Abstract
In the present study we examined the role of thymic stromal lymphopoietin (TSLP) in experimental autoimmune encephalomyelitis (EAE). Here, we report that TSLP knock-out (KO) mice display a delayed onset of disease and an attenuated form of EAE. This delayed onset was accompanied by a reduced number of encephalitogenic T helper type 1 (Th1) cells in the central nervous system (CNS) of TSLP KO mice. In addition, CD4+ and CD8+ T cells from CNS of TSLP KO mice show a reduced activation status in comparison to wild-type mice. It is noteworthy that we could also show that lymph node cells from TSLP KO mice expanded less efficiently and that interleukin (IL)-6-, interferon (IFN)-γ and tumour necrosis factor (TNF)-α levels were reduced. Furthermore, CD3+ T cells isolated in the preclinical phase from myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35–55)-immunized TSLP KO mice showed a reduced response after secondary exposure to MOG35–55, indicating that differentiation of naive T cells into MOG35–55-specific effector and memory T cells was impaired in KO mice. The addition of recombinant TSLP enhanced T cell proliferation during MOG35–55 restimulation, showing that T cells also respond directly to TSLP. In summary, these data demonstrate that expression of, and immune activation by, TSLP contributes significantly to the immunopathology of EAE.
Keywords: autoimmunity, EAE, T cells, TSLP
Introduction
Thymic stromal lymphopoietin (TSLP) is an interleukin (IL)-7 related cytokine which acts on multiple lineages, including dendritic cells (DCs), T cells, natural killer T (NK T) cells, eosinophils and mast cells, mediating proliferation and survival 1. Monocytes and DCs are known to have the highest expression of TSLP receptors 2,3. TSLP has the capacity to potently enhance the maturation and function of human and murine DCs, as evidenced by the strong induction of major histocompatibility complex (MHC)-II and co-stimulatory molecules such as CD40, CD80, CD83 and CD86, as well as by the release of T helper type 2 (Th2)-attracting chemokines 4–7. Furthermore, it has been reported several times that TSLP conditions DCs to drive proliferation and Th2 skewing of human T cells 6,8,9 and murine T cells 10,11. However, TSLP also acts directly on CD4+ T cells. Although TSLP receptor- and TSLP-deficient mice exhibited normal lymphohaematopoietic development 12–14, TSLP preferentially stimulated proliferation and survival of CD4 single positive thymocytes and peripheral T cells in vitro 12. When transferred into irradiated hosts, CD4+ T cells from TSLPR knock-out (KO) mice expanded less efficiently than wild-type (WT) CD4+ T cells, suggesting a direct effect of TSLP on CD4+ T cell homeostasis 12. In addition, activation and proliferation of TSLPR-deficient CD4+ T cells was much weaker than of WT T cells after immunization and secondary exposure to the antigen 15. Furthermore, TSLP treatment of naive CD4+ T cells resulted in immediate signal transducer and activator of transcription (STAT)-6 activation, IL4 gene transcription and Th2 differentiation in the absence of antigen-presenting cells (APCs) and exogenous IL-4 16. In addition to eliciting the proliferation of naive CD4+ T cells, TSLP has been demonstrated to act on CD8+ T cells. Both TSLPR and IL-7Rα are expressed by CD8+ T cells, and TSLP could act directly on murine CD8+ T cells to activate the STAT-5 and protein kinase B (Akt) signalling pathways in these cells 17. However, the underlying mechanisms and cell signalling pathways mediating these effects on T cells remain unclear.
Autoimmune diseases, specifically multiple sclerosis (MS), have been associated with single nucleotide polymorphisms (SNPs) in the IL-7Rα gene locus 18. Thus, in the present study using TSLP KO mice we examined the role of TSLP for the first time in the experimental autoimmune encephalomyelitis (EAE). EAE, the conventional experimental model for MS, is a demyelinating disease of the central nervous system (CNS). In our study, EAE was induced once by immunization with myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35–55), resulting in a monophasic acute form of EAE. In this model interferon (IFN)-γ-producing Th1 and IL-17-producing Th17 cells are considered to be the main players in the developing CNS inflammation 19. In contrast, regulatory T cells confer significant protection from EAE 20.
Keeping in mind that TSLP is a potent activator of DCs, which play a key role in T cell differentiation and activation, and that TSLP could also directly activate T cells, we monitored the course of EAE in TSLP KO and TSLP WT mice and investigated its effects on CNS and lymphatic organs at different stages of disease.
We found that mice deficient for TSLP showed an amelioration of EAE symptoms accompanied by reduced inflammatory infiltrates in brain and spinal cord. Interestingly, in CNS of TSLP KO mice the encephalitogenic T cells showed a reduced activation. In-vitro experiments confirmed that TSLP directly, and not only indirectly via DCs, also activates T cells and that T cells from TSLP KO mice show a reduced antigen-driven activation.
Taken together, these results suggest that in mice, TSLP may be involved not only in allergic diseases, but also in inflammatory disorders such as EAE.
Materials and methods
Animals
TSLP KO mice and TSLP WT mice were housed under specific pathogen-free conditions. TSLP KO mice were constructed as described previously 14. TSLP KO and TSLP WT mice were crossed with DEREG (DEpletion of REGulatory T cells) mice, which were kindly provided by Professor T. Sparwasser 27. All animal experiments were performed in accordance with institutional, state and federal guidelines.
EAE induction and scoring
Male TSLP KO and TSLP WT mice (12–16 weeks old) were immunized subcutaneously (s.c.) with MOG35–55 (Sigma-Genosys, The Woodlands, TX, USA) at day 0 to induce EAE as described previously 49. EAE paralysis of mice was scored as follows: 0, no disease; 1, tail weakness; 2, paraparesis; 3, paraplegia; 4, paraplegia with forelimb weakness; and 5, moribund or dead animals.
Neuropathology
For visualization of inflammatory infiltrates, brains and spinal cords were harvested, fixed in liquid nitrogen and stored at −80°C. Subsequent analyses of sections were performed according to protocols as described previously 49. Histochemistry was performed on 3-µm thick paraffin sections. Quantification of demyelinated areas was performed on Luxol fast blue-stained sections. Areas with complete demyelination were identified as lesions. Blinded quantification was performed on serial sections and included three independent spinal cross-sections per mouse.
Immunofluorescence
Immunofluorescence images of brain (and spinal cord) have been generated using the multi-epitope ligand cartography (MELC) technique. Sample preparation, data acquisition and data analysis have been performed as described previously 50.
Real time reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was prepared from tissues or cells using the RNeasy Plus Mini Kit and QIAshredder spin columns (Qiagen, Valencia, CA, USA). Traces of genomic DNA were removed by DNase digestion with the RNase-free DNase Set (Qiagen). Subsequently, 1 µg RNA was reverse-transcribed into sscDNA using the First Strand cDNA Synthesis Kit (Fermentas, Burlington, ON, Canada), as specified by the manufacturer. Realtime RT–PCR was performed in the Rotor-Gene Q (Qiagen) using the SYBR® Select Master Mix (Applied Biosystems, Carlsbad, CA, USA) and primers selected from a primerbank or previously published primers (Supporting information, Table S1).
Isolation of CNS-infiltrating cells
Mice were killed at the indicated time-points and perfused with 20 ml phosphate-buffered saline (PBS). Brain and spinal cord were cut into small pieces and incubated under constant stirring in R10 [RPMI-1640, 10% fetal calf serum (FCS), 1% L-glutamine, 1% penicillin–streptomycin, 0·1% β-mercaptoethanol] containing 2·5 mg/ml collagenase III (Sigma-Aldrich, St Louis, MO, UK) and 0·5 mg/ml DNase I (Sigma-Aldrich) for 60 min at 37°C. Then 5 mM ethylenediamine tetraacetic acid (EDTA) (Applichem, St Louis, MO, USA) was added and the tissues were passed through a 70-µm cell strainer. Cells were centrifuged, washed twice with R10, resuspended in 40% Percoll (Sigma-Aldrich) and overlaid on 70% Percoll. Gradient solutions were centrifuged at 650x g for 30 min without braking and the interphase, containing mononuclear cells, was recovered. Cells were washed twice with R10 and used for further manipulation.
Bone marrow-derived dendritic cell (BMDC) culture and stimulation
Bone marrow cells were cultured in 10 ml R10 medium containing 10% culture supernatant of a murine granulocyte–macrophage colony-stimulating factor (GM-CSF)-transfected cell line (equivalent to >200 U/ml). For subsequent flow cytometry or cytometric bead array (CBA) analyses, mature DCs were generated by adding tumour necrosis factor (TNF)-α (500 U/ml; PeproTech, Rocky Hill, NJ, USA) or lipopolysaccharide (LPS) (100 ng/ml; Sigma-Aldrich) overnight to day 9 bone marrow (BM) cultures. For subsequent real-time RT–PCR analyses, stimulation with rmTSLP (15 ng/ml; R&D Systems, Minneapolis, MN, USA) and CD40L (5% of hybridoma supernatant; clone FGK45) was additionally performed. For subsequent mixed lymphocyte reaction (MLR) analyses, TNF-α (500 U/ml; PeproTech) was added to day 9 BM cultures. All cells were harvested at day 10.
Flow cytometry
For flow cytometry, FcRs were blocked with anti-mouse CD16/CD32 (Fc-Block™; BD Biosciences, San Jose, CA, USA). The following directly fluorochrome-conjugated monoclonal antibodies [purchased from BD Biosciences, BioLegend, San Diego, CA, USA and eBioscience, San Diego, CA, USA)] were used for the specific staining of CD3 (500A2), CD4 (RM4-5), CD8a (53–6·7), CD11b (M1/70), CD11c (HL3), CD25 (7D4), CD40 (3/23), CD45 (30-F11), CD62L (MEL-14), CD69 (H1·2F3), CD80 (16-10A1), CD83 (Michel-19), CD86 (GL-1), F4/80 (BM8), forkhead box protein 3 (FoxP3) (FJK-16s), GATA binding protein 3 (GATA-3) (L50-823), IFN-γ (XMG1.2), IL-17A (TC11-18H10), lymphocyte antigen 6 complex, locus C (Ly6C) (HK1.4), Ly6G (1A8) and MHC-II (2G9). Intracellular cytokine and transcription factor staining was performed according to the manufacturer's instructions using the FoxP3/Transcription Factor Staining Buffer Set (eBioscience) and the Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (BD Bioscience). Cells were analysed with the flow cytometer fluorescence activated cell sorter (FACS)Canto (BD Bioscience). Data analysis was performed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Stimulation of lymph node cells with recombinant TSLP
Lymph node (LN) cells, 4 × 105, from WT mice were placed as triplicates in a 96-well flat-bottomed plate ± 100 ng/ml murine recombinant TSLP (R&D Systems) and ± 2 µl anti-CD3/CD28 beads (Life Technologies, Carlsbad, CA, USA). After 48 h of culture the proliferation rate was assessed using a thymidine incorporation assay.
Restimulation of CD3+ splenocytes with MOG35–55 peptide
Splenocytes were harvested from TSLP KO and TSLP WT mice at day 5 post-immunization with MOG35–55 peptide. CD3+ T cells were sorted from single cell suspensions according to the manufacturer's instructions with the Pan T cell Isolation Kit II, Mouse (Miltenyi Biotech, Bergisch Gladbach, Germany). T cells (4 × 105) were incubated with different concentrations of MOG35–55 peptide in 200 µl R10/well of a 96-well tissue culture plate. At days 0, 1 and 2, 20 ng/ml rmTSLP (R&D Systems) were added to the indicated wells. At day 3, proliferation was assessed using a thymidine incorporation assay.
Mixed lymphocyte reaction
TNF-α-stimulated, day 10 BMDCs from TSLP KO and TSLP WT mice (C57BL/6N background) were cultured as triplicates in a 96-well flat-bottomed plate in R10 medium at titrated numbers. LN cells (2 × 105 per well) from TSLP KO and TSLP WT mice (BALB/c background) were used as responder cells. After 3 days of co-culture, supernatants were analysed by CBA and the proliferation rate was assessed using a thymidine incorporation assay.
Thymidine incorporation assay
Cells were pulsed with 1 µCi/well methyl-[3H]-thymidine (Amersham Biosciences, Little Chalfont, UK) in R10 medium for 16 h and harvested onto filtermats using an ICH-110 harvester (Inotech, Dottikon, Switzerland). Filters were counted in a 1450 microplate counter (Wallac, Turku, Finland).
Cytometric bead array
Cell culture supernatants and sera were analysed regarding the presence of specific cytokines using the CBA nouse Th1/Th2/Th17 cytokine kit or the CBA mouse inflammation kit according to the manufacturer's instructions (BD Biosciences). Complexes of capture beads, analytes and detection reagents were analysed using a FACScan (BD Biosciences).
Statistics and data presentation
All statistics were calculated with Prism version 5·0 (GraphPad, La Jolla, CA, USA) using a Wilcoxon–Mann–Whitney comparison test or two-tailed unpaired Student's t-test as indicated. All data are presented as mean ± standard error of the mean (s.e.m.). P-values of *P < 0·05, **P < 0·01 and ***P < 0·001 were considered statistically significant.
Results
Delayed and ameliorated EAE development in TSLP-deficient mice
To investigate the role of TSLP in CNS autoimmunity, the clinical score of EAE in TSLP KO and TSLP WT mice was assessed. Upon EAE induction, TSLP WT mice developed classical monophasic EAE-symptoms characterized by ascending paralysis 9–11 days after immunization with MOG35–55 peptide (Fig. 1a, left). In contrast TSLP-deficient mice displayed, first, delayed onset of disease (Fig. 1a, left) and secondly, an ameliorated form of EAE when compared with TSLP WT mice (Fig. 1a, left and right). To verify that the differences in disease progression between TSLP KO and TSLP WT mice are due to differences in TSLP expression and bioavailability, recombinant murine (rm)TSLP protein was injected intraperitoneally (i.p.) into TSLP KO mice and subsequently disease progression was monitored. In this experimental setting, the disease course between TSLP WT and TSLP KO mice, which received rmTSLP, was identical (Fig. 1b).
Figure 1.
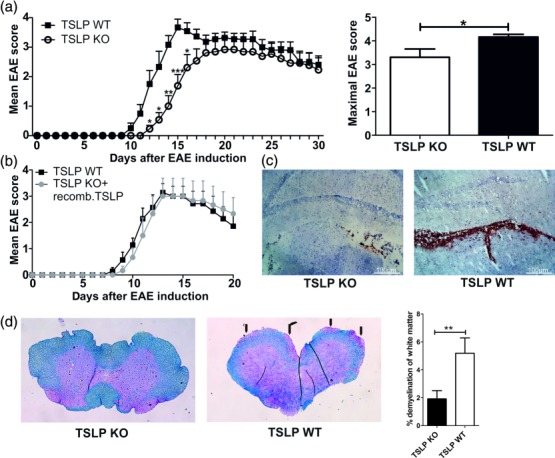
Thymic stromal lymphopoietin (TSLP) knock-out (KO) mice show a delayed experimental autoimmune encephalomyelitis (EAE) development and a reduced EAE severity. (a) EAE was induced in male TSLP KO mice or wild-type (WT) control animals by immunization with myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35–55) in complete Freund's adjuvant (CFA) and disease severity was monitored according to the classical EAE score [mean clinical EAE score ± standard error of the mean (s.e.m.); maximal clinical EAE score ± s.e.m.; TSLP KO n = 13, TSLP WT n = 12; data presented are the mean of three independent experiments; Wilcoxon–Mann–Whitney comparison test: *P < 0·05; **P < 0·01; ***P < 0·001]. (b) EAE was induced in TSLP KO mice and in TSLP WT mice. In addition, TSLP KO mice received rmTSLP [500 ng in 200 µl phosphate=buffered saline (PBS)] intraperitoneally (i.p.) on days 0–5; TSLP WT mice received 200 µl PBS i.p. on days 0–5; disease severity was monitored according to classical EAE score (mean clinical EAE score ± s.e.m.; TSLP KO n = 6, TSLP WT n = 7). (c) CD45 staining of brain harvested from TSLP KO and TSLP WT mice at day 12 after EAE induction. Results are representative of at least three independent experiments. (d) Luxol fast blue staining of spinal cord sections harvested from TSLP KO mice (left) and TSLP WT mice (middle) at day 12 after EAE induction; arrows mark demyelinated areas; bar graph (right) shows quantification of demyelinated areas (n = 8–10 mice; Wilcoxon–Mann–Whitney comparison test: **P < 0·01).
Histological analyses of brain and spinal cord derived from TSLP KO and TSLP WT mice illustrate that at day 12 after EAE induction there were significantly lower numbers of CD45+ leucocytes present as meningeal infiltrates of the brain (Fig. 1c, left) and in spinal cord (data not shown) of TSLP KO mice compared to TSLP WT mice (Fig. 1c, right). MELC images also revealed that the choroid plexus of TSLP KO mice contained fewer CD45+ leucocytes, CD11b+ macrophage/microglial cells, CD4+ T helper cells and CD4+/FoxP3+ regulatory T cells (Supporting information, Fig. S1). The number of CD11b+/Ly6G+ neutrophil granulocytes was comparable in brain of both mouse strains (Supporting information, Fig. S1). Blinded analysis of demyelinated areas in spinal cords of mice at day 12 after EAE induction also resulted in a reduction of demyelination by approximately 37% in TSLP KO mice compared to WT controls (Fig. 1d).
TSLP deficiency does not alter immune cell homeostasis in healthy mice
The requirement of TSLP for the development and maintenance of T cell populations was compared directly in TSLP KO and TSLP WT mice. In the murine as well as in the human system it has been shown previously that TSLP is involved not only in the development of Th2 cells 6,10,11,21, but also in the development of regulatory T cells (Tregs) 22–24. For humans, it even has been reported that TSLP is involved in the differentiation of CD4+ effectors producing both Th1 and Th2 cytokines 25, as well as in the development of Th17 cells 26. Therefore, we compared the numbers of Th1, Th2, Th17 and Treg cells in inguinal lymph nodes of healthy TSLP KO mice with healthy TSLP WT mice (Supporting information, Fig. S2a). However, no differences could be observed regarding the numbers of different T cell subsets. Furthermore, TSLP deficiency did not alter the numbers of macrophages (CD11b+, F4/80+, Ly6C+) or neutrophil granulocytes (CD11b+, Ly6G+) in inguinal lymph nodes (Supporting information, Fig. S2a). Also, no differences regarding the number of CD3+ T cells, CD3+/CD8+ cytotoxic T lymphocytes (CTLs), CD3+/IL-17A+ Th17 cells, macrophages (CD11b+, F4/80+, Ly6C+) or neutrophil granulocytes (CD11b+, Ly6G+) in the brain of healthy mice could be observed when compared with TSLP KO mice (Supporting information, Fig. S2b).
To test whether the generation of Tregs is impaired in mice deficient for TSLP, TSLP KO mice were crossed genetically with DEREG mice, which express a diphtheria toxin (DT) receptor-enhanced green fluorescent protein (eGFP) fusion protein under the control of the FoxP3 locus 27. After the injection of DT, FoxP3+ Tregs are specifically depleted; 3 days later FoxP3+ T cells start to rebound (not shown). However, no defects in the generation of Tregs could be observed, as similar numbers of FoxP3+ T cells could be detected in inguinal lymph nodes, spleen and thymus in TSLP KO and TSLP WT mice at day 4 after DT injection (Supporting information, Fig. S2c).
TSLP has the capacity to potently enhance the maturation and function of CD11c+ human myeloid DCs 5–7. Similarly, murine BMDCs responded to TSLP treatment by producing CCL17, CCL22 and increased expression of MHC-II and co-stimulatory proteins 4. To test whether TSLP-deficient mice display differences regarding their DCs, BMDCs from bone marrow of both healthy TSLP KO and healthy TSLP WT mice were generated. Real-time RT–PCR of TSLP WT BMDCs showed that TSLP mRNA expression was up-regulated significantly upon stimulation with TNF-α, and even more pronounced after LPS stimulation, whereas TSLP and CD40L stimulation showed no effect on TSLP mRNA expression (Fig. 2a). Furthermore, BMDCs of both genotypes were left either immature or were matured with LPS or TNF-α, and then supernatants were collected and analysed for the presence of IL-6, IL-10, IL-12p70, MCP-1 and TNF-α. No differences in terms of cytokine concentrations could be observed between supernatants from TSLP KO- and TSLP WT BMDCs in the unstimulated condition (not shown) as well as after TNF-α (not shown) or LPS stimulation (Fig. 2b). In addition, flow cytometry revealed that immature BMDCs from TSLP KO and TSLP WT mice showed no differences in MHC-II, CD25, CD40, CD80, CD83 and CD86 expression (Fig. 2c). After TNF-α stimulation, BMDCs from both groups showed an up-regulated expression of all the above-mentioned molecules, which was even more prominent after LPS stimulation. However, no differences in the expression of the analysed activation and maturation markers could be observed between the two genotypes (Fig. 2c).
Figure 2.
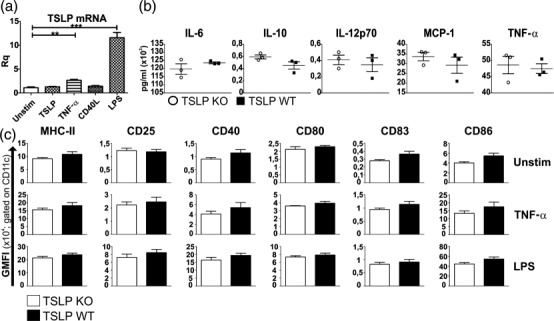
Bone marrow dendritic cells (BMDCs) from thymic stromal lymphopoietin (TSLP) knock-out (KO) and wild-type (WT) mice show no differences in cytokine secretion or maturation marker expression. (a) TSLP mRNA expression in WT BMDCs which were left unstimulated or which were stimulated overnight with TSLP, tumour necrosis factor (TNF)-α, CD40L or lipopolysaccharide (LPS) [mean ± standard error of the mean (s.e.m.)]. (b) Cytometric bead array (CBA) of supernatants from LPS-stimulated TSLP KO (o) and TSLP WT (▪) BMDCs (mean ± s.e.m.). (c) Fluorescence activated cell sorter (FACS) analysis of TSLP KO and WT BMDCs, which were left either unstimulated or were matured with TNF-α or LPS. GMFI = geometric mean fluorescence intensity. The bar charts represent the mean ± s.e.m. (a, b) TSLP KO n = 3, TSLP WT n = 3. (c) TSLP KO n = 3, TSLP WT n = 5. All data are representative of four independent experiments.
In TSLP KO mice Th2 responses are not reduced during EAE
Several previous studies, using TSLP-receptor KO mice, reported reduced Th2 cytokine levels during different inflammatory diseases, paralleled by an increase of Th1 type cytokines 28. As it has been reported that not only Th1 but also Th17 cells mediate pathogenic effects in the EAE model, whereas Treg play a regulatory role, we analysed cytokine production by these cell populations. Cytokines characteristic for Th1, Th2, Th17 cell and Treg immune responses were evaluated in the serum of mice 5 days post-MOG35–55 immunization. As anticipated, mice immunized with MOG35–55 peptide showed increased serum concentrations of IL-6, IL-17A, I TNF-α and TNF-α compared to healthy controls (Supporting information, Fig. S3b). IL-2, IL-4 and IL-10 were not detectable. It is noteworthy that no significant differences were observed between TSLP KO and TSLP WT mice during inflammation. At the same time-point, spleens from TSLP KO and TSLP WT mice showed similar numbers of CD11c+ DCs, and these cells expressed comparable amounts of MHC-II, CD25, CD40, CD80, CD83 and CD86 on their cell surface (Supporting information, Fig. S3a). The same was true for inguinal lymph nodes (not shown).
Also shortly before the peak of disease (i.e. day 12 after EAE induction), we did not detect any differences in mRNA expression levels of typical cytokines associated with T cell differentiation or activity in lymph node cells (Fig. 3a). Furthermore, no differences in the number of CD4+/IL-17A+ Th17 cells, CD4+/TNF-α+ Th1 cells and CD4+/FoxP3+ Treg could be detected by flow cytometry in the lymph nodes of TSLP KO in comparison to TSLP WT mice (Fig. 3b).
Figure 3.
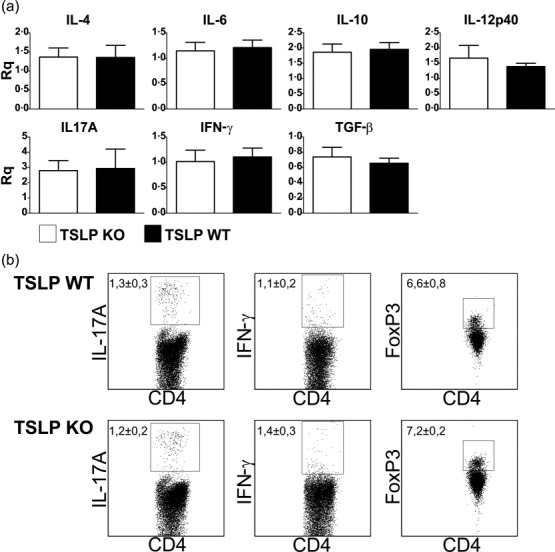
Thymic stromal lymphopoietin (TSLP) deficiency does not lead to reduced T helper type 2 (Th2) or elevated Th1 immune responses in experimental autoimmune encephalomyelitis (EAE) diseased mice. (a) Cytokine expression of lymph node cells (mixture of inguinal, axillary and cervical lymph nodes) harvested at day 12 post-EAE induction. mRNA expression of cytokines was determined by real-time reverse transcription–polymerase chain reaction (RT–PCR) analyses. TSLP KO n = 6, TSLP wild-type (WT) n = 6. Rq= relative quantity. (b) Fluorescence activated cell sorter (FACS) analyses of lymph node cells (mixture of inguinal, axillary and cervical lymph nodes) harvested at day 12 post-EAE induction. TSLP KO n = 6, TSLP WT n = 5. Data (mean ± standard error of the mean) are representative of at least three independent experiments.
Reduced CNS inflammation in TSLP KO mice at day 11 after EAE induction
Quantitative real-time RT–PCR of spinal cord samples harvested at day 11 after EAE induction showed a generally reduced mRNA expression of genes specific for a proinflammatory response in TSLP KO mice compared to TSLP WT mice. Markers specific for macrophages (CD11b), DCs (CD11c), B cells (CD19), T cells (CD3, CD4, CD8), T cell-specific transcription factors (T-bet, RORγ t, GATA-3, FoxP3) and cytokines (IFN-γ, IL-4) were clearly reduced in the TSLP KO mice compared to TSLP WT mice (Fig. 4). In contrast, mRNA expression levels of cytokines produced by Th17 cells (e.g. IL-17A, IL-17F, IL-22) were even slightly elevated in TSLP KO mice. These results demonstrate that the reduced EAE severity in the TSLP KO mice is caused mainly by a reduced infiltration of the spinal cord by macrophages and T cells (Fig. 4). Interestingly, the expression of the CC chemokine CCL1, which is expressed exclusively by T cell receptor (TCR)-activated T cells 29,30, and the expression of its receptor, CCR8, was reduced significantly in spinal cord of TSLP KO mice. Similar results were obtained for brain samples (not shown).
Figure 4.
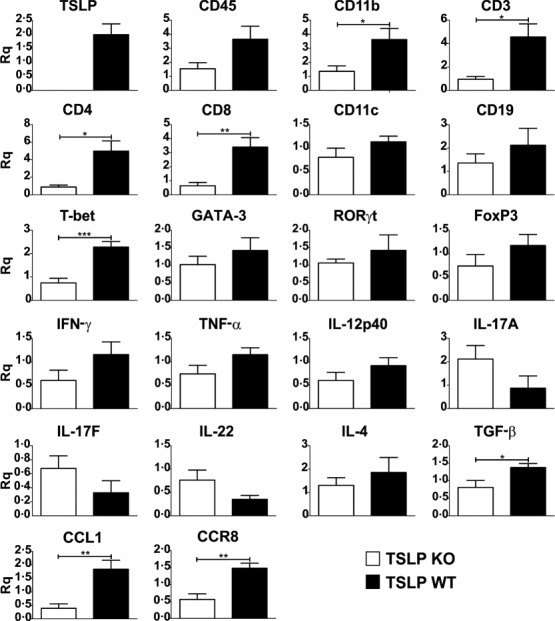
Shortly before the peak of disease thymic stromal lymphopoietin (TSLP) knock-out (KO) mice show a clearly reduced leucocyte infiltration in the spinal cord. Real-time reverse transcription–polymerase chain reaction (RT–PCR) of spinal cord, harvested at day 11 after experimental autoimmune encephalomyelitis (EAE) induction. TSLP KO mice n = 5, TSLP wild-type (WT) n = 7. Rq = relative quantity ± standard error of the mean. Results are representative of at least three independent experiments; two-tailed unpaired Student's t-test: *P < 0·05; **P < 0·01; ***P < 0·001.
According to the EAE course, cells isolated from CNS of TSLP KO mice at day 112 clearly showed lower numbers of infiltrated CD3+/CD4+ T helper cells and also lower numbers of CD3+/CD8+ CTLs in flow cytometry analyses than CNS cells isolated from TSLP WT mice (Fig. 5a,c). As Th1 and Th17 cells play a pivotal role in the pathogenesis of EAE 19, we subsequently analysed the numbers of CD45+/CD4+/IFN-γ+ Th1 cells and of CD45+/CD4+/IL-17A+ Th17 cells, revealing that the number of IFN-γ-positive Th1 cells was clearly reduced in the brain of TSLP KO mice (Fig. 5b,c).
Figure 5.
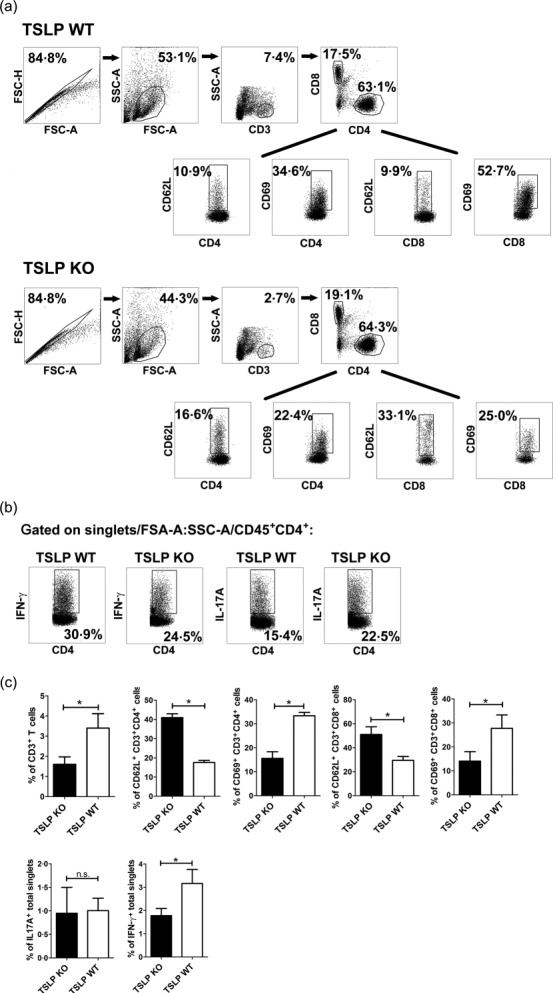
Fewer T helper type 1 (Th1) cells are present in brain of thymic stromal lymphopoietin (TSLP) knock-out (KO) mice at day 11 after experimental autoimmune encephalomyelitis (EAE) induction and infiltrating CD4+ and CD8+ T cells show an impaired activation. (a) CD45+ leucocytes isolated from pool of three brains were analysed for surface antigens CD4, CD8, CD62L and CD69 by flow cytometry. (b) Flow cytometric analyses of interferon (IFN)-γ and interleukin (IL)-17A production in singlets/forward-scatter (FSC)-A : side-scatter (SSC)-A/CD45+-gated CD4+ T cells. The figures (a) and (b) are representative of five independent experiments. (c) Statistical analyses by flow cytometric analyses of brain infiltrating total CD3+ T cells, CD69 and CD62L expression on CD4+ and CD8+ T cells, IL-17A and IFN-γ expression in brain singlets (bar graphs, n = 12–18; Wilcoxon–Mann–Whitney comparison test: *P < 0·05).
CD4+ T cells isolated at day 11 after EAE induction from brain of TSLP KO mice showed a clear reduction of the activation marker CD69 on their surface. In TSLP KO mice, the expression of the activation marker CD69 was only present in an average of 15·5% of the infiltrated CD4+ T cells, whereas approximately 33·3% of the CD4+ T cells derived from TSLP WT mice expressed CD69 (Fig. 5a,c). Accordingly, the expression of CD62L, which is a marker for naive T cells, is significantly higher on CD4+ T cells derived from TSLP KO mice when compared to TSLP WT T cells (KO: 41% versus WT: 17.5%; Fig. 5a,c). The same was true for CD8+ CTLs. Only 14·1% of the CD8+ CTLs from TSLP KO mice expressed the activation marker CD69, whereas 29·1% of CTLs from TSLP WT mice expressed CD69 (Fig. 5a,c). Thus, the expression of CD62L was almost two times higher on CD8+ CTLs derived from TSLP KO in comparison to TSLP WT mice (KO: 51% versus WT: 29·4%; Fig. 5a,c).
No differences in T cell activation at the later phase of disease
At day 20 post-EAE-induction, no significant differences regarding disease-associated paralyses could be observed between TSLP KO and TSLP WT mice (Fig. 1a). Accordingly, we did not detect any differences in the number of CD45+ leucocytes, CD3+ T cells, CD3+/CD4+ T helper cells and CD3+/CD8+ CTLs isolated at this time-point from brain tissue (Supporting information, Fig. S4a). Furthermore, no differences in the number of IFN-γ-positive Th1 cells or IL-17A-positive Th17 cells were detectable (Supporting information, Fig. S4a). Moreover, CD3+/CD4+ T helper cells, as well as CD3+/CD8+ CTLs, did not show any differences in the expression of CD69 or CD62L at this time-point (Supporting information, Fig. S4b).
TSLP stimulates proliferation and activation of T cells
Previously it has been reported that murine TSLP can regulate CD4+ 12,15,16,31 and CD8+ 17 T cell function and homeostasis in the presence as well as in the absence of APCs. To study the effect of TSLP on T cell proliferation in the presence of APCs, we performed an MLR. Thus, LN cells from TSLP KO and TSLP WT mice (Balb/c background) were cultured for 3 days with BMDCs derived from TSLP KO or TSLP WT mice (C57/BL6 background) and then supernatants were analysed using a cytometric bead array (CBA). Furthermore, cell proliferation rates were assessed using a thymidine incorporation assay. These analyses revealed clearly that LN cells derived from TSLP KO mice respond significantly more weakly to the BMDCs than lymph node cells derived from TSLP WT mice (Fig. 6a). Interestingly, the genotype of the BMDCs had no effect on the proliferation of the lymph node cells (Fig. 6a). Supernatants from MLRs showed that reduced amounts of IL-6, IFN-γ and TNF-α were produced when TSLP KO LN cells were co-cultured with BMDCs in comparison to TSLP WT LN cells (Fig. 6b). No differences regarding IL-2 and IL-17A production could be observed between TSLP KO and TSLP WT LN cells (Fig. 6b), while IL-4 and IL-10 were not detectable.
Figure 6.
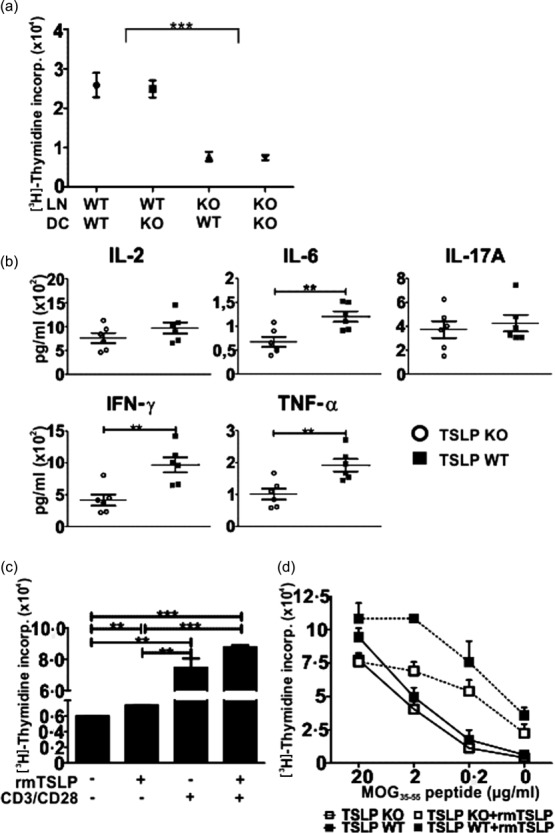
Thymic stromal lymphopoietin (TSLP) stimulates the proliferation of T cells. (a) 200 000 lymph node (LN) cells (Balb/c) were seeded together with 3000 tumour necrosis factor (TNF)-α-matured bone marrow dendritic cells (BMDCs) (C57/BL6) into a 96-well flat-bottomed plate for 72 h. Cell cultures were then pulsed with 1 μCi/well [3H]methylthymidine for 16 h and then the incorporation was assessed; TSLP KO n = 6, TSLP wild-type (WT) n = 6. (b) 200 000 KO or WT LN cells (Balb/c) were co-cultured with 3000 WT BMDCs (C57/BL6) for 72 h. Supernatants were then analysed regarding their cytokine concentrations by cytometric bead array (CBA); TSLP KO n = 6, TSLP WT n = 6. (c) LN cells from naive WT mice were seeded into a 96-well flat-bottomed plate (400 000/well) and cultured for 48 h in the presence or absence of CD3/CD28 beads and rmTSLP. Cell cultures were then pulsed with 1 μCi/well [3H]-methylthymidine for 16 h and incorporation was assessed; n = 3. (d) Splenocytes were harvested from TSLP KO and WT mice at day 5 after immunization with myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35–55) peptide. CD3+ T cells were then isolated, seeded into a 96-well flat-bottomed plate (400 000/well) and restimulated for 72 h with different concentrations of MOG35–55 peptide in the presence or absence of rmTSLP. Cell cultures were then pulsed with 1 μCi/well [3H]-methylthymidine for 16 h and incorporation was measured; TSLP KO n = 6, TSLP WT n = 3. The bar and line charts represent the mean ± standard error of the mean. Results are representative of three independent experiments; two-tailed unpaired Student's t-test: *P < 0·05; **P < 0·01; ***P < 0·001.
To analyse whether TSLP directly stimulates the proliferation of T cells, LN cells from naive TSLP WT mice were incubated for 48 h ± rmTSLP and ± CD3/CD28 beads and then proliferation rates were assessed. Thymidine incorporation shows that rmTSLP alone already promotes the proliferation LN cells (Fig. 6c). The addition of CD3/CD28 beads increased proliferation significantly, and when rmTSLP was additionally added proliferation was even higher (Fig. 6c). To determine possible differences in the myelin-specific T cell response, we compared the ability of CD3+ T cells from TSLP KO and WT mice in respect to the antigen-specific MOG35–55 response. Proliferation assays with CD3+ T cells isolated from spleen at day 5 after EAE induction and subsequent in-vitro restimulation for 72 h with different concentrations of MOG35–55 peptide, showed that CD3+ T cells from TSLP KO mice respond more weakly to MOG35–55 than CD3+ T cells from TSLP WT mice (Fig. 6d). Although proliferation of both T cell types was increased in the presence of rmTSLP, TSLP KO-derived T cells showed a lower response in comparison to TSLP WT cells (Fig. 6d).
Discussion
To date, the precise role of TSLP in autoimmune diseases is still unknown, and only very few studies have been published showing a defined role for TSLP in Th1- or Th17-driven autoimmune diseases 32. TSLP is a potent activator of myeloid DCs, enhancing Th2-mediated hypersensitivity 4,8, and it has been implicated in the pathogenesis of atopic diseases 31. Furthermore, it has been reported that TSLP produced from murine medullary thymic epithelial cells (mTEC) contributes to the expression of FoxP3 and the maturation of natural Tregs 24. However, in naive mice the absence of TSLP did not alter leucocyte balance, as shown by normal numbers of different types of leucocytes including Th1, Th2, Th17 and Tregs in lymphoid organs and CNS of TSLP KO mice (Supporting information, Fig. S2a–c). The continuous activity of IL-7, which shares overlapping activity with TSLP, may compensate for the missing TSLP signals in naive mice, and this could be a reason for normal Th2 numbers in TSLP KO mice. Accordingly, TSLP was shown previously to play only a minor role in murine T lymphopoiesis 33. Further, it was demonstrated that TSLPR KO mice exhibit normal myeloid, lymphoid, DC and NK cell numbers 12.
As it has been reported that murine BMDCs responded to TSLP treatment by producing Th2-attracting chemokines and increasing MHC-II and co-stimulatory molecules 4, BMDCs from BM of healthy TSLP KO and WT mice were generated. Real-time PCR revealed that TNF-α and especially LPS stimulation of BMDCs led to an up-regulation of TSLP mRNA expression (Fig. 2a). However, BMDCs from TSLP KO mice showed no reduced expression of activation and maturation markers after LPS- or TNF-α stimulation (Fig. 2c). Furthermore, using the MLR assay no reduction of cytokine secretion (Fig. 2b) and T cells stimulatory capacity (Fig. 6a) were observed, in comparison to BMDCs derived from TSLP WT mice. At day 5 after EAE induction, no differences were detected with regard to the numbers and activation status of DCs derived from spleen and inguinal lymph nodes of TSLP KO in comparison to TSLP WT mice (Supporting information, Fig. S3a). Neither at this time-point nor at day 12 post-immunization were reduced Th2- and/or Treg responses with Th1 or Th17 bias observed in TSLP KO mice (Fig. 3; Supporting information, Fig. S3b). Thus, there appear to be compensatory immune mechanisms during chronic antigen exposure that allow the development of Th2 responses.
To investigate the role of TSLP in CNS autoimmunity, we first compared the clinical score of EAE. Surprisingly, TSLP-deficient mice displayed a delayed onset of disease and an ameliorated form of EAE (Fig. 1a). To verify that the differences in disease progression between KO and WT mice are due to differences in TSLP expression, the TSLP deficiency in the KO mice was compensated by the injection of rmTSLP protein. Interestingly, the course of disease between TSLP WT mice and TSLP KO mice, which both received rmTSLP, was identical (Fig. 1b). This major finding indicates that TSLP is responsible for the observed differences in disease progression between TSLP KO and TSLP WT mice.
In accordance with our findings, TSLPR-deficient mice showed less severe arthritis in collagen-induced autoimmune arthritis and displayed a down-regulation of T cell activity and inhibition of proinflammatory cytokines and catabolic mediators at the site of inflammation 32. Accordingly, administration of rmTSLP exacerbated disease severity and joint damage significantly, associated with an increased T cell activation 32. These data display a direct role of TSLP in the promotion of autoimmune responses. In contrast to previous data using TSLP receptor KO mice, the usage of TSLP KO mice in this study eliminates possible remaining signalling effects of TSLP by binding to still-available IL-7Rα chains.
Histological and flow cytometry analyses of brain and spinal cord harvested at days 11–12 after EAE induction showed a strongly reduced infiltration of leucocytes, especially macrophages and T cells in TSLP KO mice, in comparison to TSLP WT mice (Figs 1c, 4 and 5). RT–PCR analyses showed that at day 11 after EAE induction, mRNA expression levels of several transcripts specific for macrophages and T cells were reduced significantly in CNS of TSLP KO mice (Fig. 4). RT–PCR and flow cytometric analyses showed that mRNA as well as protein expression of all investigated T cell markers, and especially Th1 markers, were reduced strongly in the CNS of TSLP KO mice. Thus, it was somewhat surprising to observe that the expression of cytokines produced by Th17 cells was similar in both groups, or sometimes even slightly elevated in the CNS of TSLP KO mice (Figs 4 and 5). In line with these findings, it has been reported that during colitis, TSLP is produced by DCs of the gut and acts directly on T cells, thereby reducing their capacity to produce IL-17 34. Consequently, a regulatory function of TSLP on Th17 cell cytokine production could also be relevant in the EAE model. Furthermore, our results underline the fact that the differences in disease severity are due most probably to differences in the number of Th1 cells and not to differences in the number of Th17 cells in the brain. The slightly elevated ratio of Th17 cells in the CNS of TSLP KO mice should have only minor effects on the course of EAE, if at all, as the absolute number of Th1 cells infiltrating the CNS far exceeds Th17 cell numbers 35.
In addition, TSLP KO mice showed a significantly reduced mRNA expression of the chemokine CCL1 (Fig. 2) in the CNS at day 11 after EAE induction. This indicates that CNS-infiltrating T cells from TSLP KO mice might display a less activated phenotype than T cells isolated from TSLP WT mice, as CCL1 is secreted exclusively by T cells after T cell receptor (TCR) stimulation 29. Consistent with these findings, the expression of the CCL1 receptor, i.e. CCR8, was also reduced significantly in TSLP KO mice in comparison to TSLP WT mice. CCR8 is expressed by phagocytic macrophages and activated microglia in MS lesions and is correlated directly with demyelinating activity 36. Flow cytometry demonstrated clearly that CD4+ T cells, as well as CD8+ T cells from CNS of TSLP KO mice, showed a significantly attenuated activation when compared with CD4+ and CD8+ T cells derived from CNS of TSLP WT mice (Fig. 3). As CD4+ T cells, especially Th1 and Th17 cells, are the main players in EAE 19, a reduced activation of CD4+ T cells elicited by TSLP deficiency could contribute to the reduced EAE severity observed in the TSLP KO mice. However, it is not clear whether the activation status of CD8+ T cells has an impact on EAE severity, because the role of CD8+ T cells in EAE is still controversial 37,38. Because we observed a strongly reduced activation of encephalitogenic T cells in TSLP KO mice in the early inflammatory phase of EAE, despite a normal DC composition, TSLP might directly influence the activation status of T cells in the EAE model. To date, it is still under debate whether TSLP can strongly activate CD4+ T cells only indirectly via myeloid DCs or directly 3,4,7,12,15,16. In addition to eliciting the proliferation of naive CD4+ T cells, TSLP has also been demonstrated to act directly 17 and indirectly via myeloid DCs 39 on CD8+ T cells. However, it is still unclear whether TSLP only increases CD8+ T cell survival without influencing proliferation 17 or whether it promotes survival and proliferation 40.
According to experiments performed with TSLP receptor KO mice 12,15,16, we next conducted comparable analyses with ex-vivo-derived cells from our TSLP KO mice. When co-cultured with allogeneic TSLP WT- or TSLP KO-derived BMDCs, LN cells from TSLP KO mice expanded less efficiently than lymph node cells from TSLP WT mice (Fig. 6a). Interestingly, however, the DC genotype had no influence on T cell proliferation (Fig. 6a). These results indicate that T cells generated in the thymus and differentiated in secondary lymphoid organs without the signal transduction underlying TSLP expression show a weaker antigen-driven activation than T cells generated in the presence of TSLP. The fact that at day 20 post-immunization no differences in disease severity and T cell activation could be observed between TSLP KO and TSLP WT mice (Supporting information, Fig. S4) indicates that there is an active immune response taking place in the TSLP KO mice, and this argues against a global developmental T cell defect.
The expression of several inflammatory cytokines, which play an important role in EAE and MS, seem to be impaired when TSLP is missing (Fig. 4b). As IL-6-deficient mice were completely resistant to MOG-induced EAE 41, a reduced IL-6 production in TSLP KO mice could contribute to the ameliorated form of EAE observed in these animals. IFN-γ and TNF-α, in contrast, seem to play a more complex role in EAE. Administration of IFN-γ ameliorated EAE in some cases, and treatment with anti-IFN-γ antibodies had no effect or led to an enhanced severity of the disease 42–44. Initial reports claimed that in-vivo TNF blockade in mice and rats resulted in EAE amelioration 45,46. In contrast, therapeutic administration of TNF was reported to provide protection from EAE 47. Kruglov and colleagues demonstrated that the cellular source of TNF and the stage of disease are important for the effects of TNF 48. Therefore, further experiments are necessary to clarify whether a reduced IFN-γ or TNF production contributes to disease amelioration in TSLP KO mice. However, such studies would exceed the scope of the present study.
To analyse whether TSLP stimulates the proliferation of T cells in the absence of APCs or TCR ligands, naive LN cells, which consist mainly of T cells, were incubated in medium alone or with rmTSLP. Using a thymidine incorporation assay, we could show that in the absence of TCR ligands, rmTSLP only slightly induced the proliferation of LN cells (Fig. 6c), which may be due to the very low expression of TSLP receptor on naive T cells 3. However, when the cells were stimulated with CD3/CD28 beads, rmTSLP clearly up-regulated proliferation (Fig. 6c). These observations are in accordance with previous studies on TSLP receptor KO mice 12.
Next, the MOG35–55-specific CD3+ T cell response in TSLP KO and TSLP WT mice was analysed. Proliferation assays with CD3+ T cells, isolated from spleens at day 5 after EAE induction and restimulated with MOG35–55 peptide, showed that CD3+ T cells from TSLP KO mice responded less efficiently to MOG35–55 than T cells from TSLP WT mice (Fig. 6d). This may be due to a reduced activation of naive T cells in vivo, leading to fewer T cells differentiating into MOG35–55-specific effector and memory cells. These findings are in line with previous reports showing that TSLP enhances the proliferation of TCR-stimulated CD4+ T cells and that TSLP is important for the transition of naive CD4+ T cells into activated effector cells 15. Although proliferation of both T cell types was increased when rmTSLP was added, TSLP KO T cells responded much more weakly to the combination of MOG35–55 peptide and rmTSLP than TSLP WT T cells (Fig. 6d). This finding shows that MOG35–55-specific T cells respond to TSLP during TCR activation. As the addition of rmTSLP enhances T cell responses, this could explain why TSLP KO mice which received rmTSLP showed a more severe EAE than PBS-treated TSLP KO mice (Fig. 1b). These experiments underline the fact that TSLP is indeed involved in the induction of MOG35–55-specific T cell clones in vivo, and that it is able to enhance the proliferation of these antigen-specific T cells. How TSLP directly regulates cell proliferation is beyond the scope of this study, and will be analysed in future. However, as mentioned above, immature and mature T cells do not only carry IL-7Rα but also the TSLPR chain. Thus, TSLP could directly induce STAT activation, especially STAT-5, in T cells regulating cell survival and proliferation 2,3. These possible effects in EAE need to be explored in further studies. Taken together, our data indicate that expression of, and immune activation, by TSLP contributes significantly to the immunopathology of EAE.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) via the SFB 643 projects A11, B9 and B13, the IZKF project A46 and the DFG-Graduiertenkolleg 1071.
Disclosure
The authors declare no commercial or financial conflict of interest.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's Web site:
Fig. S1. The choroid plexus of thymic stromal lymphopoietin (TSLP) knock-out (KO) mice contained fewer leucocytes in comparison to TSLP wild-type (WT) mice. Multi-epitope ligand cartography (MELC) images of brain harvested from TSLP KO mice and WT mice at day 12 after experimental autoimmune encephalomyelitis (EAE) induction. Upper row: phase contrast (white), CD45+ leucocytes (red), propidium iodide+ nuclei (green), CD31+ blood vessels (blue). Lower row: CD4+ T cells (red), CD11b+ macrophages/microglial cells (green), Ly6G+ neutrophil granulocytes (blue), CD11b+/Ly6G+ granulocytes (cyan), CD31+ blood vessels (magenta), forkhead box protein 3 (FoxP3+) regulatory T cells (red with white nuclei). Bar = 50 μm.
Fig. S2. In healthy thymic stromal lymphopoietin (TSLP) KO mice no reduction in the numbers of different T cell subtypes, macrophages or neutrophil granulocytes were observed. (a) Fluorescence activated cell sorter (FACS) analyses of inguinal lymph nodes [mean ± standard error of the mean (s.e.m.); TSLP KO n= 3, TSLP wild-type (WT) mice WT n= 4)]. (b) FACS FACS analyses of brain (TSLP KO = pool of three three mice, TSLP WT= pool of four four mice). (c) FACS analyses of inguinal lymph nodes, spleen and thymus. Diphteria toxin was injected at days 0 and 1, organs were removed at day 4 (three mice/group). Data (a,b) (mean ± s.e.m.) are representative of two and data (c) of three independent experiments.
Fig. S3. No differences in dendritic cell (DC) maturation or serum cytokine concentrations between thymic stromal lymphopoietin (TSLP) KO and wild-type (WT) mice mice at day 5 after experimental autoimmune encephalomyelitis (EAE) induction. (a) FACS analysis of spleens. GMFI = geometric mean fluorescence intensity. The bar charts represent the mean ± standard error of the mean (s.e.m.). TSLP KO n = 3, TSLP WT n = 3. Data are representative of three independent experiments. (b) Cytometric bead array (CBA) of serum. TSLP KO n = 9; TSLP WT n = 9; untreated C57/BL6 n = 4; mean (± s.e.m.; two-tailed unpaired Student's t-test: *P < 0·05; **P < 0·01; ***P < 0·001; n.s. = not significant). Data are representative of two independent experiments.
Fig. S4. At day 20 after experimental autoimmune encephalomyelitis (EAE) induction, thymic stromal lymphopoietin (TSLP) knock-out (KO) mice and TSLP wild-type (WT) mice mice show the same extent of central nervous system (CNS) inflammation. Flow cytometric analysis of brain. (a) CD45+ cells were gated on forward-/side-scatter (FSC/SSC), CD3+ cells were gated on CD45+ cells, CD4+ and CD8+ cells were gated on CD3+ cells. Interferon (IFN)-γ and interleukin (IL)-17Av cells were gated on CD4+ cells. (b) CD62L+ and CD69+ cells were gated on CD4+ or CD8 + cells. Results are representative of two independent experiments (pool of three brains each).
Table S1. Quantitative reverse transcription (qRT) primer sets used in this study.
References
- Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol 2011; 187:1207–11. doi: 10.4049/jimmunol.1100355. .] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentmeier H, Drexler HG, Fleckenstein D, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–92. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- Lu N, Wang YH, Wang YH, Arima K, Hanabuchi S, Liu YJ. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med 2009; 206:2111–9. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 2005; 6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol 2001; 167:336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Hanabuchi S, Soumelis V, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol 2004; 5:426–34. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005; 202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumelis V, Liu YJ. Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin Immunopathol 2004; 25:325–33. doi: 10.1007/s00281-003-0152-0. [DOI] [PubMed] [Google Scholar]
- Li YL, Li HJ, Ji F, et al. Thymic stromal lymphopoietin promotes lung inflammation through activation of dendritic cells. J Asthma 2010; 47:117–23. doi: 10.3109/02770900903483816. [DOI] [PubMed] [Google Scholar]
- Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–67. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shami A, Spolski R, Kelly J, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–68. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpino N, Thierfelder WE, Chang MS, et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol 2004; 24:2584–92. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon C, Lechmann M, Brustle A, et al. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity. 2011;35:223–35. doi: 10.1016/j.immuni.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shami A, Spolski R. Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;;202:829–39. doi: 10.1084/jem.20050199. KellyJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 178:1396–404. doi: 10.4049/jimmunol.178.3.1396. 2007. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 181:7699–705. doi: 10.4049/jimmunol.181.11.7699. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Schmidt S, Seth P, et al. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. 1083;39 doi: 10.1038/ng2103. Nat Genet 2007; –. [DOI] [PubMed] [Google Scholar]
- Aranami T, Yamamura T. Th17 Cells and autoimmune encephalomyelitis (EAE/MS) Allergol Int 2008; 57:115–20. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–6. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Wang YH, Lee HK, et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- Hanabuchi S, Ito T, Park WR, et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol. 2010;184:2999–3007. doi: 10.4049/jimmunol.0804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Su H, Knudsen G, Helms W, Su L. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Hanabuchi S, Marloie-Provost MA, Antonenko S, Liu YJ, Soumelis V. Human TSLP promotes CD40 ligand-induced IL-12 production by myeloid dendritic cells but maintains their Th2 priming potential. Blood. 2005;105:4749–51. doi: 10.1182/blood-2004-09-3622. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Watanabe N, Kido M, et al. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy. 2009;39:89–100. doi: 10.1111/j.1365-2222.2008.03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahl K, Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol Biol. 2011;707:157–72. doi: 10.1007/978-1-61737-979-6_10. [DOI] [PubMed] [Google Scholar]
- Shi L, Leu SW, Xu F, et al. Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin Immunol. 2008;129:202–10. doi: 10.1016/j.clim.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Iellem A, Colantonio L, Bhakta S, et al. Inhibition by IL-12 and IFN-alpha of I-309 and macrophage-derived chemokine production upon TCR triggering of human Th1 cells. Eur J Immunol. 2000;30:1030–9. doi: 10.1002/(SICI)1521-4141(200004)30:4<1030::AID-IMMU1030>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Miller MD, Krangel MS. The human cytokine I-309 is a monocyte chemoattractant. Proc Natl Acad Sci USA. 1992;89:2950–4. doi: 10.1073/pnas.89.7.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci USA. 2008;105:11875–80. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgring SA, Willis CR, Dean CE, Jr, et al. Critical proinflammatory role of thymic stromal lymphopoietin and its receptor in experimental autoimmune arthritis. Arthritis Rheum. 2011;63:1878–87. doi: 10.1002/art.30336. [DOI] [PubMed] [Google Scholar]
- Sims JE, Williams DE, Morrissey PJ, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;192:671–80. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5:184–93. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta. 2011;1812:246–51. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst C, Staugaitis SM, Kivisakk P, et al. CC chemokine receptor 8 in the central nervous system is associated with phagocytic macrophages. Am J Pathol. 2003;162:427–38. doi: 10.1016/S0002-9440(10)63837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Whitaker JN, Huang Z, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–87. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- York NR, Mendoza JP, Ortega SB, et al. Immune regulatory CNS-reactive CD8+ T cells in experimental autoimmune encephalomyelitis. J Autoimmun. 2010;35:33–44. doi: 10.1016/j.jaut.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Soumelis V, Watanabe N, et al. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med. 2003;197:1059–63. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu T, Watanabe N, Kido M, et al. Human TSLP directly enhances expansion of CD8+ T cells. Clin Exp Immunol. 2008;154:98–106. doi: 10.1111/j.1365-2249.2008.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6. [PubMed] [Google Scholar]
- Billiau A, Heremans H, Vandekerckhove F, et al. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988;140:1506–10. [PubMed] [Google Scholar]
- Duong TT, St Louis J, Gilbert JJ, Finkelman FD, Strejan GH. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J Neuroimmunol. 1992;36:105–15. doi: 10.1016/0165-5728(92)90042-j. [DOI] [PubMed] [Google Scholar]
- Voorthuis JA, Uitdehaag BM, De Groot CJ, Goede PH, van der Meide PH, Dijkstra CD. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin Exp Immunol. 1990;81:183–8. doi: 10.1111/j.1365-2249.1990.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Butler D, Scallon BJ, O'Neill JK, Turk JL, Feldmann M. Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur J Immunol. 1994;24:2040–8. doi: 10.1002/eji.1830240916. [DOI] [PubMed] [Google Scholar]
- Ruddle NH, Bergman CM, McGrath KM, et al. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193–200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Marino MW, Wong G, et al. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med. 1998;4:78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- Kruglov AA, Lampropoulou V, Fillatreau S, Nedospasov SV. Pathogenic and protective functions of TNF in neuroinflammation are defined by its expression in T lymphocytes and myeloid cells. J Immunol. 2011;187:5660–70. doi: 10.4049/jimmunol.1100663. [DOI] [PubMed] [Google Scholar]
- Zinser E, Lechmann M, Golka A, Lutz MB, Steinkasserer A. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble CD83. J Exp Med. 2004;200:345–51. doi: 10.1084/jem.20030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt J, Ostalecki C, Kuczera K, Schuler G, Pommer AJ, Lechmann M. Murine whole-organ immune cell populations revealed by multi-epitope-ligand cartography. J Histochem Cytochem. 2013;61:125–33. doi: 10.1369/0022155412470140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The choroid plexus of thymic stromal lymphopoietin (TSLP) knock-out (KO) mice contained fewer leucocytes in comparison to TSLP wild-type (WT) mice. Multi-epitope ligand cartography (MELC) images of brain harvested from TSLP KO mice and WT mice at day 12 after experimental autoimmune encephalomyelitis (EAE) induction. Upper row: phase contrast (white), CD45+ leucocytes (red), propidium iodide+ nuclei (green), CD31+ blood vessels (blue). Lower row: CD4+ T cells (red), CD11b+ macrophages/microglial cells (green), Ly6G+ neutrophil granulocytes (blue), CD11b+/Ly6G+ granulocytes (cyan), CD31+ blood vessels (magenta), forkhead box protein 3 (FoxP3+) regulatory T cells (red with white nuclei). Bar = 50 μm.
Fig. S2. In healthy thymic stromal lymphopoietin (TSLP) KO mice no reduction in the numbers of different T cell subtypes, macrophages or neutrophil granulocytes were observed. (a) Fluorescence activated cell sorter (FACS) analyses of inguinal lymph nodes [mean ± standard error of the mean (s.e.m.); TSLP KO n= 3, TSLP wild-type (WT) mice WT n= 4)]. (b) FACS FACS analyses of brain (TSLP KO = pool of three three mice, TSLP WT= pool of four four mice). (c) FACS analyses of inguinal lymph nodes, spleen and thymus. Diphteria toxin was injected at days 0 and 1, organs were removed at day 4 (three mice/group). Data (a,b) (mean ± s.e.m.) are representative of two and data (c) of three independent experiments.
Fig. S3. No differences in dendritic cell (DC) maturation or serum cytokine concentrations between thymic stromal lymphopoietin (TSLP) KO and wild-type (WT) mice mice at day 5 after experimental autoimmune encephalomyelitis (EAE) induction. (a) FACS analysis of spleens. GMFI = geometric mean fluorescence intensity. The bar charts represent the mean ± standard error of the mean (s.e.m.). TSLP KO n = 3, TSLP WT n = 3. Data are representative of three independent experiments. (b) Cytometric bead array (CBA) of serum. TSLP KO n = 9; TSLP WT n = 9; untreated C57/BL6 n = 4; mean (± s.e.m.; two-tailed unpaired Student's t-test: *P < 0·05; **P < 0·01; ***P < 0·001; n.s. = not significant). Data are representative of two independent experiments.
Fig. S4. At day 20 after experimental autoimmune encephalomyelitis (EAE) induction, thymic stromal lymphopoietin (TSLP) knock-out (KO) mice and TSLP wild-type (WT) mice mice show the same extent of central nervous system (CNS) inflammation. Flow cytometric analysis of brain. (a) CD45+ cells were gated on forward-/side-scatter (FSC/SSC), CD3+ cells were gated on CD45+ cells, CD4+ and CD8+ cells were gated on CD3+ cells. Interferon (IFN)-γ and interleukin (IL)-17Av cells were gated on CD4+ cells. (b) CD62L+ and CD69+ cells were gated on CD4+ or CD8 + cells. Results are representative of two independent experiments (pool of three brains each).
Table S1. Quantitative reverse transcription (qRT) primer sets used in this study.


