Abstract
Increase in urine albumin excretion rate (AER) precede a fall in glomerular filtration rate in patients developing diabetic chronic kidney disease (CKD). Our results have shown that 7 (50 %) of diabetic and hypertensive individuals with decreased GFR do not have increased AER. In this cross-sectional study, we measured AER of 75 patients with type 2 diabetes and hypertension by immunoturbidimetric method. We correlated the results with eGFR values obtained by Cockcroft–Gault and MDRD method. The method used was not a compensated method. We measured serum creatinine by modified Jaffe’s kinetic method in autoanalyzer XL-600. Analysis of data showed positive correlation between eGFR and microalbuminuria by both the methods with eGFR <60 mL/min/1.73 m2. Pearson’s correlation co-efficient (r) was 0.9 (p = 0.0001) by Cockcroft–Gault formula and 0.69 (p = 0.0063) by MDRD formula. Our results concluded that there was positive correlation between AER and eGFR <60 mL/min/1.73 m2. We have recognized that these two parameters provide a complimentary benefit in management of cases with CKD.
Keywords: eGFR, Cockcroft–Gault, Modification of diet in renal disease, Albumin excretion rate
Introduction
Primary hypertension and type 2 diabetes mellitus are the major causes of renal damage and cardiovascular events. The co-existence of these conditions further increase the risk of progressive renal disease, cardiovascular events and mortality. Urinary excretion of albumin (microalbuminuria) and lowered GFR are the early markers of such tendency. The importance of screening for microalbuminuria and a lowered GFR in hypertensive and diabetic patients lies in the detection of preclinical kidney disease, and identification of individuals at increased risk of progressive renal disease, cardiovascular events, and mortality. Intensive therapy that is directed at the optimal control of blood pressure, blood sugar, cardiovascular risk factors as well as interventions aimed at decreasing albuminuria and slowing the progression of renal disease have demonstrable beneficial effect. Microalbuminuria is defined as albumin excretion in the urine between 20 and 200 μg/min or 30–299 mg/24 h. Values below this range indicate normoalbuminuria, while those above are regarded as macroalbuminuria [1].
Urinary albumin excretion has assumed a central role in the diagnosis and management of kidney disease among people with hypertension and both type 1 and 2 diabetes [2]. Progressive increase in urine albumin excretion rate (AER) is thought to precede a fall in GFR in patients developing chronic kidney disease (CKD). However, some larger studies with type 2 diabetes and few smaller studies in individuals with type 1 diabetes have demonstrated that a substantial proportion of diabetic individuals with decreased GFR levels do not have increased AER [3, 4]. Earlier studies have suggested that GFR only starts to decrease when AER reaches the macroalbuminuric range [5]. Studies have also shown a spontaneous remission of microalbuminuria in patients with diabetic nephropathy [6]. Detection of early decline in GFR has been possible at the stage of new onset microalbuminuria, well before the onset of overt nephropathy. In type 2 diabetes, the onset of hypertension promotes a rise in AER and a decline in GFR [7]. In our study we have correlated the degree of albuminuria with respect to eGFR in type 2 diabetics and hypertensives. We have calculated eGFR by non-compensated creatinine methods by using the factor 186 in MDRD method as opposed to the factor 175 for Isotope dilution mass spectrometry (IDMS) traceable MDRD study.
Materials and Methods
We recruited 75 patients including 12 females and 63 males, within the age group of 25–85 years of type 2 diabetes and primary hypertension visiting the laboratory of a tertiary care center for investigation and follow up. Among these, 30 patients were of type 2 diabetes, 21 patients were of primary hypertension and the remaining 24 patients had both diabetes and hypertension. After fulfilling the inclusion and exclusion criteria, we took a detailed case summary and treatment history of the patients.
Inclusion Criteria: Patients of both sexes with type 2 diabetes and primary hypertension
Exclusion Criteria:
Pregnancy
Malignant diseases
Cases with acute and chronic infections.
Hypo and hyperthyroidism
Glomerulonephritis
Urine albumin excretion was estimated using a 24-h urine sample by Siemens DCA 2000 systems which is cartridge based point of care device. In this method purified goat anti- human albumin antiserum binds with albumin in the presence of polyethylene glycol, the turbidity produced was measured at 531 nm. Serum creatinine was measured by modified Jaffe’s kinetic method in Erba Transasia autoanalyzer (XL-600). The within run and between run C.V for the creatinine assay were 3.53 and 2.3 % respectively. The mean value of internal QC is 2.7 ± 0.1.
The calculation of eGFR by Cockcroft and Gault formula:
MDRD Formula:
Factor 186 is used for non-compensated creatinine method.
Patients were stratified by their eGFR values (mL/min/1.73 m2) into five CKD stages according to the National Kidney Foundation guidelines [8] (Table 1).
Table 1.
Staging of CKD (National Kidney Foundation guidelines)
| Stage | Description | GFR mL/min/1.73 m2 body surface area |
|---|---|---|
| 1 | Kidney damage* with normal or increased GFR | ≥90 |
| 2 | Kidney damage* with mildly decreased GFR | 60–89 |
| 3 | Moderately decreased GFR | 30–59 |
| 4 | Severely decreased GFR | 15–29 |
| 5 | Kidney failure | <15 or dialysis |
* Kidney damage defined as abnormalities on pathologic, urine, blood, or imaging tests
Pearson’s correlation coefficient was used to find the correlation between eGFR and microalbuminuria, with p value of ≤0.05 as significant. Standard deviation and variance was estimated to compare the precision between Cockcroft and Gault and MDRD methods.
Results
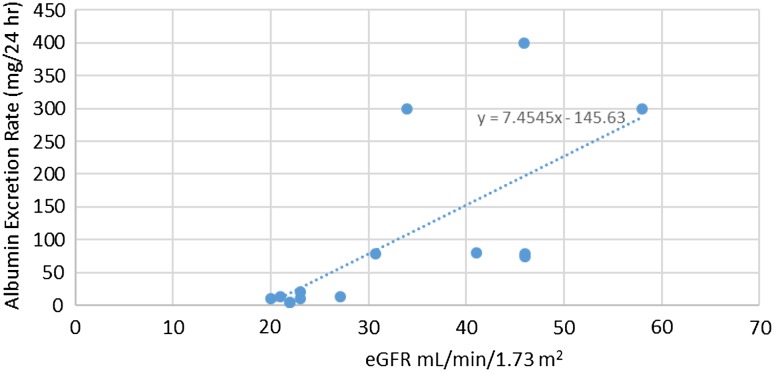
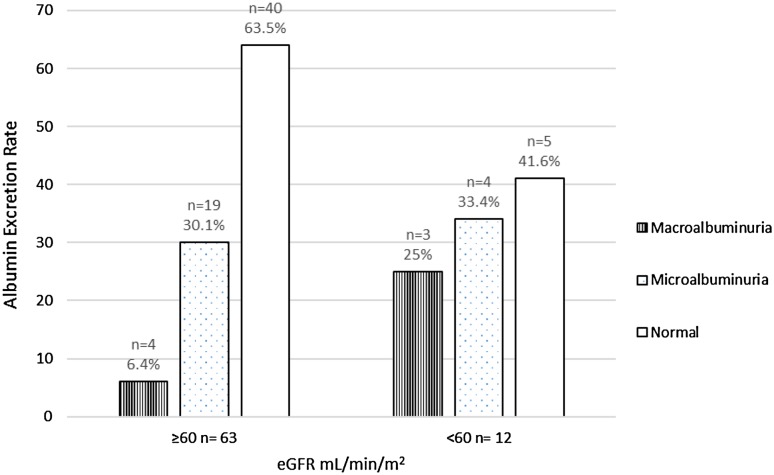
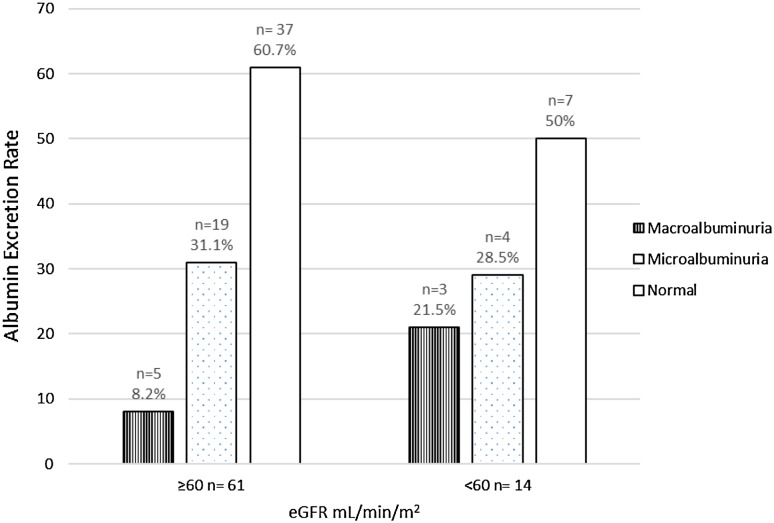
The body weight (kg), age (years), albumin excretion (mg/24 h) and serum creatinine (mg/dL) were 70.5 ± 8.6, 54 ± 13.2, 71.2 ± 128.7 and 1.16 ± 0.81 respectively. The standard deviation and variance by Cockcroft and Gault method are 32.72 and 1,056.5 respectively. The standard deviation and variance by MDRD method are 32.97 and 1,072.91 respectively. There was significant positive correlation between microalbuminuria and eGFR (<60 mL/min/1.73 m2). The Pearson’s correlation co-efficient (r) by Cockcroft–Gault formula was 0.89 (p = 0.0001) and with MDRD formula, the Pearson’s correlation co-efficient (r) was 0.69 (p = 0.006) (Fig. 1). We have found that with Cockcroft and Gault method, 19 persons (30.1 %) had microalbuminuria and 4 (6.4 %) had macroalbuminuria with normal eGFR estimate (Fig. 2). Here 5 (41.6 %) have normoalbuminuria with eGFR <60 mL/min. Similarly with MDRD method 19 persons (31.1 %) had microalbuminuria and 5 (8.2 %) had macroalbuminuria with normal eGFR estimate (Fig. 3). Here 7 persons (50 %) had normoalbuminuria with eGFR <60 mL/min.
Fig. 1.
Linear regression analysis of eGFR and Microalbuminuria
Fig. 2.
Distribution of cases with albuminuria and eGFR (Cock-croft and Gault method)
Fig. 3.
Distribution of cases with albuminuria and eGFR (MDRD method)
Discussion
This study was aimed at correlating microalbuminuria with eGFR in type 2 diabetes and hypertension. In our study there was positive correlation between microalbuminuria and eGFR estimated by both the methods within eGFR < 60 mL/min/1.73 m2. Glomerular filtration rate is commonly elevated in early diabetes and patients with this symptom are arbitrarily considered to have hyperfiltration. The prevalence of hyperfiltration in type 1 diabetes varies from <25 % to more than 75 %. This state of ‘hyperfiltration’ reflects hyperglycaemia and can be reversed with intensive insulin therapy. Biological factors that may influence GFR in the hyperfiltration range include glycaemic control, diabetes duration, BMI, sex, pubertal status in type 1 diabetes and age in type 2 diabetes. Hyperglycaemia may influence GFR and albuminuria, and may therefore confound the evaluation of hyperfiltration as an independent risk factor for diabetic nephropathy [9].
The eGFR by Cockcroft and Gault method has lower standard deviation and variance in comparison with eGFR by MDRD method. Hence Cockcroft and Gault method is more reliable (repeatable) than MDRD method also eGFR by Cockcroft and Gault method has better correlation with AER (r = 0.89) than the MDRD method (r = 0.69). However, screening with Cockcroft and Gault method alone would have missed two patients of normoalbuminuria with eGFR <60 mL/min and one case of macroalbuminuria with normal eGFR, as compared to MDRD method.
GFR can be estimated clinically from serum concentrations of endogenous creatinine or cystatin C [10]. The combined creatinine–cystatin C equation performed better than equations based on either of these markers alone and may be useful as a confirmatory test for CKD. The cystatin equation is not more accurate than creatinine equation, but the combined creatinine-cystatin equation is more precise than either of the two methods used separately [11]. Serum creatinine alone should not be used to assess the GFR or to detect the presence of CKD because it is affected by the GFR and by factors independent of GFR, including age, sex, race, body size, diet, certain drugs, and laboratory analytical methods [12]. According to Myers et al. calibration bias and measurement imprecision for serum creatinine have a much larger impact on the uncertainty in estimated GFR when serum creatinine is close to the reference value, which is the relevant range for detecting early CKD [GFR <60 mL/min/1.73 m2 [13]. This limitation applies to all estimating equations based on serum creatinine. For this reason, the NKDEP has recommended that GFR estimates above 60 mL/min/1.73 m2 be reported simply as “>60 mL/min/1.73 m2” rather than as a discrete numeric value [14].
The DCCT and EDIC study by Molitch et al. on 1,439 type 1 diabetes patients, has shown that the macroalbuminuria is a strong predictor for progression to a sustained eGFR <60 mL/min/1.73 m2 and the long-term risk of developing albuminuria and impaired GFR was lower among patients who were treated by intensive glycaemic control soon after diagnosis than among those treated by conventional glycaemic control. However screening with AER alone would have missed 20 cases (24 %) with sustained impaired eGFR [3].
The urine of healthy participants constitute more than 90–95 % of albumin-derived fragments compared with <5–10 % of intact albumin. These albumin derived fragments are not detected by conventional albumin assays [15]. In general, the proportion of intact albumin increases as renal disease progresses from normoalbuminuria to microalbuminuria to macroalbuminuria. It is possible that in people who follow the normoalbuminuric pathway to renal insufficiency, there is an exaggerated rate of urinary albumin fragmentation [16].
Conclusion
Measurement of urinary albumin excretion and eGFR has assumed a central role in the diagnosis and management of kidney disease among people with Diabetes and Hypertension. Microalbuminuria and GFR estimation help in prognosis of renal damage in these individuals. In the present study, we have found that the eGFR (<60 mL/min/1.73 m2) by Cockcroft–Gault method and MDRD method positively correlate with the urinary albumin excretion in cases with type 2 diabetes and primary hypertension. Although macroalbuminuria is a strong predictor of eGFR loss, 4 persons (6.4 %) people and 5 persons (8.2 %) with macroalbuminuria had normal eGFR by Cockcroft and Gault method and MDRD method respectively. Our findings are in agreement with Jerums et al. [9] that both eGFR and AER should be assessed in the evaluation of kidney disease in diabetic and hypertensive patients. The limitation of our study is the sample size and larger studies are recommended to derive a better correlation between eGFR and AER.
Acknowledgment
The study did not receive any specific grant from any funding agency in public, commercial or not-for-profit sector.
Conflict of interest
The authors have no conflict of interest.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–1432. doi: 10.1016/S0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 3.Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, De Boer IH, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33:1536–1543. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacIsaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens. 2011;20(3):246–257. doi: 10.1097/MNH.0b013e3283456546. [DOI] [PubMed] [Google Scholar]
- 5.Lambers Heerspink HJ, de Zeeuw D. Debate: pRO position. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am J Nephrol. 2010;31:458–461. doi: 10.1159/000292501. [DOI] [PubMed] [Google Scholar]
- 6.Chatzikyrkou C, Haller H. Hyperfiltration—a risk factor for nephropathy in T1DM? Nat Rev Endocrinol. 2012;8:385–386. doi: 10.1038/nrendo.2012.63. [DOI] [PubMed] [Google Scholar]
- 7.Mogensen CE. Microalbuminuria, blood pressure and diabetic renal disease: origin and development of ideas. Diabetologia. 1999;42:263–285. doi: 10.1007/s001250051151. [DOI] [PubMed] [Google Scholar]
- 8.Kramer H, Molitch ME. Screening for kidney disease in adults with diabetes. Diabetes Care. 2005;28:1813–1816. doi: 10.2337/diacare.28.7.1813. [DOI] [PubMed] [Google Scholar]
- 9.Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac R. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53(10):2093–2104. doi: 10.1007/s00125-010-1794-9. [DOI] [PubMed] [Google Scholar]
- 10.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58(4):682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shemesh O, Golbety H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 13.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 2002;39:S1–246. [PubMed]
- 15.Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y, Kobayashi M. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study. Nephrol Dial Transpl. 2009;24:1212–1219. doi: 10.1093/ndt/gfn603. [DOI] [PubMed] [Google Scholar]
- 16.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Non albuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]