplementation to prevent deficiencies of both nutrients.
Acknowledgments
The authors wish to thank the management of Chacha Nehru Bal Chikitsalya Hospital, Associated to Maulana Azad Medical College, New Delhi for providing facilities to carry out this work.
References
- 1.Harinarayan CV, Joshi SR. Vitamin D status in India—its implications and remedial measures. J Assoc Physicians India. 2009;57:40–48. [PubMed] [Google Scholar]
- 2.Marwaha RK, Sripathy G. Vitamin D and bone mineral density of healthy school children in northern India. Indian J Med Res. 2008;127:239–244. [PubMed] [Google Scholar]
- 3.Balasubramanian S. Vitamin D deficiency in breastfed infants & the need for routine vitamin D supplementation. Indian J Med Res. 2011;133:250–252. [PMC free article] [PubMed] [Google Scholar]
- 4.Etten EV, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev. 2008;66(2):S125–S134. doi: 10.1111/j.1753-4887.2008.00096.x. [DOI] [PubMed] [Google Scholar]
- 5.Blazsek I, Farabos C, Quittet P, Labat ML, Bringuier AF, Triana BK, et al. Bone marrow stromal cell defects and 1 alpha, 25-dihydroxyvitamin D3 deficiency underlying human myeloid leukemias. Cancer Detect Prev. 1996;20:31–42. [PubMed] [Google Scholar]
- 6.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–475. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 7.Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams hematology. 7. New York: McGraw-Hill; 2006. [Google Scholar]
- 8.Visser M, Deeg J, Puts MT, Seidell JC, Lips P. Low serum concentration of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am J Clin Nutr. 2006;84(3):616–622. doi: 10.1093/ajcn/84.3.616. [DOI] [PubMed] [Google Scholar]
- 9.Zabalza R, Múgica I, Sistiaga F, Garrido A, Emparanza JI, Zubillaga P. Assessment of vitamin D supplementation in people with intellectual disability. J Intellect Disabil Diagn Treat. 2014;2:46–53. doi: 10.6000/2292-2598.2014.02.01.6. [DOI] [Google Scholar]
- 10.Grindulus H, Scott PH, Belton NR, Wharton BA. Combined deficiency of iron and vitamin D in Asian toddlers. Arch Dis Child. 1986;61:843–884. doi: 10.1136/adc.61.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heldenberg D, Tenenbaum G, Weisman Y. Effect of iron on serum 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D concentrations. Am J Clin Nutr. 1992;56(3):533–536. doi: 10.1093/ajcn/56.3.533. [DOI] [PubMed] [Google Scholar]
- 12.Hodgkin P, Kay GH, Hine PM, Lumb GA, Stanbury SW. Vitamin D deficiency in Asian at home and in Britain. Lancet. 1973;2:167–172. doi: 10.1016/S0140-6736(73)93004-3. [DOI] [PubMed] [Google Scholar]
- 13.Hollick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Jin HJ, Lee JH, Kim MK. The prevalence of vitamin D deficiency in iron-deficient and normal children under the age of 24 months. Blood Res. 2013;48:40–45. doi: 10.5045/br.2013.48.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGillivray G, Skull SA, Davie G, Kofoed SE, Frydenberg A, Rice J, et al. High prevalence of asymptomatic vitamin D and iron deficiency in East African immigrant children and adolescents living in a temperate climate. Arch Dis Child. 2007;92:1088–1093. doi: 10.1136/adc.2006.112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meguro S, Tomita M, Katsuki T, KatoK, Oh H, Ainai A, et al. Plasma 25-hydroxyvitamin d is independently associated with hemoglobin concentration in male subjects with type 2 diabetes mellitus. Int J Endocrinol. 2011; 2011: Article ID 362981 doi:10.1155/2011/362981. [DOI] [PMC free article] [PubMed]
- 17.Yoon JW, Kim SW, Yoo G, Kim MK. Prevalence and risk factors for vitamin D deficiency in children with iron deficiency anemia. Korean J Pediatr. 2012;55(6):206–211. doi: 10.3345/kjp.2012.55.6.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sim JJ, Lac PT, Liu IL, Meguerditchian SO, Kumar VA, Kujubu DA, et al. Vitamin D deficiency and anemia: a cross-sectional study. Ann Hematol. 2010;89:447–452. doi: 10.1007/s00277-009-0850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277(5333):1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105–111. doi: 10.1542/peds.2009-1195. [DOI] [PubMed] [Google Scholar]