Abstract
Our aims were to identify the differential expression of microRNA (miR)-155, as well as to explore the possible regulatory effects of miR-155 on the differentiation and function of T helper type 17 (Th17) cells in atopic dermatitis (AD). The Th17 cell percentage and expression levels of miR-155, retinoic acid-related orphan receptor (ROR)γt, interleukin (IL)-17 and suppressor of cytokine signalling-1 (SOCS1) in peripheral CD4+ T cells, plasma and skin specimens were detected and compared in AD patients and healthy subjects. A miR-155 mimic and an inhibitor were transfected separately into AD CD4+ T cells to confirm the in-vivo data. The Th17 cell percentage, miR-155 expression, RORγt mRNA expression, IL-17 mRNA expression and plasma concentration were increased significantly in AD patients compared with healthy subjects. Conversely, SOCS1 mRNA expression and plasma concentration were decreased significantly. Similar results were detected in cultured CD4+ T cells transfected with the miR-155 mimic compared with a miR-155 inhibitor or a negative control. Additionally, there was a sequential decrease in miR-155 expression, as well as RORγt and IL-17 mRNA expression, but an increase in SOCS1 mRNA expression, from AD lesional skin and perilesional skin to normal skin. Positive correlations were found between miR-155 expression and AD severity, Th17 cell percentage, RORγt mRNA expression and IL-17 mRNA expression and plasma concentration, while negative correlations were observed between miR-155 expression and SOCS1 mRNA expression and plasma concentration in AD peripheral circulation and skin lesions. In conclusion, miR-155 is over-expressed and may be involved in AD pathogenesis by modulating the differentiation and function of Th17 cells.
Keywords: atopic dermatitis, IL-7, miR-155, suppressor of cytokine signalling-1, Th17 cells
Introduction
Atopic dermatitis (AD) is a common, chronic, relapsing, inflammatory skin disease which usually occurs in early infancy but can also start or persist in adulthood. The pathogenesis of AD has been attributed to a complex interaction including environmental factors, host susceptibility genes, altered skin barrier function and a deregulated immune system 1. MicroRNAs (miRNAs, miRs) are short, endogenous non-coding RNAs that suppress the expression of protein-coding genes by translational repression, mRNA destabilization or the combination of these two mechanisms. MiRNAs play essential regulatory roles in immune homeostasis, the development of lymphoid lineage and inflammatory reactions 2–5. Differential expression of miRNAs has been shown in several immunological and inflammatory disorders. MiR-155 is among the first miRNAs linked to inflammation and immunity by virtue of its potent up-regulation in multiple immune cell lineages, including T lymphocytes, B lymphocytes, mast cells, fibroblasts, macrophages and dendritic cells 3,6–8, several of which are implicated in the pathogenesis of chronic skin inflammation. A wide variety of immunologically relevant target genes of miR-155 have been reported, among which suppressor of cytokine signalling-1 (SOCS1) can be targeted directly by miR-155 9. The interleukin (IL)-17-producing T helper type 17 (Th17) cell is a recently identified CD4+ T helper cell subset, which plays important roles in regulating the immune system and is involved in various autoimmune and inflammatory diseases, including AD 10–14. The impact of Th17 cells was first made evident in mice, where over-expression of IL-17 led to increased granulopoiesis in vivo, while inhibition of IL-17 ameliorated several autoimmune disorders 15–17. The regulatory role of miR-155 on Th17 cell differentiation has also been demonstrated in animal models. MiR-155-deficient mice fail to develop experimental autoimmune encephalomyelitis (EAE), and miR-155 knock-out mice are resistant to antigen-specific Th17 cell responses in a collagen-induced arthritis model 18,19. Additionally, accumulating evidence suggests that miR-155 can enhance murine Th17 cell differentiation and IL-17 production by targeting SOCS1 20. MiRNAs function through binding to the 3′ untranslated regions (UTRs) of target mRNAs carrying complementary sites. In humans, SOCS1 is also the predicted target gene of miR-155 according to bioinformatic analysis, and recent studies using the TaqMan low-density array (TLDA) have demonstrated that miR-155 is over-expressed in AD lesions and expressed predominantly in infiltrating immune cells 21. In the present study, we aimed to identify the differential expression of miR-155 in the peripheral circulation and lesional skin from patients with AD, and to further explore the possible regulatory effects of miR-155 on the differentiation and function of Th17 cells in AD patients.
Materials and methods
Subjects and skin samples
Patients (n = 33) with acute AD (18 males and 15 females, aged 6–18 years) were included into the study. The diagnosis of AD was confirmed according to the criteria defined by Rajka 22, and the disease severity was evaluated using the SCORing Atopic Dermatitis (SCORAD) index 23. Prior to entering the study, none of the AD patients had been treated with systemic glucocorticoids, immunosuppressive agents or desensitization therapy within the past 6 months or local glucocorticoids or calcineurin inhibitors within the past week. None had received a vaccination within the past month, had infections within the past 2 weeks or experienced complications associated with significant abnormalities in other organs, malignant neoplasms, psychiatric disorders, autoimmune diseases or other skin diseases. Normal healthy people (n = 31, 17 males and 14 females, aged 6–17 years) who did not have the aforementioned abnormalities or atopic disorders to date served as the control group. Samples of skin tissue were acquired from 15 AD patients (6–8-mm-long excisional sample, including the papule lesion and perilesional skin) and samples of age-matched normal skin were obtained from 15 healthy subjects undertaking aesthetic or reconstructive surgery and reporting no history of atopic disorders or inflammatory skin diseases. The study was guided by the Declaration of Helsinki and approved by the Research Ethics Committee of Binzhou Medical University Hospital. Informed consent was obtained from all individuals and/or their parents.
Sample preparation
Peripheral venous blood (8 ml) was collected in the morning between 8:00 and 8:30 a.m. following an overnight fast. Plasma was isolated within 30 min after blood collection and stored at −80 °C. Peripheral blood mononuclear cells (PBMCs) were collected by Ficoll-Hypaque density gradient centrifugation, and untouched CD4+ T cells were isolated from PBMCs using immunomagnetic beads, according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA, USA). The purity of the CD4+ T cells was 96·6 ± 1·1% as assessed by flow cytometric analysis, and the cell vitality was 96·7 ± 1·7% as determined by trypan blue staining. The skin biopsy specimens were separated along the margin of each papule into lesional and perilesional skin, and then frozen in liquid nitrogen for further analysis.
MiRNA mimic or inhibitor transfection
CD4+ T cells were cultured in 12-well plates at 70–80% confluence prior to transfection with Roswell Park Memorial Institute (RPMI)-1640 medium (without serum or antibiotics) containing 100 nM of the miR-155 mimic, inhibitor or negative control (GenePharma, Shanghai, China) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) at 37 °C under a 5% CO2 environment for 5 h, according to the manufacturer's instructions.
CD4+ T cell activation and polarization
CD4+ T cells were activated by 5 μg/ml of plate-bound CD3 monoclonal antibody (mAb) and 2 μg/ml of soluble CD28 mAb, and then cultured for 96 h in the medium with 10 ng/ml recombinant IL (rIL)-1-β, 50 ng/ml rIL-6, 20 ng/ml rIL-23, 10 μg/ml anti-interferon (IFN)-γ antibody and 10 μg/ml anti-IL-4 antibody (eBioscience, San Diego, CA, USA).
Flow cytometric analysis of Th17 cell percentage
CD4+ T cells were suspended in RPMI-1640 with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 1% 1 M Hepes buffer (Sigma, St Louis, MO, USA), and stimulated with 25 ng/ml phorbol 12-myristate 13-acetate and 1 μg/ml ionomycin (Sigma) in the presence of 2 mmol/ml GolgiStop (BD Biosciences, San Jose, CA, USA) at 37°C under a 5% CO2 environment for 5 h. After harvesting and washing with phosphate-buffered saline, cells were incubated with fluorescein isothiocyanate-labelled anti-CD4 antibody (eBioscience) for 30 min at 4 °C in the dark. After fixing and permeabilization, the cell samples were then stained with phycoerythrin-labelled anti-IL-17 antibody (eBioscience) for 30 min at 4 °C in the dark and subjected to fluorescence activated cell sorter (FACS)Canto flow cytometry (BD Biosciences) for further analysis using WinMDI version 2·9 software. Isotype-matched immunoglobulin G was used as a control in all procedures.
Real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) analysis of miR-155 relative expression level, as well as SOCS1, retinoic acid-related orphan receptor (ROR)γt and IL-17 mRNA relative expression levels
Total RNA was isolated from CD4+ T cells and liquid nitrogen-frozen skin samples using Trizol reagent (Invitrogen), following the manufacturer's protocol. The quality of RNA samples was assessed by inspecting the 28S and 18S bands after 1·5% agarose gel electrophoresis; a 260/280 absorbance ratio was between 1·9 and 2·0. M-MLV reverse transcriptase (Invitrogen) was also used for complementary DNA synthesis with a miR-155 stem-loop primer (5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCCCTAT-3′) and the reverse primer of the internal reference gene (U6) and oligo (dT) primers. The relative expression levels of miR-155, as well as SOCS1, RORγt and IL-17 mRNAs were quantified by SYBR green-based real-time polymerase chain reaction (PCR) master mix (Toyobo, Osaka, Japan) with the given primers (Supporting information, Table S1). The amplification was performed on a Rotor-Gene 3000 (Corbett Research, Sydney, Australia) and analysed using the Rotor-Gene real-time analysis software version 6·0 by the two-standard curve method. U6 and β-actin were used as endogenous reference genes. The expression levels of genes of interest from the samples were presented as the fold change relative to a calibrator (baseline) sample, which was either from healthy subjects, normal skin samples or negative controls depending on the nature of the transfected cells being examined. The fold change of each sample was used for statistical analysis, and was calculated using the following formula: 2–[Ct(interest gene)–Ct(endogenous reference gene) of sample]–[Ct(interest gene)–Ct(endogenous reference gene) of calibrate].
Enzyme-linked immunosorbent assay (ELISA) for SOCS1 and IL-17
The concentrations of SOCS1 and IL-17 in plasma as well as the concentration of IL-17 in cell-free supernatant from the aforementioned cultured CD4+ T cells were measured by ELISA (USCN, Wuhan, China and R&D Systems, Minneapolis, MN, USA). All samples were measured in triplicate.
Western analysis for SOCS1
To measure SOCS1 protein secretion in AD CD4+ T cells treated with the miR-155 mimic, inhibitor or a negative control, cells were prepared using lysis buffer with ethylendiamine tetraacetic acid (EDTA)-free complete protease inhibitors (Roche, Mannheim, Germany). Total protein was extracted and subjected to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Following transfer to polyvinylidene difluoride membranes, the membranes were blocked with 5% milk powder in Tris-buffered saline, probed with an anti-SOCS1 primary antibody (at 1 : 1000 dilution), and detected with a secondary antibody (Millipore, Billerica, MA, USA). Normalization was performed by blotting the same membranes with anti-β-actin antibody. ImageJ software was used to quantify the SOCS1 protein expression levels.
Statistical analysis
According to the results of a normal distribution test (Shapiro–Wilk test), data were expressed as mean ± standard deviation, and independent-samples t-tests, one-way analysis of variance tests (followed by Holm–Šídák tests) and Pearson's correlation coefficients were used to analyse the data, with the level of significance set at P < 0·05.
Results
Th17 cell percentage in peripheral CD4+ T cells
Representative images showing the Th17 cell percentage (CD4+IL17+/CD4+ T cells%) are presented in Fig. 1. The circulating Th17 cell percentage was significantly higher in AD patients compared with healthy subjects (1·43 ± 0·31% versus 0·51 ± 0·12%, t = 15·828, P < 0·01).
Figure 1.
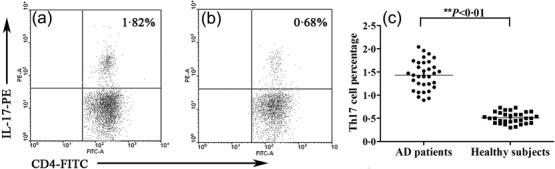
Representative images showing the T helper type 17 (Th17) cell percentage (CD4+IL17+/CD4+ T cells %). (a) A 16-year-old male atopic dermatitis (AD) patient; (b) a 17-year-old male healthy subject; (c) the Th17 cell percentage in peripheral CD4+ T cells in AD patients (n = 33) versus healthy subjects (n = 31).
Relative expression levels of miR-155, as well as SOCS1, RORγt and IL-17 mRNAs, in peripheral CD4+ T cells and skin specimens
The melting curves of miR-155, SOCS1, RORγt and IL-17 all showed a single peak, and the PCR products were specific. There was a distinct increase in the relative expression levels of miR-155 (5·46 ± 1·93 versus 1·81 ± 0·47, t = 10·529), RORγt mRNA (5·25 ± 1·22 versus 1·64 ± 0·50, t = 15·678) and IL-17 mRNA (7·10 ± 1·83 versus 1·69 ± 0·42, t = 16·506) in AD peripheral CD4+ T cells compared with healthy subjects (all P < 0·01); however, the relative expression level of SOCS1 mRNA was clearly decreased in AD patients compared with healthy subjects (0·76 ± 0·16 versus 1·71 ± 0·59, t = −8·673, P < 0·01, Fig. 2). There were significant differences in the relative expression of miR-155 (F = 42·327, P < 0·01), RORγt mRNA (F = 17·128, P < 0·01), IL-17 mRNA (F = 37·384, P < 0·01) and SOCS1 mRNA (F = 41·428, P < 0·01) among the three skin samples of AD lesional skin, perilesional skin and normal skin. Further comparisons between each two groups of samples revealed sequentially decreased expression of miR-155, and RORγt and IL-17 mRNAs from AD lesional skin and perilesional skin to normal skin (all P < 0·05). Conversely, a sequential increase was found in SOCS1 mRNA expression (all P < 0·05, Fig. 3).
Figure 2.
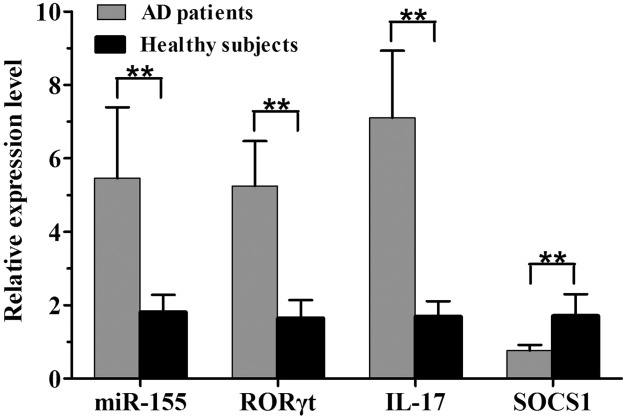
The relative expression levels of microRNA (miR)-155, as well as retinoic acid-related orphan receptor (ROR)γt, interleukin (IL)-17 and suppressor of cytokine signalling-1 (SOCS1) mRNAs, in peripheral CD4+ T cells in 33 atopic dermatitis (AD) patients versus 31 healthy subjects (**P < 0·01).
Figure 3.
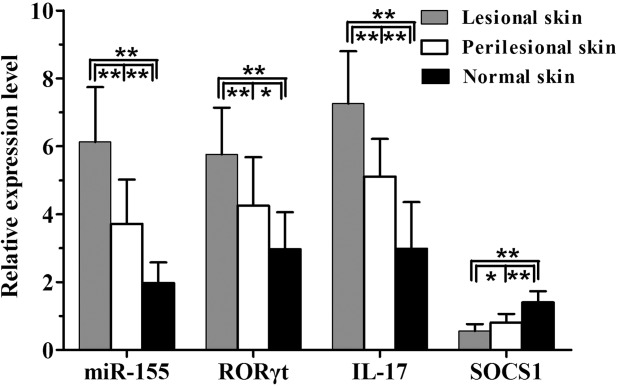
The relative expression levels of microRNA (miR)-155, as well as RORγt, IL-17 and SOCS1 mRNAs in atopic dermatitis (AD) lesional skin, perilesional skin, and normal skin (each n = 15, **P < 0·01; *P < 0·05).
Plasma concentrations of SOCS1 and IL-17
Commensurate with the mRNA expression levels found in peripheral CD4+ T cells and skin lesions, the concentrations of SOCS1 (47·15 ± 10·21 pg/ml versus 95·16 ± 23·49 pg/ml, t = −10·489) and IL-17 (32·37 ± 5·94 pg/ml versus 11·60 ± 2·15 pg/ml, t = 18·834) in plasma were obviously decreased or increased in AD patients compared with healthy subjects (both P < 0·01; Supporting information, Fig. S1).
Correlation analysis
Positive correlations were found between miR-155 expression and the SCORAD index, Th17 cell percentage, RORγt mRNA expression and IL-17 mRNA expression and plasma concentration (r = 0·412, 0·424, 0·381, 0·473 and 0·464, respectively, all P < 0·05). Conversely, negative correlations were found between miR-155 expression and SOCS1 mRNA expression (r = −0·393, P = 0·025) and plasma concentration (r = −0·392, P = 0·024, Supporting information, Fig. S2). Conversely, both the mRNA expression level and plasma concentration of SOCS1 correlated negatively with the SCORAD index, Th17 cell percentage, RORγt mRNA expression and IL-17 mRNA expression and plasma concentration (SOCS1 mRNA expression level: r = −0·438, −0·549, −0·765 and −0·586, respectively, all P < 0·05; Supporting information, Fig. S3; SOCS1 plasma concentration: r = −0·526, −0·619, −0·531 and −0·554, respectively, all P < 0·05; Supporting information, Fig. S4). In parallel with the peripheral circulation results, the miR-155 expression level also correlated positively with the RORγt and IL-17 mRNA expression levels in AD lesional and perilesional skin (r = 0·441 and 0·433, respectively, both P < 0·05), while correlating negatively with SOCS1 mRNA expression level (r = −0·499, P < 0·05, Supporting information, Fig. S5). Moreover, there was also a positive correlation of the miR-155 expression level between AD lesional skin and peripheral CD4+ T cells (r = 0·687, P < 0·05). Conversely, the SOCS1 mRNA expression level correlated negatively with the RORγt and IL-17 mRNA expression levels in AD lesional and perilesional skin (r = −0·496 and −0·419, respectively, both P < 0·05; Supporting information, Fig. S6).
Th17 cell percentage, as well as RORγt, IL-17 and SOCS1 expression levels, in transfected CD4+ T cells
CD4+ T cells from 10 AD patients were transfected with a miR-155 mimic, a miR-155 inhibitor or a negative control. The percentage of Th17 cells and the relative expression levels of RORγt, IL-17 and SOCS1 mRNA were detected. The results showed that the percentage of Th17 cells, the relative expression levels of RORγt and IL-17 mRNA in CD4+ T cells, as well as the IL-17 concentration in cell-free supernatant, were all significantly higher in CD4+ T cells transfected with the miR-155 mimic than those transfected with a miR-155 inhibitor or a negative control, and were distinctly lower in the miR-155 inhibitor group compared with the miR-155 mimic and negative control groups (F = 72·613, 34·002, 40·057 and 54·863, respectively, all P < 0·01, Fig. 4). The relative expression level of SOCS1 mRNA was obviously reduced in the miR-155 mimic group compared with the miR-155 inhibitor and negative control groups, but elevated significantly in the miR-155 inhibitor group compared with the other two groups (F = 139·706, P < 0·01, Fig. 4). Moreover, the secretion of SOCS1 protein from CD4+ T cells showed the same tendency as SOCS1 mRNA expression (F = 80·177, P < 0·01, Fig. 4).
Figure 4.
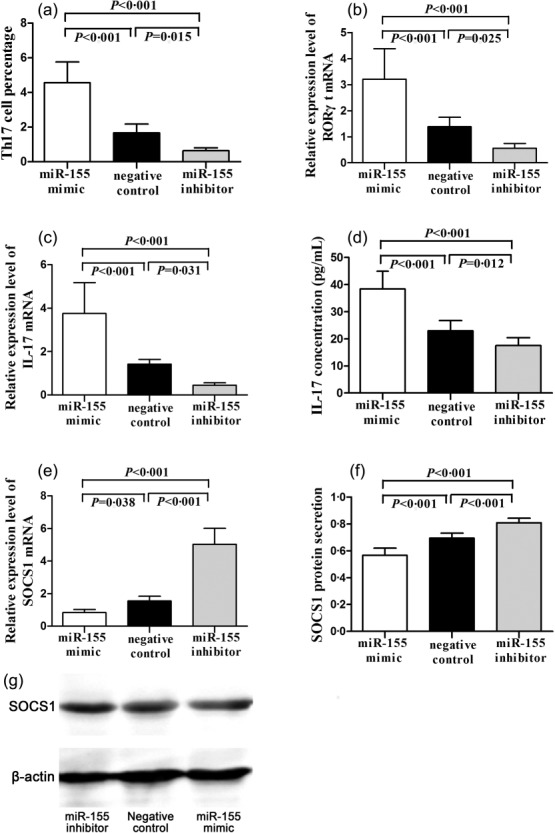
Th17 cell percentage (a), RORγt mRNA expression (b), and IL-17 mRNA expression (c) in transfected AD CD4+ T cells; IL-17 concentration in cell-free supernatant (d); SOCS1 mRNA expression (e) and SOCS1 protein secretion (f) in transfected atopic dermatitis (AD) CD4+ T cells; a representative western blot image of SOCS1 protein secretion (g).
Discussion
MiR-155 has been reported to be one of the highest-ranked up-regulated miRNAs in AD skin (4·6-fold up-regulation) by TLDA 21. In this study, we found that miR-155 was expressed highly in both AD peripheral CD4+ T cells and skin specimens, including perilesional skin. Furthermore, a comparison of the miR-155 expression levels in AD peripheral CD4+ T cells with respect to AD disease severity revealed a moderately positive correlation, indicating that miR-155 may be involved in the pathogenesis of AD. It has been shown that miR-155 was expressed predominantly in infiltrating immune cells of the skin, especially in CD4+ T helper and dendritic cells 3,21. Th17 cells are a recently identified CD4+ T helper cell subset which play a critical role in the development of autoimmunity, as well as inflammatory and allergic reactions, by producing the effective cytokine IL-17. The ROR family transcription factor RORγt is the subset-determining transcription factor of Th17 cells, and is essential for the development and function of Th17 cells 24. We found an increased percentage of Th17 cells in peripheral CD4+ T cells, enhanced expression of RORγt and IL-17 mRNA in peripheral CD4+ T cells, lesional skin and perilesional skin and elevated concentration of IL-17 in plasma in AD, all of which were commensurate with the previous reports 10,11. MiR-155 has been demonstrated to be necessary for the differentiation of Th17 cells in animal models of EAE and arthritis 18,19. To explore the possible regulatory effects of miR-155 on the differentiation of Th17 cells in AD, we correlated miR-155 expression levels with the Th17 cell percentage and the RORγt and IL-17 mRNA expression levels in the peripheral circulation, as well as in lesional and perilesional skin, and most of the results showed moderately positive relationships. For further insight into the regulatory abilities of miR-155, we transfected a miR-155 mimic or a miR-155 inhibitor into the purified CD4+ T cells of AD patients. The results showed that miR-155 clearly increased the percentage of Th17 cells and promoted the mRNA expression of RORγt and IL-17 in cultured CD4+ T cells, as well as the protein concentration of IL-17 in cell-free supernatant, while opposite changes were observed in the miR-155 inhibitor group. The differentiation of T helper cells is regulated by a complex cytokine network. The IL-6/signal transducer and activator of transcription-3 (STAT-3) signalling pathway is known to be pivotal for the development of Th17 cells and the production of IL-17 20,25. SOCS1 is a negative regulator of the Janus kinase (JAK)/STAT signalling pathway, which can regulate the activation, development and differentiation of T lymphocytes, and is involved in immune and inflammatory diseases 26,27. The effect of SOCS1 on the differentiation and function of Th17 cells was clarified recently by characterizing a mimetic of SOCS1, namely the novel tyrosine kinase inhibitor peptide (Tkip), which blocked IL-6-induced activation of STAT-3 and inhibited the development of Th17 cells and the production of IL-17 28–31. In our study, decreased SOCS1 expression was confirmed in AD peripheral CD4+ T cells and plasma concentrations, as well as in lesional and perilesional skin. Moreover, SOCS1 expression was correlated moderately or highly negatively with the changes in the Th17 cell percentage and the expression levels of RORγt and IL-17 mRNA, indicating the negative regulatory effect of SOCS1 on the differentiation of Th17 cells in AD.
MiRNAs mediate their regulatory action through binding to the 3′ UTRs of target mRNAs carrying complementary sites. According to the results of bioinformatic analyses, SOCS1 is the predicted target gene of miR-155 in humans. In-vivo studies have shown that miR-155 may enhance murine Th17 cell differentiation and IL-17 production by targeting SOCS1 20. Our study identified moderately negative relationships between miR-155 expression levels and SOCS1 expression levels in peripheral CD4+ T cells, plasma, lesional skin and perilesional skin. In addition, a miR-155 mimic inhibited the expression of SOCS1, while a miR-155 inhibitor caused a completely opposite effect. These preliminary findings may indicate the suppressive function of miR-155 on its target gene (SOCS1) during AD development.
IL-22 has been suggested as another Th17 cell-associated cytokine, which can synergize with IL-17 to regulate genes associated with innate skin immunity 32. In AD-related research, a marked synergistic effect between IL-17 and IL-22 on IL-8 production in normal human epidermal keratinocytes was detected 11. A cell-autonomous defect in the production of both IL-17 and IL-22 due to miR-155-deficient CD4+ T cells was observed 33; however, IL-17 expression, rather than IL-22 expression, in miR-155-deficient Th17 cells could be rescued by IL-1 signalling, which is critical for the regulation of early Th17 cell differentiation 34. In addition, Jarid2 was suggested as another target mRNA of miR-155 in regulating IL-22 expression in cultured Th17 cells, although it did not bind preferentially to the IL-17 promoter in miR-155-deficient Th17 cell cultures 33. Therefore, miR-155 may perform its regulatory function through different target mRNAs in a completely or partially synergistic manner. Further research regarding this possible mechanism will be required.
AD is often associated with allergic asthma and rhinitis which, together, constitute the atopic triad 35. It is interesting to note that the B cell integration cluster/miR-155 gene is located within a region on chromosome 21q2 associated with pollen sensitivity, as well as asthma and AD susceptibility 36. Recently, miR-155 has been reported to be essential for Th2-mediated allergen-induced eosinophilic airway inflammation, and miR-155 deficiency can result in diminished eosinophilic inflammation and mucus hypersecretion in the lungs of allergen-sensitized and allergen-challenged mice 37. Moreover, further research in the lung revealed that T cell-intrinsic miR-155 was required for Th2 immunity, and that miR-155 may have served as a potential therapeutic target to alleviate Th2-mediated inflammation and allergy 38,39. Therefore, we speculate that miR-155 may play a similar role in AD Th2 cells. In addition, IL-17, the effective cytokine of Th17 cells, has been demonstrated as an inducer of Th2 immune responses in murine AD models 12,13, leading to speculation that miR-155 may also indirectly affect the immunity function of Th2 cells through its regulatory effect on Th17 cells in AD.
In conclusion, miR-155 may play critical roles in driving the differentiation of Th17 cells and enhancing the function of Th17 cells by directly inhibiting SOCS1 in AD. These results, speculations and the results of future studies may serve to implicate miR-155 as a possible new therapeutic target for AD.
Acknowledgments
This work was funded by the Medical Science and Technology Development Project of Shandong Province, China (no. 2011QZ003), the Science and Technology Planning Project of Shandong Province, China (no. 2011YD18069) and the Shandong Provincial Natural Science Foundation of China (no. ZR2010HQ013)
Disclosure
The authors have no conflicts of interest to declare.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Table S1. List of microRNA (miR)-155, U6, suppressor of cytokine signalling-1 (SOCS1), retinoic acid-related orphan receptor (ROR)γt, interleukin (IL)-17 and β-actin primers for real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR)
Fig. S1. Plasma concentrations of interleukin (IL)-17 and suppressor of cytokine signalling-1 (SOCS1) in 33 atopic dermatitis (AD) patients versus 31 healthy subjects (**P < 0·01).
Fig. S2. The correlation analysis of microRNA (miR)-155 expression with SCORing Atopic Dermatitis (SCORAD) index (a), T helper type 17 (Th17) cell percentage (b), retinoic acid-related orphan receptor (ROR)γt mRNA expression (c), interleukin (IL)-17 mRNA expression (d), IL-17 plasma concentration (e), suppressor of cytokine signalling-1 (SOCS1) mRNA expression (f) and suppressor of cytokine signalling-1 (SOCS1) plasma concentration (g) in atopic dermatitis (AD) peripheral circulation (n = 33).
Fig. S3. The correlation analysis of suppressor of cytokine signalling-1 (SOCS1) mRNA expression with SCORing Atopic Dermatitis (SCORAD) index (a), T helper type 17 (Th17) cell percentage (b), retinoic acid-related orphan receptor (ROR)γt mRNA expression (c) and interleukin (IL)-17 mRNA expression (d) in atopic dermatitis (AD) peripheral circulation (n = 33).
Fig. S4. The correlation analysis of suppressor of cytokine signalling-1 (SOCS1) plasma concentration with SCORing Atopic Dermatitis (SCORAD) index (a), T helper type 17 (Th17) cell percentage (b), retinoic acid-related orphan receptor (ROR)γt mRNA expression (c) and interleukin (IL)-17 plasma concentration (d) in atopic dermatitis (AD) peripheral circulation (n = 33).
Fig. S5. The correlation analysis of microRNA (miR)-155 expression level with retinoic acid-related orphan receptor (ROR)γt mRNA expression (a), interleukin (IL)-17 mRNA expression (b) and suppressor of cytokine signalling-1 (SOCS1) mRNA expression (c) in atopic dermatitis (AD) lesional and perilesional skin (n = 30).
Fig. S6. The correlation analysis of suppressor of cytokine signalling-1 (SOCS1) mRNA expression with retinoic acid-related orphan receptor (ROR)γt mRNA expression (a) and interleukin (IL)-17 mRNA expression (b) in atopic dermatitis (AD) lesional and perilesional skin (n = 30).
References
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- O′Connell RM, Rao DS, Chaudhuri AA, et al. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–22. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BS, Nesterova TB, Thompson E, et al. T cell lineage choice and differentiation in the absence of the RNase III enzyme dicer. J Exp Med. 2005;201:1367–73. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs: novel regulators in skin inflammation. Clin Exp Dermatol. 2008;33:312–5. doi: 10.1111/j.1365-2230.2008.02804.x. [DOI] [PubMed] [Google Scholar]
- Lu TX, Rothenberg ME. Diagnostic, functional, and therapeutic roles of microRNA in allergic diseases. J Allergy Clin Immunol. 2013;132:3–13. doi: 10.1016/j.jaci.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebane A, Akdis CA. MicroRNAs: essential players in the regulation of inflammation. J Allergy Clin Immunol. 2013;132:15–26. doi: 10.1016/j.jaci.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Lu LF, Thai TH, Calado DP, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Xue HB, Guan XH, et al. Possible role of Th17 cells and IL-17 in the pathogenesis of atopic dermatitis in northern China. J Dermatol Sci. 2012;68:66–8. doi: 10.1016/j.jdermsci.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Koga C, Kabashima K, Shiraishi N, et al. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–30. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- Dhingra N, Guttman-Yassky E. A possible role for IL-17A in establishing Th2 inflammation in murine models of atopic dermatitis. J Invest Dermatol. 2014;134:2071–4. doi: 10.1038/jid.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Kitoh A, Egawa G, et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J Invest Dermatol. 2014;134:2122–30. doi: 10.1038/jid.2014.51. [DOI] [PubMed] [Google Scholar]
- Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberger P, La Russa V, Miller A, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–9. [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- O'Connell RM, Kahn D, Gibson WS, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–19. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluml S, Bonelli M, Niederreiter B, et al. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 2011;63:1281–8. doi: 10.1002/art.30281. [DOI] [PubMed] [Google Scholar]
- Yao R, Ma YL, Liang W, et al. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLOS ONE. 2012;7:e46082. doi: 10.1371/journal.pone.0046082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Janson P, Majuri ML, et al. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J Allergy Clin Immunol. 2010;126:581–9. doi: 10.1016/j.jaci.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Rajka G, Langeland T. Grading of severity of atopic dermatitis. Acta Derm Venerol. 1989;144:13–4. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Liu X, Lee YS, Yu CR, et al. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trengove MC, Ward AC. SOCS proteins in development and disease. Am J Clin Exp Immunol. 2013;2:1–29. [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Xu WD, Peng H, et al. SOCS signaling in autoimmune diseases: molecular mechanisms and therapeutic implications. Eur J Immunol. 2014;44:1265–75. doi: 10.1002/eji.201344369. [DOI] [PubMed] [Google Scholar]
- Flowers LO, Subramaniam PS, Johnson HM. A SOCS-1 peptide mimetic inhibits both constitutive and IL-6 induced activation of STAT3 in prostate cancer cells. Oncogene. 2005;24:2114–20. doi: 10.1038/sj.onc.1208437. [DOI] [PubMed] [Google Scholar]
- Jager LD, Dabelic R, Waiboci LW, et al. The kinase inhibitory region of SOCS-1 is sufficient to inhibit T-helper 17 and other immune functions in experimental allergic encephalomyelitis. J Neuroimmunol. 2011;232:108–18. doi: 10.1016/j.jneuroim.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Mahdi RR, Oh HM, et al. Suppressor of cytokine signaling-1 (SOCS1) inhibits lymphocyte recruitment into the retina and protects SOCS1 transgenic rats and mice from ocular inflammation. Invest Ophthalmol Vis Sci. 2011;52:6978–86. doi: 10.1167/iovs.11-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba MG, Flowers LO, Patel CB, et al. Treatment of mice with the suppressor of cytokine signaling-1 mimetic peptide, tyrosine kinase inhibitor peptide, prevents development of the acute form of experimental allergic encephalomyelitis and induces stable remission in the chronic relapsing/remitting form. J Immunol. 2005;175:5077–86. doi: 10.4049/jimmunol.175.8.5077. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar TM, Kanellopoulou C, Kugler DG, et al. miR-155 activates cytokine gene expression in Th17 cells by regulating the DNA-binding protein Jarid2 to relieve polycomb-mediated repression. Immunity. 2014;40:865–79. doi: 10.1016/j.immuni.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–87. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(suppl):S118–27. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Blumenthal MN, Langefeld CD, Barnes KC, et al. A genome-wide search for quantitative trait loci contributing to variation in seasonal pollen reactivity. J Allergy Clin Immunol. 2006;117:79–85. doi: 10.1016/j.jaci.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Malmhäll C, Alawieh S, Lu Y. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. 2014;133:1429–38. doi: 10.1016/j.jaci.2013.11.008. J Allergy Clin Immunol, 1438.e1–7. [DOI] [PubMed] [Google Scholar]
- Okoye IS, Czieso S, Ktistaki E, et al. Transcriptomics identified a critical role for Th2 cell-intrinsic miR-155 in mediating allergy and antihelminth immunity. Proc Natl Acad Sci USA. 2014;111:E3081–90. doi: 10.1073/pnas.1406322111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of microRNA (miR)-155, U6, suppressor of cytokine signalling-1 (SOCS1), retinoic acid-related orphan receptor (ROR)γt, interleukin (IL)-17 and β-actin primers for real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR)
Fig. S1. Plasma concentrations of interleukin (IL)-17 and suppressor of cytokine signalling-1 (SOCS1) in 33 atopic dermatitis (AD) patients versus 31 healthy subjects (**P < 0·01).
Fig. S2. The correlation analysis of microRNA (miR)-155 expression with SCORing Atopic Dermatitis (SCORAD) index (a), T helper type 17 (Th17) cell percentage (b), retinoic acid-related orphan receptor (ROR)γt mRNA expression (c), interleukin (IL)-17 mRNA expression (d), IL-17 plasma concentration (e), suppressor of cytokine signalling-1 (SOCS1) mRNA expression (f) and suppressor of cytokine signalling-1 (SOCS1) plasma concentration (g) in atopic dermatitis (AD) peripheral circulation (n = 33).
Fig. S3. The correlation analysis of suppressor of cytokine signalling-1 (SOCS1) mRNA expression with SCORing Atopic Dermatitis (SCORAD) index (a), T helper type 17 (Th17) cell percentage (b), retinoic acid-related orphan receptor (ROR)γt mRNA expression (c) and interleukin (IL)-17 mRNA expression (d) in atopic dermatitis (AD) peripheral circulation (n = 33).
Fig. S4. The correlation analysis of suppressor of cytokine signalling-1 (SOCS1) plasma concentration with SCORing Atopic Dermatitis (SCORAD) index (a), T helper type 17 (Th17) cell percentage (b), retinoic acid-related orphan receptor (ROR)γt mRNA expression (c) and interleukin (IL)-17 plasma concentration (d) in atopic dermatitis (AD) peripheral circulation (n = 33).
Fig. S5. The correlation analysis of microRNA (miR)-155 expression level with retinoic acid-related orphan receptor (ROR)γt mRNA expression (a), interleukin (IL)-17 mRNA expression (b) and suppressor of cytokine signalling-1 (SOCS1) mRNA expression (c) in atopic dermatitis (AD) lesional and perilesional skin (n = 30).
Fig. S6. The correlation analysis of suppressor of cytokine signalling-1 (SOCS1) mRNA expression with retinoic acid-related orphan receptor (ROR)γt mRNA expression (a) and interleukin (IL)-17 mRNA expression (b) in atopic dermatitis (AD) lesional and perilesional skin (n = 30).


