Abstract
Monocyte subsets with differing functional properties have been defined by their expression of CD14 and CD16. We investigated these subsets in anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) and determined their surface expression of ANCA autoantigens. Flow cytometry was performed on blood from 14 patients with active AAV, 46 patients with AAV in remission and 21 controls. The proportion of classical (CD14highCD16neg/low), intermediate (CD14highCD16high) and non-classical (CD14lowCD16high) monocytes and surface expression levels of CD14 and CD16 were determined, as well as surface expression of proteinase 3 (PR3) and myeloperoxidase (MPO) on monocyte subsets. There was no change in the proportion of monocytes in each subset in patients with AAV compared with healthy controls. The expression of CD14 on monocytes from patients with active AAV was increased, compared with patients in remission and healthy controls (P < 0·01). Patients with PR3-ANCA disease in remission also had increased monocyte expression of CD14 compared with controls (P < 0·01); however, levels in patients with MPO-ANCA disease in remission were lower than active MPO-ANCA patients, and not significantly different from controls. There was a correlation between CD14 and both PR3 and MPO expression on classical monocytes in AAV patients (r = 0·79, P < 0·0001 and r = 0·42, P < 0·005, respectively). In conclusion, there was an increase in monocyte CD14 expression in active AAV and PR3-ANCA disease in remission. The correlation of CD14 expression with ANCA autoantigen expression in AAV may reflect cell activation, and warrants further investigation into the potential for increased CD14 expression to trigger disease induction or relapse.
Keywords: anti-neutrophil cytoplasm antibody (ANCA), monocyte, myeloperoxidase (MPO), proteinase 3 (PR3)
Introduction
Anti-neutrophil cytoplasm antibody-associated vasculitis (AAV) is a form of necrotizing small vessel vasculitis, associated with anti-neutrophil cytoplasm antibodies (ANCA) 1. Most patients with AAV have ANCA specific for either proteinase 3 (PR3) or myeloperoxidase (MPO), which are glycoprotein enzymes present in neutrophil azurophilic granules and monocyte lysozymes 2,3. Monocytes have been less well studied in AAV than neutrophils, but cells of the monocyte and macrophage lineage are prominent in the kidneys of patients with AAV, as well as in the granulomas typical of upper airways and lung involvement in granulomatosis with polyangiitis (GPA) 4. In human peripheral blood, monocyte subpopulations with distinct functional properties have been defined by their expression of CD14 and CD16 into classical (CD14highCD16neg/low), intermediate (CD14highCD16high) and non-classical (CD14lowCD16high) 5–7. CD16 has been identified in two isoforms as low-affinity Fc receptors for immunoglobulin (Ig)G involved with immune complex-mediated immune cell activation and differentiation 8. Importantly, anti-neutrophil cytoplasm antibodies have been shown to require their Fc portion for cytokine release from neutrophils, suggesting a role for Fc-gamma receptors in the pathogenesis of the disease 9. CD14 is a Toll-like receptor (TLR) co-receptor and functions and as an effective pattern recognition receptor involved in innate immune response to microbial infection 10,11.
While classical monocytes have been found to respond to bacterial cues with the production of interleukin (IL)-8 and other cytokines, intermediate monocytes were shown to be the predominant producers of tumour necrosis factor (TNF)-α in the circulation in response to lipopolysaccharide (LPS) 5,12,13. The non-classical population accounts for approximately 7–10% of circulating monocytes in healthy individuals, and evidence would suggest that these cells patrol the endothelium and respond to viral antigens 5,14. Studies have demonstrated a significantly increased proportion of intermediate monocytes in various inflammatory conditions, such as in patients with rheumatoid arthritis or patients at high cardiovascular disease risk 15–17, and studies have examined some data on the monocyte populations in AAV patients 18,19.
With relevance to classical monocytes, there is an association between bacterial infection and relapse of ANCA vasculitis (AAV) 20,21, and surface expression of CD14 is relevant in cytokine responses to bacterial lipopolysaccharide 22. We have determined the mean fluorescence intensity (MFI) for CD14, CD16, PR3 and MPO on monocyte subsets in AAV. PR3-ANCA and MPO-ANCA disease are distinct genetically as well as phenotypically, in particular with respect to their tendency to relapse 23,24. We have examined the AAV cohort as a whole, as well as the individual phenotypes, as differential expression of ANCA antigens on monocyte subsets could help to explain some of the differences between PR3-ANCA and MPO-ANCA-associated disease.
Materials and methods
Patients and healthy control subjects
The study received ethical approval from Imperial College Healthcare NHS Trust's research ethics committee (04-Q0406-25). All patients were attending the multi-disciplinary vasculitis clinic at Hammersmith Hospital and fulfilled the Chapel Hill Consensus Conference (CHCC) definitions for AAV 25. All patients and controls gave written informed consent for the study. For demographic details of the subjects, including therapy, see Table1. Fourteen patients had active AAV (de novo or relapsing), defined as new clinical features of active vasculitis with a Birmingham Vasculitis Activity Score (BVAS) (2003) score > 3. All patients with active disease had new or worsening symptoms attributable to vasculitis, requiring a significant change in therapy. Forty-six remission patients with AAV were included (stable, BVAS 0, except one patient who had a BVAS of 1 due to nasal symptoms). Where possible, blood samples were collected from patients with active disease prior to starting immunosuppression, but seven of 14 were sampled within 4 days of corticosteroids at a dose greater than 20 mg/day. Five patients, who presented with active disease, subsequently gave a second sample after they were in remission. Patients were classified as having GPA or microscopic polyangiitis (MPA) based on CHCC criteria, but due to the difficulties with these clinical definitions ANCA subtype at diagnosis was used predominantly in this paper. Quantification of MPO- and PR3-ANCA was performed in the clinical immunology laboratory, Imperial College Healthcare National Health Service (NHS) Trust (Luminex, Theradiag). Patients in the remission groups were categorized as MPO or PR3-ANCA disease patients if they had been either MPO- or PR3-ANCA-positive at the time of presentation or disease flare. Complete blood counts were performed concurrently on patients, but not on healthy controls. Twenty-one healthy controls were recruited from the staff and students of Imperial College or Imperial College Healthcare NHS Trust.
Table 1.
Demographic information for anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) subjects and controls
| ANCA type | Active (n = 14) | Remission (n = 46) | Controls (n = 21) | |
|---|---|---|---|---|
| Age: median (interquartile range) | 52 (37–78) | 59 (49–68) | 42 (30–55) | |
| Gender (male : female) | 9:5 | 28:18 | 10:11 | |
| ANCA type at diagnosis (MPO : PR3) | 7:7 | 21:25 | n.a. | |
| New presentation : relapse | 11 : 3 | n.a. | n.a. | |
| Time since diagnosis (years) by serotype | MPO | 3·1 (0·9–7·2) | n.a. | |
| PR3 | 5·7 (1·5–12·5) | |||
| No. ANCA-positive at time of testing (%) | MPO | 7 (100%) | 17/21 (81%) | n.a. |
| PR3 | 7 (100%) | 17/25 (68%) | ||
| Median creatinine (µmol/l) (interquartile range) | MPO | 204 (132–356) | 128 (76–152) | Not tested |
| PR3 | 84 (61–532) | 88 (75–126) | ||
| Median eGFR ml/min/1·73 m2 (interquartile range) | MPO | 23 (11–48) | 48 (36–74) | Not tested |
| PR3 | 62 (11–90) | 72 (52–89) | ||
| Clinical phenotype | 6 MPA | 16 MPA | n.a. | |
| 8 generalized GPA | 26 generalized GPA | |||
| 3 localized GPA | ||||
| 1 EGPA | ||||
| Total WBC at time of test (interquartile range) | MPO | 11·0 (9·2–16·4) | 7·6 (6·4–8·9) | Not tested |
| PR3 | 13·2 (10·8–18·4) | 7·6 (6·4–10·0) | ||
| Neutrophil count (interquartile range) | MPO | 8·0 (7·1–12·3) | 5·5 (4·1–6·8) | Not tested |
| PR3 | 11·8 (7·7–15·7) | 5·3 (4·5–8·5) | ||
| Monocyte count (interquartile range) | MPO | 0·8 (0·5–1·4) | 0·6 (0·5–0·7) | Not tested |
| PR3 | 0·8 (0·5–1·0) | 0·6 (0·5–1·0) | ||
| CRP at test (mg/l) | MPO | 20 (3–61) | 1·2 (0·8–3·1) | Not tested |
| PR3 | 56 (39–197) | 2·9 (1·5–7·5) | ||
| BVAS at test median (interquartile range) | MPO | 14 (12–16) | 0 (0–0) | n.a. |
| PR3 | 18 (8–20) | 0 (0–0) | ||
| Median steroid dose in remission (mg) (interquartile range) | MPO | n.a. | 5 (0–5·6) | n.a. |
| PR3 | 7·5 (2·5–10) | |||
| Treatment at time of test | Nil: 5 | Nil: 2 | n.a. | |
| Pred<10 mg/day +MMF or MTX 2 | Pred < 10 mg/day alone: 4 | |||
| Aza alone: 8 | ||||
| Pred>20 mg/day (started within 4 days) 7 | MMF alone: 2 | |||
| Low-dose pred + aza: 18 | ||||
| Low-dose pred + MMF: 9 | ||||
| Low-dose pred + MTX: 3 |
Pred = prednisolone; aza = azathioprine; MMF = mycophenolate mofetil; MTX = methotrexate; Ritux = Rituximab; Methyl pred = methyl prednisolone pulse 500 mg; GPA = granulomatosis with polyangiitis; MPA = microscopic polyangiitis; MPO = myeloperoxidase ANCA; PR3 = proteinase 3 ANCA; CRP = C-reactive protein; BVAS = Birmingham Vasculitis Activity Score; eGFR = estimated glomerular filtration rate; n.a. = not available; WBC = white blood cells.
Flow cytometry
Whole blood was collected into ethylenediamine tetraacetic acid (EDTA) and was stained and fixed within 4 h. Red cells were lysed using cold-water lysis buffer (155 mM NH4Cl, 12 mM NaHCO3, 100 mM EDTA; 2 ml blood per patient). Following red cell lysis, leucocytes were resuspended in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) at a concentration of 50 × 106/ml or less. Cells were stained with the following antibodies at a concentration of 1 µl/50 µl cell suspension for all antibodies except HLA-DR (4 µl/50 µl cells), based on previous titration studies. Samples were stained in duplicate with peridinin chlorophyll-cyanin 5·5 (PerCP-Cy5·5) anti-human leucocyte antigen D-related (HLA-DR) (clone G46-6), phycoerythrin (PE)-Cy7 anti-CD16 (clone B73.1), Alexa Fluor 700 anti-CD14 (clone M5E2) (all BD Biosciences, San Jose, CA, USA). In addition, samples were stained either with PE anti-human myeloperoxidase (MPO) (clone 5B8, BD Biosciences) or fluorescein isothiocyanate (FITC) anti-PR3 (clone PR3-G2) (Cambridge Biosciences, Cambridge, UK). An isotype control was used, FITC P3, IgG1κ (Affymetrix Biosciences, Santa Clara, CA, USA). Monocytes were stained in duplicate (mix 1: CD14, CD16, HLA-DR, MPO; mix 2: CD14, CD16, HLA-DR, PR3) and isotype control mix. The mean percentages of monocytes of each subset and the MFI for CD14 and CD16 were calculated by taking the average of the readings from mix 1 and mix 2. Cells were stained for 15 min in the dark, followed by fixation with 150 µl 4% paraformaldehyde, before flow cytometry on a BD Accuri C6 flow cytometer. The machine was calibrated with quality control beads before running samples. Based on each single-stain and unstained samples, appropriate colour compensation was used for correcting spectral overlap and autofluorescence. Fluorescence activated cell sorter (FACS) analysis was performed using BD Accuri C6 software.
Monocyte gating
Monocytes were initially gated based on size and granularity (Supporting information, Fig. S1). To exclude cell doublets, cells were gated by plotting surface area (FSC) against width signal intensity. Monocytes were gated as HLA-DR-positive. Within this gate, monocytes were separated based on their expression of CD14 and CD16; this DRbrightCD14posCD16pos gating strategy has been described previously to be optimized for gating monocyte subsets 26. B cells and circulating dendritic cells expressing HLA-DR but not CD14 or CD16 were represented as CD14negCD16neg. Those cells were excluded and the percentages of monocytes of each subset were calculated. In addition, the MFI for CD14 and CD16 were calculated for each quadrant and for the total monocytes, shown in the Supporting information, Fig. S1a. The MFI for PR3 and MPO was calculated for individual subsets, and for the whole monocyte population. Isotype control expression for CD14, CD16, PR3 and MPO were subtracted from the MFI for each subject. Examples of monocyte subsets in healthy donor and active AAV patients are shown in Supporting information, Fig. S1. Note the increase in the x-axis and shift to the right (increased CD14) in the AAV patient plot.
Statistical analysis
Non-parametric statistics were used throughout. For comparing two groups, the Mann–Whitney U-test was used. For comparing three or more groups the Kruskal–Wallis test was used, with correction for multiple comparisons. Differences were not statistically significant (P < 0·05) unless indicated otherwise. The correlations were performed using Spearman's rank test. Statistics and correlations were performed using GraphPad Prism version 6. Lines on graphs represent median ± interquartile range.
Results
Demographic and clinical information
Demographic and clinical data are presented in Table1. The median age of active AAV patients was 52, remission AAV patients 59 and controls 42 years. Patients were subdivided according to ANCA specificity, clinical syndrome and extent of disease (localized or systemic). In the active group, 50% were PR3-ANCA positive, 50% MPO-ANCA positive. Overall, the median BVAS in the active patients was 16 (interquartile range = 12–19). Median BVAS in the remission patients was 0 (range = 0–1), with one patient scoring > 0 for persistent symptoms. Of the active patients, five of 14 were taking no immunosuppressive therapy at the time of testing, and two of 14 were on low-dose immunosuppression. The other seven active patients had started on high-dose prednisolone within 4 days of testing (Table1). Treatment for the remission patients is listed in Table1. It is notable that the median steroid dose for the PR3-ANCA patients in remission [7·5 mg/day (interquartile range = 2.5–10)] was slightly higher than the MPO-ANCA positive patients (5 mg, interquartile range = 0–5·6). The majority of remission patients in this cohort remained ANCA-positive at the time of sampling (81% of the patients with MPO-ANCA disease and 68% of the PR3-ANCA disease patients). The median time since diagnosis for the remission patients was 3·1 years for the MPO-ANCA patients and 5·7 years for the PR3-ANCA patients (Table1).
Monocyte subsets in AAV patients
There was no significant change in the proportion of monocytes of classical, intermediate and non-classical phenotypes as a percentage of total monocytes when comparing patients with active AAV or remission AAV to controls (Fig. 1a–c). This was also true when the patients were subdivided into MPO-ANCA and PR3-ANCA disease (Supporting information, Fig. S2). AAV patients (but not controls) had contemporaneous complete blood count measurements. The median total white blood count in the patients with active AAV was 13 × 109/l (interquartile range 10–17). The median monocyte counts for the patients with active disease [0·8 × 109/l, interquartile range = 0·5–1)] was within, but at the upper end, of the reference range for the clinical laboratory at our hospital (0·3–0·9 × 109/l), while the median neutrophil count in the active patients was raised at 9 × 109/litre (interquartile = range 7–13) (normal reference range at 2·0–7·1 × 109/l). Absolute monocyte subset counts were calculated for patients with active AAV and AAV in remission (Fig. 1d–f). There was an apparent increase in the absolute number of classical monocytes in the patients with active AAV compared with patients in remission (P < 0·05), although there was no increase in the absolute number of intermediate monocytes or non-classical monocytes. The difference in classical monocyte numbers in active disease was small, and requires confirmation in a larger cohort.
Figure 1.
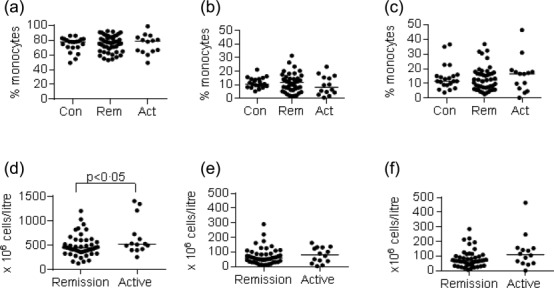
Monocyte subsets in patients with anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV). Peripheral blood was collected from healthy subjects (Con), AAV patients in remission (Rem) or AAV patients with active disease (Act). Percentages and absolute numbers of monocyte subsets were phenotyped by flow cytometry in each subject in duplicate, as described in Methods (also see Supporting information, Fig. S1). Each dot in the graphs represents an individual subject. (a–c) Percentage of total monocytes of (a) classical; (b) intermediate and (c) non-classical phenotype as a percentage of the total monocytes for each patient or healthy control. For breakdown by ANCA subtype see Supporting information, Fig. S2. (d–f) Absolute numbers of monocytes of each phenotype in patients with AAV in remission or active AAV. Horizontal line represents the median.
Expression of CD16 on monocyte populations
In order to investigate CD16 expression on monocytes in AAV, the MFI for CD16 was calculated for each monocyte subset, and the monocyte population as a whole, for controls and patients with AAV (Fig. 2). There was a small but statistically significant decrease in classical monocyte CD16 expression in patients with AAV in remission compared with those with active disease (Fig. 2b). However, the difference between MPO-ANCA patients in remission and the control subjects was not significantly different (Fig. 2b). In order to investigate a possible cause for this, monocyte CD16-MFI was correlated with steroid dose, but no correlation was seen (Supporting information, Fig. S4b). A statistically significant increase in CD16-MFI was seen in intermediate monocytes in MPO-ANCA patients in remission compared with healthy controls. However, due to the multiple comparisons and the high variance of this particular reading, this is not thought to be a biologically relevant change. Overall, the results are consistent with the results presented in Fig. 1, that there was no consistent change in the proportion of classical, intermediate or non-classical monocytes in patients with AAV based on CD16 expression.
Figure 2.
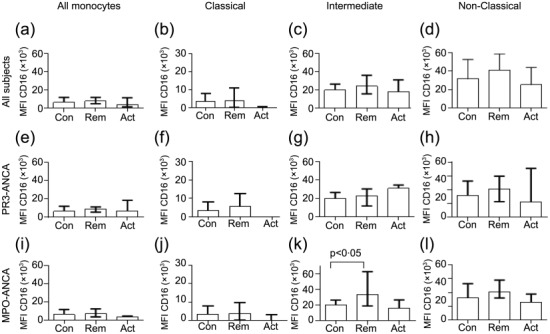
Median fluorescence intensity for CD16 in all monocytes and subsets. Blood monocytes (all monocytes, classical, intermediate and non-classical subsets) were analysed for CD16 expression by flow cytometry in all anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) patients (a–d) or patients with proteinase 3 (PR3)-ANCA disease at diagnosis (e–h) or myeloperoxidase (MPO)-ANCA disease (i–l) (see Methods). (a–d) Monocytes in all AAV patients (n = 21 controls, 46 remission, 14 active); (e–h) monocytes in PR3-ANCA patients (n = 21 controls, 25 remission and seven active); i–l) monocytes in MPO-ANCA patients (n = 21 control, 21 remission and seven active). Differences were not statistically significant by one-way analysis of variance (anova) (Kruskal–Wallis test) and post-test unless indicated otherwise. Bars represent the median ± interquartile range. Note change in y-axis scale for classical monocytes.
Expression of CD14 was increased in monocytes of patients with active AAV and PR3-ANCA vasculitis patients in clinical remission
We then went on to examine the expression of CD14 on monocytes in patients with AAV and healthy controls (Fig. 3 and Supporting information, Fig. S1a). Patients with active AAV were found to have an increased MFI for CD14 on their classical and intermediate monocytes compared with patients in remission or healthy controls (Fig. 3a–c, e–g), while there was no significant difference in the low levels of CD14 expression on non-classical monocytes (Fig. 3d, h). Regarding the remission patients, there was an interesting discrepancy between patients with PR3-ANCA disease and those with MPO-ANCA disease (Fig. 3a–h). While CD14 expression on monocytes from MPO-ANCA patients in remission was not raised compared with controls, and in fact showed an apparent decrease in classical and intermediate monocytes compared with active disease (Fig. 3e–g), the mean MFI for CD14 remained elevated on the classical and intermediate monocytes of patients with PR3-ANCA disease (Fig. 3a–c) (P < 0·01 for all monocytes, P < 0·05 for classical monocytes and P < 0·05 for intermediate monocytes). However, the MFI for CD14 in PR3-ANCA patients in remission was significantly lower than that seen in the PR3-ANCA patients with active disease (P < 0·05 for classical and intermediate monocytes, Fig. 3a–c). These results demonstrate that CD14 expression is increased in classical and intermediate monocytes in patients with active AAV, and that the phenotype does not normalize completely in patients with PR3-ANCA disease while it does in MPO-ANCA disease, which may suggest an element of ongoing monocyte activation in some patients with PR3-ANCA disease in clinical remission. In this study, the majority of the patients in remission who were tested remained ANCA-positive. Interestingly, when we subdivided the remission patients into those who were persistently ANCA-positive compared with those who were ANCA-negative at the time of testing, there was no significant difference in the MFI for CD14, suggesting that ANCA itself was not the determining factor in the CD14 MFI (Supporting information, Fig. S3). As the median steroid dose was slightly higher in the remission MPO patients than the remission PR3-ANCA patients, steroid dose was also correlated against CD14 and CD16. No correlation was seen (Supporting information, Fig. S4).
Figure 3.
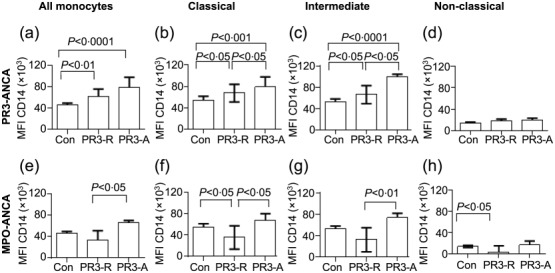
Median fluorescence intensity for CD14 in all monocytes and subsets. Blood monocytes (all monocytes, classical, intermediate and non-classical subsets) were analysed for CD14 expression by flow cytometry in anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) patients (see Methods). (a–d) Monocytes in proteinase 3 (PR3)-ANCA patients (n = 21 controls, 25 remission and seven active); (e–h) Monocytes in myeloperoxidase (MPO)-ANCA patients (n = 21 control, 21 remission and seven active). Statistics quoted were measured by one-way analysis of variance (anova) (Kruskal–Wallis test and post-test). Bars represent the median ± interquartile range.
Expression of ANCA autoantigens on monocyte subsets
The relative expression of PR3 and MPO on different monocyte subsets has not been defined previously. This may be of relevance, as the surface expression of the autoantigens may influence the ability of ANCA to interact with the cells. While most PR3 and MPO expression will be intracellular, we noted surface expression on monocyte subsets in healthy donors (Supporting information, Fig. S1b). There was differential expression of ANCA autoantigens among the monocyte subsets in both healthy controls and AAV patients (Fig. 4a–f). The expression of PR3 was highest in classical and intermediate monocytes, with lower levels of surface expression on non-classical monocytes (Fig. 4a, c). In contrast, the expression of MPO was significantly higher in intermediate monocytes than in classical monocytes (Fig. 4b, d). These differences were apparent in patients with AAV and controls, and the differences in expression levels of ANCA autoantigens may affect the ability of ANCA of different specificities to interact with monocyte subsets.
Figure 4.
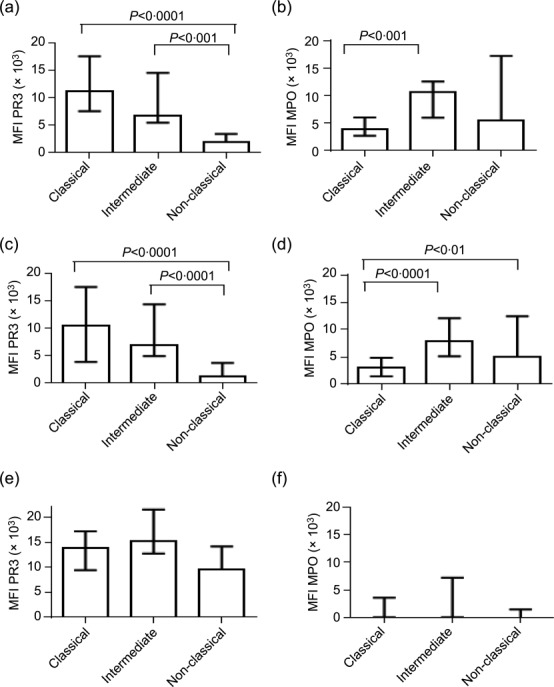
Median fluorescence intensity for proteinase 3 (PR3) and myeloperoxidase (MPO) on monocyte subsets in healthy controls and anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) patients in remission. Blood monocyte subsets (classical, intermediate and non-classical subsets) were analysed for PR3 and MPO expression by flow cytometry in all healthy subjects (controls; n = 21) and all AAV patients in remission (n = 43) and AAV patients at the time of active disease (n = 11). (a–b) Monocytes subsets in controls. (c–d) Monocyte subsets in AAV patients in remission. (e–f) AAV patients with active disease. Kruskal–Wallis test with post-test was performed for each graph. Bars represent the median ± interquartile range.
Surface expression of PR3 and MPO was quantified in 11 of 14 patients with active AAV. Due to variability in the data and the smaller cohort, the differences in this group were not statistically significant (Fig. 4e–f). Interestingly, five of 11 active patients did not have detectable MPO on the monocyte surface. In the other six patients, the pattern of expression among the monocyte subsets was broadly similar to the remission patients. The reason for the apparent reduction in MPO expression on the monocytes from active patients would require investigation in a larger cohort.
There was a correlation between monocyte CD14 expression and PR3 and MPO expression in patients with AAV
We then determined whether or not there was a correlation between monocyte CD14 expression and membrane expression of PR3 and MPO (Fig. 5a–h). In healthy controls, there was no correlation between the MFIs for PR3 and CD14 [r = 0·23, P = not significant (n.s.)] or MPO and CD14 (r = 0·36, P = n.s.) (Fig. 5a,b). However, in patients with AAV, there was a strong correlation between MFI for PR3 and CD14 (r = 0·79, P < 0·0001) and a weaker correlation between MPO and CD14 (r = 0·42, P < 0·005), Fig. 5c,d. There was a stronger correlation between CD14 and PR3 expression than CD14 and MPO expression for both PR3-ANCA and MPO-ANCA patients (Fig. 5e–h).
Figure 5.
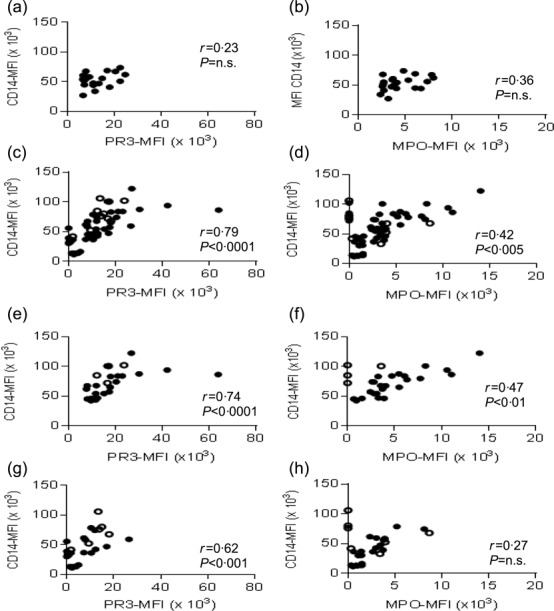
Correlation of CD14 expression with proteinase 3 (PR3) and myeloperoxidase (MPO) expression on classical monocytes in healthy controls and anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) patients. Classical monocytes were analysed for CD14, PR3 and MPO expression in healthy subjects and in AAV patients. Closed circles = remission AAV patients, open circles = active AAV patients. Each dot represents individual subject. (a–b) Correlation between CD14 and PR3 (a) or MPO (b) in healthy subjects. (c–d), Correlation between CD14 and PR3 (c) or MPO (d), in all AAV patients. (e–f) Correlation between CD14 and PR3 (e) or MPO (f), in PR3-ANCA patients. (g–h) Correlation between CD14 and PR3 (g) or MPO (h), in MPO-ANCA patients. Spearman's rank test was used for correlations.
Discussion
Monocytes have been implicated in the pathogenesis of AAV, as they localize to histological lesions 27, express ANCA autoantigens 28 and secrete proinflammatory cytokines in response to ANCA in vitro 9,29. In this study, we have demonstrated an increase in surface CD14 expression on monocytes in patients with active AAV, as well as PR3-ANCA disease in clinical remission. CD14 is a glycosyl-phosphatidyl inositol-linked receptor, and acts as a co-receptor for LPS, expressed on the surface of Gram-negative bacteria 11. Two previous studies have found that incubation of monocytes with ANCA in vitro increased monocyte expression of CD14 30,31. In one study, incubation of monocytes with monoclonal mouse anti-human PR3-ANCA increased CD14 expression, and enhanced the production of cytokines in response to LPS challenge 30. In the second study, incubation of isolated monocytes with c-ANCA- or p-ANCA-positive IgG from patients with active vasculitis increased the expression of CD14 31. Taken together with our results, monocytes in patients with active AAV may be primed to respond to Gram-negative infection or to endogenous damage-associated molecules (DAMPS) ligating TLR-4, which could potentially aggravate local inflammation in AAV.
The monocyte CD14-MFI was reduced in the MPO-ANCA patients in remission, whereas it remained higher than in healthy donors among the PR3-ANCA-positive patients. This is despite the fact that most of the remission patients recruited to this study (both MPO-ANCA and PR3-ANCA patients) were still ANCA-positive at the time of testing. There is evidence that the epitope specificity of MPO-ANCA changes between active disease to remission 32; therefore, if ANCA is directly driving the CD14 expression, a change in antibody affinity or epitope specificity may be important in the normalization of the phenotype in MPO-ANCA patients in remission. It is also possible that the increased CD14 expression reflects monocyte activation, but that it is not driven directly by ANCA. When PR3-ANCA patients in remission were subdivided into those who were ANCA-positive or -negative at the time of testing, there was no difference in CD14 MFI between the two groups, which suggests that monocyte CD14 was not related directly to ANCA status. This may present the hypothesis that PR3-ANCA AAV patients have a chronic inflammatory process during clinical remission, and that this chronic inflammation is reflected in activated monocytes. It is notable that the PR3-ANCA patients were on a higher median dose of prednisolone in remission (7·5 mg/day) compared with the MPO-ANCA patients (5 mg/day), despite a longer duration of disease in the PR3-ANCA remission patients, which suggests that their disease was less well controlled, and is consistent with this hypothesis. Another hypothesis would be that chronic infection in the sinuses, nose and upper airways affected in PR3-ANCA patients could drive chronic monocyte activation in remission PR3-ANCA patients. In this context, it is interesting to note that in addition to ANCA, peptidoglycan of Staphylococcus aureus and LPS itself have also been shown to up-regulate CD14 expression on monocytes 22,33. Infections and nasal colonization with S. aureus have been linked to relapse of GPA 20,21, and treatment with co-trimoxazole was effective in reducing relapse rates in these patients 34.
Most of the patients in this study were on immunosuppression, and glucocorticoid treatment has been reported to suppress monocyte CD14 expression 35. Five of 14 of the active patients were not yet on corticosteroids when the samples were taken, and two were on maintenance doses (10 mg per day or less). Of the remission patients, 34 of 46 were on low-dose corticosteroid treatment. It is a drawback of our study that we were unable to sample all patients off treatment; however, if it is indeed the case that corticosteroids reduce the expression of CD14 then our results are likely to be more significant, and also relevant in a real-life clinical situation. MFI for CD14, CD16, PR3 and MPO were not associated significantly with steroid dosage at the time of testing (Supporting information, Fig. S4).
No difference in the MFI for CD16 on monocytes from all subsets or in the proportion of CD16-expressing monocytes was found between AAV patients and controls. However, there was a statistically significant increase in the overall absolute numbers of monocytes in patients with active AAV compared with patients in remission. In agreement with our results, previous studies examining patients with AAV in remission also found no change in the proportion of monocyte subsets in AAV 18,19. As intermediate monocytes express ANCA antigens and activatory Fcγ receptors, and are known to be major secretors of proinflammatory cytokines such as TNF-α 5,12, it is a little surprising that this cell type is not relatively increased in AAV. However, the lack of expansion of this population does not imply that they do not respond to ANCA, or have a role in the pathogenesis of the disease. Indeed, our data demonstrate the expression of ANCA autoantigens in this cell population. In contrast to the data in AAV, the proportion of intermediate monocytes has been found to be increased in other inflammatory diseases, such as rheumatoid arthritis 15,16, and in patients on haemodialysis 36. It is of interest that renal glomerular accumulation of CD16high monocytes has been associated with lupus nephritis, and that this subset may be involved with the pathogenesis of glomerulonephritis in these patients 5. Whether a similar mechanism of CD16high-mediated kidney inflammation occurs in AAV requires further investigation.
There was a wide variation in the percentage of monocytes in each subset between individual subjects, even in the healthy control population. Each sample was stained in replicate, and there was < 10% difference in monocyte proportions between the samples (data not shown), so it is likely that this represents true biological variation. The meaning behind heterogeneous monocyte frequency and phenotype remains to be explored. However, the development and roles of the different monocyte subsets has been described 37. Reports of interchange or differentiation between mouse monocyte subsets occur 38,39, but whether this occurs in human monocytes, and its role in disease, remains to be established. Finally, the relationship between blood monocyte subsets and further effector functions of tissue macrophage phenotypes remains enigmatic and is part of ongoing investigations in many laboratories (reviewed in 38).
PR3 was expressed more highly in classical and intermediate monocytes, and was expressed at low levels in non-classical monocytes. In contrast, MPO was expressed most highly in intermediate monocytes compared with classical monocytes. It would be interesting to explore in future studies whether these differences may affect the ability of ANCA of different specificities to interact with different monocyte subsets, and consequently affect disease phenotype. There was a highly significant correlation between PR3 and CD14 MFI on classical monocytes. A similar association was also seen for intermediate monocytes (data not shown). There was a significant but lesser correlation between CD14 and MPO expression on monocytes. These data cannot imply causality, but suggest that the expression of ANCA autoantigens and CD14 on monocytes in AAV are linked. It is interesting that the correlations were not seen among healthy controls. Ciavatta et al. demonstrated epigenetic control of ANCA antigen expression in neutrophils 40. It would be of interest to examine in future studies whether or not the same is true for monocytes and, indeed, whether CD14 is regulated in a similar fashion.
In conclusion, we present a descriptive analysis of monocyte subsets in AAV. We show that AAV patients exhibit increased CD14 expression on monocytes during active disease. Monocytes in both healthy individuals and AAV express MPO and PR3 antigens; however, in AAV, CD14 correlates with ANCA autoantigen expression. Further mechanistic studies are required to investigate if the increase in CD14 has a role in innate immune response to microbial infection in AAV and how this may contribute to triggering disease or relapse.
Acknowledgments
This work was funded by the Imperial Healthcare Charity and a fellowship to R. M. T. from Arthritis Research UK. The authors acknowledge the Imperial Biomedical Research Centre.
Author contributions
R. T. and K. W. designed the study, R. T. and S. S. consented patients and collected clinical information, J. L., S. S., K. W., N. H. and R. T. performed FACS staining, J. L. and K. W. analysed flow cytometry, T. P., H. T. C. and C. P. provided advice and reviewed the manuscript, R. T. and K. W wrote the manuscript.
Disclosure
The authors have no financial conflicts of interest in this work.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's Web-site:
Fig. S1. Monocyte gating strategy. (a) Monocyte populations are gated according to forward-scatter (FSC) and side-scatter (SSC). Doublets are excluded based on width. Human leucocyte antigen D-related (HLA-DR)+ population is selected and gated on CD14 and CD16. Upper left, upper right and lower right quadrants are selected to gate on monocytes and exclude DR+ B cells and dendritic cells (DC). Percentage of classical, intermediate and non-classical populations are then calculated (see Methods). Examples of healthy and active anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) patients are given. Note the increase in x-axis and shift to the right (increased CD14) in AAV patient. (b) Example of myeloperoxidase (MPO) and proteinase 3 (PR3) expression from all DR+ monocytes in a healthy donor. Dot-plots and histogram exported from Accuri C6 software.
Fig. S2. Monocyte subsets subdivided by anti-neutrophil cytoplasm antibody (ANCA) type. As Fig. 1, except monocyte subsets are subdivided into proteinase 3 (PR3)-anti-neutrophil cytoplasm antibody (ANCA) or myeloperoxidase (MPO)-ANCA patients, at the time of diagnosis with ANCA-associated vasculitis (AAV). Each dot represents individual subjects. (a–c) Percentage of monocytes of each subset in patients with PR3-ANCA at diagnosis; (d–f) percentage of monocytes in each subset in patients with MPO-ANCA at diagnosis. Rem'n = remission. None of the differences were statistically significant by Kruskal–Wallis test.
References
- Tarzi RM, Cook HT, Pusey CD. Crescentic glomerulonephritis: new aspects of pathogenesis. Semin Nephrol. 2011;31:361–8. doi: 10.1016/j.semnephrol.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Ludemann J, Utecht B, Gross WL. Anti-cytoplasmic antibodies in Wegener's granulomatosis are directed against proteinase 3. Adv Exp Med Biol. 1991;297:141–50. doi: 10.1007/978-1-4899-3629-5_12. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Falk RJ, Hu P, Xiao H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol. 2013;8:139–60. doi: 10.1146/annurev-pathol-011811-132453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–23. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J Clin Invest. 1997;100:1416–24. doi: 10.1172/JCI119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–80. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol. 2013;3:32. doi: 10.3389/fcimb.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto H, Ono S, Hiraki S, et al. Hemoperfusion with polymyxin B-immobilized fibers reduced the number of CD16+ CD14+ monocytes in patients with septic shock. J Endotoxin Res. 2004;10:229–37. doi: 10.1179/096805104225005814. [DOI] [PubMed] [Google Scholar]
- Pedraza-Sanchez S, Hise AG, Ramachandra L, Arechavaleta-Velasco F, King CL. Reduced frequency of a CD14+ CD16+ monocyte subset with high Toll-like receptor 4 expression in cord blood compared to adult blood contributes to lipopolysaccharide hyporesponsiveness in newborns. Clin Vaccine Immunol. 2013;20:962–71. doi: 10.1128/CVI.00609-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley-Bauer LP, Roback LJ, Wynn GM, Mocarski ES. Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice. Cell Host Microbe. 2014;15:351–62. doi: 10.1016/j.chom.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–7. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- Cooper DL, Martin SG, Robinson JI, et al. FcgammaRIIIa expression on monocytes in rheumatoid arthritis: role in immune-complex stimulated TNF production and non-response to methotrexate therapy. PLoS One. 2012;7:e28918. doi: 10.1371/journal.pone.0028918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogacev KS, Cremers B, Zawada AM, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60:1512–20. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Ohlsson SM, Pettersson A, Ohlsson S, et al. Phagocytosis of apoptotic cells by macrophages in anti-neutrophil cytoplasmic antibody-associated systemic vasculitis. Clin Exp Immunol. 2012;170:47–56. doi: 10.1111/j.1365-2249.2012.04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadema H, Abdulahad WH, Stegeman CA, Kallenberg CG, Heeringa P. Increased expression of Toll-like receptors by monocytes and natural killer cells in ANCA-associated vasculitis. PLOS ONE. 2011;6:e24315. doi: 10.1371/journal.pone.0024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching AJ, Rees AJ, Pussell BA, Lockwood CM, Mitchison RS, Peters DK. Relapses in Wegener's granulomatosis: the role of infection. BMJ. 1980;281:836–8. doi: 10.1136/bmj.281.6244.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120:12–7. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- Marchant A, Duchow J, Delville JP, Goldman M. Lipopolysaccharide induces up-regulation of CD14 molecule on monocytes in human whole blood. Eur J Immunol. 1992;22:1663–5. doi: 10.1002/eji.1830220650. [DOI] [PubMed] [Google Scholar]
- Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–23. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan SL, Falk RJ, Chin H, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–31. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- Abeles RD, McPhail MJ, Sowter D, et al. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi)/CD16(neg) monocytes: Expansion of CD14(hi)/CD16(pos) and contraction of CD14(lo)/CD16(pos) monocytes in acute liver failure. Cytometry A. 2012;81:823–34. doi: 10.1002/cyto.a.22104. [DOI] [PubMed] [Google Scholar]
- Rastaldi MP, Ferrario F, Tunesi S, Yang L, D'Amico G. Intraglomerular and interstitial leukocyte infiltration, adhesion molecules, and interleukin-1 alpha expression in 15 cases of antineutrophil cytoplasmic autoantibody-associated renal vasculitis. Am J Kidney Dis. 1996;27:48–57. doi: 10.1016/s0272-6386(96)90030-x. [DOI] [PubMed] [Google Scholar]
- Muller Kobold AC, Kallenberg CG, Tervaert JW. Monocyte activation in patients with Wegener's granulomatosis. Ann Rheum Dis. 1999;58:237–45. doi: 10.1136/ard.58.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar K, Bickenbach A, Csernok E, et al. Wegener's granulomatosis: antiproteinase 3 antibodies induce monocyte cytokine and prostanoid release-role of autocrine cell activation. J Leukoc Biol. 2002;71:996–1004. [PubMed] [Google Scholar]
- Hattar K, van Burck S, Bickenbach A, et al. Anti-proteinase 3 antibodies (c-ANCA) prime CD14-dependent leukocyte activation. J Leukoc Biol. 2005;78:992–1000. doi: 10.1189/jlb.0902442. [DOI] [PubMed] [Google Scholar]
- Nowack R, Schwalbe K, Flores-Suarez LF, Yard B, van der Woude FJ. Upregulation of CD14 and CD18 on monocytes in vitro by antineutrophil cytoplasmic autoantibodies. J Am Soc Nephrol. 2000;11:1639–46. doi: 10.1681/ASN.V1191639. [DOI] [PubMed] [Google Scholar]
- Roth AJ, Ooi JD, Hess JJ, et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest. 2013;123:1773–83. doi: 10.1172/JCI65292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JS, Wang JE, Foster SJ, Thiemermann C, Hinds CJ. Peptidoglycan of Staphylococcus aureus upregulates monocyte expression of CD14, Toll-like receptor 2 (TLR2), and TLR4 in human blood: possible implications for priming of lipopolysaccharide signaling. Infect Immun. 2005;73:7613–9. doi: 10.1128/IAI.73.11.7613-7619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG. Trimethoprim–sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener's granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996;335:16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- Nockher WA, Scherberich JE. Expression and release of the monocyte lipopolysaccharide receptor antigen CD14 are suppressed by glucocorticoids in vivo and in vitro. J Immunol. 1997;158:1345–52. [PubMed] [Google Scholar]
- Nockher WA, Scherberich JE. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66:2782–90. doi: 10.1128/iai.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–95. [Google Scholar]
- Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavatta DJ, Yang J, Preston GA, et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J Clin Invest. 2010;120:3209–19. doi: 10.1172/JCI40034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Monocyte gating strategy. (a) Monocyte populations are gated according to forward-scatter (FSC) and side-scatter (SSC). Doublets are excluded based on width. Human leucocyte antigen D-related (HLA-DR)+ population is selected and gated on CD14 and CD16. Upper left, upper right and lower right quadrants are selected to gate on monocytes and exclude DR+ B cells and dendritic cells (DC). Percentage of classical, intermediate and non-classical populations are then calculated (see Methods). Examples of healthy and active anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) patients are given. Note the increase in x-axis and shift to the right (increased CD14) in AAV patient. (b) Example of myeloperoxidase (MPO) and proteinase 3 (PR3) expression from all DR+ monocytes in a healthy donor. Dot-plots and histogram exported from Accuri C6 software.
Fig. S2. Monocyte subsets subdivided by anti-neutrophil cytoplasm antibody (ANCA) type. As Fig. 1, except monocyte subsets are subdivided into proteinase 3 (PR3)-anti-neutrophil cytoplasm antibody (ANCA) or myeloperoxidase (MPO)-ANCA patients, at the time of diagnosis with ANCA-associated vasculitis (AAV). Each dot represents individual subjects. (a–c) Percentage of monocytes of each subset in patients with PR3-ANCA at diagnosis; (d–f) percentage of monocytes in each subset in patients with MPO-ANCA at diagnosis. Rem'n = remission. None of the differences were statistically significant by Kruskal–Wallis test.


