Abstract
A 41-year-old man with no previous asbestos exposure presented with 6 months of dull right lower chest pain and weight loss. The initial computed tomography (CT) scan was reported as showing a soft tissue thickening in the posterior mediastinum with non-specific nodules in the horizontal and oblique fissures. An endoscopic ultrasound-guided fine needle aspiration from the 12 × 25 mm heterogeneous posterior mediastinal mass was suspicious for a ganglioneuroma. The procedure was complicated by a large hemothorax requiring drainage. A subsequent positron emission tomographic CT revealed a moderately fluorodeoxyglucose avid area of pleural thickening extending from the sixth to ninth thoracic vertebral body in the paraspinal region along with nodules along the right horizontal and oblique fissures. A thoracoscopic biopsy of the pleural lesion confirmed a pleural epithelioid hemangioendothelioma. There was a 5-mm reduction in tumor thickness and improvement in his pain following 54 Gy of radiotherapy.
Keywords: Epithelioid hemangioendothelioma, primary pleural cancer
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare tumor of vascular endothelial origin. Although it can be found in any tissue, primary pleural EHE is less common, with only 21 cases reported to date including our patient. We report a case of pleural EHE presenting as a posterior mediastinal mass.
Case Report
A 41-year-old South African man, normally fit, and well presented with 6 months of dull right-sided anterior chest pain with 14 kg of weight loss. He was requiring codeine, paracetamol, and ibuprofen for pain control.
He served in the military for 17 years and currently works in information technology. He is a 5 pack-year ex-smoker and quit at the age of 18; he has no previous asbestos exposure.
Initial physical examination and laboratory findings were unremarkable. In view of his weight loss, gastroscopy and colonoscopy were performed and were normal. A computed tomography (CT) of his chest and abdomen showed a crescent of abnormal right-sided paraspinal soft tissue extending from the sixth to the ninth thoracic vertebral body with a lobulated soft tissue posterior mediastinal mass continuous with the right side of the distal esophagus (Fig. 1 ). There were also multiple pleural and subpleural nodules in the horizontal and oblique fissures without evidence of asbestos plaques.
Figure 1.
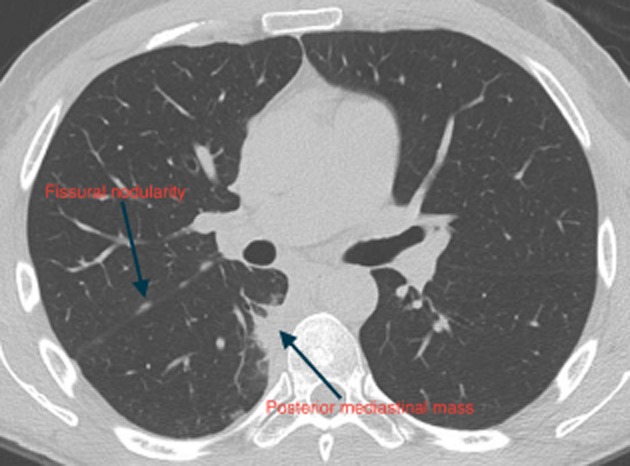
Standard computed tomography of the chest depicting the lobulated posterior mediastinal portion of the crescent of abnormal paraspinal tissue as well as the fissural nodularity.
An endoscopic ultrasound-guided fine needle aspiration (FNA) of the posterior mediastinal mass yielded bland spindle-shaped cells with “wavy” nuclei within myxoid stroma. The morphological features were suspicious for a neural lesion, possibly a ganglioneuroma. The patient experienced severe pain at the time of the FNA, which again suggested a lesion of neural origin. A chest radiograph performed the next day showed a new pleural effusion that progressed over the following 2 weeks, with associated breathlessness. He was referred to respiratory services and 800 mL of blood-stained fluid was drained during thoracentesis.
The initial CT scan was reviewed with an expert chest radiologist and it was felt that the posterior mediastinal mass and fissural nodularity were consistent with pleural malignancy. This radiological opinion was not consistent with the cytology diagnosis of possible ganglioneuroma and further investigation was undertaken. A positron emission tomographic CT demonstrated moderate fluorodeoxyglucose avidity in the right medial pleural cavity, in the fissural nodularity, and in the paraspinal pleural thickening (Figs. 2, 3).
Figure 2.
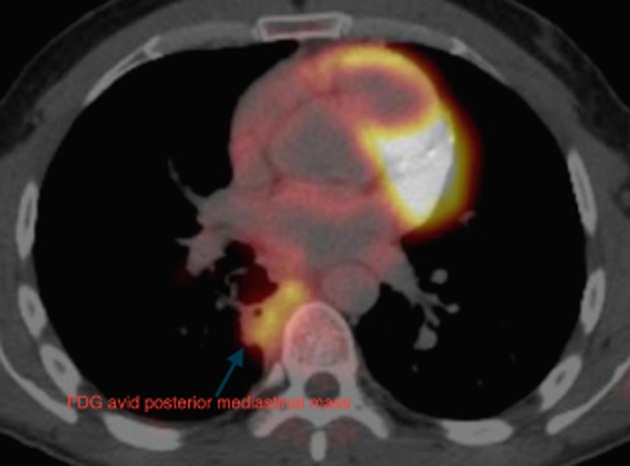
The posterior mediastinal mass is clearly fluorodeoxyglucose (FDG) avid.
Figure 3.
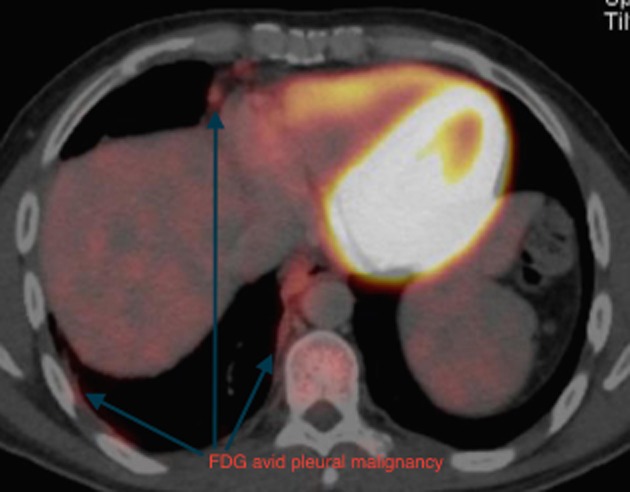
The anterolateral aspects of this pleural malignancy are also fluorodeoxyglucose (FDG) avid.
Thoracoscopy showed extensive patchy nodular plaques involving most of the chest wall with areas of nodularity in the fissures. A biopsy of the lesion in the anterolateral chest wall was performed followed by talc insufflation. The histology was consistent with an infiltrative neoplasm of vascular endothelial origin in keeping with EHE (Figs. 4, 5).
Figure 4.
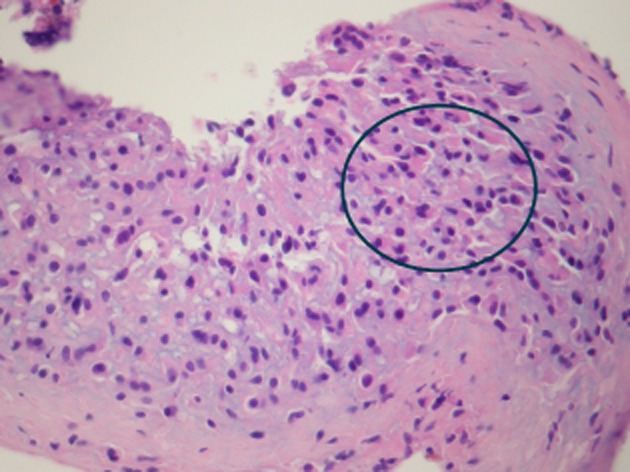
This section is heavily infiltrated with malignant cells with variable amounts of eosinophilic cytoplasm.
Figure 5.
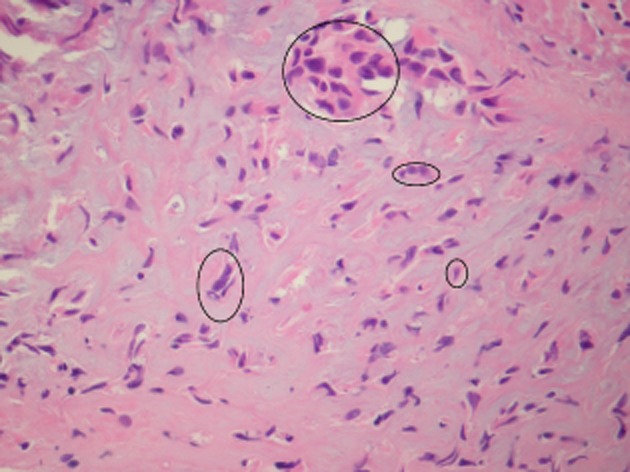
The tumor cells are variable in appearance and distribution. Circled areas show tumor cells in groups and singly. Some tumor cells have scanty cytoplasm, others more abundant (“epithelioid”) cytoplasm. Some cells have angulated nuclei, others more rounded.
He proceeded to high-dose radiotherapy (54 Gy in 30 fractions) to the right paraspinal mass. Two months following radiotherapy, a CT showed that the main paraspinal mass had reduced from 12 to 7 mm and his pain was much better controlled. The plan is for close CT surveillance and consideration of systemic therapy if there is disease progression.
Discussion
Pleural EHE is a rare endothelial tumor of vascular origin. Only 21 cases have been reported, including our patient [1], [2]. Of the cases found 15 were males and 6 were females. The mean age of presentation was 52.6, ranging from 31 to 85 years old [1], [2]. Prognosis is poor with a mean survival of 12.3 months, ranging from 4 to 24 and patients often present with unresectable disease [1], [2]. Only four patients survived longer than 12 months and all three received chemotherapy, including interferon and carboplatin plus etoposide [2]. Typical presenting symptoms include chest or shoulder pain, breathlessness, or cough [3]. Toxic exposures have been poorly recorded and of the nine cases where smoking status was documented, seven were smokers and one patient was exposed to asbestos out of the three where it was documented [1], [3], [4].
The tumor is typically composed of endothelial or hystioid cells with abundant cytoplasmic vacuoles and a typical immunohistochemistry pattern for vascular endothelium (Figs. 4, 5) [3]. Recent evidence suggests that fluorescence in situ hybridization analysis for the WWRT1/CAMTA1 fusion gene (t(1;3)(p36;q25)) is unique to EHE and may help distinguish it from hemangiomas or angiosarcomas [1].
Radiologically, this malignancy is often confused with mesothelioma [3], [4]. Typical features on CT include a pleural effusion, often with nodular pleural thickening [4]. In our case, the occurrence of a hemothorax indicated the vascular nature of this tumor [3]. The pleural rind may extend along fissures and interlobular septa and was found in one patient to involve the diaphragm [1], [3]. There seems to be a predisposition to involve the inferior aspect of the right hemithorax, with variable invasion into the mediastinum and local lymph nodes [4]. This pleurally based malignancy is distinct from “pulmonary” EHE that is similar histologically but the latter predominantly causes lung nodules with occasional pleural deposits [5]. EHEs can also be found primarily in the mediastinum, ribs and other bones, liver, and soft tissues [1].
Treatment options are limited and no consensus has been reached [1]. Chemotherapy (carboplatin, etoposide, interferon, paclitaxel, gemcitabine, and doxorubicin) is the most frequently used treatment; radiotherapy has been used rarely and in one case surgery was attempted but abandoned due to the extent of disease [2]. Our patient has so far received 54 Gy of radiotherapy with good radiological and symptomatic benefit; we feel that this should be the first-line treatment for pain control. He is well, 6 months following diagnosis, but still experiences lower chest pain.
Disclosure Statements
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
References
- Haa SY, Choia IH, Hana J, et al. Pleural epithelioid hemangioendothelioma harboring CAMTA1 rearrangement. Lung Cancer. 2014;83:411–415. doi: 10.1016/j.lungcan.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Yu L, Gu T, Xiu Z, et al. Primary pleural epithelioid hemangioendothelioma compressing the myocardium. J. Card. Surg. 2013;28:262–270. doi: 10.1111/jocs.12094. [DOI] [PubMed] [Google Scholar]
- Marquez-Medina D, Samame-Perezvargas JC, Tuset-DerAbrain N, et al. Pleural epithelioid hemangioendothelioma in an elderly patient. A case report and review of the literature. Lung Cancer. 2011;73:116–119. doi: 10.1016/j.lungcan.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Crotty EJ, McAdams HP, Erasmus JJ, et al. Epithelioid hemangioendothelioma of the pleura: clinical and radiologic features. AJR Am. J. Roentgenol. 2000;175:1545–1549. doi: 10.2214/ajr.175.6.1751545. [DOI] [PubMed] [Google Scholar]
- Chen J. Zhang S. 2014. Clinicopathological characteristics of pulmonary epithelioid hemangioendothelioma: a report of four cases and review of the literature. Oncology Letters.


