Abstract
Human leucocyte antigen (HLA)-G has a tolerogenic function and could play a role in the pathogenesis of immune-mediated diseases, including systemic sclerosis (SSc). The aim of this study was to evaluate HLA-G serum expression (sHLA-G) and the HLA-G gene 14 base pairs (bp) insertion/deletion (del−/del+) polymorphism in patients with Ssc, to search for possible associations with clinical and laboratory variables. sHLA-G was measured by enzyme-linked immunosorbent assay (ELISA) in sera from 77 patients with SSc and 32 healthy donors (HD); the 14 bp del−/del+ polymorphism was evaluated by polymerase chain reaction (PCR) amplification of peripheral blood mononuclear cells (PBMC) genomic DNA. Receiver operating characteristics (ROC) analysis identified the HLA-G cut-off that best discriminated dichotomized clinical and serological variables, that was subsequently employed to subdivide SSc patients into HLA-G high (HLA-G+) and low (HLA-G−) profile groups. sHLA-G were not statistically different between SSc patients and HD, nor between distinct SSc autoantibody subsets. Subdividing SSc patients by HLA-G positivity or negativity yielded significant differences for the modified Rodnan skin score (mRss) (P = 0·032), ‘general’ (P = 0·031) and ‘kidney’ (P = 0·028) Medsger severity scores (MSS) and disease activity index, and especially Δ heart/lung (P = 0·005). A worse ‘general’ MSS (P = 0·002) and Δ heart/lung (P = 0·011) were more frequent in the low sHLA-G group. These two variables and mRss were associated with sHLA-G levels at logistic regression analysis. Treatment had no influence on sHLA-G. Moreover, a higher frequency of scleredema was detected in the del+/del+ than the del-/del+ group (P = 0.04). These data suggest modulatory effects of sHLA-G on SSc. Prospective studies are needed to investigate a role in predicting the disease course.
Keywords: HLA-G, HLA-G genotype, systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a rare disease, with a prevalence of two to 20 cases per 105 inhabitants. SSc represents one of the most disabling and invalidating connective tissue diseases of unknown aetiology and pathogenesis; it is characterized by three main pathological processes, namely vascular abnormalities, collagen deposition and immune system activation 1,2. The disease causes widespread microvascular damage and fibrosis of the skin and internal organs, leading ultimately to an array of clinical manifestations which are quite heterogeneous from patient to patient, but that in the majority of cases (>95%) are preceded by Raynaud's phenomenon, even by several years 3.
The presence of autoantibodies, as well as tissue-infiltrating lymphocytes–monocytes–macrophages during the early stages of disease 4, indicates a pathogenic role played by the immune system. Thus, the study of immunomodulatory molecules can provide information related to the clinical course and aggressiveness of SSc.
Among immunomodulatory molecules, human leucocyte antigens (HLA)-G have the function of ‘immunosurveillance’ and, as such, can be involved directly (or indirectly) in the pathogenesis of SSc. Therefore, genetic and phenotypical analysis of this molecule may provide predictive information on the disease course.
HLA-G is a non-classical human leucocyte antigen (HLA)-class I molecule characterized by a restricted allelic polymorphism 5. It can exist as four membrane-bound (HLA-G1 -G2, -G3 and -G4) and three soluble isoforms (G5,-G6 and -G7) (sHLA-G), the latter being generated by alternative splicing of the primary transcript 6, in a similar fashion to our group's previous observations for classical soluble sHLA-class I 7. In addition, a soluble form of HLA-G1 can be generated by metalloproteinase-dependent proteolytic shedding 8 through a mechanism which appears to be, at least in part, dependent upon nitric oxide (NO) nitration of both cellular and soluble forms of the molecule 9.
HLA-G expression is restricted to certain tissues, namely adult thymus medulla, cornea, pancreatic islets 10, erythroid or endothelial cell precursors 11 and fetal tissues 12. In the latter case, the molecule would have tolerogenic functions, neutralizing potential alloreactivities at the maternal–fetal interface 13. Additional HLA-G-mediated tolerogenic functions have been reported in vitro 14–16 and in different clinical settings in humans, such as transplantation 17,18, solid or haematological malignancies 19,20 and autoimmune diseases (review in 21).
Among HLA-G gene polymorphisms, particular attention has been paid to the HLA-G gene 14 base pairs (bp) del−/del+ polymorphism in exon 8 at the 3′ untranslated region (UTR) (3′ UTR), which regulates the transcription and stability of HLA-G mRNA. Several studies have provided evidence of a relation of this polymorphism to some immune-mediated diseases. Indeed, levels of HLA-G or its gene 14 bp del−/del+ polymorphism have been evaluated in relation to the clinical activity of rheumatoid arthritis 22,23, systemic lupus erythematosus (SLE) 24, ankylosing spondylitis 25 and in some autoinflammatory diseases, namely Behçet's 26 and Kawasaki diseases 27.
In SSc, HLA-G expression has been explored only at skin levels 28, whereas no data are available on clinical correlates of sHLA-G and the HLA-G 14 bp gene del−/del+ polymorphism. In the present investigation, we have addressed this issue in 77 patients with SSc. In a smaller number of patients, clinical correlates of the HLA-G 14 bp gene del−/del+ polymorphism have also been investigated.
Material and methods
Patients
This retrospective study evaluated data from 77 patients with SSc who were being treated at the Rheumatology Units of the University of Naples and University of Bari. The patients were diagnosed according to the preliminary American College of Rheumatology criteria for the classification of the disease 29. An extensive evaluation was conducted at the entrance visit, including a routine medical history, physical examination and taking blood for laboratory tests, to assess blood cell counts, erythrocyte sedimentation rate (ESR), total serum protein (by capillary electrophoresis), C-reactive protein (CRP), titres of anti-nuclear antibodies (ANA), anti-centromere antibodies (ACA) and anti-topoisomerase-I (anti-topo-I) antibody and complement proteins (C3, C4). C3 and C4 complement levels were considered ‘low’ when below the normal range. Tests for renal (serum creatinine and urea) function were also performed.
On the basis of the presence of ACA and anti-Scl70 antibody in sera, determined on a routine basis using the anti-centromere protein B (CENP-B) and anti-Scl70 enzyme-linked immunosorbent assay (ELISA) kits (Orgentec Diagnostika GmbH, Mainz, Germany), patients were subdivided into three groups: (i) ACA+ patients (n = 40), anti-Scl70 antibody+ patients (n = 28) and patients without ACA and anti-Scl70 antibody (n = 9). None of the selected SSc patients had concomitant connective tissue diseases or vasculitis.
Disease duration was determined from the onset of the first Raynaud's manifestation 30. The SSc subtype, limited or diffuse, was determined according to LeRoy et al. 31. Skin involvement was assessed using the modified Rodnan skin score (mRss) 32, whereby the degree of skin thickness is measured in 17 areas and scored from 0 (normal skin) to 3 (severe thickening), for a total score range of 0–51. Bibasilar fibrosis for interstitial lung disease (ILD) was assessed with high-resolution computed tomography 33. For lung function, forced vital capacity (FVC) and diffusing lung capacity for carbon monoxide (DLCO) were measured and expressed as a percentage of the predicted values. Systolic pulmonary arterial pressure (sPAP) was estimated from the tricuspid regurgitant jet velocity, measured using Doppler echocardiography. Pulmonary arterial hypertension (PAH) was defined as sPAP > 35 mm Hg 34. Clinical involvement of organs and tissues was assessed and scored (from 0 to 4) according to Medsger et al. 35 in the following items: general, peripheral vascular, skin, joint/tendon, muscle, gastrointestinal tract, lung, heart and kidney. Scores for the nine items were summed to obtain the disease severity score 35. The European Scleroderma Study Group (EScSG) disease activity index was also calculated 36,37. This index includes 10 weighted items, three of which are indicated by patients, referring to possible changes in conditions over the preceding month (Δ), while seven are clinical variables recorded by the physician. The former include skin deterioration (Δskin) (score, 0 or 2), vascular deterioration (Δvasc) (score 0 or 0·5) and deterioration in heart/lung function (ΔH/L) (score 0 or 2); the latter include mRss>14 (score 0 or 1), scleredema (0 or 0·5), digital necrosis (0 or 0·5), arthritis (0 or 0·5), DLCO ≤ 80% of predicted (0 or 0·5), ESR > 30 mm/h (0 or 1·5) and hypocomplementaemia (C3, C4; 0 or 1). A disease activity index of ≥3 was used to define an active disease state 37,38. Therapy was recorded for each patient at the time when blood was drawn.
Ethical issues
Approval for the collection of sera from patients and for the use of their clinical data for research purposes was obtained from the Ethics Committees of the University of Naples and University of Bari. All subjects provided written informed consent to the use of clinical samples and data for research purposes.
Serum samples
Serum samples obtained from the 77 patients with SSc and 34 healthy donors (HD) were aliquoted and stored at −80°C until use.
ELISA for sHLA-G
Quantitative determination of sHLA-G in serum was made by a sandwich assay using the HLA-G ELISA kit (Cusabio Biotech Co. Ltd, Wuhan, China) following the manufacturer's instructions. Briefly, flat-bottomed 96-well plates precoated with HLA-G-specific antibody were incubated with 100 µl/well of serum samples (diluted 1 : 2 in sample dilution buffer) for 2 h at 37°C. Samples were harvested and 100 µl of an appropriate dilution of biotinylated antibody, to antigenic determinants of HLA-G other than the one recognized by the coated antibody, were added to each well and the incubation was prolonged for 1 h at 37°C. Then, wells were washed three times with washing buffer (200 µl/well), incubated with 100 µl/well of horseradish peroxidase (HRP)-conjugated avidin (1 h at 37°C) and washed five times. The colorimetric reaction was developed by incubating wells with 3,3',5,5'-tetramethylbenzidine (TMB)-based substrate solution (90 µl/well) for 20 min at 37° C in the dark. Colour development was stopped by adding 50 µl of ‘stop solution’. Absorbance was read at 450 nm with the Benchmark microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). HLA-G concentrations (ng/ml) in sera were determined on the calibration curve, generated by incubating wells with known concentrations of standard, up to a maximum of 80 ng/ml, and corrected for the dilution factor.
Analysis of the HLA-G gene 14 bp del−/del+ polymorphism
Analysis was performed as described previously 39, with minor modifications. In brief, peripheral blood mononuclear cells (PBMC) were isolated from heparin-treated blood by Ficoll-Hypaque gradient centrifugation and their genomic DNA was extracted, using the QIAamp DNA Mini Kit (Qiagen, Crawley, UK). The HLA-G 14 bp del−/del+ polymorphism in exon 8 at the 3′ UTR of the HLA-G gene was identified by polymerase chain reaction (PCR). The PCR forward and reverse primers were: GE14HLAG-5′-GTGATGGGCTGTTTAAAGTGTCACC-3′ and RHG4-5′-GGAAGGAATGCAGTTCAGCATGA-3′, respectively. PCR amplification was carried out in a total volume of 50 µl containing: 0·2 µl of Taq DNA polymerase, 5 µl of genomic DNA (5 ng/µl), 1 µl of each primer (final concentration 0·2 µM), deoxynucleotide triphosphate (dNTP) mixture (final concentration 0 2 mM), 1·5 µl MgCl2 (final concentration 1·5 mM), 5 µl 10× PCR buffer (Invitrogen Life Technologies, Waltham, MA, USA), using an automated PCR thermal cycler (Eppendorf, Hamburg, Germany). Thermal cycling was performed with an initial incubation step at 94°C for 2 min followed by 24 cycles each at 94°C for 15 s, 44°C for 15 s and 72°C for 30 s, and a final extension at 72°C for 30 s. PCR products were fractionated on a 4% agarose gel containing 1 mg/ml ethidium bromide. The PCR product sizes were 224 or 210 bp, according to the presence or absence of the 14 bp insertion at exon 8. Patients were classified as homozygous for deletion (del+/del+) or homozygous for the presence of the 14 bp insertion at both alleles (del−/del−), or heterozygous (del−/del+).
Statistical analyses
Receiver operating characteristics (ROC) analysis was used to find the best discriminating cut-off for dichotomizing continuous variables 40. This analysis was performed using MedCalc software (version 7.6.0.0), that automatically calculated the best discriminating cut-off.
The Mann–Whitney U-test was used with continuous variables for comparisons between groups, Fisher's exact test to define associations among dichotomized variables, multivariate backward stepwise logistic regression analysis was used to define independent associations between two or more variables and Spearman's analysis was used to find correlations between continuous variables; these statistical tests were performed using spss version 22 for Windows (SPSS, Inc., Chicago, IL, USA). A P-value < 0·05 was considered statistically significant.
For statistical analysis purposes, clinical variables retrieved from the disease severity scale and disease activity index items were dichotomized as normal (score = 0) versus abnormal (score > 0), along with subitems of the disease severity scale, namely ‘lung’ and ‘heart’, that were dichotomized as follows: FVC and DLCO <80% of the predicted value (versus ≥ 80%), ILD presence of fibrosis (ILD) (versus absence), electrocardiogram (ECG) alterations (versus absence) and left ventricular ejection fraction (LVEF) <50% (versus ≥ 50).
Results
Clinical correlates of soluble HLA-G
sHLA-G was measured in sera of 77 patients with SSc and in 34 age- and sex-matched HD. The mean level [± standard error (s.d.)] of sHLA-G in SSc (22·63 ± 19·27) was lower than in HD (30·63 ± 22·06), although with no significant differences between groups (P = 0·141). (Fig. 1a). The analysis of sHLA-G distribution levels in relation to the presence or absence of autoantibody, commonly detected in SSc, revealed no significant differences between ACA+ versus ACA− (Fig. 1b), between anti-Scl70+ versus anti-Scl70 antibody– group (Fig. 1c) and between ACA+ versus anti-Scl70 antibody+ group (Fig. 1d).
Figure 1.
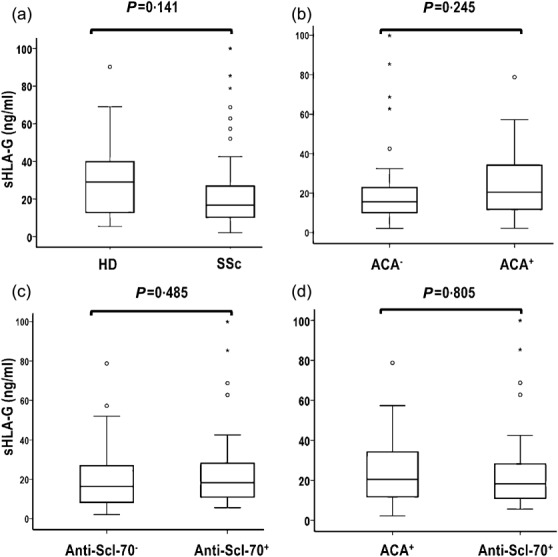
Comparison of soluble human leucocyte antigen (sHLA)-G levels (median, range) of sHLA-G between healthy donors (HD) and patients with systemic sclerosis (SSc) or in distinct autoantibody subsets of SSc. sHLA-G was measured by the enzyme-linked immunosorbent assay (ELISA) kit (Cusabio Biotech), according to the manufacturer's instructions. The proportion of difference of HLA-G levels between groups was evaluated by Mann–Whitney U-test. P < 0·05 was considered significant.
To define any clinical correlates of sHLA-G, this continuous variable was plotted by ROC analysis against dichotomized clinical and serological disease variables. The former were obtained from the 10 items of the disease activity index and nine of the disease severity scale, along with subitems of the disease severity scale ‘lung’ and ‘heart’, all scored positive (versus negative; absence of alterations). The serological variables were dichotomized as positive versus negative according to the presence (versus absence) of ACA and anti-Scl70 antibody. An acceptable ROC curve (P < 0·05) was obtained only with the dichotomization of the SSc group according to the Δ ‘heart/lung’ (i.e. the deterioration of heart/lung conditions as evaluated by the patient over the previous month), in this case using the score 0 as value of the state variable (absence of alterations) (Fig. 2). The best HLA-G cut-off discriminating patients with no change in ‘heart/lung’ conditions (i.e. score 0) (versus those who presented changes, score > 0) was 12·7 ng/ml (AUC = 0·72; P = 0·028; 67% sensitivity; 80% specificity) (Fig. 2). Using this clinical cut-off, HLA-G serum levels were dichotomized as positive (above the cut-off; high HLA-G profile) and negative (below/equal to the cut-off, low HLA-G profile). In the SSc population, 30 patients (39%) were HLA-G-negative and 47 (61%) -positive. Their clinical characteristics are reported in Table1. There were significant differences between the two groups in terms of mRss, items ‘general’ and ‘kidney’ of the Medsger disease severity scale and the disease activity index item Δ ‘heart/lung’, scores being higher in the HLA-G– than HLA-G+ (Table1).
Figure 2.
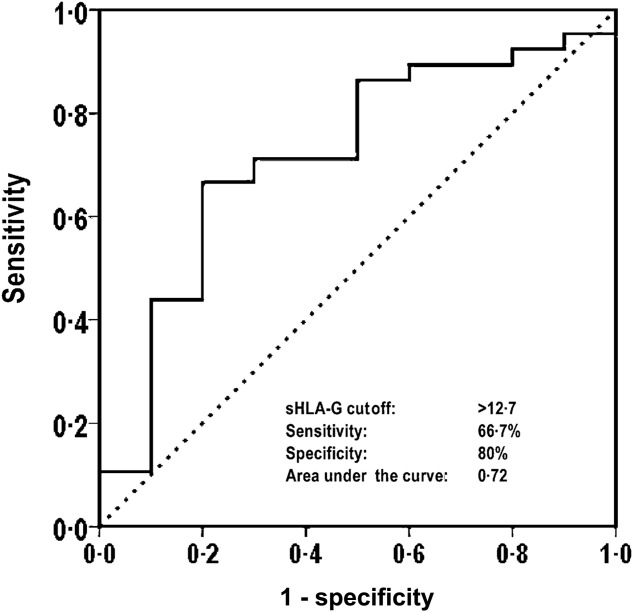
Receiver operating characteristic (ROC) analysis to define the best soluble human leucocyte antigen (sHLA)-G cut-off discriminating patients with a ‘heart/lung’ item score of 0 from those with score > 0.
Table 1.
Clinical characteristics of 77 patients with systemic sclerosis (SSc), dichotomized as human leucocyte antigen (HLA)-G-positive (>12 ng/ml) or -negative (≤12·7). Values are mean (± standard deviation) unless indicated otherwise
| Variable | All patients (N = 77) | sHLA-G negative (n = 30) | sHLA-G positive (n = 47) | P |
|---|---|---|---|---|
| sHLA-G, ng/ml | 22·63 ± 19·27 | 8·34 ± 3·22 | 31·75 ± 19·72 | 0·001* |
| Female, n (%) | 73 (94·8) | 28 (93·3) | 45 (95·7) | 0·641† |
| Age at diagnosis, years | 40·09 ± 14·49 | 37·83 ± 13·52 | 41·49 ± 15·03 | 0·201* |
| Disease duration, years | 14·80 ± 9·90 | 14·45 ± 9·86 | 15·02 ± 10·03 | 0·945* |
| Limited disease, n (%) | 54 (70·1) | 18 (60·0) | 36 (76·60) | 0·134† |
| mRss | 8·80 ± 9·13 | 11·45 ± 9·26 | 7·09 ± 8·72 | 0·032* |
| FVC, % of predicted | 93·93 ± 23·49 | 92·12 ± 23·45 | 95·12 ± 23·70 | 0·701* |
| DLCO, % of predicted | 71·67 ± 21·75 | 70·77 ± 25·32 | 72·17 ± 19·79 | 0·440* |
| sPAP, mm Hg | 31·28 ± 10·76 | 32·17 ± 11·78 | 30·78 ± 10·26 | 0·639* |
| ILD, n (%) | 31 (47·7) | 16 (42·1) | 15 (55·6) | 0·322† |
| sPAP>35 mmHg, n (%) | 16 (25) | 7 (30·4) | 9 (22·0) | 0·551† |
| Disease severity scale (total) | 7·00 ± 3·85 | 7·7 ± 4·44 | 6·55 ± 3·4 | 0·397* |
| Disease severity scale items | ||||
| General | 0·62 ± 0·92 | 1·00 ± 1·23 | 0·38 ± 0·53 | 0·031* |
| Peripheral vascular | 1·61 ± 0·83 | 1·5 ± 0·73 | 1·68 ± 0·89 | 0·447* |
| Skin | 1·13 ± 0·79 | 1·3 ± 0·84 | 1·02 ± 0·74 | 0·109* |
| Joint/tendon | 0·41 ± 1·00 | 0·57 ± 1·13 | 0·32 ± 0·91 | 0·316* |
| Muscle | 0·44 ± 0·68 | 0·47 ± 0·82 | 0·42 ± 0·58 | 0·705* |
| Gastrointestinal tract | 0·96 ± 0·56 | 1·03 ± 0·64 | 0·91 ± 0·51 | 0·472* |
| Lung | 1·46 ± 1·10 | 1·31 ± 1·07 | 1·55 ± 1·12 | 0·329* |
| Heart | 0·28 ± 0·70 | 0·31 ± 0·85 | 0·25 ± 0·61 | 0·813* |
| Kidney | 0·13 ± 0·67 | 0·33 ± 1·06 | 0·00 ± 0·00 | 0·028* |
| Disease activity index (total) | 1·68 ± 1·44 | 2 ± 1·69 | 1·48 ± 1·23 | 0·373* |
| Disease activity index items | ||||
| RSS >14 (0, 1) | 0·25 ± 0·44 | 0·37 ± 0·49 | 0·18 ± 0·39 | 0·067* |
| Scleredema (0, 0·5) | 0·21 ± 0·26 | 0·2 ± 0·25 | 0·22 ± 0·27 | 0·852* |
| Skin (0, 2) | 0·16 ± 0·54 | 0·2 ± 0·61 | 0·13 ± 0·50 | 0·585* |
| Digital necrosis (0, 0·5) | 0·09 ± 0·19 | 0·07 ± 0·17 | 0·11 ± 0·21 | 0·359* |
| Vascular (0, 0·5) | 0·08 ± 0·18 | 0·08 ± 0·19 | 0·08 ± 0·18 | 0·866* |
| Arthritis (0, 0·5) | 0·06 ± 0·16 | 0·05 ± 0·15 | 0·06 ± 0·17 | 0·690* |
| DLCO <80% of predicted (0, 0·5) | 0·30 ± 0·25 | 0·33 ± 0·24 | 0·28 ± 0·25 | 0·442* |
| Heart/lung function (0, 2) | 0·26 ± 0·68 | 0·53 ± 0·90 | 0·09 ± 0·41 | 0·005* |
| ESR >30 mm/h (0, 1·5) | 0·23 ± 0·55 | 0·15 ± 0·46 | 0·29 ± 0·60 | 0·284* |
| Low C3 or C4 (0, 1) | 0·08 ± 0·28 | 0·03 ± 0·18 | 0·12 ± 0·32 | 0·221* |
Mann–Whitney U-test
χ2 test. FVC = forced vital capacity; DLCO = diffusing lung capacity for carbon monoxide; ILD = interstitial lung disease; mRss = modified Rodnan skin score; sPAP = systolic pulmonary arterial pressure; ESR = erythrocyte sedimentation rate.
Because of the limited number of patients in our cohort, the detected clinical associations, general, kidney and Δ ‘heart/lung’, along with active disease, were explored further by Fisher's exact test in the HLA-G-positive and -negative groups, and data were analysed as the frequency of patients with particular symptoms under each item (Fig. 3). The continuous variable mRss was obviously excluded from this analysis. The HLA-G-negative group had a higher percentage of patients with a worse disease severity scale item ‘general’ [P = 0·002; odds ratio (OR) = 0 ·06] (Fig. 3a), active disease, defined by the cut-off value 3 (P = 0·046; OR = 0·34) (Fig. 3b, striped bar), with renal involvement (P = 0·05; OR = 0·36) (Fig. 3c) and (as expected) patients who underwent a deterioration of Δ ‘heart/lung’ function (P = 0.011; OR = 0·125) (Fig. 3d). After statistical correction for multiple comparisons, only ‘general’ and Δ ‘heart/lung’ remained significant. Overall, the data indicate that low levels of HLA-G are associated with a greater presence of worsening of cardiopulmonary manifestations and of the disease severity scale item ‘general’.
Figure 3.
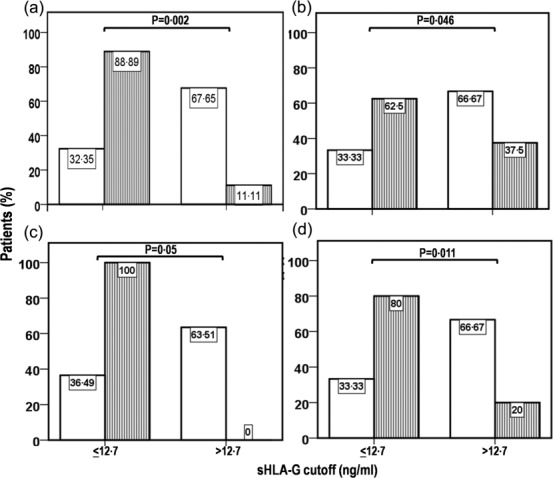
Frequency distribution of SSC disease variables in the human leucocyte antigen (HLA)-G-negative (sHLA-G ≤ 12·7 ng/ml) and positive (> 12·7 ng/ml) groups. (a) Disease severity scale, item ‘general’ score of ≤ 1 (open bar) versus score > 1 (striped bar), (b) non-active disease (open bar) versus active disease (striped bar), (c) disease severity scale, item ‘kidney’ score of 0 (open bar) versus score > 0 (striped bar) and (d) disease activity index, item ‘heart/lung’ score of 0 (open bar) versus score > 0 (striped bar). Active disease was defined as a disease activity index (DAI) ≥ 3·0. Differences between groups were assessed using Fisher's exact text.
A multivariate backward stepwise logistic regression analysis was then run by including dichotomized sHLA-G as dependent variable and mRss, the disease severity scale items ‘general’, and the disease activity index with Δ ‘heart/lung’ as covariates (Table2). The mRss, the ‘general’ disease severity score and the disease activity index item Δ ‘heart/lung’ were retained in the model with a statistically significant P-value: positivity for sHLA-G was associated with a low score of mRss (P = 0·020), ‘general’ (P = 0·040) and of the disease activity index item Δ ‘heart/lung’ (P = 0·005).
Table 2.
Multivariate backward stepwise logistic regression analysis to detect independent associations of soluble human leucocyte antigen (HLA)-G levels and clinical parameters (continuous and dichotomized) in 77 systemic sclerosis (SSc) patients
| Variable | B coefficient | s.e. | P-value* | Exp(B) |
|---|---|---|---|---|
| mRss | −0·104 | 0·045 | 0·020 | 0·902 |
| DSS item ‘general’ | −0·920 | 0·447 | 0·040 | 0·400 |
| DAI item ‘heart/lung’ | −1·817 | 0·651 | 0·005 | 0·162 |
P < 0·05 was considered statistically significant. DAI = disease activity index; DSS = disease severity scale; mRss = modified Rodnan skin score; s.e. = standard error.
To test the hypothesis that mRss and Δ ‘heart/lung’ were not influenced by disease duration and type (limited versus diffuse) in predicting high or low sHLA-G, logistic regression analysis was run by including dichotomized sHLA-G as dependent variable and mRss, Δ ‘heart/lung’ along with disease duration, and type (limited versus diffuse) as covariates. RSS (B = −0·078, P = 0·024), Δ ‘heart/lung’ (B = −1·665, P = 0·006) remained included in the model as independent variables.
Finally, given the immunomodulatory role of HLA-G, its levels were also analysed in relation to inflammatory variables, namely ESR and CRP. Spearman's correlation analysis showed that neither ESR (P = 0·346) nor CRP (P = 0·520) were correlated with sHLA-G.
HLA-G 14 bp insertion and/or deletion polymorphism distribution
Genotype analysis of HLA-G for the presence (del−) and/or absence (del+) of the 14 bp insertion at the genomic level was performed in 30 randomly selected patients with SSc and in 26 HD (representative results are shown in Fig. 4a). None of the individuals examined had the del−/del− phenotype and there was an equal distribution of the remaining two polymorphisms in the two groups (Fisher's exact test SSc versus HD: P = 1·0) (Table3). The Mann–Whitney U-test indicated no significant difference in the levels of sHLA-G between the del+/del− and del+/del+ genotype in each of the two groups examined, suggesting that genotype does not influence sHLA-G levels (Table3).
Figure 4.
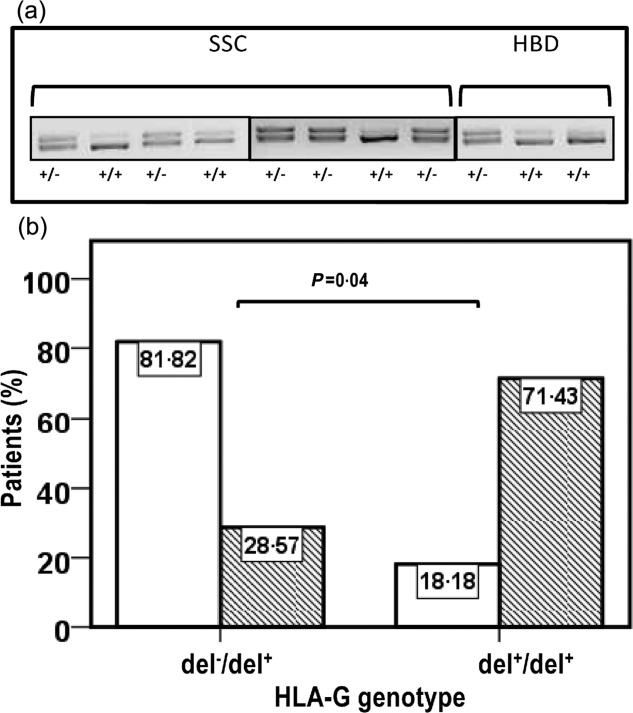
(a) Analysis of human leucocyte antigen (HLA)-G 14 base pairs (bp) polymorphisms and (b) association of the del+/del+ genotype to scleredema. (a) Polymerase chain reaction (PCR) products of exon 8 at the 3′ untranslated region (UTR) of the HLA-G gene in patients with systemic sclerosis (SSc) and healthy donors HD). Genomic DNA was extracted from heparin-treated blood using a QIAamp DNA Mini Kit (Quiagen, Crawley, UK). Exon 8 at the 3′ UTR of the HLA-G gene of each sample was amplified by polymerase chain reaction (PCR) using appropriate primers (see Materials and methods section). Then, PCR products were fractionated on a 4% agarose gel containing 1 mg/ml of ethidium bromide. The PCR product sizes were 224 or 210 bp, according to the presence or absence of the 14-bp insertion at exon 8. (b) Detection of a higher frequency of disease activity index, item scleredema score > 0 (hatched bar) in the homozygous SSc group (del+/del+) than the heterozygous group (del−/del+) for the HLA-G 14 bp polymorphisms. Differences between groups were assessed using Fisher's exact text. P < 0·05 was considered significant.
Table 3.
soluble human leucocyte antigen (HLA)-G levels in relation to the presence (del−) or absence (del+) of the 14 base pairs (bp) insertion in patients with systemic sclerosis (SSc) and in healthy donors (HD)
| Subjects | HLA-G genotype* | P† | P‡ | |||||
|---|---|---|---|---|---|---|---|---|
| Del−/del+ and del+/del+ | Del−/del+ | Del+/del+ | ||||||
| Patient no. (%) | sHLA-G, mean ± s.d. | sHLA-G, mean ± s.d. | Patient, no. (%) | sHLA-G, mean ± s.d. | Patient, no. (%) | |||
| SSc | 30 (100) | 17·4 ± 21·4 | 14·3 ± 10·4 | 18 (60·0) | 22·1 ± 32·1 | 12 (40·0) | 0·97 | |
| p=1.0 | ||||||||
| HD | 26 (100) | 34·4 ± 21·8 | 33·9 ± 22·6 | 15 (57·7) | 35·2 ± 21·7 | 11 (42·3) | 0·77 | |
Exon 8 at the 3′ untranslated region (UTR) of HLA-G gene was amplified by polymerase chain reaction (PCR), using appropriate primers and fractionated in 4% agarose gel. The PCR product sizes were 224 or 210 bp, according to the presence (del−) or absence (del+) of the 14 bp insertion. Patients were classified as heterozygous (del−/del+), homozygous for deletion (del+/del+) or homozygous for the presence of the insertion at the level of both alleles (del−/del−).
Differences of sHLA-G median concentrations between del−/del+ and del+/del+ patients in SSc and HD were evaluated by Mann–Whitney U-test. P < 0·05 was considered statistically significant.
Fisher's exact test to define a statistically different distribution of HLA-G genotypes del−/del+ and del+/del+ between the SSc and HD groups. P < 0·05 was considered statistically significant; s.d. = standard deviation.
Based on the previously detected association between HLA-G skin expression and SSc skin lesions 28, clinical correlates of the HLA-G gene 14 bp polymorphism were explored by Fisher's exact test in the SSc group, using the dichotomized clinical variable scleredema. As shown in Fig. 4b, there was a statistically higher percentage of patients with scleredema in the del+/+ than the del−/+ group (71·4 versus 28·5%; Fisher's exact test, P = 0·04, OR = 11·25).
Influence of therapy on sHLA-G levels
At the time of evaluation patients were in treatment with low-dose steroids (60·5%), azathioprine (10·5%), cyclophosphamide (13·2), methotrexate (8%), calcium channel blockers (70%), endothelin receptor antagonist (9·2%) or prostacyclin analogues (47·4%). One patient was taking rituximab and one mycophenolate mofetil. There were no significant differences in sHLA-G levels between groups dichotomized on the basis of whether or not they were taking steroids (P = 0·081), azathioprine (P = 0·146), cyclophosphamide (P = 0·420), methotrexate (P = 0·386), calcium channel blockers (P = 0·728), endothelin receptor antagonist (P = 0·308) and prostacyclin analogues (P = 0·479). Fisher's exact test showed no significant association of high/low sHLA-G with the administration of steroids (P = 0·052), azathioprine (P = 0·703), cyclophosphamide (P = 0·167), methotrexate (P = 0·670), calcium channel blockers (P = 1·00), endothelin receptor antagonist (P = 0·417) or prostacyclin analogues (P = 0·347). Overall, the data suggest that the ongoing treatment had no influence on sHLA-G levels.
Discussion
The present investigation has shown, for the first time, that levels of sHLA-G are lower in SSc than in HD, although the difference was not statistically significant. Moreover, within the SSc cohort, HLA-G serum expression or the 14 bp del−/del+ polymorphism at exon 8 is associated with mRss, disease severity scale items general or disease activity, particularly for the heart/lung, and scleredema items.
The detection of lower levels of sHLA-G in SSc, compared to HD, parallels similar observations in patients with rheumatoid arthritis 22, juvenile idiopathic arthritis 41 and SLE 42. Overall, these results are at variance with those obtained by Rosado et al. 24, who found higher levels of sHLA-G in SLE patients than in HD. The discrepancy in the results may reflect different states of disease activity at the time of HLA-G evaluation. This is supported in our study by the detection of a significantly higher percentage of patients with a worse disease severity scale item ‘general’, mRss, and deteriorations of heart/lung function in the HLA-G negative group compared to the group with a high HLA-G profile.
The association of low sHLA-G expression and high disease activity is not unique to SSc, as similar associations have been reported in patients with rheumatoid arthritis 23, juvenile idiopathic arthritis 41, ankylosing spondylitis 25 and SLE 42, albeit with some conflicting results 22,24.
The association of low sHLA-G serum expression with a greater extent of skin involvement (as indicated by logistic regression analysis) parallels similar observations by Wastowski et al. 28 in a Brazilian cohort of patients with systemic sclerosis. These investigators found an association between HLA-G skin hyperexpression with a reduced risk of developing cutaneous ulcers, telangiectasia and polyarthritis, and a better survival 28. The relationship of HLA-G and skin involvement is supported by HLA-G 14 bp polymorphism analysis, showing a higher percentage of patients who scored positive for scleredema in the del+/del+ than the del−/del+ group. Whether this association is the result of a lower HLA-G skin expression in del+/del+ group compared to the del−/del+ (or del−/del−) group remains to be determined.
The apparent discrepancy between Δ ‘heart/lung’ (significantly different between the HLA-G+ and -negative group), on one hand, and LFEV, FVC, DLCO and ILD (equally distributed between groups) on the other hand, reflects differences in the significance of Δ ‘heart/lung’ compared to other clinical variables. In fact, while the former is based on patients’ self-evaluation of the deterioration of cardiopulmonary manifestations (based mainly on the severity of effort dyspnoea), LFEV, FVC, DLCO and ILD record the degree of function deterioration not strictly related to symptoms. This conclusion was corroborated by the absence of a statistical association of these clinical variables with HLA-G after Fisher's exact test (data not shown).
A significantly higher number of patients with active disease was recorded in the group subset with a low HLA-G profile, as indicated by Fisher's exact test. However, this association most probably reflects the association of the Δ ‘heart/lung’ item of the disease activity index, as indicated by logistic regression analysis, showing that only Δ ‘heart/lung’ was retained in the model with a statistically significant P-value (P = 0·005).
The reason for the lower levels of sHLA-G and higher score for some items of the severity scale (general) or disease activity index (particularly Δ ‘heart/lung’) is unknown. It is unlikely that the levels could be dependent upon the HLA-G 14 bp polymorphism, as sHLA-G was not statistically different in SSc between the del−/del+ and del+/del+ groups.
An influence on sHLA-G levels of the ongoing therapy is also unlikely, as no association of high/low sHLA-G was found with any of the treatments, despite previous investigations indicating that in-vitro methotrexate and steroid incubation of peripheral blood mononuclear and trophoblast cells, respectively, influenced sHLA-G levels 43,44.
Alternatively, SSc patients with a high disease activity could be low producers of sHLA-G because of a decreased shedding of the molecule from the cell surface. This possibility is supported by previous findings showing, in vitro, that cellular NO can nitrate HLA-G at cellular level, making this molecule more susceptible to proteolytic cleavage by membrane metalloproteinases 9. If this is the case, then low levels of serum HLA-G may reflect low levels of cellular NO, which has been considered one of the main factors triggering vasoconstriction and favouring vasculopathy, from Raynaud's phenomenon to fully established SSc (review in 3). In this context, it remains to be evaluated whether it is the disease activation state that modulates the serum expression or, alternatively, if low HLA-G expression can set the conditions for disease activation, due to a lack of tolerogenic control by this molecule. Whatever the conclusion, the inverse relationships of HLA-G with mRss, the disease severity scale item ‘general’ and deterioration of heart/lung function indicate that this molecule can have modulatory effects on the disease, thus behaving as an anti-inflammatory molecule.
Caution must be exercised in interpreting these findings. As this is, to our knowledge, the first explorative two-centre study on sHLA-G in SSc, prospective studies need to be carried out in a larger number of patients, possibly of multi-ethnic origin, focusing on the HLA-G clinical correlates highlighted here. In this context, it will also be of interest to assess whether HLA-G may be a predictive marker of the clinical course, either in very early stages of the SSc, along with autoantibody and the capillaroscopy pattern 45, or in fully established disease.
Acknowledgments
The authors are grateful to Mr Vito Iacovizzi for his excellent secretarial assistance. They thank Mary V. Pragnell BA for language assistance. This work was supported by a grant from the ‘Italian Group against Systemic Sclerosis’, GILS.
Author contributions
F. P. and E. F. conceived and designed the study; F. P. and E. F. drafted the manuscript. G. V. and F. P. participated in critically revising the manuscript for important intellectual content. G. V., S. V., F. P. and M. P. undertook recruitment of patients and collection of clinical data. E. F., I. E. F. and C. V. carried out the assays and acquired the data. I. E. F., S. V. and C. V. participated in the analysis and interpretation of data. M. P. and F. P. performed statistical analysis. All authors read, revised and approved the final version of the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
- Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy S, Kong J, Cheema GS, Keen CL, Wick G, Gershwin ME. The immunobiology of systemic sclerosis. Semin Arthritis Rheum. 2008;38:132–60. doi: 10.1016/j.semarthrit.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Prete M, Fatone MC, Favoino E, Perosa F. Raynaud's phenomenon: from molecular pathogenesis to therapy. Autoimmun Rev. 2014;13:655–67. doi: 10.1016/j.autrev.2013.12.001. [DOI] [PubMed] [Google Scholar]
- De Palma R, Del Galdo F, Lupoli S, Altucci P, Abbate G, Valentini G. Peripheral T lymphocytes from patients with early systemic sclerosis co-cultured with autologous fibroblasts undergo an oligoclonal expansion similar to that occurring in the skin. Clin Exp Immunol. 2006;144:169–76. doi: 10.1111/j.1365-2249.2006.03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven K, Pfeiffer K, Skrablin S. HLA-G polymorphisms and molecule function–questions and more questions–a review. Placenta. 2000;21(Suppl. A):S86–92. doi: 10.1053/plac.1999.0515. [DOI] [PubMed] [Google Scholar]
- Kirszenbaum M, Moreau P, Gluckman E, Dausset J, Carosella E. An alternatively spliced form of HLA-G mRNA in human trophoblasts and evidence for the presence of HLA-G transcript in adult lymphocytes. Proc Natl Acad Sci USA. 1994;91:4209–13. doi: 10.1073/pnas.91.10.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perosa F, Prete M, Luccarelli G, Favoino B, Ferrone S, Dammacco F. Serum levels of beta-2-microglobulin-free heavy chain of HLA class I antigen in healthy individuals: relationship to their class I allotype. Hum Immunol. 1999;60:1058–66. doi: 10.1016/s0198-8859(99)00081-6. [DOI] [PubMed] [Google Scholar]
- Park GM, Lee S, Park Betal. Soluble HLA-G generated by proteolytic shedding inhibits NK-mediated cell lysis. Biochem Biophys Res Commun. 2004;313:606–11. doi: 10.1016/j.bbrc.2003.11.153. [DOI] [PubMed] [Google Scholar]
- Diaz-Lagares A, Alegre E, LeMaoult J, Carosella ED, Gonzalez A. Nitric oxide produces HLA-G nitration and induces metalloprotease-dependent shedding creating a tolerogenic milieu. Immunology. 2009;126:436–45. doi: 10.1111/j.1365-2567.2008.02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Zalatan J, McMaster M, et al. The class I HLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen HLA-G. Diabetes. 2006;55:1214–22. doi: 10.2337/db05-0731. [DOI] [PubMed] [Google Scholar]
- Menier C, Rabreau M, Challier JC, Le DM, Carosella ED, Rouas-Freiss N. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood. 2004;104:3153–60. doi: 10.1182/blood-2004-03-0809. [DOI] [PubMed] [Google Scholar]
- Le Bouteiller P, Solier C, Proll J, guerre-Girr M, Fournel S, Lenfant F. Placental HLA-G protein expression in vivo: where and what for? Hum Reprod Update. 1999;5:223–33. doi: 10.1093/humupd/5.3.223. [DOI] [PubMed] [Google Scholar]
- Riteau B, Menier C, Khalil-Daher I, et al. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol. 1999;43:203–11. doi: 10.1016/s0165-0378(99)00034-0. [DOI] [PubMed] [Google Scholar]
- Le Rond S, Azema C, Krawice-Radanne I, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/regulatory T cells. J Immunol. 2006;176:3266–76. doi: 10.4049/jimmunol.176.5.3266. [DOI] [PubMed] [Google Scholar]
- Feger U, Tolosa E, Huang YH, et al. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110:568–77. doi: 10.1182/blood-2006-11-057125. [DOI] [PubMed] [Google Scholar]
- Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc Natl Acad Sci USA. 2008;105:8357–62. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creput C, Durrbach A, Menier C, et al. Human leukocyte antigen-G (HLA-G) expression in biliary epithelial cells is associated with allograft acceptance in liver–kidney transplantation. J Hepatol. 2003;39:587–94. doi: 10.1016/s0168-8278(03)00354-4. [DOI] [PubMed] [Google Scholar]
- Qiu J, Terasaki PI, Miller J, Mizutani K, Cai J, Carosella ED. Soluble HLA-G expression and renal graft acceptance. Am J Transplant. 2006;6:2152–6. doi: 10.1111/j.1600-6143.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- Bukur J, Rebmann V, Grosse-Wilde H, et al. Functional role of human leukocyte antigen-G up-regulation in renal cell carcinoma. Cancer Res. 2003;63:4107–11. [PubMed] [Google Scholar]
- Nuckel H, Rebmann V, Durig J, Duhrsen U, Grosse-Wilde H. HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood. 2005;105:1694–8. doi: 10.1182/blood-2004-08-3335. [DOI] [PubMed] [Google Scholar]
- Brenol CV, Veit TD, Chies JA, Xavier RM. The role of the HLA-G gene and molecule on the clinical expression of rheumatologic diseases. Rev Bras Reumatol. 2012;52:82–91. [PubMed] [Google Scholar]
- Verbruggen LA, Rebmann V, Demanet C, De CS, Grosse-Wilde H. Soluble HLA-G in rheumatoid arthritis. Hum Immunol. 2006;67:561–7. doi: 10.1016/j.humimm.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Farina I, Bortolotti D, et al. HLA-G may predict the disease course in patients with early rheumatoid arthritis. Hum Immunol. 2013;74:425–32. doi: 10.1016/j.humimm.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Rosado S, Perez-Chacon G, Mellor-Pita S, et al. Expression of human leukocyte antigen-G in systemic lupus erythematosus. Hum Immunol. 2008;69:9–15. doi: 10.1016/j.humimm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang JB, Wang ZY, Chen J, Wu XD, Zhou B, Yie SM. The expression of human leukocyte antigen G (HLA-G) is associated with sacroiliitis stages of ankylosing spondylitis. Immunol Lett. 2013;152:121–5. doi: 10.1016/j.imlet.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Park KS, Park JS, Nam JH, Bang D, Sohn S, Lee ES. HLA-E*0101 and HLA-G*010101 reduce the risk of Behcet's disease. Tissue Antigens. 2007;69:139–44. doi: 10.1111/j.1399-0039.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Hong SJ, Hong YM, et al. Genetic variants in the HLA-G region are associated with Kawasaki disease. Hum Immunol. 2008;69:867–71. doi: 10.1016/j.humimm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Wastowski IJ, Sampaio-Barros PD, Amstalden EM, et al. HLA-G expression in the skin of patients with systemic sclerosis. J Rheumatol. 2009;36:1230–4. doi: 10.3899/jrheum.080552. [DOI] [PubMed] [Google Scholar]
- 29; Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- Walker UA, Tyndall A, Czirjak L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research Group database. Ann Rheum Dis. 2007;66:754–63. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- Clements P, Lachenbruch P, Siebold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- Steen VD, Owens GR, Fino GJ, Rodnan GP, Medsger TA., Jr Pulmonary involvement in systemic sclerosis (scleroderma) Arthritis Rheum. 1985;28:759–67. doi: 10.1002/art.1780280706. [DOI] [PubMed] [Google Scholar]
- Hsu VM, Moreyra AE, Wilson AC, et al. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol. 2008;35:458–65. [PubMed] [Google Scholar]
- Medsger TA, Jr, Bombardieri S, Czirjak L, Scorza R, Della RA, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol. 2003;21:S42–6. [PubMed] [Google Scholar]
- Valentini G, Della RA, Bombardieri S, et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis. 2001;60:592–8. doi: 10.1136/ard.60.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini G, Bencivelli W, Bombardieri S, et al. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann Rheum Dis. 2003;62:901–3. doi: 10.1136/ard.62.9.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini G, Medsger TA, Jr, Silman AJ, Bombardieri S. Conclusion and identification of the core set of variables to be used in clinical investigations. Clin Exp Rheumatol. 2003;21:S47–8. [PubMed] [Google Scholar]
- Boiocchi C, Bozzini S, Zorzetto M, Pelissero G, Cuccia M, Falcone C. Association between two polymorphisms in the HLA-G gene and angiographic coronary artery disease. Mol Med Rep. 2012;5:1141–5. doi: 10.3892/mmr.2012.825. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, De Rosa SC, Dubs JG, et al. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci USA. 1997;94:1967–72. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigione I, Penco F, Martini A, Gattorno M, Pistoia V, Morandi F. HLA-G and HLA-E in patients with juvenile idiopathic arthritis. Rheumatology (Oxf) 2011;50:966–72. doi: 10.1093/rheumatology/keq418. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Hviid TV, Govoni M, et al. HLA-G genotype and HLA-G expression in systemic lupus erythematosus: HLA-G as a putative susceptibility gene in systemic lupus erythematosus. Tissue Antigens. 2008;71:520–9. doi: 10.1111/j.1399-0039.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Rubini M, Govoni M, et al. HLA-G 14-bp polymorphism regulates the methotrexate response in rheumatoid arthritis. Pharmacogenet Genomics. 2006;16:615–23. doi: 10.1097/01.fpc.0000230115.41828.3a. [DOI] [PubMed] [Google Scholar]
- Akhter A, Faridi RM, Das V, Pandey A, Naik S, Agrawal S. In vitro up-regulation of HLA-G using dexamethasone and hydrocortisone in first-trimester trophoblast cells of women experiencing recurrent miscarriage. Tissue Antigens. 2012;80:126–35. doi: 10.1111/j.1399-0039.2012.01884.x. [DOI] [PubMed] [Google Scholar]
- Valentini G, Marcoccia A, Cuomo G, et al. Early systemic sclerosis: analysis of the disease course in patients with marker autoantibody and/or capillaroscopic positivity. Arthritis Care Res (Hoboken) 2014;66:1520–7. doi: 10.1002/acr.22304. [DOI] [PubMed] [Google Scholar]


