Abstract
Functional ovarian hyperandrogenism (FOH) is a form of polycystic ovary syndrome (PCOS) characterized by elevated circulating levels of androgens derived from the ovary. Insulin resistance (IR) is the most common etiological factor in women with FOH. IR causes the generation of increased oxidative stress (OS) and diminished antioxidant status. OS is directly correlated with both IR and testosterone levels, which consequently contribute to endocrine and biochemical alterations in FOH women. In the current study, elevations in total testosterone, free testosterone and luteinizing hormone (LH) levels accompanied by a decrease in follicle stimulating hormone (FSH) level leading to higher LH:FSH ratio were the prominent endocrine changes observed in women with FOH. A significant increase in fasting blood levels of glucose and insulin, as well as an elevated IR were also seen in FOH women, as compared to their age matched controls. Women with FOH have higher pro-oxidant and lower anti-oxidant levels in blood than their age matched controls. In FOH women, elevations in LH:FSH ratio and OS are correlated more with hyperandrogenemia than with IR. Of the androgens, free rather than total testosterone has better positive correlations with elevated LH:FSH ratio and OS, and hence, the former is a better predictive marker for the development of biochemical PCOS in women with FOH.
Keywords: Functional ovarian hyperandrogenism, Polycystic ovary syndrome, Insulin resistance, Total and free testosterone, LH:FSH ratio, Oxidative stress
Introduction
Functional ovarian hyperandrogenism (FOH) is characterized by hyperandrogenism which is associated with impaired glucose tolerance, hypertension, dyslipidemia and elevated endothelial dysfunction, and these abnormalities contribute to increased risks for future cardiovascular diseases and type 2 diabetes mellitus (T2DM) [1]. FOH is an ovarian dysfunction caused by excess circulating levels of androgens, which inhibit folliculogenesis and lead to poly follicular morphology. These ovarian changes then disturb the menstrual cycle leading to anovulatory infertility [2]. Apart from infertility, other common clinical symptoms of hyperandrogenism are hirsutism, acne, and male pattern alopecia [3]. The clinical and biochemical features of FOH can arise as a consequence of hyper secretion of androgen by the ovary. FOH can result either from luteinizing hormone (LH) excess or from abnormal modulation of ovarian androgen responsiveness to LH [4]. Chronic LH stimulation in FOH induces sustained hyper secretion of androgens by the theca compartment, probably augmented by insulin and insulin like growth factor [5].
Most data suggest that the primary defect may be at the ovarian level or all manifestations of the syndrome may occur secondary to hyperinsulinemia [6, 7]. Insulin resistance (IR) is, thus the main factor involved in the pathophysiology of ovarian hyperandrogenism. IR causes hyperandrogenism by activating the enzyme cytochrome p450-c17α-hydroxylase (cyt. p450c). An imbalance in the factors responsible for the up and down-regulation of cyt. p450c in theca cells may cause ovarian hyperandrogenism despite normal LH secretion [8, 9]. The excessive serine phosphorylation of insulin receptors was shown to stimulate cyt. p450c enzyme activity leading to hyperandrogenism in insulin-resistant FOH women [8, 10]. IR also potentiates the effects of LH on theca interstitial cells, resulting in increased androgen production while arresting the follicular maturation process which leads to PCOS [11, 12]. Oxidative stress (OS), which is marked by increased oxidant and decreased anti-oxidant levels, has been documented in infertile women with FOH [13–15]. OS is demonstrated to be directly correlated with testosterone and androstenedione which may consequently contribute to hyperandrogenism in PCOS women. OS is involved in altered steroidogenesis in the ovaries, thus contributing to increased androgen production, disturbed follicular development and, ultimately infertility [16, 17]. OS has also been shown to correlate with IR [18].
Materials and Methods
Subjects
This cross-sectional, case–control study was conducted at the Department of Physiology and Biochemistry, Educare Institute, Malappuram, Kerala. Women were included in the FOH and Control groups upon getting informed consent from the outpatient volunteers of the study, and after obtaining permission from the Institutional Ethics Committee. The blood samples collected from the volunteers were processed and analyzed at Dianova Laboratories, an NABL accredited fully automated specialty clinical laboratory at Kottayam. The following inclusion–exclusion criteria were used for the selection of women with FOH [19].
Married, non-pregnant woman in the age group of 20 to 33 years, having elevated total testosterone (TT) levels (>0.8 ng/ml) were screened and selected for the FOH group (n = 192). Women with TT levels >2.0 ng/ml were excluded. From the 192 women screened as above, women with FOH were finally selected, using a five-step diagnostic work-up. Women with normal serum prolactin (PRL), dehydro epi-androsterone sulphate (DHEAS), 17-hydroxy progesterone (HPrg), tri-iodo thyronine (T3), tetra-iodo thyronine (T4), and thyroid stimulating hormone (TSH) levels and elevated LH:FSH ratio were included in the FOH group (n = 100). Women with normal PRL levels but with a history of amenorrhoea were excluded [19, 20].
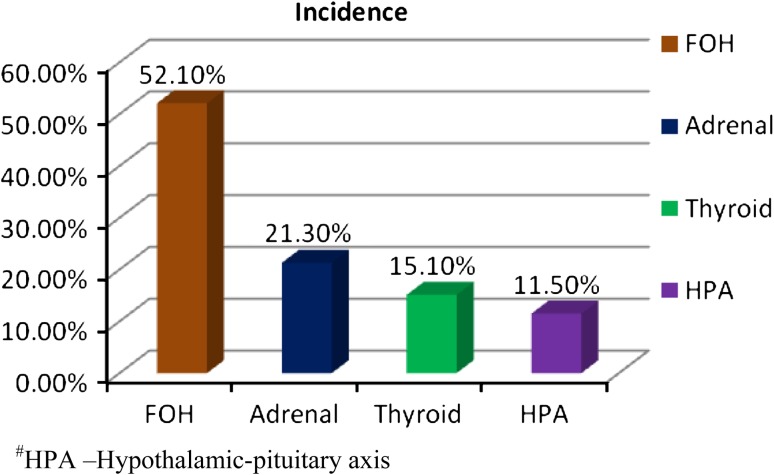
Out of the total of 192 women with hyper-androgenemia (TT >0.8 but not >2.0 ng/ml), 52.1 % (n = 100) were due to FOH. Functional adrenal hyperandrogenism (FAH), which is marked by elevated DHEAS and HPrg levels, accounted for 21.3 % (n = 41), hypothyroidism (elevated TSH and lowered T3 and T4 levels) for 15.1 % (n = 29) and hypothalamic-pituitary causes (elevated PRL) for 11.5 % (n = 22) of hyperandrogenemia in women (Fig. 1). Married non-pregnant women in the age group of 20 to 33 years who had normal serum TT levels (<0.8 ng/ml), and who were not taking any oral contraceptives, selected from among the siblings of the patients and the hospital staff, were included in the Control group (n = 50).
Fig. 1.
Incidence of ovarian and non ovarian causes of hyperandrogenemia in women
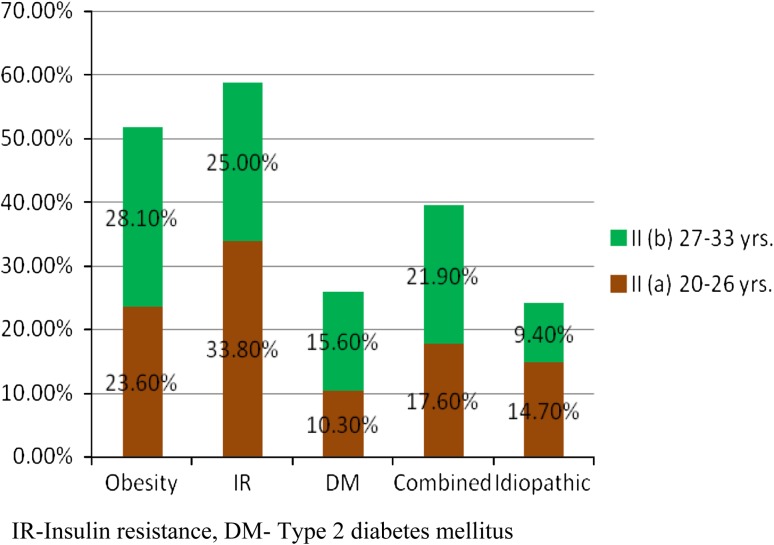
Women in the Control and FOH groups were designated respectively as Groups I and II. Based on the mean age of the study population (26.5 ± 6.5 years), the women in each group (Control and FOH groups) were sub divided into two groups viz. the 20–26 years age group {Group I (a) and Group II (a)} and the 27–33 years age group {Group I (b) and Group II (b)} respectively. Group I (a) had 22.1 % (n = 33) and Group II (a) had 11.3 % (n = 17) women, while Group I (b) and Group II (b) had respectively 45.3 % (n = 68) and 21.3 % (n = 32) women. Among the FOH women of the 20–26 years age group {Group II (a)}, 33.8 % had IR, 23.6 % had obesity, 10.3 % had T2DM and 17.6 % had a combination of two or more of these disorders. Idiopathic causes accounted for by14.7 % of these women. The corresponding figures for the 27–33 years age group {Group II (b)} were 25.0, 28.1, 15.6, 21.9 and 9.4 % respectively (Fig. 2).
Fig. 2.
Age-wise incidence of obesity and carbohydrate derangement in women with FOH
Methods
Blood samples of all subjects were collected in the morning, on days 7–10 of their menstrual cycles after 12 h fasting. Plasma samples were used for the determinations of glucose and insulin. Determination of fasting blood glucose (FBG) was carried out using enzymatic end-point assay in Daytona Fully Automated Biochemistry Analyzer of M/s. Randox Diagnostics Ltd [21]. Insulin, PRL, DHEAS, HPrg, T3, T4, TSH, TT, free testosterone (FT), LH and FSH were assayed using Chemi luminescent immuno assay (CLIA) in Advia Centaur Fully Automated CLIA analyzer of M/s. Siemens Health Care Diagnostics India Ltd [22–25]. Quality control (QC) was performed by participating in the BIO-RAD EQUAS international QC programmes. Erythrocyte catalase, super oxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GPx) and malon di-aldehyde (MDA) were determined using Spectrophotometric Kinetic assays in AU 2701 Single Beam UV–Vis. Spectrophotometer made by M/s. Dynamica (Australia) and marketed by M/s. Systronics (India) Pvt Ltd [26].
The homeostasis model assessment for insulin resistance (HOMA-IR) was used for the determination of IR. IR was calculated by multiplying the FBG values (mg/dl) with the fasting plasma insulin values (µU/ml), and then dividing by 405 [27]. The data from the above investigations were statistically analyzed by employing the multiple pair-wise comparison procedures of One Way Anova (Holm-Sidak method) for inter-group comparisons. For correlation analysis, Pearson product moment correlation was carried out using IR, TT and FT as the independent variables and LH:FSH ratio, erythrocyte catalase, SOD, GSH, GPx and MDA as the dependent variables. Since this study had an overall critical confidence level of 95 %, results with p values <0.05 were interpreted as statistically significant [28]. Data processing and data analysis were conducted using Sigma Stat 6.5 Version Software (M/s. Sigma-Aldrich Co., St. Louis, USA).
Results
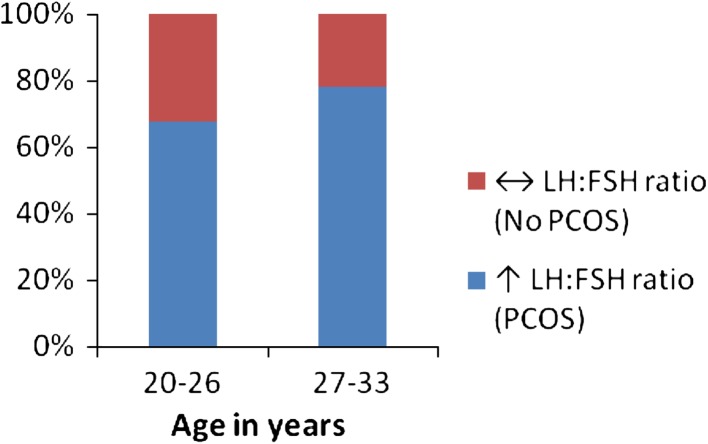
The results obtained from the present study are summarized in Fig. 3 and in Tables. Serum LH levels were increased and FSH levels were decreased resulting in an elevated LH:FSH ratio in both the age groups of FOH women. The majority of women in the FOH group (71.0 %) had elevated LH:FSH ratio. Figure 3 shows that this elevation was more pronounced in the upper age group (78.1 %) than in the lower age group (67.7 %). Serum TT and FT levels were significantly higher in both the age groups of FOH women, compared to the corresponding Controls (p < 0.001), while no significant difference between the two age groups was observed (Tables 1, 2). Fasting glucose and insulin levels were elevated significantly (p < 0.001) in the FOH women compared to their age-matched Control women. IR of both the age-groups of FOH women were significantly higher (p < 0.001) than that of the corresponding Controls. MDA levels in erythrocytes were significantly higher while the anti-oxidant system involving catalase, SOD, GSH and GPx were lower in FOH women than that of their age-matched Controls. Table 3 shows that serum TT and FT levels in women with FOH showed statistically significant positive correlations with IR (p < 0.01). Serum levels of LH and FSH in hyperandrogenic women showed positive and negative correlations respectively with IR, TT and FT. Moreover, LH:FSH ratio showed positive correlations with IR, TT and FT. However, of all these associations, serum LH levels and LH:FSH ratio correlated better with TT and FT (r = 0.999 and 1.0, p < 0.001) than with IR (r = 0.995 and 0.991, p < 0.01). The correlation coefficient (r) of LH:FSH ratio with FT had a higher value (r = 1.0, p < 0.001) than that with TT (r = 0.999, p < 0.01) showing that FT is better correlated with LH:FSH ratio than TT. Erythrocyte MDA had a statistically significant positive correlation with IR, TT and FT (p < 0.05) while the antioxidant markers GSH, GPx, catalase and SOD had significant negative correlations with IR, TT and FT. The correlations of erythrocyte SOD with both TT and FT were stronger (r = 1.0, p < 0.001) than that with IR (r = 0.994, p < 0.01).
Fig. 3.
Elevation of LH:FSH ratio (biochemical PCOS) in women with FOH
Table 1.
Parameters of hyperandrogenemia, biochemical PCOS, IR and OS in FOH women and their age-matched Control subjects (Mean ± SD)
| Parameters | Reference range | Subjects | |||
|---|---|---|---|---|---|
| Control (Group I) | FOH (Group II) | ||||
| Group I (a) | Group I (b) | Group II (a) | Group II (b) | ||
| TT | 0.20–0.80 ng/mla | 0.58 ± 0.14 | 0.63 ± 0.15 | 1.33 ± 0.35 | 1.37 ± 0.38 |
| FT | 0.3–2.0 pg/mla | 1.46 ± 0.57 | 1.53 ± 0.66 | 3.63 ± 0.48 | 3.72 ± 0.54 |
| LH | 0.50–16.5 µIU/mla | 4.29 ± 0.47 | 4.41 ± 0.56 | 8.57 ± 1.23 | 8.82 ± 1.45 |
| FSH | 1.5–9.1 µIU/mla | 6.99 ± 1.76 | 7.13 ± 1.82 | 3.46 ± 0.35 | 3.62 ± 0.41 |
| LH/FSH | 0.3–1.80 | 0.61 ± 0.27 | 0.62 ± 0.31 | 2.38 ± 0.04 | 2.41 ± 0.05 |
| FBG | 70–105 mg/dl | 84.46 ± 6.15 | 85.22 ± 6.34 | 92.81 ± 8.55 | 95.43 ± 9.76 |
| Insulin | 1–10 µIU/ml | 3.19 ± 1.24 | 3.32 ± 1.27 | 14.63 ± 4.41 | 16.81 ± 5.18 |
| IR | 0.3–1.8 | 0.665 ± 0.019 | 0.699 ± 0.020 | 3.35 ± 0.093 | 3.96 ± 0.121 |
| MDA | 3.5–4.5 µmol/l | 3.63 ± 1.04 | 3.98 ± 1.35 | 5.22 ± 2.41 | 5.97 ± 2.73 |
| Catalase | 4.5–9.7 IU/l | 5.62 ± 0.94 | 5.17 ± 0.86 | 4.33 ± 0.72 | 4.15 ± 0.67 |
| SOD | 70–150 IU/ml | 118.32 ± 14.13 | 114.87 ± 12.94 | 70.15 ± 10.86 | 67.23 ± 9.75 |
| GSH | 10.5–17.5 mg/dl | 12.66 ± 3.41 | 11.83 ± 2.76 | 9.01 ± 1.57 | 8.32 ± 1.06 |
| GPx | 30–65 IU/l | 44.85 ± 10.61 | 41.25 ± 9.34 | 32.46± 7.18 | 30.77 ± 6.82 |
Groups I (a) and II (a)—20 to 26 years, Groups I (b) and II (b)—27 to 33 years
aLuteal phase values
Table 2.
Anova statistics of the hyperandrogenemia, LH:FSH ratio, IR and OS parameters in FOH women and their age-matched Control subjects
| Parameters | Multiple pair-wise comparisons of ANOVA | |||
|---|---|---|---|---|
| I (a) versus II (a)1 | I (b) versus II (b)2 | I (a) versus I (b)3 | II (a) versus II (b)4 | |
| TT | <0.001* | <0.001* | 0.249 | 0.544 |
| FT | <0.001* | <0.001* | 0.698 | 0.434 |
| LH | <0.001* | <0.001* | 0.427 | 0.293 |
| FSH | <0.001* | <0.001* | 0.793 | 0.484 |
| LH/FSH | <0.001* | <0.001* | 0.907 | 0.402 |
| FBG | <0.001* | <0.001* | 0.684 | <0.05* |
| Insulin | <0.001* | <0.001* | 0.729 | <0.01* |
| IR | <0.001* | <0.001* | 0.221 | <0.01* |
| MDA | <0.001* | <0.01* | 0.588 | 0.108 |
| Catalase | <0.001* | <0.001* | 0.055 | 0.283 |
| SOD | <0.001* | <0.001* | 0.324 | 0.245 |
| GSH | <0.001* | <0.001* | 0.204 | 0.145 |
| GPx | <0.001* | <0.001* | 0.142 | 0.340 |
Group I (a)—Control group (20–26 years), Group I (b)—Control group (27–33 years), Group II (a)—FOH group (20–26 years), Group II (b)—FOH group (27–33 years. Degrees of freedom 1: 99, 2: 47, 3: 98 and 4: 48. Statistically significant p values are indicated by asterisk mark (*)
Table 3.
Correlations of the LH:FSH ratio and OS parameters with IR, TT and FT in FOH women
| Dependent variables | Pearson product moment correlation statisticsa | |||||
|---|---|---|---|---|---|---|
| IR | TT | FT | ||||
| r | p | r | p | r | p | |
| TT | 0.994 | <0.01 | – | – | – | – |
| FT | 0.993 | <0.01 | 1.00b | <0.001c | – | – |
| LH | 0.995 | <0.01 | 1.00b | <0.001c | 1.00b | <0.001c |
| FSH | −0.984 | <0.05 | −0.995 | <0.01 | −0.997 | <0.01 |
| LH:FSH ratio | 0.991 | <0.01 | 0.999 | <0.01 | 1.00b | <0.001c |
| MDA | 0.982 | <0.05 | 0.966 | <0.05 | 0.961 | <0.05 |
| Catalase | −0.966 | <0.05 | −0.973 | <0.05 | −0.967 | <0.05 |
| SOD | −0.994 | <0.01 | −1.00b | <0.001c | −1.00b | <0.001c |
| GSH | −0.988 | <0.05 | −0.989 | <0.05 | −0.985 | <0.05 |
| GPx | −0.977 | <0.05 | −0.983 | <0.05 | −0.978 | <0.05 |
r correlation coefficient, p p value
aFor correlation analysis, there were no age-wise split up, and all values of the above parameters in all the FOH group women were taken as a single group
bShows the strongest correlations
cIndicates the highest level of significance
Discussion
The present study showed that the major endocrine alterations in women with FOH were the elevations of TT, FT, LH and the decrease in FSH. The results of our study are consistent with previous studies reporting similar endocrine changes in FOH [2–4]. Previous studies had unequivocally documented the correlations of IR with hyperandrogenemia and elevated LH:FSH ratio [5–9]. This study also showed results comparable to these reports. One of the prominent outcomes of this study is that we could establish that in women with FOH, LH and LH:FSH ratio has stronger positive correlations with TT and FT than that with IR. More importantly, blood oxidant levels were increased while the anti oxidant system was diminished in women with FOH. The associations of ovarian hyperandrogenism with the oxidant-anti oxidant status had been reported earlier [16–18]. IR has long been known to be correlated with the generation of OS and depression of anti-oxidative mechanisms [13–15]. Interestingly, we have shown in the present study that SOD, a very important component of the blood anti-oxidant system, is more strongly correlated with TT and FT than with IR. The implications of the present study is that even though hyperinsulinemia and/IR are recognized as important etiological factors for the pathogenesis of FOH, androgen levels better correlate with biochemical PCOS and OS, than the former in women with FOH. Several authors reported that the measurement of FT rather than TT is more clinically useful in the primary screening and definitive diagnosis of hypogonadism in men as well as in the evaluation of androgen excess in women [29–31]. The present study not only agrees with these reports, but also shows that FT is a better predictive marker, than TT for the development of biochemical PCOS, in hyperandrogenic women.
Conclusions
The conclusions drawn from the present study are that women with FOH have elevations in TT and FT levels along with high LH:FSH ratio, IR and OS as compared to their age matched controls. Though hyperandrogenemia and IR correlate with LH:FSH ratio and OS in women with FOH, the former has a better correlation with LH:FSH ratio and OS. Finally, FT rather than TT is a more useful predictive marker of biochemical PCOS in FOH women.
Directions for Future Research
The finding of this study assumes importance in the screening of high risk group girls in the pre and peri adolescent period, because if treatment can be instituted for the androgen excess or IR at this age, many of the reproductive as well as metabolic consequences of FOH can be reversed if not totally prevented. This study can serve as the platform for future studies on the selective advantage of early screening in teenage girls using FT levels as a good predictor of the reproductive and metabolic consequences of FOH on a time scale.
Abbreviations
- Cyt. p450c
Cytochrome p450-c17α-hydroxylase
- DHEAS
Dehydro epi androsterone sulphate
- FBG
Fasting blood glucose
- FOH
Functional ovarian hyperandrogenism
- FSH
Follicle stimulating hormone
- FT
Free testosterone
- GPx
Glutathione peroxidase
- GSH
Glutathione (reduced)
- HPrg
17-Hydroxy progesterone
- IR
Insulin resistance
- LH
Luteinizing hormone
- MDA
Malon di-aldehyde
- OS
Oxidative stress
- PCOS
Polycystic ovary syndrome
- PRL
Prolactin
- SOD
Super oxide dismutase
- T3
Tri-iodo thyronine
- T4
Tetra-iodo thyronine
- TSH
Thyroid stimulating hormone
- TT
Total testosterone
- T2DM
Type 2 diabetes mellitus
References
- 1.Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:671–683. doi: 10.1016/j.bpobgyn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R. Diagnostic criteria for polycystic ovary syndrome: a reappraisal. Fertil Steril. 2005;83:1343–1346. doi: 10.1016/j.fertnstert.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 3.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 4.Huang A, Brennan K, Azziz R. Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil Steril. 2010;93:1938–1941. doi: 10.1016/j.fertnstert.2008.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baptiste CG, Battista MC, Trottier A, Baillargeon JP. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2010;122:42–52. doi: 10.1016/j.jsbmb.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauffman RP, Baker VM, DiMarino P, Castracane VD. Hyperinsulinemia and circulating dehydroepiandrosterone sulfate in white and Mexican American women with polycystic ovary syndrome. Fertil Steril. 2006;85:1010–1016. doi: 10.1016/j.fertnstert.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Siklar Ζ, Ocal G, Adiyaman P, Ergur A, Berberoglu M. Functional ovarian hyperandrogenism and polycystic ovary syndrome in prepubertal girls with obesity and/or premature pubarche. J Pediatr Endocrinol Metab. 2007;20:475–481. doi: 10.1515/JPEM.2007.20.4.475. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzo A, Amato MC, Giordano C. Insulin resistance and polycystic ovary syndrome. Nutr Metab Cardiovasc Dis. 2008;18:511–518. doi: 10.1016/j.numecd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Nisenblat V, Norman RJ. Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2009;16:224–231. doi: 10.1097/MED.0b013e32832afd4d. [DOI] [PubMed] [Google Scholar]
- 10.Sakumoto T, Tokunaga Y, Tanaka H, Nohara M, Motegi E, Shinkawa T, et al. Insulin resistance/hyperinsulinemia and reproductive disorders in infertile women. Reprod Med Biol. 2010;9:185–190. doi: 10.1007/s12522-010-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baptiste CG, Battista MC, Trottier A, Baillargeon JP. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2010;122:42–52. doi: 10.1016/j.jsbmb.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambineri A, Patton L, Prontera O, Fanelli F, Ciampaglia W, Cognigni GE, Pagotto U, Pasquali R. Basal insulin-like factor 3 levels predict functional ovarian hyperandrogenism in the polycystic ovary syndrome. J Endocrinol Investig. 2011;34:685–691. doi: 10.3275/7726. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;14:3–28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336–340. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 15.Verit FF, Erel O. Oxidative stress in non-obese women with polycystic ovary syndrome: correlations with endocrine and screening parameters. Gynecol Obstet Investig. 2008;65:233–239. doi: 10.1159/000113046. [DOI] [PubMed] [Google Scholar]
- 16.Kuscu NK, Var A. Oxidative stress but not endothelial dysfunction exists in non-obese, young group of patients with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2009;88:612–617. doi: 10.1080/00016340902859315. [DOI] [PubMed] [Google Scholar]
- 17.Kandasamy S, Sivagamasundari RI, Bupathy A, Sethubathy S, Gobal V. Evaluation of insulin resistance and oxidative stress in obese patients with polycystic ovary syndrome. Int J Appl Biol Pharm Technol. 2010;1:391–397. [Google Scholar]
- 18.Kurdoglu Z, Ozkol H, Tuluce Y, Koyuncu I. Oxidative status and its relation with insulin resistance in young non-obese women with polycystic ovary syndrome. J Endocrinol Investig. 2011;26:134–142. doi: 10.3275/7682. [DOI] [PubMed] [Google Scholar]
- 19.Insler V, Lunenfeld B. Polycystic ovarian disease. In: Insler V, Lunenfeld B, editors. Infertility—male and female. 2. London: Churchill Livingstone; 1993. pp. 661–675. [Google Scholar]
- 20.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 21.Burtis CA, Ashwood ER, Bruns DE. Tietz text book of clinical chemistry and molecular diagnostics. 4. Philadelphia: WB Saunders; 2004. pp. 96–405. [Google Scholar]
- 22.Clark PM. Assays for insulin, proinsulin(s) and C-peptide. Ann Clin Biochem. 1999;36:541–564. doi: 10.1177/000456329903600501. [DOI] [PubMed] [Google Scholar]
- 23.Whitley RJ, Meikle AW, Watts NB. Endocrinology, part VI: adrenocortical steroids. In: Burtis CA, Ashwood ER, editors. Textbook of clinical chemistry. 4. Philadelphia: WB Saunders; 2004. pp. 508–531. [Google Scholar]
- 24.Wartofsky L, Handelsman DJ. Standardization of hormonal assays for the 21st century. J Clin Endocrinol Metab. 2010;95:4971–5143. doi: 10.1210/jc.2010-2369. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler MJ. The determination of bio-available testosterone. Ann Clin Biochem. 1995;32:345–351. doi: 10.1177/000456329503200401. [DOI] [PubMed] [Google Scholar]
- 26.Abuja PM, Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta. 2001;306:1–17. doi: 10.1016/S0009-8981(01)00393-X. [DOI] [PubMed] [Google Scholar]
- 27.Emoto M, Nishizawa Y, Maekawa K, Hiura Y, Kanda H, Kawagishi Y, et al. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care. 1999;22:818–822. doi: 10.2337/diacare.22.5.818. [DOI] [PubMed] [Google Scholar]
- 28.Mahajan BK. Methods in biostatistics for medical students and research workers. 7. New Delhi: Jaypee Brothers; 2010. pp. 80–194. [Google Scholar]
- 29.Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. The Laboratory Diagnosis of Testosterone Deficiency, Am Urol Assoc Educ Res; 2013:7–12. [DOI] [PubMed]
- 30.Azziz R, Carmina E, Dewailly D, Kandarakis DE, Morreale EHF, Futterweit W, et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]