Abstract
Mucopolysaccharidoses, a group of inherited disorders are associated with defects in glycosaminoglycan metabolism. Thus, assessment of urinary glycosaminoglycan is used as a screening test for mucopolysaccharidoses. The detection methods range from qualitative spot tests to quantification using metachromatic dyes. In our laboratory we optimized a spectrophotometric quantitative method using a metachromatic dye, dimethylmethylene blue. Heparan sulfate was used for quantification. The glycosaminoglycan–dye complex showed a marked shift in color with increase in concentration. The color complex was quantified at 520 nm. The method was linear from 10–89 mg/L. An age matched normal range was obtained in 177 healthy individuals, grouped in 8 different age groups from neonates to adults. Urinary glycosaminoglycan concentration varied distinctly amongst the study population wherein the lowest range in healthy neonates was more than 3 times the upper limit of healthy adults. Urine samples from 10 patients with mucopolysaccharidoses were also included in the study for clinical validation. The method qualified both analytical and clinical validation and was found to be simple, robust and ideal to be offered as a screening test for mucopplysaccharidoses in a routine clinical chemistry laboratory.
Keywords: Glycosaminoglycan; Mucopolysaccharidoses; 1,9-Dimethymethylene blue; Age matched reference range
Introduction
Glycosaminoglycans (GAGs) are complex polysaccharides, which form major components of extracellular matrix. Chondroitin sulfate, keratan sulfate, dermatan sulfate, heparan sulfate, heparin and hyaluronate are the major GAGs [1]. They are involved in various cell–cell and cell–matrix interactions and migration. These processes are essential for embryonic and fetal development and are responsible for the high turnover of GAGs in early stages of life. The urinary excretion amongst healthy individuals decreases with age [2].
Glycosaminoglycans are metabolized in the lysosomes by a cascade of enzymes and any defect in respective pathways leads to their tissue accumulation and increased urinary excretion. These inherited disorders of metabolic defects are grouped as mucopolysaccharidoses (MPSs) [3]. There are around 8 sub categories (MPS I–MPS VIII) based on the type of enzyme defect and/or substrate/product accumulated. They have a broad-spectrum continuum of clinical severity often overlapping with non-lysosomal conditions. Clinical severity may be partly ascribed to different mutations within the same gene [4].
Increased urinary excretion is a consistent finding in MPS however the type of GAG excreted is characteristic to the sub categories. MPS I and II result due to deficiency of alpha-l-iduronidase and iduronate sulfatase respectively wherein, dermatan sulfate and heparan sulfate are primarily excreted. MPS IIIa–IIId result due to deficiency of four different enzymes mainly associated with degradation of heparan sulfate. Deficiency of several other enzymes are responsible for MPS type IV–VIII causing increased excretion of keratan sulfate, chondroitin sulfate and dermatan sulfate either individually or in different combinations [3]. The amount of glycosaminoglycan excreted varies in different types of MPS and is also dependent on severity of the condition. For instance, deficiency of alpha-l-iduronidase results in three subtypes of MPS-I ranging from Scheie which is a milder form to Hurler, the most severe form. Thus, a test sensitive enough to detect even slight variation in glycosaminoglycan concentration is required [5].
Several methods such as berry spot test which includes staining dried urine spot with dyes like Azure A, toluidine blue etc., turbidimetry which depends on interaction of GAGs with cationic detergent or albumin, carbazole precipitation mainly based on uronic acid precipitation, metachromatic dye binding techniques and others have been recently utilized for estimation of urinary GAGs. Each of these test results are affected by concentration, pH, ionic strength and type of glycosaminoglycan accumulated in the urine [6, 7]. These methods are cumbersome and require large volume of urine sample thus limiting their availability in routine clinical laboratories. Complex chromatographic techniques like gas chromatography–mass spectrometry (GC–MS), liquid chromatography are also used for estimation however the availability of these in routine laboratory is limited [7].
Rapid and sensitive method using dimethylmethylene blue (DMB) dye has been reported [6, 8]. We have optimized the above method for routine use in a clinical chemistry laboratory. The method was validated and used to assess the excretion of urinary GAGs amongst healthy individuals. To the best of our knowledge this data is not available for healthy Indians and hence the results reported would help to interpret the test results and diagnose patients with MPSs.
Materials and Methods
Method optimization and validation for urinary GAG estimation was carried out in the Biochemistry section of our hospital. Random urine samples were obtained from 177 healthy subjects ranging from neonates to adults. Since urinary GAG excretion decreases significantly with age particularly in the earlier years of life we have included several sub groups in the younger age group. The sub groups are categorized based on the American Association for Clinical Chemistry (AACC) publication [9] wherein a characteristic variation in the mean values is obtained in each defined age group. The excretion of GAGs does not vary significantly beyond 10 years and hence all these individuals are in one group of 10 years and above. Our aim was to verify the literature reported reference range in our population and hence as per the third edition of the Clinical and Laboratory Standard Institute (CLSI) document C28-A3 [10] around 20 individuals or more were included in each group. As a part of clinical validation urine samples from 10 patients with MPS diagnosed based on clinical and/or laboratory and radiological findings were analysed. The clinical efficacy of the method to diagnose MPS was assessed by comparing GAG results obtained in these patients.
The demographic information, antenatal and family history of metabolic disorders was collected from each participant using a questionnaire. The subjects had no psychomotor and/or skeletal disability. Urine samples were collected in sterile containers without any preservative and were immediately stored at −20 °C until analysis.
The method reported by de Jong et al. [6, 14] was used for GAG estimation except for the DMB dye reagent preparation. The dye preparation was modified for ion concentration as per Whitley et al. [8] wherein DMB stock reagent containing 0.35 mmol of 1,9-dimethylmethylene blue was prepared in sodium formate buffer (pH 3.5, 0.2 mmol/L) and stored in an amber color bottle. A working DMB reagent was diluted 10 times with Na-formate prior to use. The dye was then buffered by adding 1 part of 2 M Tris buffer (pH 10) to 9 parts of working DMB reagent for estimation [6]. The pH of this Tris buffered dye was 8.5–8.8. The matrix effect due to these buffers was blanked by using Tris-formate buffer containing 9 parts of 55 mM of formic acid and 1 part of 2 M Tris. Linearity verification was performed up to 160 mg/L of heparan sulfate standard. A stock standard of 100 mg/L heparan sulfate was used to prepare five working standards—ranging from 11.1 to 89 mg/L. Spectrophotometric quantification of the color complex formed due to the binding of GAGs to DMB dye was done at 520 nm. Two set of experiments, one with Tris formate buffer and the other with DMB-Tris dye were performed. A volume of 100 μl standards and samples were mixed with 1,000 μl of Tris-formate buffer and working DMB-Tris buffer respectively. The color complex formed was estimated at 520 nm within 15 min.
Dimethylmethylene blue was preferred due to its high binding efficacy and stability of the color complex as compared to other dyes. The dye exhibits classical metachromasia in the presence of sulfated GAGs. The method was assessed for linearity, specificity, sensitivity, precision, inter person and inter equipment variations. The method was also evaluated using chondroitin sulfate and dermatan sulfate standards at the same concentrations as above. The urinary GAG concentration was reported as mg/mmol creatinine. Normalization to creatinine is required to account for the hydration status of an individual. Urinary creatinine estimation was performed using Jaffe rate method on Beckman DXC 800 auto-analyzer [11]. The calibration for this method is traceable to isotope dilution mass spectrometry. As a part of the quality assurance program the laboratory enrolled for the BGL proficiency-testing scheme of College of American Pathologists (CAP). Samples received in the second cycle of 2012 and both cycles of 2013 were analyzed and reported to CAP. The results of our finding were compared with those reported by peer laboratories.
Results
In the study we have optimized and reported the methodology for urinary GAG estimation. As a part of standardization we have assessed several analytical and clinical validation parameters such as analytical measurement range (AMR)/linearity, limit of detection (LOD)/limit of quantification (LOQ), analytical sensitivity including accuracy and precision, external proficiency testing, reference range verification and clinical validation with known samples.
Method Validation
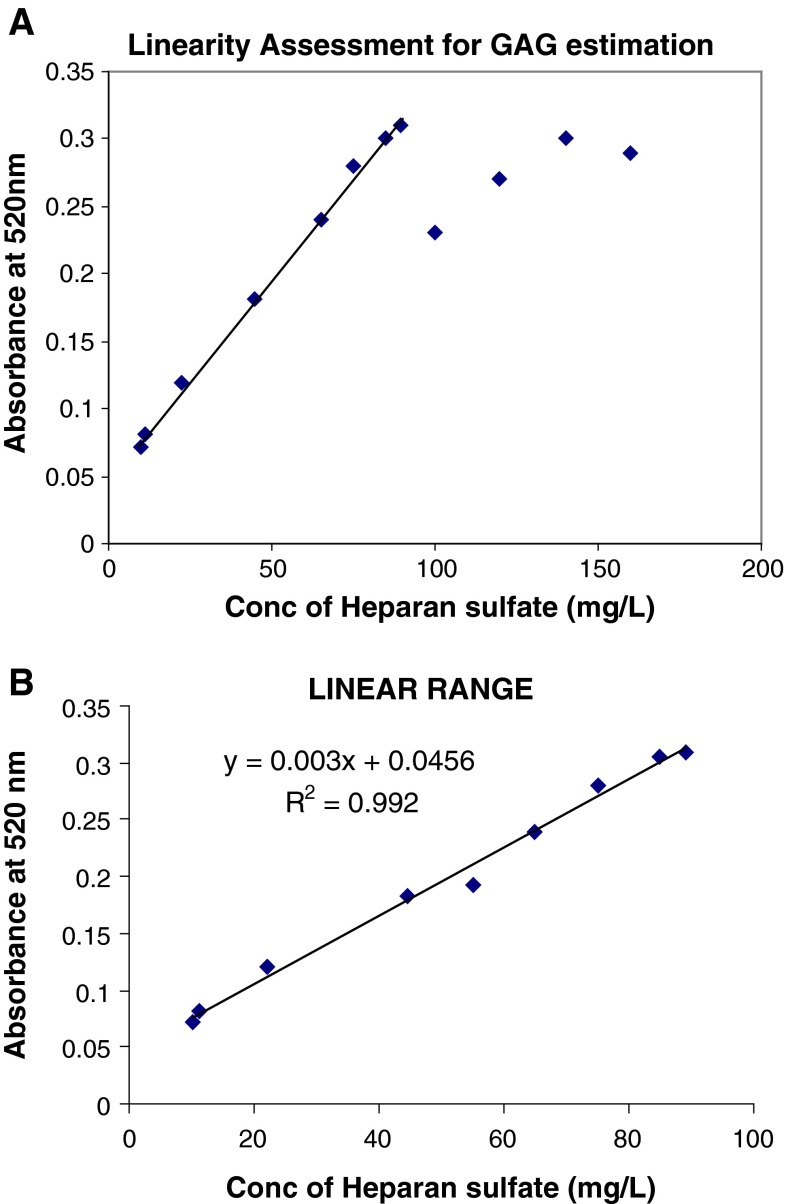
Test linearity was checked in the concentration range from 10 to 160 mg/L (Fig. 1a) and it was observed that heparan sulfate concentration showed reproducible results up to 89 mg/L while the limit of detection and quantification was 2 and 10 mg/L respectively. The analytical range of 10–89 mg/L showed an R2 = 0.992 (Fig. 1b). The reproducibility of the test results were validated on two different spectrophotometers, Shimadzu (UV 1650 PC) and Beckman Coulter (DU 800) and also by two different individuals.
Fig. 1.
a Linearity assessment for GAG estimation. b Method linearity
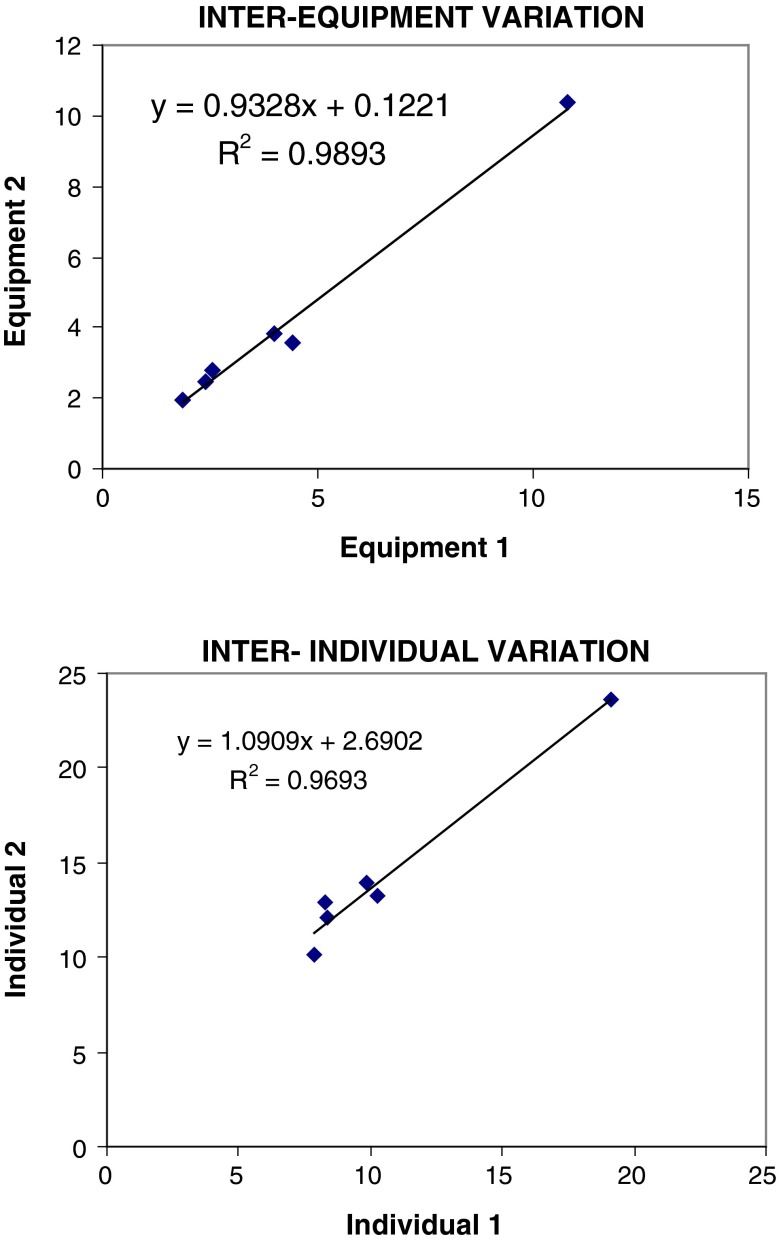
Inter equipment and inter individual variations showed R2 of 0.989 and 0.969 respectively (Fig. 2). The color complex with chondroitin and dermatan sulfate standards was unstable and showed variations in absorbance with time. The color complex with heparan sulfate standard and urine samples was stable for at least 15 min.
Fig. 2.
Inter equipment and inter individual comparison
Quality Control
Urine from healthy individuals was aliquoted in one-time use vials and each vial was used with every batch as a quality control sample. The proficiency testing samples, BGL-08 (2012) and BGL-03 (2013) received from CAP were also processed and the results obtained from CAP were assessed. The internal quality control Levy Jenning chart showed a CV of 15 % while a 100 % concurrence was obtained with results of peer laboratories participating in the CAP proficiency-testing program. Urine samples from ten known MPSs patients were analyzed for clinical validation. The results showed diagnostic efficacy for these samples.
Urinary GAGs in Healthy Individuals
We have also verified the reported reference range in our population by estimating urinary GAGs in the above mentioned sub groups including 20 subjects each. Concentration of urinary GAGs in different age groups is given in Table 1. It was seen that the excretion of GAGs decreased with age and did not show any remarkable gender variation (p > 0.05) in all study groups.
Table 1.
Comparison of literature and laboratory obtained reference range
| Age groups | Urinary GAGs | Reference range (mg/mmol creat) | ||
|---|---|---|---|---|
| Mean ± SD (mg/mmol creat) | ||||
| Males | Females | Laboratory (males + females) | Literature [9] | |
| 0–6 months | 42.4 ± 11.2 (n = 11) | 37.2 ± 13.8 (n = 4) | 18.6–53.7 | 15.2–52.2 |
| 6–12 months | 25.1 ± 6.4 (n = 13) | 19.27 ± 10.2 (n = 4) | 9.2–33.3 | 15.1–31.5 |
| 1–2 years | 18.8 ± 7.4 (n = 7) | 22.5 ± 6.6 (n = 13) | 7.6–29.7 | 9.1–29.9 |
| 2–4 years | 13.6 ± 3.3 (n = 11) | 18.3 ± 4.6 (n = 9) | 10.0–24.0 | 7.7–21.3 |
| 4–6 years | 12.5 ± 2.0 (n = 9) | 12.9 ± 2.6 (n = 11) | 9.9–17.1 | 7.6–14.4 |
| 6–8 years | 11.3 ± 1.9 (n = 9) | 12.2 ± 2.5 (n = 11) | 8.0–18.5 | 5.7–12.9 |
| 8–10 years | 9.3 ± 2.3 (n = 12) | 10.4 ± 1.2 (n = 8) | 6.9–13.0 | 5.2–11.6 |
| >10 years | 3.5 ± 1.6 (n = 29) | 3.73 ± 1.1 (n = 16) | 1.0–7.7 | 1.5–5.1 |
Clinical Validation
The GAG values in 10 MPS patients were as reported in Table 2. It is evident from the table that in 7 patients the GAG excretion was significantly high i.e. ranging from 2.2 to 7.9 fold above the upper limit of the age matched reference range while in 3 patients the GAG concentration was marginally elevated than the normal limit.
Table 2.
Urinary GAGs in patients with mucopolysaccharidoses
| Patient | Age (years) | Urine GAG (mg/mmol of creat) | Age matched reference range (mg/mmol of creat) | |
|---|---|---|---|---|
| Laboratory | Literature reference range [9] | |||
| 1 | 1 | 67.8 | 7.6–29.7 | 9.1–29.9 |
| 2 | 2 | 26.2 | 10.0–24.0 | 7.7–21.3 |
| 3 | 3 | 21.6 | 10.0–24.0 | 7.7–21.3 |
| 4 | 4 | 49.4 | 9.9–17.1 | 7.6–14.4 |
| 5 | 5 | 114.6 | 9.9–17.1 | 7.6–14.4 |
| 6 | 6 | 46.3 | 8.0–18.5 | 5.7–12.9 |
| 7 | 6 | 31 | 8.0–18.5 | 5.7–12.9 |
| 8 | 8 | 51.5 | 6.9–13.0 | 5.2–11.6 |
| 9 | 9 | 15 | 6.9–13.0 | 5.2–11.6 |
| 10 | 12 | 35 | 1.0–7.7 | 1.5–5.1 |
Discussion
Amongst the various methods applied for urinary GAG determination, dye-binding techniques are widely applicable due to their potential for quantitative results. DMB dye forms a stable soluble complex with sulfated GAGs and is used to determine GAGs from cartilage culture media, synovial fluid and in purification procedures [12, 13]. Alcian blue dye has also been used for quantification however the dye complex forms fine precipitate which affects the reproducibility of the test results. Urinary proteins may interfere with the dye binding and affect the GAG quantification [14]. Tris buffered DMB dye used in the method has been reported to have a minimal protein interference up to 5 g/L for IgG and up to 1 g/L of albumin [14]. Drugs likely to be administered to infants produce no interference except for formulations containing artificial coloring agents [8]. In the present study the patient and control samples were collected only after cessation of medicines.
The commercially available chondroitin, dermatan and heparan sulfate are isolated from the tissues. The characteristic composition of tissue GAG is different from the urine and it is this urinary composition which favors stable DMB-dye complex. Since heparan sulfate is a low molecular weight compound, tissue extracted heparan sulfate standard becomes an exception [6]. Irrespective of its source it forms a stable complex with DMB-dye similar to urinary GAGs and hence can be used as a standard for total urinary GAG estimation.
The dye complex was found stable and soluble during the assay in the analytical range from 10 to 89 mg/L of heparan sulfate. The concentration >89 mg/L did not show a proportional increase in the absorbance. The linearity reported by de Jong et al. [6] and Whitley et al. [8] ranged from 1 to 70 mg/L and 10 to 150 mg/L respectively. An aqueous DMB solution is used by de Jong et al. [6] while we have prepared DMB in sodium formate buffer. The sample to dye ratio in our method is 1:10 while a ratio of 1:2.5 is used by Whitley et al. These variations in the method have possibly contributed to difference in linearity between the methods. The present method has been thoroughly validated for accuracy, precision, linearity, ruggedness and robustness. The method has also qualified the proficiency testing program of CAP and has been adapted for clinical service.
Glycosaminoglycan concentration in healthy individuals decrease with age and the amount excreted in children is observed to be more than 2 SD of the adult range in our subjects. These values suggest a substantial overlap in normal healthy individuals of different age groups and diagnostic GAG concentration. The age matched sub groups and the GAG concentrations obtained in our study are similar to those reported by Soldin et al. [9] suggesting that GAG excretion may not be affected by ethnicity. A similar trend of decrease in excretion with age has also been reported by de Jong et al. [14] however the GAG concentrations are not comparable due to an age overlap in each sub groups in all studies.
Glycosaminoglycan excretion in different MPSs varies with type and severity of the disease in each phenotype. Amongst our MPS patients, 3 had GAG concentration towards upper normal limit while significantly increased excretion was seen in others. GAG quantification only screens the patients with MPS while further analysis is required for identification of the type of proteoglycan by separation techniques like chromatography, electrophoresis [7] etc. or an enzyme or molecular diagnosis to confirm the genetic defect. Thus, an age matched reference ranges established or verified in each laboratory are essential for interpretation of test results.
The method reported is reliable and simple and can be easily adapted by routine clinical chemistry laboratories. The service offered would help to rule out MPS in suspected cases and would minimize sample send outs to referral Biochemical Genetics laboratories.
Acknowledgments
We acknowledge support extended by the National Health & Education society for the funding, healthy volunteers for the study sample. Dr. Dhanashri Shetty for reviewing the manuscript and Dr. Vrajesh Udani for referring MPS patients for validation.
References
- 1.Berg J, Tymoczko J, Stryer L. Biochemistry. 5. New York: W.H.Freeman and Company; 2002. [Google Scholar]
- 2.Willen M, Sorrell M, Lekan C, Davis B, Caplan A. Patterns of glycosaminoglycans/proteoglycans immunostaining in human skin during aging. Cleveland: The Society for Investigative Dermatology, Inc.; 1991. pp. 968–974. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho M, Lacerda L, Alves S. Glycosaminoglycan storage disorders: a review. Biochem Res Int. 2012 doi: 10.1155/2012/471325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meikle P, Fiezt M, Hopwood J. Diagnosis of lysosomal storage disorders: current techniques and future directions. Expert Rev Mol Diagn. 2004;4(5):677–691. doi: 10.1586/14737159.4.5.677. [DOI] [PubMed] [Google Scholar]
- 5.Manley G, Williams u. Urinary excretion of glycosaminoglycans in the various forms of gargoylism. J Clin Pathol. 1969;22:67–75. doi: 10.1136/jcp.22.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong J, Wevers R, Laarakkers C, Poorthuls B. Dimethylmethylene blue-based spectrometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin Chem. 1989;35(7):1472–1477. [PubMed] [Google Scholar]
- 7.Pennock C. A review and selection of simple laboratory methods used for the study of glycosaminoglycan excretion and the diagnosis of the mucopolysacharidoses. J Clin Pathol. 1976;29:111–123. doi: 10.1136/jcp.29.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitley C, Ridnour M, Draper K, Dutton C, Neglia J. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin Chem. 1989;35(3):374–379. [PubMed] [Google Scholar]
- 9.Soldin S, Brungara C, Hicks J. Pediatric reference ranges. 3. Washington, DC: AACC Press; 1999. [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Defining, estabilishing, and verifying reference intervals in the clinical laboraotry; Approved Guideline. 3rd ed. CLSI document C28-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- 11.Beckman Coulter. Synchron system(s). Chemistry information sheet. Creatinine REF A40920.
- 12.Panin G, Nala S, Dall’Amico R, Chlandetti L, Zachello F, Catassi C, et al. Simple spectrophotometric quantification of urinary excretion of glycosaminoglycan sulfates. Clin Chem. 1986;32(11):2073–2076. [PubMed] [Google Scholar]
- 13.Carroll G. Spectrophotometric measurement of proteoglycans in osteoarthritic synovial fluid. Ann Rheumatic Dis. 1987;46:375–379. doi: 10.1136/ard.46.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong J, Wevers R, Liebrand-van Sambeek R. Measuring urinary glycosminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidoses based on dimethylmethylene blue. Clin Chem. 1992;38(6):803–807. [PubMed] [Google Scholar]