Abstract
Diabetes and tuberculosis are world’s most deadly epidemics. People suffering from diabetes are susceptible to tuberculosis. Molecular link between the two is largely unknown. It is known that Vitamin A receptor (RXR) heterodimerizes with Vitamin D receptor (VDR) and Peroxisome proliferator-activator receptor-γ (PPARγ) to regulate Tryptophan-aspartate containing coat protein (TACO) expression and fatty acid metabolism respectively, so it would be interesting to check the expression of these genes in diabetes mellitus (DM) patients which might explain the susceptibility of diabetics to tuberculosis. In this study, we checked the expression of RXR, VDR, TACO and Interferon-γ (IFNγ) genes in type-2 DM patients for understanding the link between the two diseases. We observed down regulation of RXR gene and corresponding up regulation of TACO gene expression. We have not observed significant change in expression of VDR and IFNγ genes in type-2 DM patients. Repression of RXR gene could hamper VDR-RXR heterodimer formation and thus would up regulate TACO gene expression which may predispose the type-2 DM patients to tuberculosis. Also, decrease in RXR-PPARγ heterodimer could be involved in DM.
Keywords: Diabetes, Tuberculosis, Vitamin D receptor (VDR), Vitamin A receptor (RXR), PPARγ, Molecular link, Risk factor
Introduction
Diabetes mellitus (DM) and tuberculosis are major killers of the man kind and both of the diseases are widely prevalent across the globe. The incidence of each is growing at over whelming pace and is the matter of concern in the current scenario [1]. This is particularly true for developing countries of South East Asia and Indian subcontinent [2]. Certain segments of the population still remain at increased risk for TB. Major risk factors for TB include poverty, starvation, immunodeficiency diseases such as AIDS, smoking, DM and many others [3, 4]. DM has also emerged as a common disease of the modern world so it is regarded as a potential risk factor for TB [5–11]. It is believed that diabetic patients have compromised immunity, which makes them more susceptible to bacterial infections such as tuberculosis [12, 13]. But still the molecular link between tuberculosis and diabetes is not clearly understood [14]. Our investigation was aimed to understand the molecular link between DM and TB.
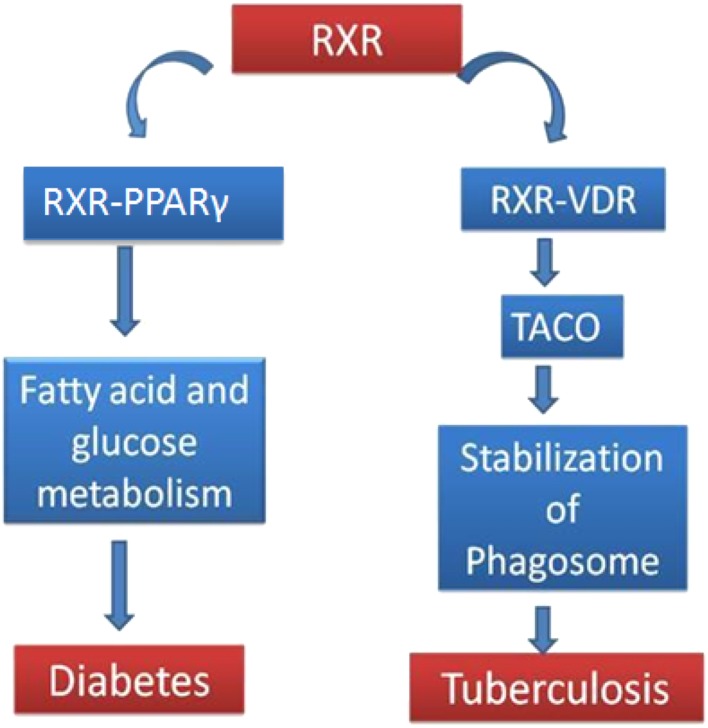
The maturation arrest of mycobacterial phagosome has been shown to play a key role in the survival of pathogenic mycobacteria within macrophages. It results from the retention of tryptophan-aspartate-containing coat protein (TACO) (also known as p57 or coronin-1) on phagosome which further allows the replication of the pathogen. Presence of TACO protein on phagosomes depends on cholesterol, a key factor associated with mycobacterial uptake by macrophages. Role of TACO in maturation arrest and tuberculosis is well understood [15, 16]. It has been shown that VDR-RXR heterodimer regulates the expression of phagosome coat protein TACO, a protein important for pathogenesis of tuberculosis [17]. PPAR- γ is involved in metabolic functions like glucose/fatty acid metabolism. VDR/Vitamin D3 play significant role in immunity against tuberculosis [18–20].It is noteworthy that RXR forms heterodimer with both PPAR- γ and VDR for their physiological functions [21] (Fig. 1). VDR, RXR and TACO are key players in tuberculosis. It has been reported that VDR expression decreases in pulmonary tuberculosis patients. Therefore it can be hypothesized that RXR, VDR, & TACO gene expression profile in diabetes may help in understanding the molecular pathology behind the increased predisposition of diabetes patients to tuberculosis.
Fig. 1.
Schematic view: RXR heterodimerizes with PPARγ and VDR to affect fatty acid/glucose metabolism and TACO protein expression respectively. Decrease in RXR- PPARγ heterodimer is responsible for diabetic state and increase in TACO expression is crucial for tuberculosis. RXR-VDR negatively regulates TACO expression. For details refer text
Materials and Methods
Materials
The reagents employed throughout the study were of analytical grade. Fresh blood sample from confirmed diabetic patient was collected just before an experiment. cDNA was synthesized by the kit manufactured by Fermentas (#K1622). DNA amplification was done by polymerase chain reaction kit manufactured by Qiagen (#201203). Primers were designed (Table 1) and synthesized by Genei, Bangalore.
Table 1.
Primers sequence for Interferon-γ, TACO, VDR, RXR and GAPDH genes
| S. No | Gene | Primer |
|---|---|---|
| 1 | Interferon-γ (Gene ID: 3458) | Forward: 5′CGTTTTGGGTTCTCTTGGCTGTT3′ |
| Reverse: 5′CTCCTTTTTCGCTTCCCTGTTTT3′ | ||
| 2 | TACO (Gene ID: 11151) | Forward: 5′CCAGTGCTATGAGGATGTGCGCG3′ |
| Reverse: 5′GACACGACTCGCTTGTCACGGC3′ | ||
| 3 | VDR (Gene ID: 7421) | Forward: 5′GGAGACTTTGACCGGAACGTG3′ |
| Reverse: 5′ATGAGGCTGAAGACGGTCAAG3′ | ||
| 4 | RXR (Gene ID: 6256) | Forward: 5′GATTTCCTGCCGCTCGATTC3′ |
| Reverse: 5′TACTTGGGGCAGTCGTCGTC3′ | ||
| 5 | GAPDH (Gene ID: 2597) | Forward: 5′CCGTCTAGAAAAACCTGCC3′ |
| Reverse: 5′GCCAAATTCGTTGTCATACC3′ |
Study Population
The study population comprised of fifteen cases of patients suffering from DM (who fulfilled the below mentioned inclusion and exclusion criteria) and eight age and sex matched healthy controls (Table 2). Random blood glucose levels more than 200 mg/dl [22] and glycated Hemoglobin (HbA1c) levels more than 8 % were taken as inclusion criteria. Patients suffering from any disease other than diabetes as reported by the OPD card of PGIMER, patients on any medication including antidiabetics, vitamins etc. prior to one month of blood sample collection and evidence of tuberculosis served the purpose of prompt exclusion.
Table 2.
Subject details (from whom samples were collected). The table showing basic characters/parameters of the human subjects participated in the study
| Age (years) | Sex: Male(M), Female(F) | Fasting blood glucose (mg/dl) | Random blood glucose (mg/dl) | HbA1c % | Ethnicity | |
|---|---|---|---|---|---|---|
| Diabetic Patient Cases. n = 15 | 25–60 | M = 10 | 158 ± 10 | 220 ± 5 | 8.3 ± 0.25 | Indian |
| F = 5 | ||||||
| Healthy Volunteer Controls. n = 8 | 25–60 | M = 5 | 84 ± 7 | 120 ± 20 | 6.2 ± 0.6 | Indian |
| F = 3 |
Family and Medical History of Patients
All patients and healthy volunteers were of Indian origin. They have not received any medication or Vitamin supplements in last 3 months. They have not undergone any major surgery in last 1 year. Out of 15 diabetic cases, three had direct relatives with diabetes. two of the diabetes cases reported blurred vision. None of the patients was diagnosed with symptoms of nephropathy. For healthy controls, there was no history of diabetes and tuberculosis. None of the diabetes patients or their direct family relatives had the history of tuberculosis.
Biological Sample Collection
5 ml of blood was collected in falcon tube containing 5 mg EDTA, from median cubital vein of the anticubital fossa of the patient by an experienced phlebotomist in the endocrinology outpatient department.
Ethical Note
The above study is cleared by Institute Ethics Committee (Micro/2008/2564). The study has been performed according to ethical standards as formulated in the Helsiniki Declaration of 1975 (revised in 1983).
Methods
5 ml of venous blood samples was drawn from proved cases of DM patients and healthy human volunteers. Random glucose levels were measured by glucose oxidase method [22]. Blood samples were subjected to standard HPLC protocol for determination of glycated hemoglobin levels. Peripheral blood mononuclear cells were isolated from whole blood by ficoll based density gradient centrifugation followed by total RNA isolation as per referred protocol [23]. Briefly, pellet of cells was suspended in 1 ml of denaturing solution (25 mM Sodium citrate, 0.5 % N-lauryl sarkosine, 0.1 M 2-Mercaptoethanol, 4 M Guanidium thiocyanate) followed by addition of 100 µl sodium acetate solution (2 M, pH 4). Subsequently, 1 ml phenol:chloroform reagent(5:1) pH 4.7 and 4 µl isoamyl alcohol was added to sample to segregate DNA/proteins and RNA into phenol phase and aqueous phase respectively. The sample was then centrifuged at 10,000 rpm at 4 °C for 30 min. Upper aqueous phase was transferred to DEPC treated eppendorf tube. RNA was precipitated by addition of 1 ml chilled isopropanol. Further, precipitated RNA was pelleted down by centrifugation at 10,000 rpm for 30 min at 4 °C. RNA pellet was re-suspended in 75 % ethanol, sedimented and vaccum dried. Latter step was repeated twice. Pellet was vaccum dried and re-suspended in 17 µl of DEPC treated water and incubated at 60 °C on heat block for 10 min to dissolve RNA pellet properly in aqueous phase. Integrity of RNA was verified by electrophoretic size separation in 1 % ethidium-bromide stained agarose gel. Then, cDNA was synthesized from the isolated total RNA using a cDNA synthesis kit as per manufacturer’s instructions. Subsequently TACO, VDR, RXR and IFN-γ gene expression analysis was carried out by amplification of the respective cDNA’s by gene specific primers (Tables 2, 3). The ethidium bromide stained PCR products were photographed and densitometry analysis of gels was done for estimation of expression levels by the application of SCION image analysis software. Bioinformatics database “STRING” (Version 9.0) was further explored for finding the potential functional and binding partners of RXR (http://string-db.org/).
Table 3.
PCR method used for gene amplification
| Initial Denaturation | Denaturation | Annealing | Extension | Repetitions | |
|---|---|---|---|---|---|
| VDR | 94 °C for 4 min | 94 °C for 45 s | 58 °C for 30 s | 72 °C for 45 s | 20 times |
| TACO | 94 °C for 4 min | 94 °C for 45 s | 60 °C for 45 s | 72 °C for 90 s | 20 times |
| Interferonγ | 94 °C for 4 min | 94 °C for 1 min | 60 °C for 1 min | 72 °C for 1 min | 20 times |
| RXR | 94 °C for 4 min | 94 °C for 45 s | 58 °C for 30 s | 72 °C for 45 s | 20 times |
| GAPDH | 94 °C for 4 min | 94 °C for 45 s | 55 °C for 45 s | 72 °C for 90 s | 20 times |
Results
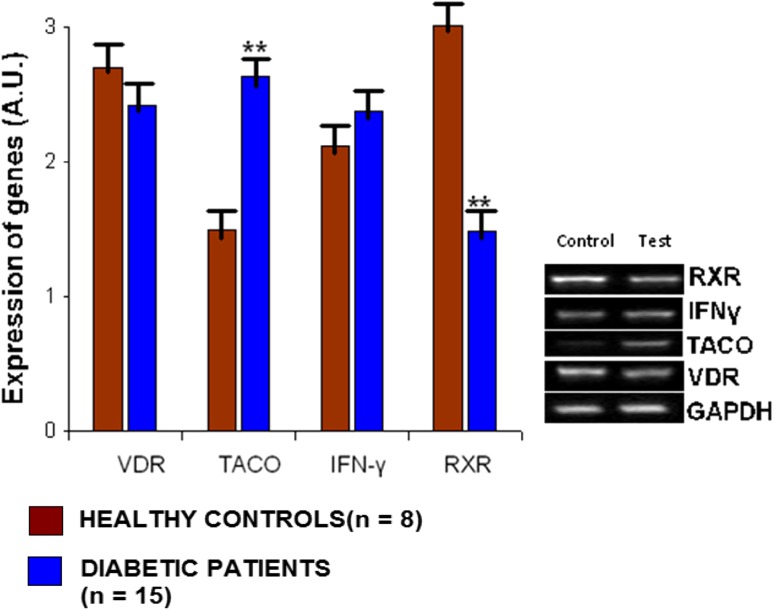
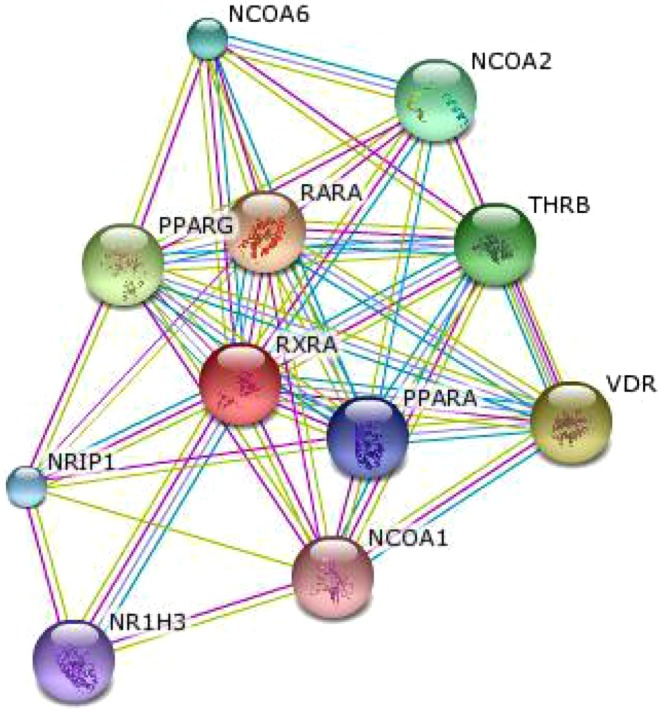
In this study, we observed that in a group of diabetic patients, there is a significant reduction of RXR gene expression along with concomitant increase in TACO gene expression in comparison to healthy controls. It was reported that VitaminD3–Retinoic acid combination via VDR-RXR heterodimer formation down regulate TACO gene expression and can confer protection against tuberculosis [17]. Reduction in RXR gene expression can hinder VDR-RXR heterodimer formation and thus may lead to up regulation of TACO gene expression. So it is expected that RXR gene repression would result in elevated TACO gene expression which has been observed in this study (Fig. 2). It is now well understood that an increase of TACO gene expression may predispose to tuberculosis since the persistence of M. tuberculosis within the macrophage is TACO dependent [16]. STRING database was searched for finding functional partners of RXR, which gave significant score to VDR and PPARγ (Fig. 3), thus, supporting our proposed link. We found insignificant change in expression of VDR and IFNγ genes in diabetes patient group in comparison to healthy controls (Fig. 2).
Fig. 2.
Densitometry analysis for estimation of expression levels of VDR, TACO, IFN-γ and RXR genes in peripheral blood mononuclear cells of diabetic patients (n = 15) in comparison to healthy controls (n = 8)
Fig. 3.
RXR functional binding partners network (www.string-db.org/)
Discussion
With reduction in RXR gene expression, TACO gene expression is enhanced in PBMCs of diabetic patients. It is apparent that reduction in RXR gene expression and increase in TACO gene expression which has been observed in the PBMCs of diabetic patients, can account for the increased incidence of tuberculosis in diabetic state. This study validates the negative regulation of TACO by VDR-RXR heterodimer, as observed by the analysis of gene expression in healthy controls and DM patients [24]. In the unpublished data, we have observed an increase in TACO expression in tuberculosis patients. This further relates with the stabilization of phagosome in tuberculosis patients (Fig. 4).
Fig. 4.

Proposed link: If RXR expression is less then VDR would have less RXR to heterodimerize, thus reducing the levels of VDR-RXR heterodimer. Less RXR-VDR means more TACO expression which is known to be involved in pathogenesis of tuberculosis (For details, please refer text)
It has been shown that VDR competes with the other receptors like PPARγ for heterodimerizing with RXR and there can be transcriptional interference due to this competition [25, 26]. This competition implies the dynamic equilibrium between VDR, RXR, PPARγ, VDR-RXR and PPARγ-RXR heterodimer. So if RXR expression goes down, it will further limit the heterodimer formation and thus limiting the function of VDR and PPARγ. Therefore in latter condition where RXR expression is less, the physiological function of VDR-RXR heterodimer and PPARγ-RXR heterodimer could be compromised. It is noteworthy that PPARγ agonists are important blood sugar lowering agent and loss of function mutation of PPARγ is known to predispose diabetes [27]. PPARγ-RXR form heterodimers that are directly involved in regulation of transcription of genes involved in insulin action, adipocyte differentiation and lipid metabolism [28].Therefore any reduction in RXR gene expression will hinder PPARγ-RXR heterodimer formation which may predispose individuals to hyperglycemic state and thereby causing DM.
Also, in this study, it is shown that there is a reduction in the VDR gene expression in the diabetic patients which is not statistically significant. It is possible that in the background of significant RXR repression in diabetic state, a minor decrease of VDR expression may be biologically significant and can impede VDR-RXR heterodimer formation and thus induce TACO gene expression. It may also account for increased association of tuberculosis with diabetic state. We have also observed that there is no significant change in IFNγ gene expression in diabetic state. It is known that IFNγ response confers protection against tuberculosis [29] but the minor difference in IFNγ gene expression in both the groups should be physiologically insignificant.
STRING database search also supports the proposed link. STRING database search further provides us with many more binding partners of RXR which could be further explored for understanding the common pathophysiology of TB and DM in near future. This study provides insights about the plausible molecular link between DM and TB. As DM is a complex disease, we presume that there may be many other pathways which could be involved in increasing the susceptibility of DM patients to TB. Verification of the results should be done at large sample size. Also, studies should be verified at protein levels. Conclusions drawn from results of gene expression analysis fit well with known facts and further research should be done to further validate this study.
Contribution
Author KS did this work under the guidance of DB. KS, AS and DB developed the concept and gone through the final version of the manuscript.
Acknowledgments
Authors acknowledge Prof. A. Bhansali, Department of Endocrinology, PGIMER for providing clinical samples and DST, New Delhi for funding DB under SERC FAST Track Scheme for young scientists. DB thanks Department of Biotechnology, Government of India for financial assistance. Authors also acknowledge Prof. A Surolia, MBU, IISc and Mr. Likhesh Sharma, MBU, IISc for suggestions and support.
Abbreviations
- PBMC
Peripheral blood mononuclear cells
- RNA
Ribonucleic acid
- RXR
Retinoid-X-receptor
- TACO
Tryptophan-aspartate containing coat protein
- VDR
Vitamin-D-receptor
- Vit.D
Vitamin D
- WHO
World Health Organization
- PPARγ
Peroxisome proliferator- activated receptor gamma
- IFNγ
Interferon-γ
References
- 1.WHO. Global Prevalence of Diabetes, Estimates for the year 2000 and projections for 2030. 2004.
- 2.Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273(3):220–226. doi: 10.1001/jama.1995.03520270054031. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Tuberculosis Fact sheet N°104. 2013.
- 4.Oni T, Stoever K, Wilkinson RJ. Tuberculosis, HIV, and type 2 diabetes mellitus: a neglected priority. Lancet Respir Med. 2013;1(5):356–358. doi: 10.1016/S2213-2600(13)70116-4. [DOI] [PubMed] [Google Scholar]
- 5.Root HF. The association of diabetes and tuberculosis. N Engl J Med. 1934;210(3):127–147. doi: 10.1056/NEJM193401182100304. [DOI] [Google Scholar]
- 6.Boucot KR, Dillon ES, Cooper DA, Meier P, Richardson R. Tuberculosis among diabetics: the Philadelphia survey. Am Rev Tuberc. 1952;65(1:2):1–50. [PubMed] [Google Scholar]
- 7.Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis. 1995;76(6):529–533. doi: 10.1016/0962-8479(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 8.Rahim Z, Momi MS, Saha SK, Zaman K, Uddin KN, Jamil SN, et al. Pulmonary tuberculosis in patients with diabetes mellitus in Bangladesh. Int J Tuberc Lung Dis. 2012;16(8):1132–1133. doi: 10.5588/ijtld.11.0846. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y, Li L, Mi F, Du J, Dong Y, Li Z, et al. Screening patients with diabetes mellitus for tuberculosis in China. Trop Med Int Health. 2012;17(10):1302–1308. doi: 10.1111/j.1365-3156.2012.03069.x. [DOI] [PubMed] [Google Scholar]
- 10.Syed Suleiman SA, Ishaq Aweis DM, Mohamed AJ, Razakmuttalif A, Moussa MA. Role of diabetes in the prognosis and therapeutic outcome of tuberculosis. Int J Endocrinol. 2012; 645362. [DOI] [PMC free article] [PubMed]
- 11.Rosu V, Ahmed N, Paccagnini D, Gerlach G, Fadda G, Hasnain SE, et al. Specific immunoassays confirm association of Mycobacterium avium Subsp. paratuberculosis with type-1 but not type-2 diabetes mellitus. PLoS One. 2009;4(2):e4386. doi: 10.1371/journal.pone.0004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44(3):617–626. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis (Edinb) 2013;93(Suppl):S10–S14. doi: 10.1016/S1472-9792(13)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Attiyah RJ, Mustafa AS. Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovis bacilli Calmette-Guerin (BCG)-vaccinated healthy subjects. Clin Exp Immunol. 2009;158(1):64–73. doi: 10.1111/j.1365-2249.2009.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, Huygen K, et al. Survival of Mycobacteria in Macrophages Is Mediated by Coronin 1-Dependent Activation of Calcineurin. Cell. 2007;130(1):37–50. doi: 10.1016/j.cell.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 16.Anand PK, Kaul D. Downregulation of TACO gene transcription restricts mycobacterial entry/survival within human macrophages. FEMS Microbiol Lett. 2005;250(1):137–144. doi: 10.1016/j.femsle.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 17.Anand PK, Kaul D. Vitamin D3-dependent pathway regulates TACO gene transcription. Biochem Biophys Res Commun. 2003;310(3):876–877. doi: 10.1016/j.bbrc.2003.09.087. [DOI] [PubMed] [Google Scholar]
- 18.Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14(1):15–23. [PubMed] [Google Scholar]
- 19.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47(4):507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 20.Hamza MS, Pott S, Vega VB, Thomsen JS, Kandhadayar GS, Ng PW, et al. De novo identification of PPARgamma/RXR binding sites and direct targets during adipogenesis. PLoS One. 2009;4(3):e4907. doi: 10.1371/journal.pone.0004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balasubramanyam MMV. Current concepts of PPAR-γ signaling in Diabetes mellitus. Curr Sci. 2000;79:1440–1446. [Google Scholar]
- 22.Rosevear JW, Pfaff KJ, Service FJ, Molnar GD, Ackerman E. Glucose oxidase method for continuous automated blood glucose determination. Clin Chem. 1969;15(8):680–698. [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 24.Anand PK, Kaul D, Sharma M. Synergistic action of vitamin D and retinoic acid restricts invasion of macrophages by pathogenic mycobacteria. J Microbiol Immunol Infect. 2008;41(1):17–25. [PubMed] [Google Scholar]
- 25.Stolzt VD. New topics in vitamin D research. New York: Nova Publishers; 2006. [Google Scholar]
- 26.Alimirah F, Peng X, Yuan L, Mehta RR, von Knethen A, Choubey D, et al. Crosstalk between the peroxisome proliferator-activated receptor gamma (PPARgamma) and the vitamin D receptor (VDR) in human breast cancer cells: PPARgamma binds to VDR and inhibits 1alpha,25-dihydroxyvitamin D3 mediated transactivation. Exp Cell Res. 2012;318(19):2490–2497. doi: 10.1016/j.yexcr.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 28.Guleria RS, Choudhary R, Tanaka T, Baker KM, Pan J. Retinoic acid receptor-mediated signaling protects cardiomyocytes from hyperglycemia induced apoptosis: role of the renin-angiotensin system. J Cell Physiol. 2011;226(5):1292–1307. doi: 10.1002/jcp.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reljic R. IFN-gamma therapy of tuberculosis and related infections. J Interferon Cytokine Res. 2007;27(5):353–364. doi: 10.1089/jir.2006.0103. [DOI] [PubMed] [Google Scholar]