Abstract
To refer to metabolomics as a new field is injustice to ancient doctors who used ants to diagnose the patients of diabetes having glycosuria. Measuring the levels of molecules in biological fluids believing them to be the representatives of biochemical pathways of carbohydrates, fats, proteins, nucleic acids or xenobiotic metabolism and deciphering meaningful data from it is what can be called as metabolomics, just as high glucose in urine suggests diabetes mellitus. Genomics, epigenetics, proteomics, transcriptomics finally converge to metabolomics, which are the signatures of mechanisms of bodily processes which is why understanding this science can have many applications. Just as a heap of stones does not make a house, having data of metabolite levels does not make it a science. Analyzing this data would help us in constructing biochemical pathways and their interactions. Analyzing the changes caused by a drug in the metabolite levels would help us in deriving the mechanisms by which the drug acts. Comparing metabolite levels in diseased with non-diseased, good-responders with poor-responders to a particular drug can help in identifying new markers of a disease or response to a drug respectively. Also, metabolite levels of an endogenous substrate can tell us the status of a person’s metabolizing enzymes and help in drug dose titration. Generating hypothesis by identifying the new molecular markers and testing their utility in clinics seems to be the most promising approach in future. This review narrates the modes of quantifying and identifying metabolome, its proposed applications in diagnosis, monitoring and understanding the diseases and drug responses. We also intend to identify hindrances in using metabolomics in clinical studies or experiments.
Keywords: Metabolomics, Drug response, Disease markers, Metabolites in diagnosis
Introduction
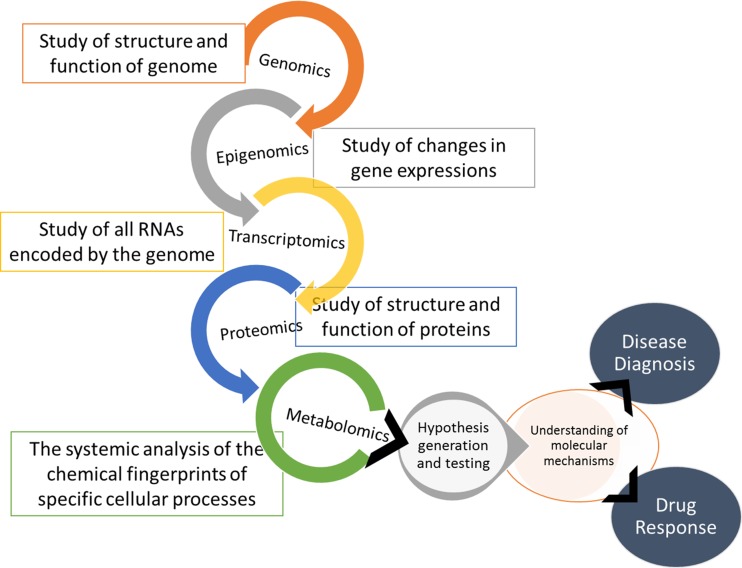
Genes code for proteins. Epigenetics regulates gene expression. Transcriptomics regulates RNA synthesis in turn regulating protein synthesis. Proteomics deals with the protein synthesis and function. The most dynamic functions of proteins are served as enzymes taking part in various metabolic pathways, using up substrates to form end products. Each reaction is therefore governed by epigenomics, genomics, transcriptomics and proteomics. The chemical signatures that each reaction leaves behind can therefore give important insights into various metabolic pathways of our body. Thus, metabolomics as defined by Oliver Fiehn is-‘A comprehensive analysis in which all the metabolites of a biological system are identified and quantified’ [1]. Quantitatively analyzing each metabolite in samples of biological fluids is what metabolomics deals with, in a way being the integration of all ‘omics’ (Fig. 1). Another such term-‘Metabonomics’ as defined by Jeremy Nicholson is-‘Quantitative measurement of the multiparametric time-related metabolic responses of a complex (multicellular) system to a pathophysiological intervention or genetic modification’ [2].
Fig. 1.
Metabolomics in hypothesis generation and testing
In as early as 1951, this concept was born in the mind of Williams R.J. [3] who tried to identify patterns of metabolite excretion by paper chromatography, i.e. qualitatively by analyzing data from over 2 lakh chromatograms. With the advent of gas chromatography (GC) and liquid chromatography, quantification of metabolites began. One of the first papers, published in 1971 was ‘Quantitative analysis of urine vapor and breath by gas liquid partition chromatography’ [4]. The first draft of the human metabolome project was put in 2007. At present, the software contains information about 41,514 metabolites which is freely accessible [5]. For each metabolite chemical, clinical, biochemical and where possible protein/genetic linkage is given. It is supported by David Wishart and ‘The Metabolomics Innovation Centre’, Canada.
Quantifying the Metabolites
Any biological fluid will have several different chemicals/proteins. The first step is to separate each substance after which each one is separately quantified. The first step of separation can be achieved by chromatographic techniques like GC, high performance liquid chromatography (HPLC), ultrahigh performance liquid chromatography (UPLC) and capillary electrophoresis (CE). HPLC is the most commonly used method since it can analyze a wide range of metabolites, though having a lower resolution compare to GC.
The second step of detection and quantification is done by mass spectrometry (MS). Here, the first step is to ionize the compounds by spray ionization or matrix associated laser desorption and ionization (MALDI), after which either a magnet or an electric field (time of flight MS) deflects them towards a detector. The strength of magnetic field required to deflect or the time taken after applying electric field to reach the detector gives an idea of the mass of the molecule [6]. The second step is collision induced fragmentation and determining the mass of each fragment, each compound fragmenting in its own characteristic daughter compounds.
Tandem mass spectrometers do not need prior separation of compounds in the biological fluid sample [6], neither does nuclear magnetic resonance (NMR) spectrometer. In an NMR spectrometer, a strong magnetic field aligns the protons in a parallel manner. This is opposed by a tiny field created by electrons and thus gives rise to resonance. The change in energy when the protons go back to their original position is detected and visualized as peaks which are characteristic of each compound [6]. Some of the differences between NMR and MS are summarized in Table 1. The human body produces such a variety of metabolites that none of the instruments is in itself sufficient to detect all of them.
Table 1.
Differences between nuclear magnetic resonance (NMR) and mass spectrometry (MS)
| Parameters | NMR | MS |
|---|---|---|
| Detection limit | Micro moles | Pico moles |
| Molecular analysis spectrum | Analyses less variety of compounds (should have H+) | Analyses wide range of compounds after chromatographic separation |
| Recovery of sample | Non-destructive, sample can be recovered | Fragments the sample |
| Preparation before running | Not much preparation | Preparation of sample needed |
| Identification of molecules | Easy to identify molecules from analysis of spectra | Difficult to identify unknown compound |
| Availability of databases of metabolites | Still comprehensive databases not available | Databases available |
Identifying the Molecular Signatures
The pillars on which this science relies are MS and NMR spectrometry and the complex data generated by them is being stored in global databases like the METLIN, from where retrieval is easy. Compounds are identified on the basis of mass, mass/charge ratios and retention time. For identifying unknown and known compounds when we have these parameters, online databases are available which store thousands of metabolite’s characteristics from which we can identify the metabolite of our interest. Two such online databases are the Metlin/XCMS and the human metabolome database. There are still many compounds whose MS or NMR characteristics are not known in which case an ‘untargeted’ approach can be used and ‘chemometrics’ can be applied where the intent is to identify the pattern of metabolites using statistics and link pattern to diseases by looking at all metabolites at a time.
Applications (Fig. 2)
Fig. 2.
Summary of proposed applications of metabolomics in diagnosis, monitoring and understanding disease and drug response; given in brackets are the respective metabolic markers; DHEA-Dehydroepiandrosterone, BCAA-Branched chain aminoacids, AAA-Aromatic aminoacids
Understanding Mechanisms of Drug Response
Aspirin
An example to identify a marker of drug response was a study done to explain variability in aspirin response [7]. They hypothesized that the variation is due to genetic variation in pathways other than thromboxane synthesis. 165 volunteers from the hereditary and phenotype intervention (HAPI) heart study [8] were given 81 mg of aspirin once a day for 2 weeks. GC–MS and LC–MS were performed on serum samples before and after 2 weeks of aspirin ingestion. Out of 165 metabolites measured in the serum, 49 were significantly altered by aspirin exposure and belonged to various pathways like purine, fatty acid, glycerol, amino acid and carbohydrate. Later, volunteers belonging to first quartile of post-aspirin collagen-stimulated platelet aggregation were taken as good responders (n = 40) and those belonging to fourth quartile of response were taken as poor-responders (n = 36). After drawing a metabolic profile of these 76 volunteers, it was found that the poor responders had significant increase in levels of adenosine and inosine after 2 weeks of aspirin exposure. So a new hypothesis gets generated that aspirin affects the purine pathway. To explain variation in aspirin response, a metabolomics informed pharmacogenomics study was done. Single Nucleotide Polymorphisms (SNPs) associated with synthesis, transport and degradation of purines were screened in 718 patients of HAPI study. A SNP in adenosine kinase (ADK) gene was found to be associated with anti-platelet response. To confirm the role of this SNP, all the participants having this SNP were screened for their purine levels post-aspirin and the levels of inosine and guanosine were found to be associated with this SNP. Thus, in this study a novel pathway was found to be associated with anti-platelet action of aspirin and a potential marker of its action was identified.
Citalopram
Another example where ‘pharmacometabolomics informed pharmacogenomics’ screening of SNPs was done, was in a study to evaluate variation in antidepressant treatment with citalopram [9]. Here, metabolomics first hinted that glycine levels were negatively associated with treatment outcomes and pharmacogenomics then revealed that a SNP in glycine dehydrogenase (GLDC) gene was what led to the variation in glycine levels.
Sertraline
Recent metabolomics study [10] on patients suffering from major depression started on sertraline found not only markers of drug response but also a new insight into sertraline’s mechanism of action. Tryptophan has four metabolic end points- kynurenine, 5-methoxy tryptamine, melatonin and 5-hydoxyindoleaceticacid (5-HIAA), the latter three formed after tryptophan gets converted into serotonin. It was found that after a month of therapy, the good responders had lower levels of serotonin (5-HT) and 5-HIAA, the 5-HT being diverted to melatonin synthesis (the melatonin analogue, agomelatine has anti-depressant activity). Also a biomarker of good response was 5-methoxy tryptamine, higher pre-treatment levels were associated with good response, which lowered with therapy.
Predicting Paracetamol Toxicity
Two studies done by Bhattacharyya et al. [11, 12] found that in both animals as well as humans, elevations of long chain acylcarnitines can predict hepatotoxicity due to paracetamol before the transaminases get elevated. Since these metabolites are involved in the β-oxidation of fatty acids and since β-oxidation takes place in mitochondria, their elevations can mean mitochondrial toxicity. Such predictions of drug induced toxicity can be used in preclinical studies in drug development to identify potential adverse drug reactions and prevent unnecessary human clinical trials.
Anti-tubercular Drugs
In a prospective study on patients of tuberculosis started on anti-tuberculosis drugs [13], urine samples were collected at start and at 1 month of therapy. The two sets of samples differed statistically with respect to six metabolites. These metabolites could be used as biomarkers for evaluating drug response, their estimation being faster than sputum culture. Also, early recognition of response can hasten clinical trials of anti-Tb drugs.
Anti-diabetics
It is possible not just to assess response to the anti-diabetic drugs but also, based on the response, to characterize the drug. A study by Walford et al. [14] involved administering either metformin, glipizide or glucose challenge to type 2 diabetes patients. In patients who were insulin sensitive, branched chain amino acids and aromatic amino acids decreased in response to release of insulin, as happened when they were given either glipizide or glucose challenge. In patients who were insulin resistant, the same metabolite levels increased when they were given metformin. Also, it is previously proven that elevated branched chain and aromatic amino acids levels are associated with insulin resistance and type 2 diabetes [15].
Anti-hepatitis C Therapy
A study was done by Saito et al. [16] to differentiate responders from non-responders in patients of hepatitis C receiving interferon + ribavirin therapy. In good responders, the levels of the enzyme gamma glutamyl transferase (GGT) decreased more than those of non-responders as the therapy brought down the oxidative stress. This reduced the need for generating glutathione to combat oxidative stress. Also, the pretreatment levels of tryptophan, glycine and γ-butyrobetaine were found to be more in good responders.
Statins
The ‘pleiotropic effects’ of statins are well known. These may be due to the reduced synthesis of isoprenoids and prenylation of proteins like rho/rab kinases. A study conducted by Krauss et al. [17] identified some statin influenced pathways that may contribute to variability in clinical efficacy and adverse effects. Samples were taken from participants of the ‘cholesterol and pharmacogenetics study’ [18] in which 40 mg of simvastatin was given to 944 patients for 6 weeks. Samples for metabolomics assessment were taken from 48 individuals, half categorized as good responders (the upper 10 % of LDL-C response) and the remaining half as poor responders. Both these type of responders demonstrated increase in levels of arachidonic acid and decrease in levels of linoleic acid. Since arachidonic acid was increased in both categories, this action was independent of HMG-CoA inhibition and since arachidonic acid is precursor for both pro-inflammatory and anti-inflammatory molecules, affecting this pathway would contribute to clinical efficacy as well as adverse reactions. Another inference derived in the same study was the ability of lithocholic acid, taurocholic acid, glycolithocholic acid and coprostanol serum levels to predict statin response. Higher levels of these metabolites before treatment were associated with a better response. This is expected as both, secondary bile acids as well as statins; share the same transporter for absorption, i.e. SLCO1B1. Higher levels of bile acids before starting statin meaning that the transporters are functioning well and would help in better statin absorption, both into the circulation as well as into the hepatocytes. Another metabolite negatively correlated with response was the baseline value of 2-hydroxy valeric acid. This metabolite represents activity of intestinal bacteria and the same bacteria causes simvastatin degradation, hence lower levels of this metabolite predict lesser degradation of simvastatin and hence better efficacy.
Anti-protozoal Drugs
The mechanism of most anti-protozoals is not exactly known. This has hindered drug development in this field and also has prevented modification of drugs already in use for better pharmacodynamic/kinetic profiling or reducing their toxic effects. A review of metabolomics of anti-protozoals [19] done on parasite metabolome threw light on some newly discovered facts like the inhibition of ornithine decarboxylase by eflornithine, a drug used against trypanosomiasis. It was seen that the substrate, ornithine accumulates while depletion of products like putrescine and spermidine occurs [20]. In case of nifurtimox, metabolomics identified perturbations in parasitic nucleotide and glycolytic pathways [20]. Likewise for the anti-leishmaniasis drugs, amphotericin B and miltefosine, interaction with the sterol and phospholipid metabolism was found respectively [19]. For the antimalarial, atovaquone, its inhibition of cytochrome bc(1), results in loss of mitochondrial membrane potential, which inhibits dihydroorotate dehydrogenase, thus inhibiting pyrimidine synthesis. Metabolomics confirmed this by showing accumulation of dihydroorotate and carbamoyl-l-aspartate, its precursor [21].
Metabolomics as a Marker of Metabolic Activity of CYP3A4/5 Activity
CYP3A4/5 metabolizes over 50 % of the commonly used drugs. Endogenous metabolites of steroidal pathways make use of CYP3A4/5 for their metabolism and their levels can give an idea of the status of CYP3A activity. A study was done to find endogenous markers which can predict CYP3A activity [22]. The control used was midazolam clearance and measuring its metabolites 1′-hydroxy midazolam and 4-hydroxy midazolam. Before giving midazolam, urine samples and plasma samples were collected. Midazolam and its metabolite’s levels in plasma were measured and serial pharmacokinetic analysis was done. Metabolomic analysis found that levels of plasma 4β-hydroxy cholesterol (a known marker), urinary 6β-hydroxycortisone, cortisone, dehydroepiandrosterone (DHEA) and 16α-hydroxy DHEA correlated well with the CYP3A activity as predicted by midazolam clearance. Also the ratios of metabolite (16α-hydroxy DHEA) to parent compound (DHEA) increased after inducing enzymes by rifampicin and vice versa happened after giving ketoconazole, an inhibitor, thus confirming the validity of these markers. The authors also generated an equation to predict clearance by CYP3A using the levels of these metabolites as markers of CYP3A activity.
As Biomarkers of Various Diseases
Diabetic Nephropathy
A recent study done by Makinen et al. [23] investigated metabolic markers for nephropathy in type 1 diabetes patients and found that sphingomyelin was elevated in type 1 diabetes patients with nephropathy as compared to those without nephropathy and the same set of patients also had increased VLDL levels and decreased HDL levels. This might mean that the excess fat causes ceramide/sphingomyelin accumulation causing nephropathy (Palmitoyl CoA and serine form sphingosine which combines with long chain fatty acids to form ceramide. which then combines with phosphatidyl choline to form sphingomyelin [24]).
Chronic Inflammation
A review was done to understand the interaction of inflammation and metabolomics by Fitzpatrick et al. [25]. As expected, due to reactive species produced by inflammatory cells, plasma glutathione was reduced and lipid peroxides were found to be increased in patients of rheumatoid arthritis (RA) [26]. An analysis of synovial fluid in patients of RA showed increased levels of lactate which is due to increased number of inflammatory cells which increase the energy requirements and levels of lactate were shown to be correlated with active inflammation and damage to the joints [27]. Also in the same study increased metabolic intermediates of fatty acid oxidation were found which included ketones and glycerol. This may be due to altered permeability in inflamed tissues and thereby increased influx of fatty acids. In inflammation increased protein breakdown can be judged by the increased levels of essential amino acids as shown by the increased valine levels in patients of osteoarthritis [28]. In Crohn’s disease and ulcerative colitis, analysis of fecal extracts showed decreased levels of butyrate, acetate and methylamine [29], suggesting that reduced amounts of gut bacteria may have a role to play in these diseases.
Coronary Artery Disease
With a specificity of >90 %, metabolomics can help in diagnosis of triple vessel stenosis, an approach much more simpler than angiography [30].
Metabolic Unwellness
If elevated fasting plasma glucose and triglycerides; hypertension, insulin resistance and low HDL is considered as being metabolically unwell, then can metabolomics help in diagnosing them? A study done on this concept [31] proved that elevated branched chain amino acids, acylcarnitines, fatty acids and ornithine can discriminate metabolically unwell irrespective of their BMI. This way branched chain amino acid levels can be monitored for treatment response in high risk patients.
Chronic Kidney Disease (CKD)
A study was done in rats by Zhao et al. [32] in search for a marker that could determine onset of chronic kidney disease (CKD) before urea and creatinine levels in plasma get raised. The model for CKD induced by adenine was well replicated. The two groups of rats (CKD and control) differed metabolically as seen by the increased levels of p-cresolsulfate, indoxyl sulfate and allantoin amongst others in the CKD group. Not only was the clearance of these metabolites decreased but also they played a role in aggravating the inflammation in kidneys.
Metabolomics of Gut Bacteria
Our body bacterial flora plays a significant role in generating metabolites as their cells outnumber our cells by 10:1 [33]. In a study it was demonstrated that when healthy volunteers were given paracetamol, those with high urinary p-cresol levels before taking paracetamol showed lower ratios of sulfate conjugate/glucuronide conjugate. p-cresol is formed in the body by Clostridium difficile residing in the gut and this p-cresol competes with paracetamol for sulfation by SULT1A1 [34]. Reduced sulfation of paracetamol could then lead to increased N-acetyl para benzoquinoneimine resulting in hepatotoxicity. A study revealed that metabolites produced by gut microflora-phosphatidylcholine, trimethylamine N-oxide and betaine predispose to cardiovascular diseases and hence can be used as markers [35].
Gut microflora metabolites are a part of our metabolome and thereby are a source of variation in each person’s metabolome as everyone has a different microbial flora. Not only do these bacteria help in degrading some drugs but also the metabolites they produce compete with hepatic metabolism of some drugs too, thereby altering the response to drugs differently in different people.
Metabolomics in Food Industry
Here, metabolomics has surged in with a lot of applications. An example of application in checking food quality is the measurement of diacetyl and 2,3 pentadione compounds in beer to determine endpoints of fermentation [36]. Also accidental contamination by allergic compounds or by bacteria can be recognized, e.g. E. coli in spinach, putrescine, cadaverine and histamine in spoiled fish [36]. Compounds can be identified which determine the taste of some foods. Metabolomics has shown that genetically modified potatoes and tomatoes differ from normal ones by just a few compounds like fructans and flavonoids respectively [37, 38]. Also, verification of labelled ingredients which we believe to be true can be achieved by metabolomics.
Factors Affecting Metabolome
Genetics determine the status of drug metabolizing enzymes and hence the metabolome. Another source which determines a person’s metabolome is the gut microflora. Environment naturally plays a major role, since diet, exposure to xenobiotics and pollutants alter the metabolites produced [39]. Also people only differing in age and gender also tend to have a different metabolome [39].
Standardizing Experiments in Metabolomics
If we want to see the effect of a single drug or intervention on the metabolome, it is a necessity that all experimental subjects should have the same metabolome before intervention. As noted above, the metabolome is affected by factors which are hard to control or standardize. If the study is being done in human subjects, all can be chosen who have the same age, gender and from the same environment. Their diet can be standardized few weeks before the experiment and exposure to xenobiotics and smoking should be avoided.
In case of animal studies all these standardizations are easy by controlling living conditions. To nullify genetic differences, their breeding can also be controlled. But differences exist between humans and mice in the way which drugs and metabolites are handled. To overcome these differences, genetically modified mouse models were made [39], where, for example, PXR (pregnane X receptor) humanized mice were used to standardize CYP3A experiments [40], PPAR-α humanized mice for studying fibrates and CYP2E1 humanized mice for paracetamol toxicity studies [41]. To overcome differences in gut flora, human microbiome can be set up in a mouse which is germ free at birth.
Conclusion
Though technically a lot of progress has been made in measuring metabolite levels, the understanding needed to make use of the data generated is still lacking. Metabolomics holds an important place in ‘hypothesis generation’ as exemplified by the discovering of new pathways in understanding drug action. Also it can help categorize poor and good responders, find new markers for diseases like cancer, predicting status of drug metabolizing enzymes. Just like the genome each person has a unique metabolome. The major advantage with metabolome over genome is that it reflects the environmental influences and gives us a snapshot of the current pathophysiological status of an individual. New applications will come up as we test these metabolomic approaches in clinics.
References
- 1.Fiehn O. Metabolomics–the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–171. doi: 10.1023/A:1013713905833. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson JK, Lindon JC, Holmes E. “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 3.Williams RJ. Individual metabolic patterns and human disease: an exploratory study utilizing predominantly paper chromatographic methods. 1951. http://repositories.lib.utexas.edu/handle/2152/7023. Cited 25 Feb 2014.
- 4.Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc Natl Acad Sci USA. 1971;68:2374–2376. doi: 10.1073/pnas.68.10.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Human Metabolome Database (HMDB). http://www.hmdb.ca/. Cited 17 Apr 2014.
- 6.Kennelly PJ, Rodwell VW. Proteins: determination of primary structure. In: Murray RK, Bender DA, Botham KM, Kennelly PJ, Rodwell VW, Weil PA, editors. Harper’s illustrated biochemistry. New York: Lange Medical-McGraw-Hill; 2009. pp. 26–28. [Google Scholar]
- 7.Lewis JP, Yerges-Armstrong LM, Ellero-Simatos S, Georgiades A, Kaddurah-Daouk R, Hankemeier T. Integration of pharmacometabolomic and pharmacogenomic approaches reveals novel insights into antiplatelet therapy. Clin Pharmacol Ther. 2013;94:570–573. doi: 10.1038/clpt.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell BD, McArdle PF, Shen H, Rampersaud E, Pollin TI, Bielak LF, et al. The genetic response to short-term interventions affecting cardiovascular function: rationale and design of the HAPI heart study. Am Heart J. 2008;155:823–828. doi: 10.1016/j.ahj.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji Y, Hebbring S, Zhu H, Jenkins GD, Biernacka J, Snyder K, et al. Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: pharmacometabolomics-informed pharmacogenomics. Clin Pharmacol Ther. 2011;89:97–104. doi: 10.1038/clpt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu H, Bogdanov MB, Boyle SH, Matson W, Sharma S, et al. Pharmacometabolomics of response to sertraline and to placebo in major depressive disorder—possible role for methoxyindole pathway. PLoS One. 2013;8:e68283. doi: 10.1371/journal.pone.0068283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya S, Pence L, Beger R, Chaudhuri S, McCullough S, Yan K, et al. Acylcarnitine profiles in acetaminophen toxicity in the mouse: comparison to toxicity metabolism and hepatocyte regeneration. Metabolites. 2013;3:606–622. doi: 10.3390/metabo3030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya S, Yan K, Pence L, Simpson PM, Gill P, Letzig LG, et al. Targeted liquid chromatography–mass spectrometry analysis of serum acylcarnitines in acetaminophen toxicity in children. Biomark Med. 2014;8:147–159. doi: 10.2217/bmm.13.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahapatra S, Hess AM, Johnson JL, Eisenach KD, DeGroote MA, Gitta P, et al. A metabolic biosignature of early response to anti-tuberculosis treatment. BMC Infect Dis. 2014;14:53. doi: 10.1186/1471-2334-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walford GA, Davis J, Warner AS, Ackerman RJ, Billings LK, Chamarthi B, et al. Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism. 2013;62:1772–1778. doi: 10.1016/j.metabol.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai ES, Tan MLS, Stevens RD, Low YL, Muehlbauer MJ, Goh DLM, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito T, Sugimoto M, Igarashi K, Saito K, Shao L, Katsumi T, et al. Dynamics of serum metabolites in patients with chronic hepatitis C receiving pegylated interferon plus ribavirin: a metabolomics analysis. Metabolism. 2013;62:1577–1586. doi: 10.1016/j.metabol.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Krauss RM, Zhu H, Kaddurah-Daouk R. Pharmacometabolomics of statin response. Clin Pharmacol Ther. 2013;94:562–565. doi: 10.1038/clpt.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, Shiboski S, et al. Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the cholesterol and pharmacogenetics (CAP) study. Am J Cardiol. 2006;97:843–850. doi: 10.1016/j.amjcard.2005.09.134. [DOI] [PubMed] [Google Scholar]
- 19.Creek DJ, Barrett MP. Determination of antiprotozoal drug mechanisms by metabolomics approaches. Parasitology. 2014;141:83–92. doi: 10.1017/S0031182013000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent IM, Creek DJ, Burgess K, Woods DJ, Burchmore RJS, Barrett MP. Untargeted metabolomics reveals a lack of synergy between nifurtimox and eflornithine against Trypanosoma brucei. PLoS Negl Trop Dis. 2012;6:e1618. doi: 10.1371/journal.pntd.0001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biagini GA, Fisher N, Shone AE, Mubaraki MA, Srivastava A, Hill A, et al. Generation of quinolone antimalarials targeting the plasmodium falciparum mitochondrial respiratory chain for the treatment and prophylaxis of malaria. Proc Natl Acad Sci USA. 2012;109:8298–8303. doi: 10.1073/pnas.1205651109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin K-H, Choi MH, Lim KS, Yu K-S, Jang I-J, Cho J-Y. Evaluation of endogenous metabolic markers of hepatic CYP3A activity using metabolic profiling and midazolam clearance. Clin Pharmacol Ther. 2013;94:601–609. doi: 10.1038/clpt.2013.128. [DOI] [PubMed] [Google Scholar]
- 23.Mäkinen V-P, Kangas AJ, Soininen P, Würtz P, Groop P-H, Ala-Korpela M. Metabolic phenotyping of diabetic nephropathy. Clin Pharmacol Ther. 2013;94:566–569. doi: 10.1038/clpt.2013.158. [DOI] [PubMed] [Google Scholar]
- 24.Botham KM, Mayes PA. Metabolism of acylglycerols and sphingolipids. In: Murray RK, Bender DA, Botham KM, Kennelly PJ, Rodwell VW, Weil PA, editors. Harper’s illustrated biochemistry. Lange Medical-McGraw-Hill: New York; 2009. pp. 208–210. [Google Scholar]
- 25.Fitzpatrick M, Young SP. Metabolomics–a novel window into inflammatory disease. Swiss Med Wkly. 2013;143:w13743. doi: 10.4414/smw.2013.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan MQ, Hadi RA, Al-Rawi ZS, Padron VA, Stohs SJ. The glutathione defense system in the pathogenesis of rheumatoid arthritis. J Appl Toxicol JAT. 2001;21:69–73. doi: 10.1002/jat.736. [DOI] [PubMed] [Google Scholar]
- 27.Naughton DP, Haywood R, Blake DR, Edmonds S, Hawkes GE, Grootveld M. A comparative evaluation of the metabolic profiles of normal and inflammatory knee-joint synovial fluids by high resolution proton NMR spectroscopy. FEBS Lett. 1993;332:221–225. doi: 10.1016/0014-5793(93)80636-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhai G, Wang-Sattler R, Hart DJ, Arden NK, Hakim AJ, Illig T, et al. Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dis. 2010;69:1227–1231. doi: 10.1136/ard.2009.120857. [DOI] [PubMed] [Google Scholar]
- 29.Marchesi JR, Holmes E, Khan F, Kochhar S, Scanlan P, Shanahan F, et al. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J Proteome Res. 2007;6:546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- 30.Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HWL, et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8:1439–1444. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- 31.Batch BC, Shah SH, Newgard CB, Turer CB, Haynes C, Bain JR, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism. 2013;62:961–969. doi: 10.1016/j.metabol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y-Y, Cheng X-L, Wei F, Bai X, Tan X-J, Lin R-C, et al. Intrarenal metabolomic investigation of chronic kidney disease and its TGF-β1 mechanism in induced-adenine rats using UPLC Q-TOF/HSMS/MS E. J Proteome Res. 2013;12:692–703. doi: 10.1021/pr3007792. [DOI] [PubMed] [Google Scholar]
- 33.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 34.Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci USA. 2009;106:14728–14733. doi: 10.1073/pnas.0904489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cevallos-Cevallos JM, Reyes-De-Corcuera JI, Etxeberria E, Danyluk MD, Rodrick GE. Metabolomic analysis in food science: a review. Trends Food Sci Technol. 2009;20:557–566. doi: 10.1016/j.tifs.2009.07.002. [DOI] [Google Scholar]
- 37.Catchpole GS, Beckmann M, Enot DP, Mondhe M, Zywicki B, Taylor J, et al. Hierarchical metabolomics demonstrates substantial compositional similarity between genetically modified and conventional potato crops. Proc Natl Acad Sci USA. 2005;102:14458–14462. doi: 10.1073/pnas.0503955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Gall G, DuPont MS, Mellon FA, Davis AL, Collins GJ, Verhoeyen ME, et al. Characterization and content of flavonoid glycosides in genetically modified tomato (Lycopersicon esculentum) fruits. J Agric Food Chem. 2003;51:2438–2446. doi: 10.1021/jf025995e. [DOI] [PubMed] [Google Scholar]
- 39.Johnson CH, Patterson AD, Idle JR, Gonzalez FJ. Xenobiotic metabolomics: major impact on the metabolome. Annu Rev Pharmacol Toxicol. 2012;52:37–56. doi: 10.1146/annurev-pharmtox-010611-134748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, et al. The PREgnane X receptor gene-humanized mouse: a model for investigating drug–drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos Biol Fate Chem. 2007;35:194–200. doi: 10.1124/dmd.106.012831. [DOI] [PubMed] [Google Scholar]
- 41.Cheung C, Yu A-M, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, et al. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos Biol Fate Chem. 2005;33:449–457. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]