Abstract
Although mast cells were discovered more than a century ago, their functions beyond their role in allergic responses remained elusive until recently. However, there is a growing appreciation that an important physiological function of these cells is the recognition of pathogens and modulation of appropriate immune responses. Because of their ability to instantly release several pro-inflammatory mediators from intracellular stores and their location at the host–environment interface, mast cells have been shown to be crucial for optimal immune responses during infection. Mast cells seem to exert these effects by altering the inflammatory environment after detection of a pathogen and by mobilizing various immune cells to the site of infection and to draining lymph nodes. Interestingly, the character and timing of these responses can vary depending on the type of pathogen stimulus, location of pathogen recognition and sensitization state of the responding mast cells. Recent studies using mast cell activators as effective vaccine adjuvants show the potential of harnessing these cells to confer protective immunity against microbial pathogens.
Mast cells are a key cell type of the haematopoietic lineage that has evolutionarily conserved functions in pathogen surveillance. They are dispersed throughout most tissues but are crucially located at the host’s interfaces with the environment, such as the skin and mucosae, supporting a role in the recognition of pathogens or other signs of infection (FIG. 1). Mast cells and many of their products are best known for their association with pathological conditions such as asthma, allergy and anaphylaxis, in which aberrant, chronic or systemic activation of mast cells promotes harmful inflammatory sequellae and damage to host tissues. However, despite the potential detrimental effects that mast cells can have on immune homeostasis, these cells are indispensable to the host, as suggested by the observations that they are evolutionary preserved across many species and that humans that lack mast cells have never been described1. The first strong evidence that mast cells function in a protective capacity against infectious disease came from studies of host–parasite interactions2,3, and an increasing amount of work supports their essential contribution to controlling a wide range of pathogenic infections, including those by parasites, bacteria and probably viruses. We now understand that mast cells function not only as sentinels but also as modulators of innate and adaptive immune responses, ultimately influencing disease outcomes.
Figure 1. Mast cells are strategically located in host peripheral tissues.
a | A partially degranulated mast cell is visualized by electron microscopy, releasing granules near a blood vessel in mouse ear tissue activated by topical application of phorbol 12-myristate 13-acetate (PMA). The arrow indicates a granule that seems to be in the process of being released. b | Mouse ear tissue was stained in whole mount for CD31-expressing blood vessels (red) and mast cell granules, using a mast cell-specific fluorescent conjugated probe (green), after topical PMA treatment to activate mast cells. c | PMA-treated mouse ear tissue is stained to visualize LYVE1-expressing lymphatic vessels (blue) and mast cells (green). d | This image depicts tyrosine hydroxylase-expressing neurons (red) in close proximity to activated mast cells in compound 48/80-treated whole mounted bladder. e | Mouse ears were treated with PMA and prepared in whole mount for imaging by staining mast cells (green), CD31-expressing blood vessels (red) and CD11c-expressing dendritic cells (blue). For images in b–d, tissue was imaged at x10 magnification. Owing to whole mount preparation and large depth of field, overlap of stained elements can be seen.
In this Review, we discuss recent advances in our understanding of mast cell responses to pathogens. We first discuss the potential mechanisms by which mast cells can be activated by pathogens. We then describe the responses of mast cells, particularly with regard to the timing of the responses and the various roles they have in host defence, as sensors of pathogens, as effectors of adaptive immune responses and as modulators of local inflammation. Finally, we examine the evidence indicating that mast cells make meaningful contributions to controlling infectious challenges and discuss how mast cells might be harnessed during vaccination.
Cell biology of mast cells
Mast cells arise from bone marrow-derived precursors that circulate in the blood and become differentiated after entering tissues. They are long-lived cells, able to survive for months or years and, despite being terminally differentiated, they can proliferate in response to appropriate signals4. All mature mast cells reside in the body’s tissues and have a common fundamental morphology with prominent electron dense granules in their cytoplasm. At the earliest stages of infection, mast cells are important for communicating the presence of a pathogen to many cell types located nearby in the site of infection and distally in draining lymph nodes (FIG. 2). To facilitate these interactions, mast cells are strategically located at the host–environment interface, proximal to both blood vessels (FIG. 1a, b) and lymphatic vessels (FIG. 1c), as well as to nerve fibres (FIG. 1d) and tissue-resident immune cells, including dendritic cells (DCs) (FIG. 1e).
Figure 2. Cellular communication by mast cells promotes host defence.
Mast cells ‘communicate’ with various cell types, including immune cells (such as lymphocytes50,67,71, macrophages51, dendritic cells41,46,49,61,68–70 and neutrophils34,35,43,44,48,52), epithelial cells66, smooth muscle cells45,63 and endothelial cells49,57–60. These interactions contribute to pathogen surveillance, antipathogen immunity and other mechanisms of eliminating microorganisms from the host. These cellular targets of mast cells are located both in the site of infection and in distant draining lymph nodes. Examples of functional consequences of mast cell communication are shown, as are examples of mast cell mediators that have been shown to contribute to the target cell response. BCR, B cell receptor; CCL5, CC-chemokine ligand 5; IL, interleukin; LT, leukotriene; MCP1, mast cell protease 1; PG, prostaglandin; TCR, T cell receptor; TNF, tumour necrosis factor.
Despite having a common lineage, granulated morphology and functions, mast cells are highly heterogeneous and phenotypically malleable cells5,6, the intricacies of which have only begun to be defined, with little known about their distinct functionality. However, it is likely that this heterogeneity is shaped by the requirements of residing in a particular tissue or encountering unique pathogen challenges. On the basis of distinct staining properties, it was quickly recognized that rodent mast cells fall into two broad categories: mucosal and connective tissue mast cell types. These distinct mast cell types can now be further distinguished by several features, including granule composition, differing degranulation responses to pharmacological stimulation and the ability to proliferate in response to parasitic challenge7. This suggests that responses to other stimuli, including pathogens, might differ depending on the mast cell type.
The main protein components of mast cell granules are proteases. In mice, the granules of connective tissue mast cells contain two types of protease — tryptases and chymases — that are bound to heparin, whereas mucosal mast cells contain only chymases, which are bound to chondroitin sulphate7. Human mast cells also show heterogeneity with regard to these two main protease types, although with less stringent tissue-type specificity8. The storage of proteases varies not only between mast cell subtypes but also within an individual mast cell depending on the stimuli it receives. For example, in mouse mast cells, the expression of these proteases has been shown to be modulated at a transcriptional level by interleukin-10 (IL-10)9, and treatment of human mast cells in vitro with IL-4 increased the relative amount of chymase incorporated into granules10. Two types of human mast cell, defined by relative tryptase and chymase content, also vary with respect to their expression of the receptor for complement component C5a (C5aR)11. Although other inflammatory mediators and surface receptors might also have tissue-type or activation-specific specificity, the varied composition of granules (particularly well characterized for proteases) shows the heterogeneity of mast cells.
Armed with granules containing preformed mediators, mast cells have the potential to be the first responders (within seconds to minutes) following recognition of an invading pathogen. The findings that mast cells can respond to their environment — not only by producing appropriate mediators for the pathogen they have encountered, such as selective cytokine production12, but also by altering the transcription and storage of preformed mediators9,10 — suggest that they can modulate their phenotype during the course of infection. They also have the ability to replenish their granules during an infection or after its resolution7,13. Altering the production of preformed mediators and, thereby, the composition of their granules, if shown to be beneficial in preventing or controlling reinfection, could be considered a form of immunological memory. This would allow the responses of pathogen-experienced mast cells to be refined by the infectious challenges they have previously encountered.
Mast cells as sentinels of infection
Direct recognition of microorganisms
At the initiation of infection, the first responsibility of mast cells is to recognize that pathogen invasion has occurred. Many cells, including DCs, epithelial and endothelial cells, can alert the host immune system to the presence of pathogens, whether in the skin, gut or other sites in the body that are exposed to the environment or susceptible to pathogen encounter. This can be achieved by directly recognizing pathogens through pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), which are activated in response to conserved pathogen-associated molecular patterns (PAMPs)14. Mast cells are well equipped for this task as, in addition to being able to recognize PAMPs, they can detect a range of products through the expression of other receptors that sense pathogens (for example, Fc receptors (FcRs), which bind pathogen-specific antibodies) and receptors for inflammatory factors produced at the site of infection. Direct pathogen recognition by mast cells occurs both in response to factors that are common to classes of pathogens (such as through TLRs) and those that are specific to only a certain infectious challenge (such as through binding of antibodies specific for pathogen-associated epitopes). Interestingly, mast cell responses to TLR triggering alone can vary depending on the PAMP stimulus. For example, lipopolysaccharide (LPS) stimulation of rodent mast cells through TLR4 promoted cytokine production in the absence of degranulation, whereas stimulation through TLR2 by peptidoglycan induced both degranulation and cytokine production15. In this study, responses to individual PAMP stimulation overlapped for some cytokines (stimulation of either TLR4 or TLR2 promoted the production of tumour necrosis factor (TNF), IL-6 and IL-13), but diverged for other cytokines (TLR4 stimulation resulted in IL-1β production but not IL-4 or IL-5 production, whereas TLR2 stimulation resulted in IL-4 and IL-5 production but not IL-1β production)15. Similarly, for mast cells derived from human cord blood, both peptidoglycan and LPS were shown to induce a T helper 2 (TH2)-type cytokine response, however only peptidoglycan resulted in histamine release from intracellular stores16. In addition to conventional PRRs, other receptors on the surface of mast cells can be activated in response to pathogens; for example, CD48, which can detect the presence of fimbriated Escherichia coli, Mycobacterium tuberculosis and Staphylococcus aureus17–19.
Activation by FcRs
Owing to the expression of multiple FcRs, including FcγRII receptors and the high-affinity receptor for IgE, FcεRI, mast cells can bind both IgG and IgE and become sensitized to antigens that have been previously encountered by the host. Subsequently, mast cells can become activated, resulting in degranulation following receptor cross-linking by polyvalent antigen20,21. Antibody-mediated mast cell recognition of specific antigens and the signalling events downstream of receptor cross-linking have been most thoroughly characterized in models of asthma and allergy, but are also likely to be relevant in the context of infection as suggested by one study examining parasite clearance22. Interestingly, it has been shown that FcεRI activation and TLR stimulation can have synergistic effects on cytokine production by mast cells, enhancing cytokine transcription through the cumulative increase in activity of mitogen-activated protein kinases (MAPKs)23. Mast cells can also be activated by FcR signalling triggered by bacterial superantigens, such as S. aureus protein A, which can to bind certain classes of antibodies, independent of antigen specificity24.
Activation by pathogen-associated substances
Mast cells can also undergo degranulation in response to some exogenous stimuli that accompany pathogen injection into the skin or breaching of the skin barrier, such as components of wasp venom25 or mosquito saliva26. Mastoparan, for example, is a 14-amino acid peptide found in wasp venom that efficiently induces mast cell degranulation25. Degranulation in response to mosquito saliva could have implications for immune defence against arboviruses or vector-transmitted parasitic diseases such as malaria, although this remains to be investigated.
Activation by endogenous inflammatory factors
Several host endogenous peptides, including neurotensin, substance P27 and endothelin 1 (REF. 28), and by-products of inflammation, such as complement components, can also activate mast cells. Activation of mast cells through complement receptors, particularly C5aR, can result in degranulation29. This receptor was also identified as the main receptor responsible for mast cell detection of, and degranulation in response to, the yeast product zymosan, rather than TLR2 (which is generally ascribed the function of recognizing zymosan)30. The inflammation marker endothelin 1, which can have toxic side-effects during inflammation, is degraded by mast cell-derived proteases, showing an important feedback mechanism of mast cells in limiting potential detrimental effects of inflammatory processes through granule exocytosis28.
Two waves of mediator release
As mentioned, activation of mast cells by pathogens can result in both degranulation and de novo cytokine synthesis. Degranulation involves the rapid (beginning within seconds to minutes following stimulation) release of pre-packaged, insoluble mediators into the surrounding tissue, a strategy that gives mast cell-derived products a temporal advantage over those produced by other immune surveillance cells (FIG. 3). Other sentinel cells, for example, Langerhans cells in the skin, tissue-resident DCs and various subtypes of epithelial cells show a comparatively delayed secretory response to pathogens owing to the requirement for de novo production of mediators. The two-phase process of mast cell secretion can affect the nature, duration and specificity of the host’s responses to pathogen, for example through the contribution of mast cells to promoting the function of antigen-specific lymphocytes (discussed later).
Figure 3. Timing of mast cell responses to pathogens.
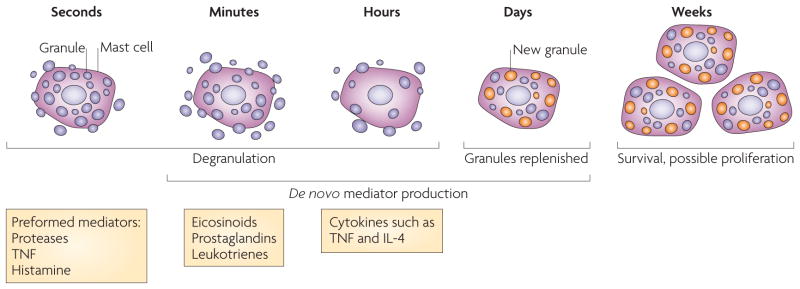
Mast cells can respond quickly to pathogen challenges owing to the presence of preformed mediators in cytoplasmic granules that can be quickly released at a site of infection through the process of degranulation. Mast cells also begin to produce lipid-derived eicosinoid mediators in the initial minutes of activation, as transcription is not required for these mediators to be converted to an active form. In a second wave of the response, consistent with the processes that are initiated by other cells involved in pathogen surveillance, mast cells begin to release de novo synthesized mediators, including a large number of cytokines — such as tumour necrosis factor (TNF) and interleukin-4 (IL-4) — that are transcribed and translated in response to pathogens. They can also replenish their granules, possibly with altered contents, in response to inflammatory signals. Mast cells are unique in their ability to survive for prolonged periods after activation compared with other innate immune cell types that may begin to die during the contraction of the innate response. Finally, mast cells can survive in the tissues and might proliferate in response to appropriate stimuli.
Degranulation
As Paul Ehrlich, the first scientist to visualize and describe mast cells, noted, “the cell is chiefly of a chemical nature”31, and so the earliest responses of mast cells depend on the structure and composition of their granules, which are formed and maintained as discrete insoluble structures in the cytoplasm by virtue of charge interactions. Following granule exocytosis, some granule-associated mediators, such as histamine, become soluble immediately, whereas most of the structure remains in an insoluble, particulate form. These exocytosed nanoparticles maintain their structure through tight interactions between negatively charged carbohydrate components, such as heparin, and positively charged proteins, most of which are proteases7. Granule proteins, such as TNF, can remain associated with the insoluble particles and be released slowly and for a prolonged time32. Moreover, these exocytosed particles can travel from the site of infection through lymphatic vessels to draining lymph nodes32, suggesting that these structures have been physiologically tailored for long-distance delivery of inflammatory mediators. As the activity of a given amount of cytokine can be greatly enhanced when protected from degradation and dilution in the insoluble particulate structure, other cytokines may be identified that remain encapsulated within these particles and have functional effects beyond the site of inflammation, although limited quantities may make detection difficult.
Studies have begun to establish the importance of mast cell proteases to immune defence, including one study showing a role for mast cell protease 6 (MCP6; also known as tryptase-β2) in the host response to peritonitis33. Importantly, however, as proteases are structural components of granules, deficiencies in these proteins can result in altered granule contents and mediator storage. Despite this caveat to studying mast cell protease function, several lines of evidence implicate mast cell proteases in the recruitment of neutrophils to sites of infection34,35. Indeed, exogenous MCP6 can lead to the recruitment of neutrophils into the peritoneal cavity32, and MCP6-deficient mice have impaired mast cell-mediated recruitment of eosinophils, resulting in defects in the control of a chronic parasitic infection36. Other mast cell-derived proteases, including human chymase, have also been shown to influence neutrophil recruitment33. Furthermore, mast cell proteases may have an important role in limiting the harmful effects of toxic host-derived by-products of inflammation, such as neurotensin (which causes hypotension during sepsis37) and endothelin 1 (REF. 28) (discussed earlier).
Many signalling mediators upstream of degranulation have been identified and these converge on a common requirement for generating a Ca2+ flux in the responding cell (reviewed in REF. 38). In vivo, integrated signalling pathways triggered by several stimuli contribute to degranulation. Indeed, exposure to a single stimulus, such as a PAMP, may not be sufficient to induce degranulation in experimental models, despite being able to contribute to degranulation in vivo and at the site of infection where many pathogen-derived products and pro-inflammatory endogenous products are present.
De novo mediator production
Similar to other cells, activation of mast cells by pathogens also induces de novo production of mediators such as cytokines and eicosinoids. Many studies have shown that de novo production of these mediators can vary greatly depending on the stimulus and the experimental conditions39,40. For example, IgE-mediated activation of human mast cells was shown to result in higher production of eicosinoids than the application of neurogenic peptides or pharmacological stimuli40. Leukotrienes, which are a type of eicosinoid, can be generated and quickly released by mast cells, owing to their lipid rather than protein structure and the requirement for only a catalytic conversion to the active form41. They function predominately at the local vascular endothelium, promoting the rolling and recruitment of neutrophils through the actions of P-selectin and neutrophil chemotaxis42,43, and they contribute to defence against bacterial infection in mice44. Prostaglandins are another class of mast cell-produced, lipid-derived products that are produced quickly after mast cell stimulation. Few functions have been ascribed to them during infection, although in other models they, like leukotrienes, have been shown to contribute to vascular permeability, chemotaxis of various cells, mucus production and activation of nerve cells41,45.
The list of cytokines and chemokines that mast cells can produce following stimulation is extensive and has been reviewed previously, with TNF, IL-4, IL-5, IL-6 and IL-3 being well characterized examples identified in several species39. Functions in host defence have not been ascribed to each mast cell-derived cytokine, so more work is needed to determine the individual contributions of these mast cell-derived factors to host defence as well as any synergistic effects they may have when produced in unique combinations. It is clear, however, that mast cell-produced cytokines and chemokines act both to activate local cells and to promote cell recruitment to sites of infection. For example, mast cell-derived TNF has been shown to promote DC activation and antigen presentation46. Mast cells are most often associated with TH2-type inflammatory responses; however, more recently, mast cells were shown to be an important source of the TH1-type cytokine IL-12 during peritonitis, which promoted neutrophil function and survival of infected mice47. Further actions of mast cell-derived cytokines can involve direct chemotactic responses, such as the TLR3-stimulated induction of cytotoxic T lymphocyte chemotaxis, or increased adhesion of leukocytes to the vascular endothelium, which occurs during E. coli infection through mast cell-derived TNF48–50. Recent studies have also shown that mast cell-derived cytokines can enhance bacterial killing: mast cell-derived IL-4 can promote macrophage killing of intracellular Francisella tularensis51 and mast cell-derived IL-6 can enhance neutrophil killing of Klebsiella pneumoniae52. Furthermore, there is evidence that mast cell-derived cytokines can have an inhibitory function during infection; for example, intracellular IL-15 antagonizes the production of the chymase MCP2 (also known as MCPT2), which, in turn, adversely affected survival in a mouse model of sepsis53. The anti-inflammatory cytokine IL-10 is also produced by mast cells and can limit the extent of lymphocyte infiltration during contact hypersensitivity54; however, the potential contribution of mast cell production of IL-10 during infection has not been adequately investigated. As mast cells are increasingly being recognized for their negative regulatory or feedback responses55, the contribution of this and other anti-inflammatory cytokines to counteracting either an already initiated immune response or other mast cell-associated processes, such as wound healing4,56, should be a goal of future studies. Cumulatively, the range of possible mediators produced by mast cells allows them to follow a degranulation response with the production of factors that are suited to the activating stimulus, such as cytokines associated with the clearance of viral or bacterial infections. This flexibility allows mast cells to not only activate surrounding cells but also direct them towards appropriate containment and clearance of the particular pathogenic challenge.
Innate immune functions of mast cells
In models of bacterial pathogenesis, it has become clear that immune cell recruitment to sites of infection is facilitated by the location of mast cells in tissues. For the many mast cells located proximal to blood vessels, the release of factors, such as histamine, TNF, vascular endothelial growth factor (VEGF) and proteases, contributes to increased local vascular permeability and oedema at the site of infection57–60. Chemokine production by mast cells has been implicated in the recruitment of other participants in the inflammatory response, including eosinophils and natural killer (NK) cells, through CC-chemokine ligand 11 (CCL11; also known as eotaxin) and CXC-chemokine ligand 8 (CXCL8; also known as IL-8), respectively12 (FIG. 4). Both mast cell-derived TNF and MCP6 have been reported to promote neutrophil recruitment in bacterial peritonitis models34 and other inflamed tissues52,61. Recruitment of innate immune cells by mast cell products also probably occurs during viral infections, as it seems that the double-stranded RNA analogue polyinosinic–polycytidylic acid (polyI:C) promotes the production of a wide range of chemokines by human cord blood-derived mast cells, which in turn can induce NK cell chemotaxis12 (FIG. 4).
Figure 4. Cell trafficking responses induced or increased by mast cells.
Host control and clearance of invading pathogens requires the mobilization of many cell types, both into the site of infection for effective innate immune responses and into draining lymph nodes to initiate appropriate adaptive immune responses. The diverse and divergent cell types that are recruited into infected sites during various models of pathogenesis as a result of mast cell products collectively show the specificity of mast cell-promoted trafficking responses to individual pathogen challenges. a | After entry of a bacterial pathogen, mast cells can become activated and release products that promote many of the necessary cell trafficking events. In models of bacterial pathogenesis, neutrophils are recruited, which are largely responsible for pathogen clearance. Mast cells also enhance the trafficking of dendritic cells (DCs) through infected tissues by mobilizing the DC precursors from the blood and into infected tissues. The activation of several subclasses of DCs has been shown to occur as a result of mast cell activation during bacterial infections, resulting in enhanced trafficking of these cells from infected sites and into draining lymph nodes to initiate adaptive immune responses. Mast cell-derived particles from exocytosed granules can also flow into the lymphatics and travel to draining lymph nodes, where they promote the retention of lymphocytes during the process of lymph node hypertrophy. b | During infection with parasites, eosinophils, basophils and mast cell precursors have been reported to be recruited into sites of infection, such as in the gut. In addition, there is evidence that mast cells proliferate in parasite infection models. c | In viral infection, mast cell activation can promote the chemotaxis of CD8+ T cells and natural killer (NK) cells to the peritoneal cavity or in vitro.
Mast cells also produce products that have direct bactericidal activity. Both human and mouse mast cells can produce antimicrobial peptides known as cathelicidins. Mast cells from mice lacking the genes encoding these products were found to have defects in their ability to kill group A streptococci. Interestingly, these products seem to be produced constitutively by mast cells as well as induced in response to stimuli such as LPS62. In addition, mast cells produce compounds to aid bacterial killing after phagocytosis, including reactive oxygen species4.
It is also important to consider the influence of mast cells on processes that impede pathogen colonization and promote their expulsion. For example, mast cells can influence the behaviour of nerve cells, the first cell type recognized as a target of mast cell signals, through products such as histamine (and serotonin in the case of mouse mast cells)27. Mast cells also communicate with smooth muscle cells63. Together, these interactions could potentially promote the elimination of pathogens from the body, such as that which occurs during mast cell-promoted expulsion of bacteria and fluids from the gut in response to cholera and clostridium toxins64,65. In addition, mast cells can promote mucus production by epithelial cells66, a key process that immobilizes pathogens and aids in their clearance from surfaces such as the nasal mucosa, gut and bladder.
Promoters and effectors of adaptive immunity
After stimulation, mast cells shape the inflammatory milieu and control the activation state of many cells crucial for adaptive immunity67. In the site of infection, mast cell-derived TNF induces the upregulation of E-selectin expression by the local vascular endothelium, promoting the influx of monocyte-derived DCs, which are subsequently increased in draining lymph nodes49. The production of CCL20 by mast cells probably contributes to the recruitment of DC precursors from the blood and into the tissues39. Mast cells have also been shown to promote activation of Langerhans cells, a skin-resident DC subset, in response to the bacterial product peptidoglycan68 or Gram-negative bacteria49, which leads to increased numbers of Langerhans cells in the draining lymph nodes49,68. In addition, mast cell products can directly modulate DC activation and antigen presentation. For example, histamine has been suggested to promote antigen uptake and cross-presentation69 and the upregulation of co-stimulatory molecules required for T cell activation46. Furthermore, mast cell products can promote DCs to acquire a TH2 cell-inducing phenotype70.
Mast cells might also promote the recruitment of effector T cells to sites of infection. In a viral peritonitis model, TLR3 activation on mast cells resulted in the upregulation of CXCL10 (also known as IP10) and CCL5 (also known as RANTES) and to the recruitment of CD8+ T cells50 (FIG. 4). This observation adds another facet to our understanding of how mast cells orchestrate the coordinated mobilization of immune cells during infection.
Moreover, mast cells themselves can present antigen to T cells. Early evidence suggesting that mast cells function as antigen-presenting cells (APCs) was provided by the findings that activated mast cells upregulate expression of MHC class II and co-stimulatory molecules and that they have been visualized in vivo physically interacting with T cells7. However, the functional requirement of MHC class II-dependent antigen presentation by mast cells has yet to be fully evaluated in vivo. By contrast, there is now evidence that mast cells function efficiently as APCs for MHC class I-restricted CD8+ T cells in vivo71. In this recent study, antigen-pulsed mast cells were shown to promote CD8+ T cell activation, proliferation and production of T cell products such as IL-2 and granzyme B. This is the first report describing an important functional role for mast cells in antigen presentation71 and, although shown in a model of autoimmunity, it raises the possibility that mast cells, acting as APCs, directly promote and modulate CD8+ T cell function during infection.
Together with local responses to pathogens, mast cells have long-distance and long-term effects in the host by modulating draining lymph nodes and promoting the development of adaptive immunity to pathogens. As mentioned, mast cells can also influence cell trafficking to draining lymph nodes (FIG. 4). In an E. coli infection model, we showed that mast cell-derived TNF is required for normal lymph node hypertrophy, a crucial event in which draining lymph nodes double in size during the first 24 hours of infection owing to the retention of lymphocytes49. This process increases the probability that rare antigen-specific lymphocytes will be present in draining lymph nodes during the induction of adaptive immunity, probably improving the specificity of adaptive responses. Together, these two processes of DC trafficking from or through infected tissues to lymph nodes and lymphocyte sequestration in draining lymph nodes should increase the magnitude and specificity of the adaptive immune response by ensuring that appreciable peripheral antigen is shuttled to the draining lymph nodes and that, simultaneously, rare antigen-specific lymphocytes are retained there. We observed functional consequences of mast cell-promoted responses in enhanced humoral immunity to E. coli in wild-type mice compared with mast cell-deficient mice, including increased E. coli-specific antibody titres and protection after passive immunization49, although it is currently unclear if mast cells have direct effects on B cells or antibody production.
As discussed previously, and best characterized in models of allergy, mast cells can function as effectors of adaptive immunity through their ability to become sensitized by binding antibodies through FcRs7. After a primary response, in which high-affinity pathogen-specific antibodies are present, antibody cross-linking of mast cell FcRs can result in faster responses of greater magnitude, allowing mast cells to participate in the enhanced immunity that results after immunological memory formation (FIG. 5). This may be particularly important during infection with some viral pathogens against which degranulation may not occur or may not be an immediate response. At least during chronic parasite infections, this sensitization can be important for ongoing control of latent infections7,22,72, indicating that mast cells can translate a functional antibody response into protection against a pathogenic challenge.
Figure 5. Pathogen-specific sensitization of mast cells enhances immune responses.
Tissue-resident mast cells are activated by many pathogens, and their products, through innate receptors and, as a result, promote adaptive immunity by modulating dendritic cell migration and cellular events occurring in distant lymph nodes. In addition, they can also be sensitized with various classes of immunoglobulins through Fc receptors (FcRs), enabling them to support more specific, amplified or quicker responses. This ability of mast cells to bind antigen-specific antibodies may be most crucial in secondary challenges, during chronic infection or in situations where innate recognition of a particular pathogen is not sufficient to initiate strong responses by mast cells. Furthermore, mast cells can replenish their granules after being activated and may alter the production of preformed mediators in response to the inflammatory milieu and the cytokines present there. This might result in a different degranulation response during the secondary challenge than during the initial challenge. IL, interleukin; SCF, stem cell factor.
Conversely, T cells can modulate mast cells, particularly through the production of chemokines such as CCL3 (also known as MIP1α) and CCL2, which probably contribute to mast cell degranulation, as well as through physical contact between mast cells and T cells73. In vitro, mast cell production of TNF and release of histamine can be enhanced by contact with T cells73, suggesting that another feedback mechanism exists, by which the adaptive immune system might regulate mast cell function during an ongoing inflammatory process or infection.
Pathogen-specific functional responses
The initial events following infection are key to containing rapidly replicating or host-adapted pathogens. Any delay in the initiation of antimicrobial efforts could potentially shift the advantage from the host to the pathogen, resulting in morbidity or mortality. Mast cells have been shown to initiate pathogen-specific programmes to functionally contribute to pathogen clearance and, in some experimental models, they have been shown to promote the survival of the host during an infectious challenge48,74 (TABLE 1).
Table 1.
Evidence for functional mast cell responses to pathogens
| Species | Tissue | Functional observation | Implicated factors | Refs |
|---|---|---|---|---|
| Parasites | ||||
| Trichinella spiralis (nematode) | Gut | Increased mast cell precursors in the gut | ND | 93 |
| Parasite expulsion coincides with mucosal mast cell activation in rats | Parasite expulsion coincides with peak systemic MCP2 levels | 2 | ||
| Reconstitution of mast cell-deficient mice with mast cells hastens parasite expulsion | Parasite expulsion delayed in mice lacking MCP1 | 72 | ||
| Mast cell defects result in increased numbers of larvae deposited in muscle cells | Eosinophil recruitment, but not parasite expulsion, delayed in the absence of MCP6 | 36 | ||
| Nippostrongylus brasiliensis (hookworm) | Gut | Mast cell precursor mobilization from the blood and proliferation in the gut | IL-3 and IL-4 | 76,94,95 |
| Parasite expulsion coincides with mucosal mast cell activation in rats | Worm expulsion coincides with peak systemic MCP2 levels | 2 | ||
| Accelerated expulsion of parasites during primary but not secondary infections in mast cell-sufficient compared with mast cell-deficient mice | ND | 76 | ||
| Strongyloides ratti (nematode) | Gut | Mast cell-deficient mice showed delayed parasite expulsion and higher peak larval numbers | ND | 3 |
| Strongyloides venezuelensis (nematode) | Gut | Mast cell-promoted parasite expulsion | IL-3 contributes to protection | 96 |
| Haemaphysalis longicornis (larval tick) | Skin | Decreased resistance to ticks in mast cell-deficient skin grafts compared with mast cell-sufficient skin grafts | ND | 97 |
| Leishmania major (protozoa) | Skin | Larger skin lesions with higher parasite burden and decreased cell recruitment in mast cell-deficient mice | Associated with reduced IL-12 in lesions | 77 |
| Plasmodium berghei (protozoa) | Parasitaemia | Mast cell-deficient mice have increased parasitaemia | Mast cell-derived TNF is protective | 98 |
| Bacteria | ||||
| Escherichia coli | Peritoneum | Mast cell-dependent recruitment of neutrophils and bacterial clearance | Dependent on mast cell-derived TNF and leukotrienes | 44,48,74 |
| Skin | Mast cell-dependent lymph node hypertrophy, recruitment of DC precursors to site of infection and egress to draining lymph node | Mast cell-derived TNF increases E-selectin expression on vascular endothelium and lymph node hypertrophy | 49,67 | |
| Bladder | Enhanced bacterial clearance from bladder after passive immunization with sera from infected mast cell-sufficient mice compared with infected mast cell-deficient mice | Attributed to higher antigen-specific antibody titres in post-immune mast cell-sufficient animals | 49,99 | |
| Citrobacter rodentium | Gut | Mast cell-deficient mice have decreased survival, increased histopathology and increased bacterial spread | ND | 75 |
| Mycoplasma pneumoniae | Airways | Mast cell-deficient mice have increased bacterial burden associated with increased lung pathology | ND | 100 |
| Francisella tularensis | Airways | Mast cell inhibition of bacterial replication in macrophages | Contact-dependent events and secreted IL-4 | 51 |
| Klebsiella pneumoniae | Lung | Mast cell-dependent recruitment of neutrophils and bacterial clearance | Mast cell-derived TNF | 48 |
| Peritoneum | Survival decreased in mice with mast cells lacking IL-6 | Mast cell-derived TNF and IL-6 promotes neutrophil killing | 52 | |
| Clostridium difficile | Gut | Toxin A from C. difficile induces mast cell-dependent neutrophil recruitment and intestinal fluid secretion based on studies with mast cell-deficient mice | Substance P-mediated activation of mast cells | 82 |
| Pseudomonas aeruginosa | Skin | Larger skin lesions in mast cell-deficient mice | ND | 81 |
| Pronounced mast cell degranulation in wild-type mice and neutrophil accumulation | ND | |||
| Caecal microflora | Peritoneum | Feedback inhibition of chymase transcription to constrict innate immunity | Intracellular IL-15 | 53 |
| Mast cell-dependent recruitment of neutrophils and bacterial clearance | Complement component C3 activation; mast cell-derived TNF is protective | 101 | ||
| Decreased survival of mast cell-deficient mice | Reconstitution with wild-type but not IL-12-deficient mast cells corrects defect | 47 | ||
| Viruses | ||||
| Sendai virus | Lung | Mast cells increase after infection and airway hyperresponsiveness | ND | 86,87 |
| HIV | Systemic | gp120 protein acts as a superantigen and binds to IgE, activating FcεRI+ cells | Release of IL-4 and IL-13 by FcεRI+ cells | 84,88 |
| Infected mast cells are an inducible virus reservoir | 88 | |||
| Newcastle virus | Peritoneum | Recruitment of CD8+ T cells | Chemokine production including CCL5 | 50 |
CCL, CC-chemokine ligand; DC, dendritic cell; FcεRI, high affinity Fc receptor for IgE; IL, interleukin; MCP, mast cell protease; ND, not determined; TNF, tumour necrosis factor.
Parasites
The first observations that mast cells could have a role in the control of infection came from the use of models of helminth infection in the gut2,3. In these early studies, mast cells were observed to cluster around sites with parasites, although it was unclear at the time if these cells were proliferating or newly recruited, and they displayed an activated phenotype, with many cells undergoing degranulation2. It is now clear that the control or clearance of parasites by mast cells involves various mechanisms, including the recruitment of key immune cells, regulation of gut permeability and parasite expulsion, and containment of chronic infection22,72,75–77. Proliferation of mast cells during gut helminth infection was observed in one model to depend on the key mast cell growth factor stem cell factor (SCF; also known as KIT ligand)78, and in another model to depend on IgE79. Studies of the requirement for mast cells during parasite infections have established that responses to pathogens vary greatly depending on the type of challenge. For example, a recent study examining hookworm infection indicated that expulsion of the parasite from the gut during a secondary challenge depended on basophils rather than mast cells, in contrast to expulsion during a primary challenge, which occurred much more quickly in mast cell-sufficient mice than their deficient counterparts76. In the case of infection with the parasite Trichinella spiralis, it was shown (using IgE-deficient mice) that IgE production by the host contributed to parasite expulsion from the gut22. In this study, the authors suggested that decreased levels of MCP1, previously shown to influence the speed of parasite expulsion from the gut, might explain these observations22. Mast cells also seem to be crucial for cutaneous immunity during the parasitic skin infection leishmaniasis, promoting protective immunity, including T cell function, and resulting in decreased skin lesion size77. The distinct functional roles of mast cells in various tissues are shown by these examples; however, the fundamental differences between responses require further characterization.
Bacteria
Mast cells are clearly essential for initiating both innate and adaptive immune responses to many bacterial pathogens and products, and for protecting the host from lethal infection48,74. Several of the earliest studies showed this by determining that mice deficient in mast cells show increased mortality after E. coli injection into the peritoneum than their wild-type counterparts48,74. In these peritonitis models, mast cells are functionally important for initiating innate immune responses to enterobacteria, particularly through their ability to recruit neutrophils to promote bacterial clearance48. These observations were reinforced by a recent study examining peritonitis caused by caecal ligation, however, during the most experimentally severe conditions, the mast cells in this study were no longer protective and the TNF they produced contributed to pathology80. Mast cells are crucial for containing bacterial infection and preventing dissemination in other tissues, and have been shown, for example, to promote the clearance of E. coli from the peritoneum and bladder48,49 and K. pneumoniae from the lungs48,52, as well as to limit skin lesions during Pseudomonas aeruginosa infection81. Based on the studies discussed above and others, the strongest functional evidence for the necessity of mast cell function to survival comes from studies involving bacterial challenges. Indirect activation of mast cells by host endogenous proteins can also highly influence the host response during bacterial infection, as shown by the contribution of mast cells to fluid production and neutrophil influx in the gut in response to neuron production of substance P, which occurs in the presence of toxin A from Clostridium difficile82.
Viruses
The role of mast cells in viral infections is more enigmatic and has been less well studied. Many viral products can activate mast cells, in particular to promote cytokine production; however, the extent of mast cell degranulation in response to viral infection and any resulting functional implications are less clear. In support of direct recognition of viruses by mast cells, it has been shown that histamine release can occur in response to Sendai virus83 and that the gp120 envelope protein of HIV can induce cytokine production by mast cells84. The ability of mast cells to promote the recruitment of CD8+ T cells during viral challenge50 (as previously discussed) or promote the production of type I interferons85 suggests that mast cell recognition of virus may promote the types of cell-mediated responses that are associated with the clearance of intracellular viral infection. In both rodents and humans, mast cell numbers in the lungs are higher than uninfected controls following respiratory viral infections86,87. However, the interaction between viruses and mast cells does not always clearly favour the host. HIV, for example, is known to infect mast cells, and these cells may constitute a reservoir for latent virus within the host88. Other viruses that seem to infect mast cells include respiratory syncytial virus and dengue virus89,90; however, these studies were carried out in neoplastic cell lines and the consequences of these interactions remain to be explored in vivo.
Targeting mast cells in vaccines
Many parallels exist between the functions of mast cells in vivo, in particular their role in enhancing adaptive immune responses, and our requirements for efficient vaccine adjuvants. Thus, it was hypothesized that activation of mast cells during vaccination could potentially promote protective immunity. It has now been shown that addition of a mast cell activator, compound 48/80, to vaccine formulations can result in increased humoral immunity, which gives protection against a lethal viral challenge. When administered intranasally, these compounds can also promote antigen-specific IgA production, a key goal in the search for effective mucosal adjuvants91,92. It is likely that the mechanism of the enhanced responses is multifaceted and may involve many of the known interactions between mast cells and functional outcomes of adaptive responses including cellular mobilization, communication with draining lymph nodes and establishment of an environment at the site of vaccine administration that is similar to the environment in an infected tissue. These results indicate that mast cells can be intentionally activated to enhance protective host responses, including the production of high-affinity antibodies and immunological memory, and raise the possibility of incorporating mast cell activators in vaccine formulations to harness the inherent adjuvant activity of mast cell activation.
Concluding remarks
An increasing amount of experimental evidence supports the hypothesis that mast cells are essential for pathogen containment and/or clearance. They have the ability not only to affect immediate innate processes to clear pathogens but also to influence the long term host responses to pathogens. These mast cell contributions to adaptive immune processes occur directly (for example, through their antigen-presentation capabilities) or indirectly (such as through lymph node potentiation). The ability of mast cells to orchestrate complex cellular migration within tissues, from the blood and in distant lymph nodes (FIG. 3) is a key mechanism by which they bridge the processes of both innate and adaptive immunity and function to accelerate essential host programmes of defence. Further questions remain, however, particularly with regard to the functional contributions of mast cells acting as effectors of immunological memory during secondary challenges or during an ongoing process of chronic infection, topics that few studies have addressed. Future studies are also likely to provide more insight into the effects that the heterogeneity of mast cells within and between different tissues has on the functional outcomes of pathogen challenge, as current evidence discussed here suggests that mast cell responses may be highly specific, depending not only on the type of pathogen encountered but also on the tissue in which it is encountered. In addition, it will be important to assess the influence that a mast cell’s experience of chronic or acute infection has on subsequent responses to pathogens, considering their long lifespan and phenotypic plasticity. The interaction between mast cell responses to pathogens and their involvement in other host inflammatory processes could be one area where these concepts are particularly applicable, as the complex interactions between chronic respiratory infections and airway hyperresponsiveness may show.
Mast cells have a kinetic advantage over other sentinel cells in initiating both innate and adaptive immune responses through their ability to store preformed mediators and release them nearly instantaneously into a site of infection. Furthermore, exocytosed granules seem to function as long distance delivery devices for their cargo, including inflammatory mediators. Long distance communication of cells is classically thought to be autocrine, in which large amounts of proteins, such as hormones, are released in a soluble form to achieve high enough serum concentrations to affect distant cell types. Yet, it now seems that mast cells have a newly discovered form of long distance communication that involves the targeted delivery of small quantities of mediators. Understanding this unique feature of mast cell biology raises the possibility of using this communication strategy for prophylactic or therapeutic potential. Thus, successful use of mast cell activators to enhance immune responses during vaccination confirms the potential contributions of mast cells to protection against subsequent challenges and opens the possibilities of targeting mast cells, or the processes they control, during rational vaccine design.
Acknowledgments
The authors’ work is supported by the US National Institutes of Health grants R01 AI35678, R01 DK077159, R01 AI50021, R37 DK50814 and R21 AI056101. We thank M. M. Ng and H. Yap of the National University of Singapore for their help in acquiring the image in FIG. 1a and Z. Swan for reviewing the manuscript.
Footnotes
Competing interests statement.
The authors declare no competing financial interests.
References
- 1.McNeil HP, Adachi R, Stevens RL. Mast cell-restricted tryptases: structure and function in inflammation and pathogen defense. J Biol Chem. 2007;282:20785–20789. doi: 10.1074/jbc.R700017200. [DOI] [PubMed] [Google Scholar]
- 2.Woodbury RG, et al. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature. 1984;312:450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- 3.Nawa Y, Kiyota M, Korenaga M, Kotani M. Defective protective capacity of W/Wv mice against Strongyloides ratti infection and its reconstitution with bone marrow cells. Parasite Immunol. 1985;7:429–438. doi: 10.1111/j.1365-3024.1985.tb00088.x. References 2 and 3 are early reports of a functional involvement of mast cells in the expulsion of intestinal parasites. [DOI] [PubMed] [Google Scholar]
- 4.Abraham SN, Malaviya R. Mast cells in infection and immunity. Infect Immun. 1997;65:3501–3508. doi: 10.1128/iai.65.9.3501-3508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe T, Swieter M, Imai T, Hollander ND, Befus AD. Mast cell heterogeneity: two-dimensional gel electrophoretic analyses of rat peritoneal and intestinal mucosal mast cells. Eur J Immunol. 1990;20:1941–1947. doi: 10.1002/eji.1830200911. [DOI] [PubMed] [Google Scholar]
- 6.Vliagoftis H, Befus AD. Rapidly changing perspectives about mast cells at mucosal surfaces. Immunol Rev. 2005;206:190–203. doi: 10.1111/j.0105-2896.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 8.Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997;61:233–245. doi: 10.1002/jlb.61.3.233. [DOI] [PubMed] [Google Scholar]
- 9.Ghildyal N, McNeil HP, Gurish MF, Austen KF, Stevens RL. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J Biol Chem. 1992;267:8473–8477. [PubMed] [Google Scholar]
- 10.Toru H, et al. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood. 1998;91:187–195. [PubMed] [Google Scholar]
- 11.Oskeritzian CA, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke SM, et al. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–5476. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
- 13.Burwen SJ. Recycling of mast cells following degranulation in vitro: an ultrastructural study. Tissue Cell. 1982;14:125–134. doi: 10.1016/0040-8166(82)90012-x. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nature Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 15.Supajatura V, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. One of several papers from this group that shows the crucial in vivo role of mast cell-expressed TLRs in mobilizing immune cells following exposure to microbial products. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varadaradjalou S, et al. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 17.Malaviya R, Gao Z, Thankavel K, van der Merwe PA, Abraham SN. The mast cell tumor necrosis factor α response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc Natl Acad Sci USA. 1999;96:8110–8115. doi: 10.1073/pnas.96.14.8110. This study describes the identification of a unique receptor on mast cells that recognizes bacteria, triggering mast cell exocytosis of granules and uptake of bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz S, Hernandez-Pando R, Abraham SN, Enciso JA. Mast cell activation by Mycobacterium tuberculosis: mediator release and role of CD48. J Immunol. 2003;170:5590–5596. doi: 10.4049/jimmunol.170.11.5590. [DOI] [PubMed] [Google Scholar]
- 19.Rocha-de-Souza CM, Berent-Maoz B, Mankuta D, Moses AE, Levi-Schaffer F. Human mast cell activation by Staphylococcus aureus: interleukin-8 and tumor necrosis factor α release and the role of Toll-like receptor 2 and CD48 molecules. Infect Immun. 2008;76:4489–4497. doi: 10.1128/IAI.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nature Rev Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 21.Woolhiser MR, Okayama Y, Gilfillan AM, Metcalfe DD. IgG-dependent activation of human mast cells following up-regulation of FcγRI by IFN-γ. Eur J Immunol. 2001;31:3298–3307. doi: 10.1002/1521-4141(200111)31:11<3298::aid-immu3298>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Gurish MF, et al. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–1145. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 23.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεR1 and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese A, et al. Bacterial immunoglobulin superantigen proteins A and L activate human heart mast cells by interacting with immunoglobulin E. Infect Immun. 2000;68:5517–5524. doi: 10.1128/iai.68.10.5517-5524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai Y, et al. A new mast cell degranulating peptide “mastoparan” in the venom of Vespula lewisii. Chem Pharm Bull (Tokyo) 1979;27:1942–1944. doi: 10.1248/cpb.27.1942. [DOI] [PubMed] [Google Scholar]
- 26.Demeure CE, et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J Immunol. 2005;174:3932–3940. doi: 10.4049/jimmunol.174.7.3932. This study shows how mosquito bites can trigger dermal mast cell degranulation and how this can affect the recruitment of immune cells to the site of the insect bite and to the draining lymph node. This may be highly relevant to many insect borne diseases. [DOI] [PubMed] [Google Scholar]
- 27.Johnson D, Krenger W. Interactions of mast cells with the nervous system — recent advances. Neurochem Res. 1992;17:939–951. doi: 10.1007/BF00993271. [DOI] [PubMed] [Google Scholar]
- 28.Maurer M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson G, et al. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996;157:1693–1698. [PubMed] [Google Scholar]
- 30.Mullaly SC, Kubes P. Mast cell-expressed complement receptor, not TLR2, is the main detector of zymosan in peritonitis. Eur J Immunol. 2007;37:224–234. doi: 10.1002/eji.200636405. [DOI] [PubMed] [Google Scholar]
- 31.Ehrlich P. Nobel Lecture, December 11, 1908. Elsevier; Amsterdam: 1967. [Google Scholar]
- 32.Kunder CA, et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206:2455–2467. doi: 10.1084/jem.20090805. This paper reveals a new mechanism of how peripheral mast cells can regulate distal draining lymph nodes after degranulation by long distance communication through lymphatics using particle-packaged cytokines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakurdas SM, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 34.Huang C, et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160:1910–1919. This paper describes a role for proteases in neutrophil recruitment. [PubMed] [Google Scholar]
- 35.Tani K, et al. Chymase is a potent chemoattractant for human monocytes and neutrophils. J Leukoc Biol. 2000;67:585–589. doi: 10.1002/jlb.67.4.585. [DOI] [PubMed] [Google Scholar]
- 36.Shin K, et al. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piliponsky AM, et al. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nature Med. 2008;14:392–398. doi: 10.1038/nm1738. This study reveals a protective role for mast cells following sepsis, through decreasing levels of an endogenous peptide, neurotensin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nature Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nature Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 40.Benyon RC, Robinson C, Church MK. Differential release of histamine and eicosanoids from human skin mast cells activated by IgE-dependent and non-immunological stimuli. Br J Pharmacol. 1989;97:898–904. doi: 10.1111/j.1476-5381.1989.tb12030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 42.Datta YH, et al. Peptido-leukotrienes are potent agonists of von Willebrand factor secretion and P-selectin surface expression in human umbilical vein endothelial cells. Circulation. 1995;92:3304–3311. doi: 10.1161/01.cir.92.11.3304. [DOI] [PubMed] [Google Scholar]
- 43.McIntyre TM, Zimmerman GA, Prescott SM. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc Natl Acad Sci USA. 1986;83:2204–2208. doi: 10.1073/pnas.83.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malaviya R, Abraham SN. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. J Leukoc Biol. 2000;67:841–846. doi: 10.1002/jlb.67.6.841. [DOI] [PubMed] [Google Scholar]
- 45.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 46.Caron G, et al. Histamine induces CD86 expression and chemokine production by human immature dendritic cells. J Immunol. 2001;166:6000–6006. doi: 10.4049/jimmunol.166.10.6000. [DOI] [PubMed] [Google Scholar]
- 47.Nakano N, et al. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood. 2007;109:4846–4855. doi: 10.1182/blood-2006-09-045641. [DOI] [PubMed] [Google Scholar]
- 48.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. This was one of the first studies to provide evidence that mast cells promote survival during bacterial infections by promoting innate immune cell recruitment. It also identified TNF as a crucial mast cell-derived factor for neutrophil recruitment. [DOI] [PubMed] [Google Scholar]
- 49.Shelburne CP, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–342. doi: 10.1016/j.chom.2009.09.004. This paper shows the key role of mast cells in recruiting DCs to sites of bacterial infection and in the resulting protective immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orinska Z, et al. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood. 2005;106:978–987. doi: 10.1182/blood-2004-07-2656. This report shows that mast cells can respond to virus with a chemotactic response characteristic of CD8+ T cell recruitment, suggesting pathogen-specific responses by mast cells. [DOI] [PubMed] [Google Scholar]
- 51.Ketavarapu JM, et al. Mast cells inhibit intramacrophage Francisella tularensis replication via contact and secreted products including IL-4. Proc Natl Acad Sci USA. 2008;105:9313–9318. doi: 10.1073/pnas.0707636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orinska Z, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nature Med. 2007;13:927–934. doi: 10.1038/nm1615. This study reveals the complex intracellular interplay of various mast cell products in the regulation of mast cell activity during sepsis. [DOI] [PubMed] [Google Scholar]
- 54.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nature Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 55.Palker TJ, Dong G, Leitner WW. Mast cells in innate and adaptive immunity to infection. Eur J Immunol. 2010;40:13–18. doi: 10.1002/eji.200990325. [DOI] [PubMed] [Google Scholar]
- 56.Peranteau WH, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 57.Boesiger J, et al. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fcε receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sendo T, et al. Involvement of proteinase-activated receptor-2 in mast cell tryptase-induced barrier dysfunction in bovine aortic endothelial cells. Cell Signal. 2003;15:773–781. doi: 10.1016/s0898-6568(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 59.Heltianu C, Simionescu M, Simionescu N. Histamine receptors of the microvascular endothelium revealed in situ with a histamine-ferritin conjugate: characteristic high-affinity binding sites in venules. J Cell Biol. 1982;93:357–364. doi: 10.1083/jcb.93.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. A pivotal report showing that mast cells generate and store TNF, suggesting that they have a role in many inflammatory responses, including microbial infections. [DOI] [PubMed] [Google Scholar]
- 61.Biedermann T, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. This study shows for the first time that antimicrobial actions of mast cells could also be attributable to their expression of antimicrobial peptides. [DOI] [PubMed] [Google Scholar]
- 63.Margulis A, et al. Mast cell-dependent contraction of human airway smooth muscle cell-containing collagen gels: influence of cytokines, matrix metalloproteases, and serine proteases. J Immunol. 2009;183:1739–1750. doi: 10.4049/jimmunol.0803951. [DOI] [PubMed] [Google Scholar]
- 64.Klimpel GR, et al. A role for stem cell factor and c-kit in the murine intestinal tract secretory response to cholera toxin. J Exp Med. 1995;182:1931–1942. doi: 10.1084/jem.182.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pothoulakis C, Castagliuolo I, LaMont JT. Nerves and intestinal mast cells modulate responses to enterotoxins. News Physiol Sci. 1998;13:58–63. doi: 10.1152/physiologyonline.1998.13.2.58. [DOI] [PubMed] [Google Scholar]
- 66.Bischoff SC. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol. 2009;31:185–205. doi: 10.1007/s00281-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 67.McLachlan JB, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nature Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. This paper indicates that peripheral mast cells, and specifically their product TNF, activate distal draining lymph nodes promoting hypertrophy in response to infection. [DOI] [PubMed] [Google Scholar]
- 68.Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol. 2006;177:1755–1762. doi: 10.4049/jimmunol.177.3.1755. This is the first demonstration that mast cells can mobilize a subset of DCs to draining lymph nodes in response to bacterial products. [DOI] [PubMed] [Google Scholar]
- 69.Amaral MM, et al. Histamine improves antigen uptake and cross-presentation by dendritic cells. J Immunol. 2007;179:3425–3433. doi: 10.4049/jimmunol.179.6.3425. [DOI] [PubMed] [Google Scholar]
- 70.Mazzoni A, Siraganian RP, Leifer CA, Segal DM. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J Immunol. 2006;177:3577–3581. doi: 10.4049/jimmunol.177.6.3577. [DOI] [PubMed] [Google Scholar]
- 71.Stelekati E, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 72.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mekori YA, Metcalfe DD. Mast cell-T cell interactions. J Allergy Clin Immunol. 1999;104:517–523. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- 74.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. This study was one of the first to provide in vivo evidence of the crucial role of mast cells and TNF in promoting survival of the host during bacterial infections. [DOI] [PubMed] [Google Scholar]
- 75.Wei OL, Hilliard A, Kalman D, Sherman M. Mast cells limit systemic bacterial dissemination but not colitis in response to Citrobacter rodentium. Infect Immun. 2005;73:1978–1985. doi: 10.1128/IAI.73.4.1978-1985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184:344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 77.Maurer M, et al. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006;20:2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 78.Newlands GF, Coulson PS, Wilson RA. Stem cell factor dependent hyperplasia of mucosal-type mast cells but not eosinophils in Schistosoma mansoni-infected rats. Parasite Immunol. 1995;17:595–598. doi: 10.1111/j.1365-3024.1995.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 79.Asai K, et al. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 80.Piliponsky AM, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6–KitW-sh/W-sh mice. Am J Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siebenhaar F, et al. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am J Pathol. 2007;170:1910–1916. doi: 10.2353/ajpath.2007.060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wershil BK, Castagliuolo I, Pothoulakis C. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 1998;114:956–964. doi: 10.1016/s0016-5085(98)70315-4. [DOI] [PubMed] [Google Scholar]
- 83.Sugiyama K. Histamine release from rat mast cells induced by Sendai virus. Nature. 1977;270:614–615. doi: 10.1038/270614a0. One of the early suggestions of a possible role for mast cells in modulating immune responses to viruses. [DOI] [PubMed] [Google Scholar]
- 84.Patella V, Florio G, Petraroli A, Marone G. HIV-1 gp120 induces IL-4 and IL-13 release from human FcεRI+ cells through interaction with the VH3 region of IgE. J Immunol. 2000;164:589–595. doi: 10.4049/jimmunol.164.2.589. [DOI] [PubMed] [Google Scholar]
- 85.Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 86.Castleman WL, Sorkness RL, Lemanske RF, Jr, McAllister PK. Viral bronchiolitis during early life induces increased numbers of bronchiolar mast cells and airway hyperresponsiveness. Am J Pathol. 1990;137:821–831. [PMC free article] [PubMed] [Google Scholar]
- 87.Sorden SD, Castleman WL. Virus-induced increases in bronchiolar mast cells in Brown Norway rats are associated with both local mast cell proliferation and increases in blood mast cell precursors. Lab Invest. 1995;73:197–204. [PubMed] [Google Scholar]
- 88.Sundstrom JB, et al. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood. 2007;109:5293–5300. doi: 10.1182/blood-2006-11-058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shirato K, Taguchi F. Mast cell degranulation is induced by A549 airway epithelial cell infected with respiratory syncytial virus. Virology. 2009;386:88–93. doi: 10.1016/j.virol.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Legrand LF, Hotta H, Hotta S, Homma M. Antibody-mediated enhancement of infection by dengue virus of the P815 murine mastocytoma cell line. Biken J. 1986;29:51–55. [PubMed] [Google Scholar]
- 91.McLachlan JB, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nature Med. 2008;14:536–541. doi: 10.1038/nm1757. The first demonstration of small molecular activators of mast cells as highly effective vaccine adjuvants. [DOI] [PubMed] [Google Scholar]
- 92.McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009;27:3544–3552. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dillon SB, MacDonald TT. Limit dilution analysis of mast cell precursor frequency in the gut epithelium of normal and Trichinella spiralis infected mice. Parasite Immunol. 1986;8:503–511. doi: 10.1111/j.1365-3024.1986.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 94.Kasugai T, et al. Infection with Nippostrongylus brasiliensis induces invasion of mast cell precursors from peripheral blood to small intestine. Blood. 1995;85:1334–1340. [PubMed] [Google Scholar]
- 95.Madden KB, et al. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387–1391. [PubMed] [Google Scholar]
- 96.Lantz CS, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 97.Matsuda H, et al. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornis ticks in mice. J Immunol. 1990;144:259–262. [PubMed] [Google Scholar]
- 98.Furuta T, Kikuchi T, Iwakura Y, Watanabe N. Protective roles of mast cells and mast cell-derived TNF in murine malaria. J Immunol. 2006;177:3294–3302. doi: 10.4049/jimmunol.177.5.3294. This study provides the first in vivo evidence of a protective role for mast cells against a blood-borne parasite. [DOI] [PubMed] [Google Scholar]
- 99.Malaviya R, Ikeda T, Abraham SN. Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli. Immunol Lett. 2004;91:103–111. doi: 10.1016/j.imlet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 100.Xu X, et al. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173:219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]






