Abstract
Objectives
Case reports have described a syndrome of cyclic vomiting associated with chronic marijuana use, termed cannabinoid hyperemesis syndrome. The primary objective was to determine the prevalence of patients presenting with cyclic vomiting before and after the liberalization of medical marijuana in Colorado in 2009. The secondary objective was to describe the odds of marijuana use among cyclic vomiting visits in these same time periods.
Methods
This was a cross-sectional study of cyclic vomiting visits to the emergency department (ED) before and after marijuana liberalization. ED visits with International Classification of Diseases, ninth revision, coding for cyclic vomiting or that met diagnostic criteria for cyclic vomiting by the Rome III criteria were included.
Results
The authors reviewed 2,574 visits and identified 36 patients diagnosed with cyclic vomiting over 128 visits. The prevalence of cyclic vomiting visits increased from 41 per 113,262 ED visits to 87 per 125,095 ED visits after marijuana liberalization, corresponding to a prevalence ratio of 1.92 (95% confidence interval [CI] = 1.33 to 2.79). Patients with cyclic vomiting in the postliberalization period were more likely to have marijuana use documented than patients in the preliberalization period (odds ratio = 3.59, 95% CI = 1.44 to 9.00).
Conclusions
The prevalence of cyclic vomiting presentations nearly doubled after the liberalization of medical marijuana. Patients presenting with cyclic vomiting in the postliberalization period were more likely to endorse marijuana use, although it is unclear whether this was secondary to increased marijuana use, more accurate marijuana reporting, or both.
In November 2000, Colorado amended its constitution to allow for the use of medical marijuana in patients with debilitating medical conditions. However, few patients used medical marijuana until October 2009, when the U.S. Attorney General ceased its prosecution of marijuana users and suppliers nationwide,1 effectively liberalizing its use and sale in Colorado. As a result, the number of medical marijuana licenses in the state rose from 5,051 in January 2009 to 118,895 in January 2011 (unpublished data, Colorado Department of Public Health and Environment, January 2014). This sudden increase in marijuana availability represents an opportunity to study the adverse effects of cannabis use among patients in our emergency departments (ED).
Marijuana is the most commonly used illicit substance in the United States, with 18.9 million users in 2012.2 Unfortunately, there is little information on the deleterious effects of chronic use and its implications for public health. Recently, a number of case reports have suggested a novel syndrome of cyclic vomiting associated with repeated marijuana use, termed cannabinoid hyperemesis syndrome (CHS). These reports describe patients with habitual marijuana use presenting with cyclic vomiting, abdominal pain, compulsive showering, and improvement of symptoms with cessation of cannabis.3–12 While these reports have generated interest in this phenomenon, there have been no epidemiologic studies associating marijuana use with CHS. This deficit is likely multifactorial due to the lack of formal diagnostic criteria for CHS, the relatively low prevalence of this syndrome, and the social stigma regarding marijuana use that discourages self-reporting.
The recent liberalization of medical marijuana serves as an opportune natural experiment to study CHS. If CHS exists, one would expect an association between increased marijuana availability and an increase in the number of ED visits for cyclic vomiting. The primary objective of this study was to describe the prevalence of cyclic vomiting visits before and after the liberalization of medical marijuana in Colorado. The secondary objective of this study was to determine the odds of patient-reported marijuana use among cyclic vomiting visits for the same time periods.
METHODS
Study Design
This was a retrospective cross-sectional study of cyclic vomiting visits. The study was approved by the local institutional review board and informed consent was waived.
Study Setting and Population
The visits occurred at two urban academic EDs with a combined annual volume of approximately 120,000 visits. Medical records at Denver Health (Denver, CO; DH) are hand-written and scanned into an electronic system where they can be reviewed; medical records at the University of Colorado Hospital (Aurora, CO; UCH) are entered and reviewed electronically (EPIC 2010; Epic Systems, Verona, WI).
The study periods were selected to bookend the date of medical marijuana liberalization in Colorado: October 19, 2009.1 The preliberalization period was November 1, 2008, to October 31, 2009, and the postliberalization period was June 1, 2010, to May 31, 2011. A gap between the policy change and the post-liberalization period was incorporated to account for the expected time required to open new dispensaries and for patients to acquire medical marijuana prescriptions.
Eligible ED visits at each hospital were retrieved via computer algorithm, searching for International Classification of Diseases, ninth revision (ICD-9), codes of “cyclical vomiting” (ICD-9 536.2) or “nausea and vomiting” (ICD-9 787.0, 787.01) in either the primary or the secondary diagnosis. ED visits with ICD-9 coding for nausea and vomiting were included for analysis only if the patient satisfied the Rome III diagnostic criteria for cyclic vomiting syndrome: three or more visits for nausea and vomiting within the past year,13 in addition to lack of an obvious anatomic explanation for illness. ED visits with ICD-9 coding for cyclic vomiting were included for analysis regardless of the number of total visits.
Patient visits for nausea and vomiting were initially excluded if there was only a single visit for nausea and vomiting at a single hospital in the time period of interest, as it would be mathematically impossible to satisfy the Rome III criteria of three visits at both hospitals combined. Each remaining patient with at least two visits for nausea and vomiting at either hospital was then manually queried in the other hospital’s records for ED visits in the same time period, and patients with fewer than three total visits for nausea and vomiting were subsequently excluded.
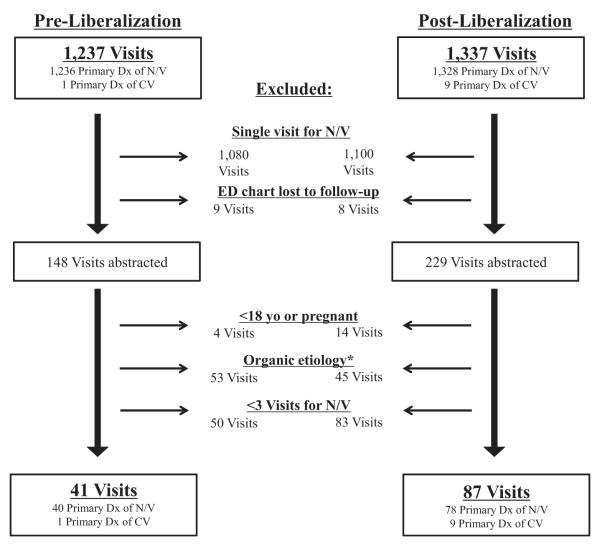
Additionally, we excluded patients who were pregnant, to exclude hyperemesis gravidarum, and patients under the age of 18 years old. Finally, visits were excluded if the ED record reflected an obvious pathophysiologic etiology for nausea and vomiting (e.g., cancer undergoing chemotherapy, bowel obstruction, uremia in emergent dialysis dependents). Visit diagnoses of “gastroenteritis” were excluded only if there was documentation of nausea and vomiting and diarrhea with either subjective or objective complaint of fever. Visit diagnoses of “gastroparesis” were excluded only if there was a positive gastric emptying study at any time in the patient’s medical record (see Figure 1 for a summary of inclusion and exclusion criteria).
Figure 1.
Flow chart. *Seven visits from each of the pre- and postliberalization periods were excluded due to a positive gastric emptying study. CV = cyclic vomiting; Dx = diagnosis; N/V = nausea/vomiting.
Study Protocol
Documentation from each ED visit was reviewed, including the initial three defining visits for cyclic vomiting. The following clinical and demographic variables were abstracted: patient sex, age, race, insurance status, having a primary care physician, marijuana use, compulsive hot showering, ED medications received, serum bicarbonate, length of stay (LOS), and final disposition. All notes from each visit were reviewed, including the triage, nursing, resident physician, and attending physician notes. Insurance status was coded as federal (Medicare or Medicaid), indigent assistance (state indigent discount program), private insurance, or self-pay. The administration of intravenous fluids, ondansetron, metoclopramide, prochlorperazine, promethazine, droperidol or haloperidol, and lorazepam were abstracted as binary variables. Serum bicarbonate was captured as a surrogate for severity of nausea and vomiting.
A positive history of marijuana use was considered any report of marijuana use in the ED chart or if there was a positive urine drug screen during the ED visit. The patient was considered a nonmarijuana user if there was no record of use in the ED chart. Compulsive hot showering was considered positive if documented in the ED note; if there was no mention of showering in the note, this was considered negative. Primary data abstraction was performed by the study authors using a standardized data collection sheet. Abstracters were not formally blinded to the study period (i.e., before/after liberalization) due to the requirements of thorough chart review. Sixteen percent of charts were double-abstracted and inter-rater reliability was measured using Cohen’s kappa; we selected the first 4% of visits in four separate groups to total 16% to calculate inter-rater reliability: preliberalization DH, preliberalization UCH, postliberalization DH, and postliberalization UCH. In cases of disagreement, the ED chart was reviewed by the lead author and reconciled.
The primary outcome of interest was a comparison of the prevalence of cyclic vomiting visits in the post- and preliberalization periods. The secondary outcome was the prevalence of positive marijuana use among cyclic vomiting visits in the post- and preliberalization periods. Finally, we conducted a hypothesis-generating exploratory analysis of the adjusted odds of admission and treatments associated with marijuana use for all cyclic vomiting visits in the study.
Data Analysis
All analyses were performed using JMP 10 (SAS, Inc.). For the primary outcome, we calculated the prevalence of cyclic vomiting visits in both periods and determined the prevalence ratio of visits in the postliberalization period compared to the preliberalization period. For the secondary outcome, we calculated the crude odds ratio (OR) for positive marijuana use among cyclic vomiting visits in the postliberalization period compared to the preliberalization period. Finally, in the exploratory analysis we applied a direct nominal logistic regression to the study cohort to predict hospital admission and calculated ORs for the following covariates, determined a priori: age, sex, and ED medications received (ondansetron, metoclopramide, prochlorperazine, promethazine, droperidol/haloperidol, and lorazepam). We then used a similar multivariate model to explore if there were any differences in treatment given to patients with positive and negative marijuana use. Covariates were assessed for colinearity using the inverse of the correlation matrix, and this was not found to be an issue. All p-values were two-sided with a threshold of p < 0.05 for statistical significance.
RESULTS
At both institutions combined there were a total of 113,262 ED visits in the preliberalization period and 125,095 visits in the postliberalization period. A total of 2,574 visits (63% from UCH) included ICD-9 codes for “cyclic vomiting” or “nausea and vomiting” and were reviewed. Thirty-six patients met the criteria for cyclic vomiting over 128 unique visits; 10 visits were included based on a primary diagnosis of cyclic vomiting, and 118 visits for nausea and vomiting were included after they satisfied the Rome III criteria for cyclic vomiting. The demographic characteristics of the study population are listed in Table 1. The kappa values for all variables were 0.77 (95% confidence interval [CI] = 0.47 to 1.00) and 0.89 (95% CI = 0.68 to 1.00) at DH and UCH, respectively. The kappa value for marijuana use only was 1.00 (95% CI = 1.00 to 1.00) at both hospitals.
Table 1.
Baseline Characteristics of Study Population, by Visits
| Characteristic | Total (N = 128) | Preliberalization (n = 41) | Postliberalization (n = 87) |
|---|---|---|---|
| ICD-9 CV (%)* | 27 (21) | 7 (17) | 20 (22) |
| Visits per patient, median (range) | 3 (1–9) | 3 (1–9) | 3 (1–9) |
| Age, mean (±SD) | 31.4 (±8.0) | 31.0 (±8.0) | 31.6 (±8.1) |
| Female, n (%) | 91 (71) | 26 (63) | 65 (75) |
| Race, n (%) | |||
| Black | 36 (28) | 16 (39) | 20 (23) |
| White† | 92 (72) | 25 (61) | 67 (77) |
| Insurance | |||
| Self-pay | 31 (24) | 6 (14) | 25 (29) |
| Private | 15 (12) | 4 (10) | 11 (13) |
| Medicare/Medicaid | 72 (56) | 24 (59) | 48 (55) |
| Indigent assistance | 10 (8) | 7 (17) | 3 (3) |
| No PCP, n (%) | 37 (29) | 16 (39) | 21 (24) |
| Bicarbonate (mmol/L), mean (±SD) | 21.6 (±4.0) | 21.2 (±4.3) | 21.8 (±3.8) |
| Marijuana use, n (%) | 44 (34) | 7 (17) | 37 (43) |
Includes both primary and secondary discharge diagnoses of CV, as designated by the treating emergency physician.
Both Hispanic and non-Hispanic patients were merged into the “white” variable, as one hospital (Denver Health) did not differentiate between these two ethnicities.
CV = cyclic vomiting; ICD-9 = International Classification of Disease, ninth revision; PCP = primary care physician.
Primary Outcome
In the pre- and postliberalization periods, there were 41 versus 87 visits for cyclic vomiting, respectively (Figure 1). Thus, the prevalence for cyclic vomiting visits increased from 41 per 113,262 ED visits to 87 per 125,095 ED visits after liberalization, corresponding to a prevalence ratio of 1.92 (95% CI = 1.33 to 2.79).
Secondary Outcome
In the pre- and postliberalization periods, there were seven versus 37 cyclic vomiting visits with positive marijuana use, respectively. Visits were more likely to have marijuana use documented in the postliberalization period compared to the preliberalization period (crude OR = 3.59, 95% CI = 1.44 to 9.00). Individual patient endorsement of marijuana use was highly consistent between visits; only two of the 36 patients who endorsed marijuana use on one visit had other visits with negative use. Both of these patients were in the postliberalization period, resulting in two of seven total visits in these patients who were positive for marijuana use.
Exploratory Analysis
ED visits during which patients received promethazine therapy were significantly more likely to result in hospital admission (adjusted OR = 5.06, 95% CI = 2.01 to 13.63, p = 0.0008). Of the 35 total visits resulting in hospitalization, 24 visits received promethazine therapy (four visits as monotherapy, 15 as one of two antiemetic medications, and five as one of three antiemetic medications). Documentation of marijuana use was not associated with admission (crude OR = 1.66, 95% CI = 0.74 to 3.69). There were no differences in the treatments given to marijuana users and nonusers, and median LOS did not differ between these two groups. No patients had documentation of compulsive hot bathing or showering in the ED record.
DISCUSSION
The prevalence of cyclic vomiting presentations to our EDs nearly doubled after marijuana liberalization. Additionally, patients presenting with cyclic vomiting after marijuana liberalization were more likely to have marijuana use documented in the ED record, although it is unclear whether this effect was secondary to increased use, more accurate self-reporting, or both. The most parsimonious explanation of this increased prevalence in an area with no other major socioeconomic or environmental changes is that increased marijuana use contributed to an increased rate of cyclic vomiting presentations. It should be noted, however, that despite a high rate of marijuana use in our community, the absolute prevalence of cyclic vomiting remained low, underscoring that CHS is a relatively uncommon condition.
As the number of new and chronic marijuana users grows annually, it is important to measure its effect on public health.2 While marijuana-associated pulmonary disease may take years to manifest, if associated with marijuana at all, the rate of cyclic vomiting seems to have increased acutely. Paradoxically, the association of marijuana use with cyclic vomiting contrasts its well-touted antiemetic properties. This effect may be in part modulated by the concentration of cannabidiol, a cannabinoid molecule in marijuana, rather than the more psychoactive delta-9-tetrahydrocannabinol (THC). In animal models, cannabidiol is antiemetic in low doses but has a proemetic effect in higher doses.14
We found that only one patient was seen in both the pre- and the postliberalization periods. This low rate of recidivism is likely due to the limited prevalence of CHS in the preliberalization period as well as the strict inclusion criteria of at least three visits, which would exclude a single or repeat visit for nausea and vomiting in the postliberalization period. Alternatively, the low rate of recidivism may indicate that the pathophysiology of CHS is similar to that of cyclic vomiting syndrome, which is defined by an absence of nausea and vomiting between episodes.13
We did not find documentation of the typical behavioral features of CHS described in case reports, such as compulsive hot bathing or showering.3–5 This may be due to the retrospective study design leading to lack of clinical detail in ED documentation. Furthermore, case reports of this syndrome only began surfacing in 2004, with more popular dissemination in the EM literature in 2011,3,15 predating our study period. The associated compulsive showering habits were likely not realized by physicians during the study period. Alternatively, these symptoms may not be present in all patients with CHS.
Patients who presented with cyclic vomiting in the study periods were predominantly female (71%) and white (72%). It is unclear whether these numbers indicate that this specific phenotype is at increased risk for cannabinoid hyperemesis, although it should be noted that a recent characterization of our ED population in metropolitan Denver showed that 57% of all ED patients were female, and 49% were white.16
In our exploratory analysis, we found that the use of promethazine for symptomatic relief was associated with an increased risk of admission. Although the magnitude of this increased risk was sizeable, we hesitate to apply this to clinical practice before understanding the pathophysiology of this effect. It is more likely that promethazine was associated with increased odds of admission due to its use as a second- or third-line antiemetic therapy in our ED settings.
To date, the literature on CHS has been limited to case reports, and thus our study represents a critical and novel contribution to the understanding of this syndrome. This study does not demonstrate causation or definitive quantification of the exposure, but instead represents a preliminary association and should serve as the foundation for future prospective studies on the association between marijuana use and cyclic vomiting, the eventual establishment of formal diagnostic criteria for CHS, and study of various interventions for symptomatic treatment. Foremost among these interventions should be counseling of patients toward marijuana cessation.
LIMITATIONS
The internal validity of this study is limited by potential biases in identifying cases of cyclic vomiting. Our results may have underestimated the prevalence of cyclic vomiting in either study period by imposing restrictive inclusion criteria based on the Rome III criteria; patients may have been excluded if they visited our EDs only twice but other community EDs other times. However, there is no reason why this bias would preferentially affect one time period over the other, and this bias is likely to exist in any study of CHS. It is also possible that the unblinded nature of chart review may have biased the exclusion of some nausea/vomiting visits with an “obvious pathophysiologic etiology.” We sought to minimize the risk of subjective interpretation of this exclusion criterion by establishing rigid definitions of gastroenteritis and gastroparesis. Although we intended to establish an a priori list of organic exclusion criteria, this proved to be impractical due to the emergence of unanticipated but obvious pathophysiologic diagnoses, such as stage IV gastric carcinoma undergoing weekly chemotherapy. Nevertheless, it is possible that unblinded review may have biased the recognition of potential cases of cyclic vomiting, which speaks to the difficulty of studying a syndrome without formal diagnostic criteria or ICD coding.
Conversely, our results may have overestimated the prevalence of cyclic vomiting in either period by including any visit with a diagnosis of cyclic vomiting by ICD-9 coding. Although it is possible that the prevalence of cyclic vomiting in the postliberalization period may reflect increasing awareness of CHS among ED providers over time, we feel that this is less likely given that the first known ED literature on this syndrome post-dates the conclusion of our study period.3,15 Although there were several case reports of CHS in non-ED literature prior to6,9,11 and during7 the preliberalization period, these reports prominently feature compulsive hot bathing as a key aspect of this syndrome. Given that none of the 128 visits included in this study included a mention of “cannabinoid hyperemesis syndrome” or compulsive bathing, it is unlikely that ED physicians were aware of these reports. Finally, the number of visits that met inclusion criteria by a primary diagnosis of cyclic vomiting was quite small (see Figure 1), and the adjusted numbers for visits included by Rome III criteria remain impressive: 78 versus 40 in the post- and preliberalization periods, respectively. However, it remains a possibility that increasing awareness of CHS over the study period resulted in an increased frequency of primary cyclic vomiting diagnoses.
The study’s secondary outcome is limited by reliance on patient self-report of marijuana use, which may be inaccurate in the preliberalization period due to fear of judgment or failure of providers to inquire among other social pressures. Although we found that patients in the postliberalization period were more likely to endorse marijuana use, it is unclear whether marijuana use increased, whether these patients were more willing to report their use, or whether providers were more likely to inquire about and document use. However, the marked increase of marijuana availability during this time makes it likely that there was a true increase in exposure.
This study’s external validity is limited by the high marijuana availability in our region. This may result in more use due to greater availability of marijuana overall, and of cannabis products with higher THC concentrations, leading to higher rates of cyclic vomiting. This may limit the broader applicability of our results, but it is what makes our patient population a unique opportunity for study of this syndrome. The liberalization of medical marijuana in Colorado in 2009 resulted in a dramatic rise in permits and dispensaries over a short period of time, which represents a natural experiment that is impossible to engineer.
CONCLUSIONS
In this retrospective study, we observed that the prevalence of cyclic vomiting presentations nearly doubled after the liberalization of medical marijuana in Colorado. This increase was accompanied by an increase of self-report of marijuana usage and serves as a crucial first step in establishing a formal diagnosis of cannabinoid hyperemesis syndrome.
Acknowledgments
AAM receives funding from NIH 1 K23 GM110516 01 and NIH UL1 TR001082.
Footnotes
No other authors received financial support for any aspect of the submitted manuscript, and no authors have conflicts of interest to disclose.
Dr. Heard, an associate editor for this journal, had no role in the peer-review process or publication decision for this paper.
Presented at the North American Congress of Clinical Toxicology Annual Meeting, New Orleans, LA, October 2014.
References
- 1.Stout D, Moore S. U.S. Won’t Prosecute in States That Allow Medical Marijuana. The New York Times. 2009 Oct 19; [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration . Results from the 2012 National Survey on Drug Use and Health: summary of national findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. SAMHSA; Rockville, MD: 2013. [Google Scholar]
- 3.Donnino MW, Cocchi MN, Miller J, Fisher J. Cannabinoid hyperemesis: a case series. J Emerg Med. 2011;40:e63–6. doi: 10.1016/j.jemermed.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566–70. doi: 10.1136/gut.2003.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc. 2012;87:114–9. doi: 10.1016/j.mayocp.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh E, Coyle W. Cannabinoid hyperemesis. Am J Gastroenterol. 2008;103:1048–9. doi: 10.1111/j.1572-0241.2007.01772_11.x. [DOI] [PubMed] [Google Scholar]
- 7.Sontineni SP, Chaudhary S, Sontineni V, Lanspa SJ. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15:1264–6. doi: 10.3748/wjg.15.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Digestive Dis Sci. 2010;55:3113–9. doi: 10.1007/s10620-010-1131-7. [DOI] [PubMed] [Google Scholar]
- 9.Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry. 2007;15:156–8. doi: 10.1080/10398560701196778. [DOI] [PubMed] [Google Scholar]
- 10.Nicolson SE, Denysenko L, Mulcare JL, Vito JP, Chabon B. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics. 2012;53:212–9. doi: 10.1016/j.psym.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Chepyala P, Olden KW. Cyclic vomiting and compulsive bathing with chronic cannabis abuse. Clin Gastroenterol Hepatol. 2008;6:710–2. doi: 10.1016/j.cgh.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Patterson DA, Smith E, Monahan M, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med. 2010;23:790–3. doi: 10.3122/jabfm.2010.06.100117. [DOI] [PubMed] [Google Scholar]
- 13.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 14.Parker LA, Kwiatkowska M, Burton P, Mechoulam R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew) Psychopharmacology (Berl) 2004;171:156–61. doi: 10.1007/s00213-003-1571-2. [DOI] [PubMed] [Google Scholar]
- 15.Shipsey P, Nordt S, Herbert M. In: Cannabis hyperemesis syndrome [podcast] Herbert M, editor. EMRAP; [Accessed Feb 4, 2015]. Available at: https://www.emrap.org/episode/2011/august/cannabis. [Google Scholar]
- 16.Valley MA, Heard KJ, Ginde AA, Lezotte DC, Lowenstein SR. Observational studies of patients in the emergency department: a comparison of 4 sampling methods. Ann Emerg Med. 2012;60:139–45. doi: 10.1016/j.annemergmed.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]