Abstract
Crohn's disease (CD) is a chronic inflammatory disease associated with a dysregulated T cell response towards intestinal microflora. Vitamin D has immune modulatory effects on T cells through the nuclear vitamin D receptor (VDR) in vitro. It is unclear how oral vitamin D treatment affects VDR expression. The aim of this study was to establish a flow cytometry protocol, including nuclear and cytoplasmic VDR expression, and to investigate the effects of vitamin D treatment on T cell VDR expression in CD patients. The flow cytometry protocol for VDR staining was developed using the human acute monocytic leukaemia cell line (THP-1). The protocol was evaluated in anti-CD3/CD28-stimulated peripheral blood mononuclear cells (PBMCs) from vitamin D3- (n = 9) and placebo-treated (n = 9) CD patients. Anti-VDR-stained PBMCs were examined by flow cytometry, and their cytokine production was determined by cytokine bead array. VDR, CYP27B1 and RXRα mRNA expression levels in CD4+ T cells were measured by quantitative reverse transcriptase polymerase chain reaction. The flow cytometry protocol enabled detection of cytoplasmic and nuclear VDR expression. The results were confirmed by confocal microscopy and supported by correlation with VDR mRNA expression. VDR expression in CD4+ T cells increased following stimulation. This VDR up-regulation was inhibited with 30% by vitamin D treatment compared to placebo in CD patients (P = 0·027). VDR expression was correlated with in-vitro interferon-γ production in stimulated PBMCs (P = 0·01). Flow cytometry is a useful method with which to measure intracellular VDR expression. Vitamin D treatment in CD patients reduces T cell receptor-mediated VDR up-regulation.
Keywords: Crohn's disease, flow cytometry, T cells, VDR, vitamin D
Introduction
Crohn's disease (CD) is characterized by a transmural intestinal mucosal inflammation that results from an inappropriate immune response towards the intestinal commensal microbiota in a genetically predisposed host 1. CD inflammation is dominated by an imbalanced T helper type 1 (Th1) and Th17 response, resulting in increased production of tumour necrosis factor (TNF)-α, interferon (IFN)-γ 2, interleukin (IL)-17 and IL-22 3,4.
Vitamin D deficiency is common in CD 5,6; it is associated with a lower quality of life in CD patients 7 and increases the risk of developing CD 8. Vitamin D is produced in the skin and is hydroxylated twice. The second hydroxylation is mediated by hydroxyvitamin-D3-1-α-hydroxylase (1α-hydroxylase), which is encoded by CYP27B1. The active form of vitamin D, 1.25-dihydroxyvitamin D3 (1·25-vitD), has a complex signal-transducing pathway. Together with one of the isoforms of retinoid X receptor (RXR), 1·25-vitD binds to the nuclear and cytoplasmic vitamin D receptor (VDR) 9. Then, the complex of 1·25-vitD and VDR-RXR translocate to the nucleus 10, where it binds to vitamin D receptor response elements (VDREs) 11. By binding to nuclear vitamin D-responsive genes, this vitamin D complex has great immune-modulating potential 12. This potential has been observed in animal models of inflammatory bowel disease (IBD) and in human studies of CD. In animal IBD models, vitamin D3 reduces colitis symptoms 13,14. In humans, a prospective study showed that patients with active CD had lower vitamin D levels than CD patients in remission 12. In a placebo-controlled CD trial we demonstrated a reduced relapse rate in vitamin D3-treated CD patients 15. This effect may be due to decreased expression of CD80 in lipopolysaccharide (LPS)-matured monocyte-derived dendritic cells, together with decreased production of IL-6 and IL-1β 16 and an increase in the synthesis of the anti-bacterial peptide cathelicidin 17. In T cells, oral vitamin D treatment increases the proliferation and IL-6 production ex vivo 18.
Several studies have reported an association between intestinal inflammation and the expression of vitamin D-related genes. In mice, dextran sodium sulphate (DSS)-induced colitis is associated with increased CYP27B1 mRNA but not with VDR mRNA expression in colonic tissue 19. DSS colitis in mice was associated with lower 1·25-vitD serum levels. In human in-vitro studies, the expression of VDR and CYP27B1 mRNA is induced by 1·25-vitD-stimulated T cells 9,20. Ham et al. showed that inflammation (active CD) increases CYP27B1 mRNA expression in T cells compared to CD patients in remission 12. Unlike reports from mice studies 19, patients with active CD exhibited increased VDR mRNA expression in blood T cells compared with CD in remission. A positive association between VDR expression and T cell activation in vitro has been reported 10.
VDR expression has been investigated with polymerase chain reaction (PCR), immunohistochemistry, flow cytometry and Western blot analyses. Gene expression studies may not reflect the actual cellular expression of VDR, and in the study using flow cytometry it was not specified whether the applied permeabilization procedure included the nucleolemma 10. Considering that VDR is mainly a nuclear receptor 11, the inclusion of nuclear VDR expression may be of importance. With Western blot the relative VDR expression can be measured in both cytosol and nucleus of fractioned cells 13,21–23. However, to detect the VDR expression by Western blot in different cell subsets, isolation of the specific cell type of interest from the tissue should be performed. Therefore, this procedure will be extremely time-consuming compared with a flow cytometry analysis, and increases the need for necessary cellular material. We present a flow cytometry protocol that enables the investigation of both cytoplasmic and nuclear VDR expression developed on the human monocytic human acute monocytic leukaemia (THP1) cell line. THP1 cells express VDR and have a large nucleus, which is optimal for verifying nuclear staining for VDR by confocal microscopy 24.
We hypothesized that vitamin D treatment modulates T cell VDR expression in CD patients. We investigated this hypothesis by using the newly described flow cytometry protocol for the measurement of VDR expression in T cells from vitamin D- and placebo-treated CD patients.
Materials and methods
Patients
A total of 108 patients were enrolled in a randomized placebo-controlled clinical study 15. Each patient was treated for 1 year with 30 μg vitamin D3 or placebo daily. All patients received 1200 mg calcium daily. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient separation (Ficoll-Paque, Amersham Biosciences, Uppsala, Sweden) at weeks 0 and 26 and stored at −140°C.
In the present study, nine patients receiving vitamin D3 were selected from the described clinical study, on the basis of the largest increase in the serum levels of 25-vitD from baseline to week 26. Nine placebo-treated patients seasonally matched by inclusion date were included as controls. Table1 shows the characteristics of the patients. No differences in patient characteristics were detected other than the 25-vitD levels at week 26.
Table 1.
Patient characteristics
| Vitamin D3 | Placebo | |||
|---|---|---|---|---|
| Week 0 | Week 26 | Week 0 | Week 26 | |
| Numbers of patients | 9 | 9 | 9 | 9 |
| Female patients, n | 6 | – | 6 | – |
| Age (years), median (range) | 36 (28–65) | – | 42 (23 – 66) | – |
| Enrolled during the winter*, n | 8 | – | 8 | – |
| Relapse during 26 weeks† | – | 0 | – | 1 |
| Azathioprine users, n | 6 | – | 3 | – |
| Biologicals prior to inclusion, n‡ | 1 | – | 1 | – |
| 25-vitD levels nmol/l, median (range) | 36 (16–66) | 111 (62–154) | 66 (22–105) | 45 (27–97) |
| HBI, median (range) | 2 (1–4) | 2 (1–4) | 2 (1–7) | 1 (0–6) |
| CDAI, median (range) | 35 (8–111) | 33§ (23–53) | 22 (0–187) | 27 (0–273) |
| CRP nmol/l, median (range) | 61 (6–247) | 17 (6–166) | 20 (12–276) | 18 (0–83) |
Winter enrolment corresponded to enrolment between 1 November and 30 April.
Relapse was defined as an increase in the Crohn's disease activity index (CDAI) of > 70 and a total CDAI score > 150.
Infliximab or natalizumab. None of the patients were treated with biologicals during the intervention.
n = 8. 25-vitD = 25-hydroxyvitamin D3; CRP = C-reactive protein; HBI = Harvey–Bradshaw Index. No differences were observed between the two groups except increased 25-vitD in the vitamin D group within 26 weeks of treatment, which was expected.
THP1 cell culture
Human monocytic leukaemia THP1 cells (ATCC TIB-202; LGC Standards, Teddington, UK) (1 × 106/ml) were cultured in culture medium (RPMI-1640 (25 mM HEPES, 2 mM ι-glutamine), penicillin and streptomycin, 10% heat-inactivated human AB serum and 1% glutamine). The cells were cultured in culture medium ± 10−8 M 1·25-vitD3 (Sigma-Aldrich, St Louis, MO, USA) for 3 days in 24-well plates (TPP, Trasadingen, Switzerland). On day 2, 100 ng/ml phorbol-12-myristate-13-acetate (PMA) (Sigma-Aldrich) was added to wells selected for PMA stimulation, and 200 μl of culture medium was added to all wells. The cells were harvested on day 3 and washed twice before resuspension in phosphate-buffered saline (PBS) (bovine serum albumin 0·5%, natriumazid 0.09%; Aarhus University, Aarhus, Denmark) to a concentration of 5 × 106/ml.
Confocal microscopy of THP1 cells
THP1 cells (1 × 106/ml) were cultured on acetone-processed glass cover slips in culture medium ± 10−8 M 1·25-vitD3 for 3 days in 24-well plates. Cells were stained with unconjugated mouse anti-VDR antibody (clone D-6; Santa Cruz Biotechnologies Inc., Dallas, Texas, USA) or matching isotype (Biolegend, San Diego, CA, USA), followed by staining with a secondary antibody, 0.75 μg/μl donkey anti-mouse Alexa Fluor 488 (Life Technologies, Carlsbad, CA, USA), which was diluted to 1 : 400. The nucleus was stained with 4',6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich). Confocal microscopy was performed within 48 h.
Culture and stimulation of PBMCs
PBMCs were thawed and diluted in RPMI-1640 and washed twice. Cells (2 × 106/ml) were cultured in RPMI-10 medium (2 × 106 cells/well) (RPMI-1640, penicillin and streptomycin, 10% human heat-inactivated AB serum and 1·5 ml HEPES) and stimulated with 1 μg/ml immobilized anti-CD3 (Orthoclone OKT3; a kind gift from Cilag AG International, Schaffhausen, Switzerland) and 1 μg/ml soluble anti-CD28 (BD Biosciences, San Diego, CA, USA) for 3 days. On day 3, the PBMCs were harvested, washed twice and suspended in PBS (5 × 106/ml).
Cytokines
After 3 days of culture with or without anti-CD3/anti-CD28 stimulation (described above), the supernatants were harvested and frozen at −20°C. The supernatants were thawed for the measurement of cytokine concentration (IL-2, IL-4, IL-6, IL-10, IL-17A, TNF-α and IFN-γ) using cytometric bead array (BD Biosciences). The detection limits were 10 pg/ml for all cytokines.
VDR antibody conjugation
The anti-VDR antibody (clone D-6; Santa Cruz Biotechnologies, Inc.) was conjugated with a phycoerythrin (PE) lighting link according to the manufacturer's description (Innova Biosciences, Cambridge, UK) and suspended to a final concentration of 0·4 μg/μl, confirmed with a NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
Nuclear and cytoplasmic VDR staining
One hundred μl PBMC suspension was added each tube and stained with 2·5 μl of anti-CD4 antibody fluorescein isothiocyanate (FITC) (BD Bioscience), 2·5 μl of anti-CD8 antibody allophycocyanin (APC) (BD Bioscience) and 2 μl of live-dead antibody (Life Technologies, Carlsbad, CA, USA) and incubated for 20 min in the dark at room temperature (RT). The THP1 cells were stained only with 2 μl of live-dead antibody. Then, 2 ml of PBS was added and the cells were washed. The cells were resuspended by vortexing in 100 μl of 4% freshly prepared formaldehyde solution (37% formaldehyde diluted in PBS) (J. T. Baker, Deventer, Holland) and incubated for 20 min in the dark at RT. Thereafter, the cells were washed twice in 2 ml of PBS and resuspended in 100 μl of cold, freshly prepared staining buffer [5% inactivated human AB serum, 0.5% Triton X (Sigma-Aldrich) and PBS] and incubated for 30 min in the dark at 4°C. Anti-VDR PE antibody (0·8 μg) or phycoerythrin (PE) isotype (0·8 μg) (clone eBM2a; eBioscience) were added, and the cells were incubated for 30 min in the dark at 4°C. The cells were then washed twice in cold stain buffer before resuspension in 250 μl of stain buffer. Flow cytometry analysis was performed within 2 h. The VDR-positive gate was set on the basis of the isotype PE control in each experiment. VDR expression is given as the number of positive VDR-stained cells in percentage, and the VDR median fluorescence index (MFI) was measured for VDR-positive cells. The delta-VDR MFI was also calculated (delta-VDR MFI = MFI anti-CD3CD28 stimulated cells minus MFI non-stimulated cells).
Separation of CD4+ T cells for PCR
CD4+ T cells (> 95% purity) were isolated from the PBMCs by magnetic bead separation using autoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). The PBMCs (4–13 × 106 cells) were thawed and diluted in RPMI (25 mM HEPES and 2 mM L-glutamine) and washed twice with RPMI. The cells were resuspended according to the manufacturer's instructions and labelled with CD4+ MicroBeads (Miltenyi Biotec). The autoMACS separation program ‘possel’ was chosen. The CD4+ cell suspensions were washed twice, and the supernatants were completely aspirated with a pipette. The cell pellet was resuspended immediately in 350 μl of RLT RNeasy Plus lysis buffer (Qiagen, Copenhagen, Denmark) supplemented with 3·5 μl of 2-mercaptoethanol (14·3 M, Sigma Aldrich, Brøndby, Denmark) and vortexed for 1 min. The cell suspensions were stored on ice for a maximum of 20 min before RNA and DNA isolation.
Isolation of RNA for PCR
RNA was extracted using the Qiagen Allprep DNA/RNA mini kit (Qiagen) from the CD4+ T cell suspensions according to the manufacturer's instructions and quantified by measuring the absorbance at 260 nm with a NanoDrop ND-8000 8-sample spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The 260/280 nm ratio was used to determine the purity of the samples and was found to be 1·8 or higher. The RNA concentration was too low to generate reverse transcription–PCR (RT–PCR) products for the one week 0 sample and the one week 26 sample; these samples were from two different patients, both in the vitamin D group.
Gene expression using RT–PCR
The cDNA was generated from the stored RNA using the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA), according to the manufacturer's protocol. Predesigned gene-specific primers and TaqMan MGB probe sets were obtained from Applied Biosystems (Life Technologies). Real-time PCR was performed in triplicate in reaction mixtures consisting of 1 µl of 380 ng/20 μl of cDNA, 0·5 µl of 20× assay mix, 5 µl of TaqMan® Fast Universal PCR MasterMix (both Applied Biosystems, Foster City, CA, USA) and 3·5 µl of ddH2O (Milli-Q H2O). The thermocycling was performed using a 7500 fast real-time PCR system (Applied Biosystems) using the following protocol: 95°C for 20 s, followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. Probes were tested and housekeeping genes selected as described previously 25. The housekeeping genes (HKGs) expressed most consistently during the vitamin D3 treatment were identified using the geNorm Excel sheet developed by Vandesompele et al. 26; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), beta glucuronidase (GUSB) and phosphoglycerate kinase (PGK-1) were the three most consistently expressed HKGs in our analyses. The analysis of the variation between the normalization factors calculated using two and three HKGs (V2/3) was 0·07.
The expression levels of the target genes were normalized to the levels of three HKGs. The calculations were performed using 7500 software version 2·0 ·1 (Applied Biosystems).
Statistical analysis
The median and total range gives the descriptive statistics for the patient characteristics. We used the non-parametric Wilcoxon rank sum test to compare unpaired data and the non-parametric Wilcoxon signed rank test with paired data. An association between two variables was estimated using the Spearman's rank correlation coefficient (Spearman's rho).
The RT–PCR results of gene expression are presented in relative quantification (RQ) values and median RQ. A P-value below 0·05 was considered statistically significant. All the statistical analyses and graphs were completed using GraphPad Prism version 6 (GraphPad Software Inc., La Jolla, CA, USA).
Ethics
The clinical study (which provided patient samples to the present study) and additional studies based on the collected patient samples were approved by the National Committee on Health Research Ethics (j. no. 2004/0149) and the Danish Medicines Agency (EudraCT no. 2005/001216/50) and were registered at ClinicalTrial.gov (NCT 0013 2184). In the randomized clinical trial that the patients signed and approved, the blood samples were stored for further studies on the effects of vitamin D in CD. The clinical trial conformed to the Helsinki Declaration and to Danish legislation. Patients gave informed consent to participate in the study 15.
Results
Establishing and validating a flow cytometry protocol for nuclear and intracellular VDR expression
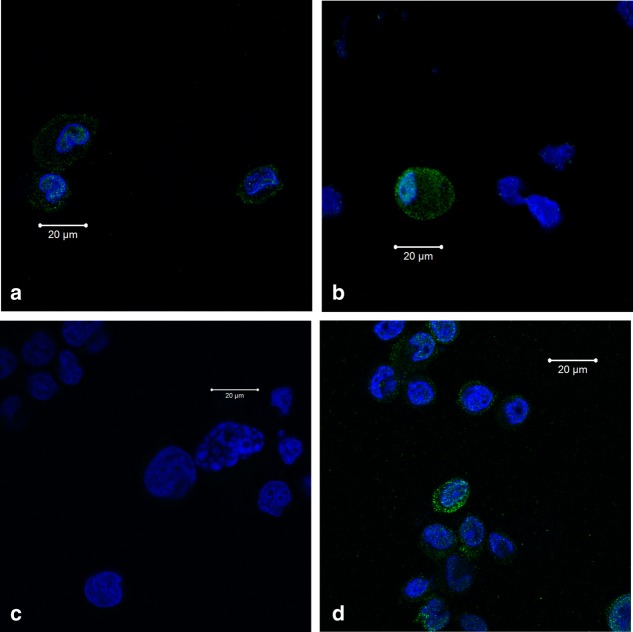
We developed and validated a flow cytometry protocol for cytoplasmic and nuclear VDR expression using the cell line THP1. Flow cytometry analysis revealed that approximately 30% of non-stimulated THP1 cells express VDR compared to isotype control. To verify the protocol's sensitivity to in-vitro changes in cellular VDR activation, THP1 cells were stimulated with 1·25-vitD, which doubled the VDR expression (data not shown). To show that the protocol included both nuclear and cytoplasmic VDR expression, we performed a confocal microscopic evaluation of THP1 cells labelled with anti-VDR antibody (Fig. 1). Confocal microscopy demonstrated that VDR staining was associated with both the nucleus and the cytoplasm of the THP1 cells. There was no staining with the isotype control. Confocal microscopy verified that 1·25-vitD stimulated THP1 cells had an increased VDR expression compared to non-stimulated cells. 1.25-vitD stimulation resulted in a minor translocation of VDR into the nucleus compared to no stimulation.
Figure 1.
Confocal microscopy of vitamin D receptor response (VDR) expression in human acute monocytic leukaemia cell line (THP1) cells. (a1 and a2) Confocal microscopy photos of non-stimulated THP1 cells labelled with VDR antibody and nuclear 4',6-diamidino-2-phenylindole (DAPI). VDR is present in some THP1 cells both in the nucleus and cytosol with different intensity. (b) Confocal microscopy photo of 25-hydroxyvitamin D3 (1·25-vitD)-stimulated THP1 cells labelled with isotype and nuclear DAPI. (c) Confocal microscopy photograph of 1·25-vitD-stimulated THP1 cells labelled with VDR antibody and nuclear DAPI. 1·25-vitD increases the VDR expression and induces a minor shift of VDR towards the nucleus compared to non-stimulated cells. Blue colour = nuclear DAPI; green colour = VDR.
Verifying the VDR flow protocol in PBMCs
We used the VDR staining protocol in PBMCs from CD patients. To substantiate the specificity of the flow protocol to VDR on PBMCs, we correlated the VDR mRNA expression in magnetically separated CD4+ T cells with the percentage of CD4+VDR+ T cells. Flow VDR expression was correlated with mRNA VDR expression in CD4+ T cells (Fig. 2a). To substantiate the specificity of the mRNA VDR quantification we examined the mRNA expression of RXRα, which heterodimerizes with VDR. As expected, RXRα was correlated with mRNA VDR (Fig. 2b) and with the percentage of CD4+VDR+ T cells measured by flow cytometry (Spearman's rho = 0·78, P = 0·0004) (Fig. 2c).
Figure 2.
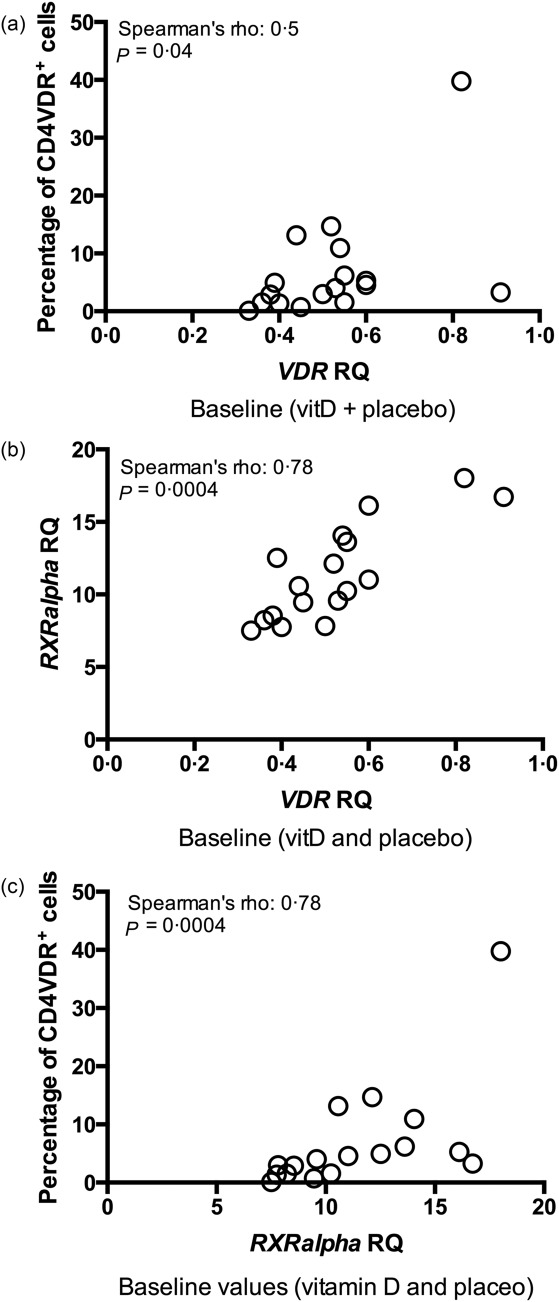
Correlations between flow cytometry and mRNA levels. (a) Baseline association between the percentage of flow cytometry CD4+ vitamin D receptor response (VDR)+ T cells and mRNA VDR expression in CD4+ T cells, including both the vitamin D and placebo group (n = 17; data are missing from one vitamin D-treated patient). VDR mRNA is measured as relative quantification (RQ). Cells for VDR expression measurement [flow cytometry and polymerase chain reaction (PCR)] were unstimulated. VitD = vitamin D-treated patients; placebo = placebo-treated patients. (b) Baseline correlation between mRNA RXRα RQ and mRNA VDR RQ in unstimulated CD4+ T cells at baseline (n = 17; data are missing from one vitD-treated patient). RXRα = retinoid X receptor alpha; VDR = vitamin D receptor. (c) Correlation between the percentage of CD4+VDR+ T cells and mRNA RXRα RQ in non-stimulated CD4+ T cells at baseline (n = 17; data are missing from one vitD-treated patient.
T cell receptor activation increases VDR expression in T cells
The sensitivity of the VDR flow cytometry protocol was determined by detecting changes in VDR expression in T cells following T cell receptor (TCR) stimulation. TCR activation increased VDR expression in both CD4+ and CD8+ T cells by four- to fivefold compared to unstimulated T cells (for both CD4+ and CD8+ T cells, P < 0·0001; Fig. 3a,b). Flow cytometry VDR expression in stimulated lymphocytes was also associated with an increased in-vitro production of the proinflammatory cytokine IFN-γ (Spearman's rho 0·58, P = 0·01; Fig. 3c). VDR expression may be influenced by in-vivo inflammation in CD. This finding was supported by a trend towards an association between faecal calprotectin and mRNA VDR expression in CD4+ T cells at baseline (P = 0·055, n = 13; Fig. 3d).
Figure 3.
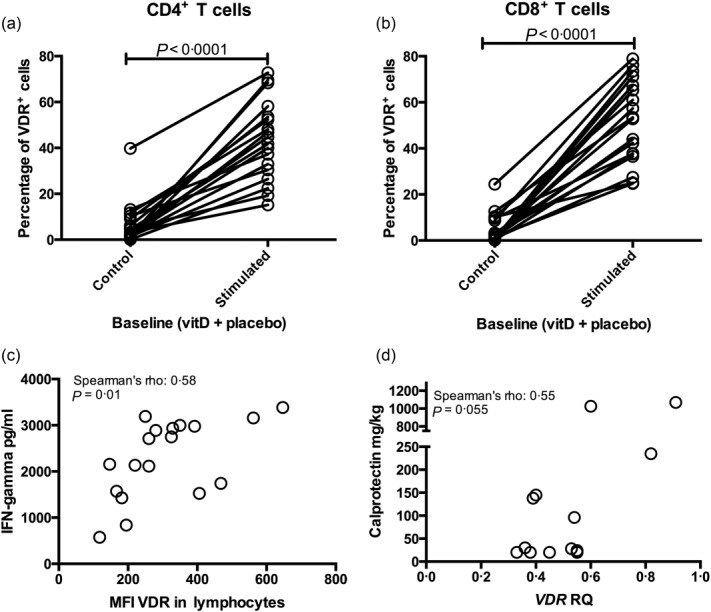
T cell receptor (TCR) stimulation increases vitamin D receptor response (VDR) expression. (a) The percentage of CD4+VDR+ T cells measured by flow cytometry is induced by T cell receptor activation with anti-CD3 and anti-CD28 stimulation. (a) Comprises vitamin D- and placebo-treated patients at baseline; n = 18. Stimulated = anti-CD3 and anti-CD28 stimulation. (b) The percentage of CD8+VDR+ T cells (flow cytometry) is also induced by T cell receptor activation. (b) Comprises vitamin D- and placebo-treated patients at baseline; n = 18. Stimulated = anti-CD3 and anti-CD28 stimulation. (c) Interferon gamma production in supernatant from TCR stimulated peripheral blood mononuclear cells (PBMCs) correlated with the median fluorescence index of VDR in lymphocytes at baseline; n = 18 comprising both the vitamin- and placebo-treated CD patients. (d) When combining the vitamin D and placebo group at baseline, VDR mRNA expression in unstimulated CD4+ T cells tended to correlate with faecal calprotectin; n = 13 mRNA data were missing from one vitamin D patient and faecal calprotectin data was missing from one vitamin D patient and three placebo-treated patients. RQ = relative quantification.
Oral vitamin D treatment is associated with lower VDR up-regulation following in-vitro T cell receptor activation
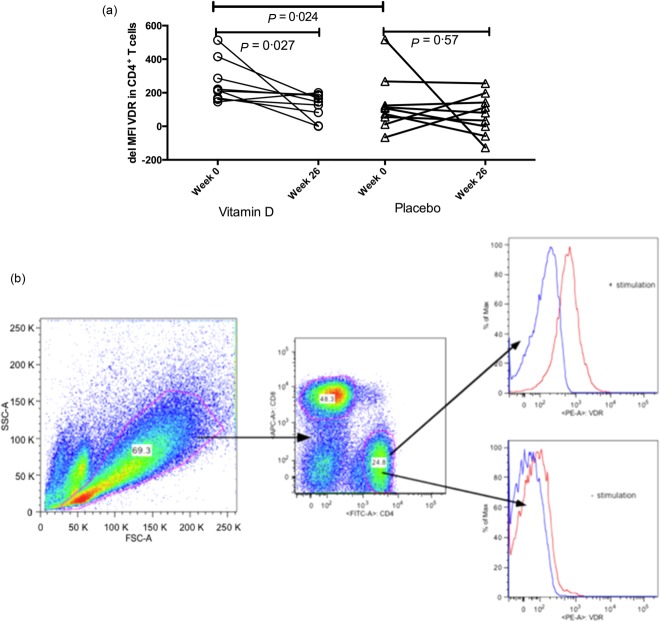
Flow cytometry revealed that vitamin D treatment did not influence the flow cytometry VDR expression in non-stimulated T cells or the cytokine secretion in PBMC supernatants (data not shown). However, 26 weeks of vitamin D treatment reduced CD4+ T cell VDR up-regulation by 30% in response to TCR stimulation compared to the placebo (P = 0·027, Fig. 4a,b). Vitamin D-treated patients exhibited a higher TCR-induced up-regulation of VDR than the placebo-treated patients at baseline. The baseline CD4+VDR+ delta expression was independent of the gender (data not shown). There was no statistically significant effect of vitamin D on CD8+ T cell VDR up-regulation following TCR stimulation (data not shown). We examined whether these effects were due to a decreased ability to synthesize mRNA VDR and CYP27B1 after 26 weeks of vitamin D treatment. Neither vitamin D nor placebo treatment changed the mRNA VDR and CYP27B1 expression in separated CD4+ T cells (data not shown).
Figure 4.
Vitamin D receptor response (VDR) expression is influenced by vitamin D treatment. (a) Delta median fluorescence index (MFI) VDR (anti-CD3- and anti-CD28-stimulated MFI VDR–non-stimulated MFI VDR) expression at baseline and after 26 weeks of vitamin D or placebo treatment in CD4+ T cells. Vitamin D treatment decreases the ability to up-regulate VDR in CD4+ T cells compared to placebo. Delta VDR values at baseline are higher in vitamin D-treated patients compared to the placebo group. (b) The gating strategy of a representative patient after 26 weeks of vitamin D treatment, ± anti-CD3 and anti-CD28 stimulation. The histograms shown in red represent isotype controls; the histograms shown in blue represent CD4+VDR+ cells.
Discussion
In the present study, we used a flow cytometry protocol to determine cytoplasmic and nuclear VDR expression and to investigate the effects of vitamin D treatment on T cell VDR expression in CD. Vitamin D treatment decreased the activation-induced VDR up-regulation in CD4+ T cells by 30% compared to placebo.
Studies of VDR expression have been limited previously to gene expression analyses, immunohistochemistry, Western blot and flow cytometry investigation. Gene expression may not reflect cellular and functional VDR protein expression. The relative VDR expression in cytosol and nucleus can be measured by Western blot in separated fractionated cells. However, we aimed to develop a method to detect VDR expression in small amounts of patient material and in different cell subsets. Several studies have reported intracellular VDR protein expression using different VDR antibodies 10,27–29. These VDR antibodies may be associated with significant non-specific staining. Wang et al. reported that the VDR antibody clone D-6 from Santa Cruz Biotechnologies was the only specific VDR antibody in a test panel of nine VDR antibodies 21. We therefore chose to use that VDR D-6 antibody in the present study. Joseph et al. performed an intracellular flow cytometry protocol for VDR staining, using the VDR D-6 antibody from Santa Cruz Biotechnologies and validated the results with Western blot 10. Fluorescence activated cell sorter (FACS) Lysing/Perm II Solution from BD was used for permeabilization. However, Joseph et al. have not described whether the permeabilizaton procedure also included the nucleolemma. We used Triton X for permeabilization because of the ability to permeabilize both the cell membrane and nucleolemma 30,31. We validated the ability of the flow protocol to stain for both cytoplasmic and nuclear VDR expression with confocal microscopy of THP-1 cells. We observed a minor but definite vitamin D-dependent shift of VDR towards the nucleus, as described earlier 13.The sensitivity of the flow protocol in PBMCs was indicated strongly by the correlation between percentages of CD4+VDR+ T cells and the mRNA VDR levels in magnetically separated CD4+ T cells. To minimize the effects of non-specific staining, we used an isotype control and 4% formaldehyde fixation.
TCR activation induced VDR expression in CD4+ and CD8+ T cells in accordance with observations in previous studies 13,32,33. At baseline, the patients who received vitamin D demonstrated a higher VDR up-regulation following TCR stimulation than the placebo-treated patients. This may reflect a selection bias. The vitamin D group was selected on the basis of the largest increase in 25-vitD and patients with a low baseline 25-vitD are more likely to have a large increase in 25-vitD compared to patients with a normal baseline 25-vitD. Twenty-six weeks of vitamin D treatment reduced the ability of TCR-stimulated CD4+ T cells to up-regulate VDR by 30% compared with placebo. This finding is in contrast to previous in-vitro studies, demonstrating that vitamin D stimulation induced VDR expression in CD4 T cells 13,34. Long-term vitamin D supplementation might modulate the inflammatory response in T cells, resulting in less responsive T cells and thereby a secondary decrease in VDR up-regulation. This tolerant response is in good agreement with the anti-inflammatory effects of vitamin D reported from clinical settings, where vitamin D reduces the relapse rate of CD 15.
VDR expression in non-stimulated T cells was not influenced by vitamin D treatment when measured by flow cytometry or by mRNA levels. VDR was present in non-stimulated T cells, both measured by flow and mRNA, but at very low levels. There have been conflicting results regarding the expression of VDR in non-stimulated CD4+ T cells. Some studies have reported VDR expression in non-stimulated T cells 20,35, whereas other studies have reported no VDR expression 13,32. Our results support the low expression of VDR in unstimulated CD4+ T cells. However, our study shows that the primarily modulatory effect on CD4+ T cells from oral vitamin D treatment occurs through decreased up-regulation of VDR following T cell activation.
VDR expression in CD might be influenced by inflammation, as suggested by the association between IFN-γ levels in cell culture supernatants and VDR expression in PBMCs. Furthermore, VDR expression tended to be correlated with faecal calprotectin levels, a sensitive marker for mucosal inflammation in CD 36. These results are in agreement with other studies reporting a negative correlation between 25-vitD levels and inflammation 37,38.
Conclusion
Flow cytometry is a valid and reliable method to examine the nuclear and cytoplasmic expression of VDR in different cell subsets. The method is sensitive and can be used to detect the influence of vitamin D treatment on the VDR expression of T cells. Long-term vitamin D treatment of CD patients reduces activation-induced VDR up-regulation in CD4+ T cells.
Acknowledgments
We thank laboratory technician Rikke Andersen for expert laboratory technical support. Financial support was granted from the Danish Colitis Crohn Foundation.
Author contributions
M. B., B. D., A. D., T. H. and L. H. performed the experiments. M. B., B. L., B. D. and J. A. designed the study. All authors contributed to writing the paper.
Disclosure
None.
References
- Baumgart DC,WJ, Sandborn Crohn's disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- Niessner M, Volk BA. Altered T helper type 1 (Th1)/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT–PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veny M, Esteller M, Ricart E, Pique JM, Panes J, Salas A. Late Crohn's disease patients present an increase in peripheral Th17 cells and cytokine production compared with early patients. Aliment Pharmacol Ther. 2010;31:561–72. doi: 10.1111/j.1365-2036.2009.04209.x. [DOI] [PubMed] [Google Scholar]
- Holtta V, Klemetti P, Sipponen T, et al. IL-23/IL-17 immunity as a hallmark of Crohn's disease. Inflamm Bowel Dis. 2008;14:1175–84. doi: 10.1002/ibd.20475. [DOI] [PubMed] [Google Scholar]
- de Bruyn JR, van Heeckeren R, Ponsioen CY, et al. Vitamin D deficiency in Crohn’ disease and healthy controls: a prospective case–control study in the Netherlands. J Crohns Colitis. 2014;8:1267–73. doi: 10.1016/j.crohns.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Bours PH, Wielders JP, Vermeijden JR, van de Wiel A. Seasonal variation of serum 25-hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos Int. 2011;22:2857–67. doi: 10.1007/s00198-010-1484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. J Parenter Enteral Nutr. 2011;35:308–16. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology. 2012;142:482–9. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–60. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- Joseph RW, Bayraktar UD, Kim TK, et al. Vitamin D receptor upregulation in alloreactive human T cells. Hum Immunol. 2012;73:693–8. doi: 10.1016/j.humimm.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Klopot A, Hance KW, Peleg S, Barsony J, Fleet JC. Nucleo-cytoplasmic cycling of the vitamin D receptor in the enterocyte-like cell line, Caco-2. J Cell Biochem. 2007;100:617–28. doi: 10.1002/jcb.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham M, Longhi MS, Lahiff C, Cheifetz A, Robson S, Moss AC. Vitamin D levels in adults with Crohn's disease are responsive to disease activity and treatment. Inflamm Bowel Dis. 2014;20:856–60. doi: 10.1097/MIB.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsbak M, von Essen MR, Boding L, et al. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLOS ONE. 2014;9:e96695. doi: 10.1371/journal.pone.0096695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn's disease – a randomised double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- Bartels LE, Bendix M, Hvas CL, et al. Oral vitamin D3 supplementation reduces monocyte-derived dendritic cell maturation and cytokine production in Crohn's disease patients. Inflammopharmacology. 2014;22:95–103. doi: 10.1007/s10787-013-0197-1. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Bendix-Struve M, Bartels LE, Agnholt J, Dige A, Jorgensen SP, Dahlerup JF. Vitamin D3 treatment of Crohn's disease patients increases stimulated T cell IL-6 production and proliferation. Aliment Pharmacol Ther. 2010;32:1364–72. doi: 10.1111/j.1365-2036.2010.04463.x. [DOI] [PubMed] [Google Scholar]
- Liu N, Nguyen L, Chun RF, et al. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- Wang Y, Becklund BR, DeLuca HF. Identification of a highly specific and versatile vitamin D receptor antibody. Arch Biochem Biophys. 2010;494:166–77. doi: 10.1016/j.abb.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177:686–97. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Zhang YG, Lu R, et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2014 doi: 10.1136/gutjnl-2014-307436. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik R, Asplin JR, Lindeman C, Favus MJ, Bai S, Coe FL. Lipopolysaccharide negatively modulates vitamin D action by down-regulating expression of vitamin D-induced VDR in human monocytic THP-1 cells. Cell Immunol. 2004;232:137–43. doi: 10.1016/j.cellimm.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Harslof T, Husted LB, Carstens M, et al. The expression and regulation of bone-acting cytokines in human peripheral adipose tissue in organ culture. Horm Metab Res. 2011;43:477–82. doi: 10.1055/s-0031-1277156. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT–PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:0034.1–11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V ON, Asani FF, Jeffery TJ, Saccone DS, Bornman L. Vitamin D receptor gene expression and function in a South African population: ethnicity, vitamin D and I. PLOS ONE. 2013;8:e67663. doi: 10.1371/journal.pone.0067663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto G, Giordano RJ, Marti LC, et al. Identification of novel immunoregulatory molecules in human thymic regulatory CD4+CD25+ T cells by phage display. PLOS ONE. 2011;6:e21702. doi: 10.1371/journal.pone.0021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo G, La Manna G, Cappuccilli ML, et al. VDR expression on circulating endothelial progenitor cells in dialysis patients is modulated by 25(OH)D serum levels and calcitriol therapy. Blood Purif. 2011;32:161–73. doi: 10.1159/000325459. [DOI] [PubMed] [Google Scholar]
- Prufer K, Hernandez C, Gilbreath M. Mutations in the AF-2 region abolish ligand-induced intranuclear immobilization of the liver X receptor alpha. Exp Cell Res. 2008;314:2652–60. doi: 10.1016/j.yexcr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Tercel M, McManaway SP, Liyanage HD, Pruijn FB. Preparation and properties of clickable amino analogues of the duocarmycins: factors that affect the efficiency of their fluorescent labelling of DNA. ChemMedChem. 2014;9:2193–206. doi: 10.1002/cmdc.201402169. [DOI] [PubMed] [Google Scholar]
- Baeke F, Korf H, Overbergh L, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem Mol Biol. 2010;121:221–7. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–9. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Nakane M, Ma J, Dixon D, Gagne G. Vitamin D receptor (VDR) localization in human promyelocytic leukemia cells. Leuk Lymph. 2006;47:727–32. doi: 10.1080/10428190500398898. [DOI] [PubMed] [Google Scholar]
- Diaz L, Martinez-Reza I, Garcia-Becerra R, Gonzalez L, Larrea F, Mendez I. Calcitriol stimulates prolactin expression in non-activated human peripheral blood mononuclear cells: breaking paradigms. Cytokine. 2011;55:188–94. doi: 10.1016/j.cyto.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407–15. doi: 10.1097/MIB.0000000000000057. [DOI] [PubMed] [Google Scholar]
- Waldron JL, Ashby HL, Cornes MP, et al. Vitamin D: a negative acute phase reactant. J Clin Pathol. 2013;66:620–2. doi: 10.1136/jclinpath-2012-201301. [DOI] [PubMed] [Google Scholar]
- Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–11. doi: 10.3945/ajcn.110.008490. [DOI] [PubMed] [Google Scholar]