Abstract
Neutrophil is a key cell in pathophysiology of granulomatosis with polyangiitis. Recently, neutrophil extracellular traps were described in this disease. Mitochondrial DNA is also released during traps formation. We measured circulating cell-free mitochondrial and genomic DNA in serum of patients with granulomatosis with polyangiitis. Subjects with the disease (14 active and 11 in remission stage) and 10 healthy controls were enrolled. Quantitative real-time polymerase chain reaction (PCR) was used to measure 79 base pairs (bp) and 230 bp mtDNA fragments. Alu repeats were quantified to evaluate abundance of nuclear DNA in serum at the presence of plasmid control. Both fragments of mtDNA (79 bp and 230 bp) and genomic DNA were elevated significantly in granulomatosis with polyangiitis compared to controls. Only the shorter 79bp mtDNA correlated with active stage of granulomatosis with polyangiitis and clinical symptoms. A mechanism of extracellular release of mitochondrial DNA accompanies the active stage of the disease. Circulating mtDNA is extremely high in untreated patients. This suggests that biomarker properties of mtDNA are useful for monitoring of treatment.
Keywords: circulating mtDNA, GPA, real-time PCR
Introduction
Neutrophils are the key cells participating in the inflammatory process of granulomatosis with polyangiitis (GPA). The presence of circulating autoantibodies against cytoplasmatic neutrophil proteins (cANCA), especially reactive with the neutrophil serine protease, proteinase 3 (PR3), is detectable in approximately 85% of GPA patients 1. ANCA-mediated neutrophil activation seems to involve both Fc and Fab fragments of the antibody, but the exact mechanism of this process is not completely understood 2–4. Ohlsson et al. reported that neutrophils in patients with ANCA-associated vasculitis exhibited an increased propensity for activation by cANCAs 5. Studies by Pankhurst et al. showed that different immunoglobulin G subclasses of ANCA had different abilities for neutrophil activation 6.
The lifespan of non-activated blood neutrophils is 5–6 days on average. In addition to activation, a unique and irreversible transformation of the neutrophil has been described. By active release of chromatin fibre traps, named neutrophil extracellular traps (NETs) 7, the cell can enhance its anti-microbial capacity. NET formation involves the rearrangement of nuclear and granular architecture. It requires dissolution of internal cell membranes followed by chromatin decondensation and cytolysis. Besides infectious diseases, NETs formation has been described in pre-eclampsia 8 and small-vessel vasculitis 9. Kessenbrock et al. described neutrophil activation followed by in-vitro NET formation in response to c-ANCA stimulation. c-ANCA-induced NETs contained DNA and cytoplasmatic antigens for c-ANCA antibodies, potentially perpetuating the autoimmune process 9. Studies by Yousefi et al. and more recently by Keshari et al. proved that neutrophil extracellular traps contain mitochondrial DNA 10,11. Elevated levels of circulating cell-free genomic DNA (ccf nDNA) were observed in autoimmune diseases 12,13. In addition, mitochondrial DNA (ccf mtDNA) have been described in several types of cancer and proposed as a promising non-invasive diagnostic or prognostic biomarker 14,15. However, diagnostic performance of ccf mtDNA measurements in autoimmune diseases has not been well studied. The hypothesis of this study was that ccf mtDNA could compare with the current laboratory assays in monitoring the activity of GPA. Moreover, by quantification of ccf mtDNA we aimed to explain if findings of activated blood neutrophils could link the disease with an extracellular traps formation.
Materials and methods
Patients and sample collection
In an observational non-randomized study we enrolled 25 consecutive subjects with GPA: 14 subjects in the active stage of the disease (10 exacerbated and four new treatment-naive cases) and 11 subjects in remission of the disease. A control group included 10 healthy volunteers matched by age and sex. Disease activity was ascertained using the Birmingham Vasculitis Activity Score (BVAS). Peripheral blood samples were collected without anti-coagulant, using the same collection system (S-Monovette, Sarstedt, Nümbrecht, Germany). Serum was separated using a standard laboratory method, aliquoted and frozen in −80°C for further analysis. Other laboratory tests [complete blood count (CBC), C-reactive protein (CRP) level, anti-proteinase 3 (PR3) immunoglobulin (Ig)G level, procalcitonine, creatinine, lactate dehydrogenase (LDH)] were performed in all participants using validated assays. Written informed consent was obtained from all participants in the study. The protocol was approved by the Jagiellonian University Bioethical Committee.
DNA isolation
Total ccf DNA was isolated from serum by the phenol/chloroform method. Briefly, 500 µl of serum was added as an internal standard of DNA [pGEM-3Zf(+) plasmid, 0·34 ng; Promega, Madison, WI, USA] to compensate for errors in the sample processing, diluted with 1 m of ultrapure water, and extracted four times with equal volume of phenol/chloroform mixture. DNA was recovered by precipitation using 1 : 10 v/v 3 M sodium acetate and 2 : 1 v/v ice-cold 96% ethanol. After rinsing in 70% ethanol and drying the pellet, DNA was resuspended in 20 µl of water.
Genomic and mitochondrial DNA quantification
The abundance of circulating-cell free genomic (ccf nDNA) and mitochondrial DNA (ccf mtDNA) was measured by a quantitative real-time polymerase chain reaction (qPCR) (ABI Prism 7900HT Real-Time PCR System; Applied Biosystems, Foster City, CA, USA). Conditions for qPCR and sequences of primers used for amplification of mitochondrial gene encoding 16s-RNA (mtDNA79 and mtDNA230) and genomic DNA [Alu repetitive sequence 124 base pairs (bp) product] have been described previously 15,16. Quantification of internal standard plasmid was performed using M13 primers, as recommended by the manufacturer (Promega, Madison, WI, USA). Amplifications were performed using SYBR Green Master Mix (Life Technologies, Carlsbad, CA, USA), 1 µM forward/reverse primer and 5 µl of ccf DNA sample. nDNA, mtDNA79 and mtDNA230 quantification cycle values were adjusted to the internal standard and calculated as relative expression (RE) using the 2−ΔCt formula 17. To confirm specificity of PCR amplification products, melting curve analysis was used (Supporting information, Fig. S1).
Statistical analyses
Data are presented as mean and standard deviation or median and 25–75th percentile depending on the distribution. A non-parametric Mann–Whitney U-test and Kruskal–Wallis with post-hoc Dunn's tests were used to compare between the studied groups because of their small size. Correlations between serum ccf DNA and clinical or laboratory data were calculated by Spearman's rank method. Calculations were performed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). A type I statistical error P < 0·05 was considered significant.
Results
Demography and clinical characteristics of GPA and control subjects are summarized in Table1. The two fragments of mitochondrial genome, mtDNA79 and mtDNA230, had a significantly higher abundance in GPA subjects than in controls [RE mtDNA230: GPA-0·16 (0·07–0·26) versus HC-0·04 (0·03–0·09); P < 0·05; RE mtDNA79: GPA-1·35 (0·42–3·05) versus HC-0·40 (0·27–0·76); P < 0·05; median (25–75th percentile)]. The longer mtDNA230 fragment did not differ between subjects with active and remission GPA [RE: 0·18 (0·11–0·35) versus 0·11 (0·05–0·19); Fig. 1C]. The highest level of mtDNA230 was detected in treatment-naive GPA [RE: 0·32 (0·12–2·0); Fig. 1C]. The short mtDNA79 fragment was more abundant in subjects with active GPA than in remission of the disease or in controls [RE: 2·0 (1·4–2·8) versus 0·4 (0·3–0·9) or 0·4 (0·2–0·70; P < 0·05; Fig. 1a]. The highest expression of mtDNA79 was observed in serum of four treatment-naive GPA subjects [RE: 66·2 (10·8–131·0)]. The integrity index calculated as mtDNA230 to mtDNA79 was significantly lower in patients with treatment-naive or active GPA (0·019 ± 0·013 or 0·1 ± 0·05) than in remission (0·22 ± 0·1; P < 0·05; Fig. 1d). The highest level of ccf nDNA was observed in serum of treatment-naive GPA [RE: 16·4 (8·0–106·7)]. nDNA level was significantly higher both in serum of active and remission GPA [RE: 2·15 (1·0–4·3) and 1·66 (0·6–4·43)] than in controls (RE: 0·37 (0·17–0·46); P < 0·05). nDNA serum level did not discriminate between active GPA or remission GPA (P > 0·05; Fig. 1e). A correlation between abundance of ccf DNA (mtDNA or nDNA) and BVAS was present only for the short mtDNA79 in active disease (ρ = 0·89; P = 0·001; Fig. 1b). No other clinical or laboratory data correlated with ccf mtDNA or nDNA.
Table 1.
Selected characteristics of the study participants
| Naive GPA | Active GPA | Inactive GPA | Control | |
|---|---|---|---|---|
| n | 4 | 10 | 11 | 10 |
| Age (mean ± s.d.) | 52·6 ± 10·9 | 49·7 ± 12·6 | 48·1 ± 13·4 | 50·7 ± 12·6 |
| Gender (female/male) | 3/1 | 5/5 | 5/6 | 4/6 |
| BVAS (min–max) | 14–24 | 6–28 | 0 | 0 |
| GC treatment (yes/no) | 0/4 | 4/6 | 6/5 | – |
| GC dose (mg/day) (min–max) | 0 | 10–20 | 4–8 | – |
| cANCA (RU/ml) (min–max) | 64–156 | 37–200 | <20–140 | <20 |
| CRP (mg/ml) | 108 ± 82·6 | 20 ± 14 | 5·8 ± 3·8 | <5·0 |
| PBMC (103/µl) | 13·9 ± 7·4 | 10·5 ± 4·3* | 7·03 ± 2·74 | 5·4 ± 2 |
| PMN (103/µl) | 11·6 ± 7·3 | 5·9 ± 5·4* | 4·73 ± 3 | 2·83 ± 1·5 |
| PLT (103/µl) | 416·7 ± 155·6 | 294·5 ± 102·3* | 235 ± 80 | 207 ± 45·7 |
| Procalcitonine (ng/ml) | <0·05 | <0·05 | <0·05 | <0·05 |
| Creatinine (µmol/l) | 230·3 ± 62 | 103 ± 79 | 136·2 ± 110 | 99 ± 24 |
| LDH (U/Ll) | 645 ± 250 | 505 ± 149·5* | 512·5 ± 128* | 370 ± 52 |
Naive GPA = patients with newly diagnosed not treated GPA; active GPA = patients with BVAS>2; inactive GPA = patients with BVAS = 0; BVAS = Birmingham Vasculitis Activity Score
P < 0·05, in comparison with controls; GC = glucocorticosteroids.
Figure 1.
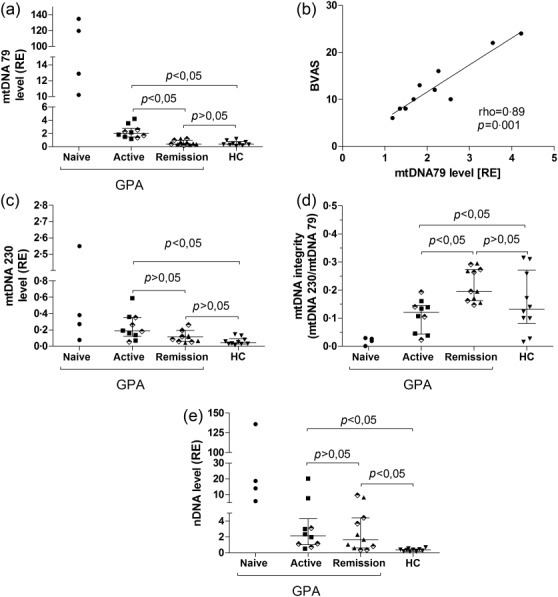
Serum abundance of circulating cell free genomic and mitochondrial DNA in patients with granulomatosis with polyangiitis. Results are presented as median and 25–75th percentile of relative expression (RE) calculated using 2−ΔCt formula (ΔCt = CtmtDNA − CtpGEM) from the DNA plasmid spike-in standard (pGEM). (a) Expression of mtDNA79 in treatment-naive, active or remission granulomatosis with polyangiitis (GPA) and healthy control subjects; (b) correlation between serum mtDNA79 abundance and disease activity in subgroup of active GPA subjects; (c) mtDNA230; (d) mtDNA integrity index; (e) abundance of nDNA Alu repeats in the study subgroups. ⋄ = patients treated with GC.
Discussion
Despite advances in understanding the pathophysiology of GPA, the disease treatment is still difficult and prognosis uncertain. Since the discovery of ANCA, and identification of PR3 as their highly specific target, diagnostics of GPA have improved, but the clinical assessment and longitudinal management remain complex. A major problem involves discriminating between damage caused by GPA activity or treatment adverse effects. ANCA can be found in the serum of more than 80% patients with GPA, but seems not particularly useful as a biomarker of disease activity because it does not predict flares or remission 1. Consequently, the gold standard in defining these end-points is based on evaluation of consensus-derived clinical scores, possibly biasing estimation of the disease activity 18. Therefore, a search for more accomplished biomarkers of GPA activity remains one of the top investigative priorities.
Circulating cell-free genomic DNA was shown to be an universal diagnostic marker in several malignancies 19,20. Recent studies have demonstrated that mitochondrial DNA fragments might have biomarker properties 14,21. In this preliminary study we analysed the serum level of ccf nDNA and mtDNA in patients with GPA. We used validated methods to quantify ccf nDNA and two different sizes of mtDNA. In conclusion, ccf DNA levels in the serum of GPA patients are elevated; however, the active stage of GPA is accompanied exclusively by a high serum level of mtDNA79, almost four times greater than in remission or in healthy controls. The highest levels of mtDNA79 were observed in four treatment-naive GPA patients, surpassing healthy controls by two orders of magnitude (164-fold increase). Moreover, we observed that serum ccf mtDNA79 correlated with disease activity evaluated as BVAS. The diagnostic performance of nDNA fragments measured as an abundance of Alu repeats or the long mtDNA230 fragment was inferior to mtDNA79, although both treatment-naive and active-stage GPA subjects had higher serum levels than controls. In our study, the active phase of GPA, and especially treatment-naive subjects, had the highest fragmentation of mitochondrial DNA (lowest integrity index), whereas their shorter ccf mtDNA79 fragment remained elevated. It could be explained either by an unknown mechanism of protection of small DNA fragments against DNAse I activity or more simply by augmented release of mtDNA into the bloodstream. Jahr et al. proposed that nDNA could be released during cell apoptosis or necrosis 22. Little is known about a source of mtDNA. Opinions about neutrophils’ apoptosis in GPA are contradictory. In patients with ANCA-associated vasculitis, including GPA, Harper et al. described an accelerated ratio of neutrophil apoptosis 23. However, Abdgawad et al. observed that spontaneous apoptosis of neutrophils is decreased in these diseases 24. Our results also support decreased neutrophil apoptosis in GPA (Supporting information, Fig. S2). Another neutrophil deletion mechanism is NETs formation, also described in autoimmune diseases 9. As NETs contain mtDNA 11,25, this might become a source of the biomarker. Moreover, in some recent studies, NETs formation was described without cell death 10,26,27; a recent study by McIlroy et al. also showed a sustained elevation of both ccf mtDNA and nDNA following trauma and trauma surgery, but only mtDNA levels were independent of the cell necrosis 28. Yet another source of ccf mtDNA should be considered in GPA. Platelets participate in NET formation 29, and we found a significantly increased frequency of neutrophil–platelet aggregates in the disease, especially during its active phase (Supporting information, Fig. S3). Boudreau et al. described the release of mitochondria by activated platelets and hydrolysis of mitochondrial membrane-releasing damage-associated molecular patterns (DAMPs) which promoted leucocyte activation 30. Thus, the mechanism of the release of mtDNA in GPA will require further elucidation.
In our study, serum ccf mtDNA was a suitable marker of treatment-naive or active GPA. In particular, small mtDNA fragments can predict GPA remission or flare more accurately than autoantibodies because of a fast decay in serum. However, a relatively small group size and lack of longitudinal observation is a limitation of this study.
Acknowledgments
The work was supported by National Centre of Science in Poland, grant number: DEC-2011/03/N/NZ6/01578
Author contributions
M. P. S planned, performed experiments and wrote the draft article, M. H. M. performed experiments, K. W. A., W. S. and J. M. collected clinical characteristic of patients, M. S. planned experiments and wrote the paper.
Disclosures
The authors declare no conflicts of interest.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's Web site:
Fig. S1. (a) Exemplary dissociation curve of real-time polymerase chain reaction (PCR) products. mtDNA79 coloured blue and mtDNA230 coloured red. (b) Exemplary dissociation curve of nDNA Alu repeats real-time PCR products.
Fig. S2. Neutrophil apoptosis in patients with granulomatosis with polyangiitis and healthy controls. Twelve patients with granulomatosis with polyangiitis (GPA) were enrolled into the study: 11 in inactive state of disease (remission) and one in active state. Control group consisted of 10 healthy volunteers. Neutrophil apoptosis was measured using flow cytometry and cells were stained with annexin V for early apoptosis and with 7-aminoactinomycin D (7AAD) for late apoptosis/necrosis. Results were presented at the 9th International Congress on Autoimmunity, 26–30 March, Nice, France 2014. Abstract available online at: http://autoimmunity.meetingxpert.net/AUTOIMMUNITY_475/poster_87589/program.aspx
Fig. S3. Neutrophil/platelet aggregates in patients with granulomatisis with polyangiitis and healthy controls. Neutrophils/platelet aggregates were analysed in fresh-sampled sodium citrate blood. Neutrophils were gated based on forward-/side-scatter and CD16-positive staining. Neutrophil-platelet aggregates index was calculated as a percentage of CD16/CD42a-positive cells to total number of neutrophils (CD16-positive). Results were presented at the 9th International Congress on Autoimmunity, 26–30 March, Nice, France 2014. Abstract available online at: http://autoimmunity.meetingxpert.net/AUTOIMMUNITY_475/poster_87588/program.aspx
References
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147:611–9. doi: 10.7326/0003-4819-147-9-200711060-00005. [DOI] [PubMed] [Google Scholar]
- Kettritz R. How anti-neutrophil cytoplasmic autoantibodies activate neutrophils. Clin Exp Immunol. 2012;169:220–8. doi: 10.1111/j.1365-2249.2012.04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JJ, Preston GA, Alcorta DA, et al. Expression profile of leukocyte genes activated by anti-neutrophil cytoplasmic autoantibodies (ANCA) Kidney Int. 2002;62:1638–49. doi: 10.1046/j.1523-1755.2002.00619.x. [DOI] [PubMed] [Google Scholar]
- Surmiak M, Kaczor M, Sanak M. Expression profile of proinflammatory genes in neutrophil-enriched granulocytes stimulated with native anti-PR3 autoantibodies. J Physiol Pharmacol. 2012;63:249–56. [PubMed] [Google Scholar]
- Ohlsson SM, Ohlsson S, Söderberg D, et al. Neutrophils from vasculitis patients exhibit an increased propensity for activation by anti-neutrophil cytoplasmic antibodies. Clin Exp Immunol. 2014;176:363–72. doi: 10.1111/cei.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst T, Nash G, Williams J, Colman R, Hussain A, Savage C. Immunoglobulin subclass determines ability of immunoglobulin (Ig)G to capture and activate neutrophils presented as normal human IgG or disease-associated anti-neutrophil cytoplasm antibody (ANCA)-IgG. Clin Exp Immunol. 2011;164:218–26. doi: 10.1111/j.1365-2249.2011.04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–82. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–54. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Krumbholz M, Scho U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–44. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- Keshari RS, Jyoti A, Kumar S, et al. Neutrophil extracellular traps contain mitochondrial as well as nuclear DNA and exhibit inflammatory potential. Cytometry A. 2012;81:238–47. doi: 10.1002/cyto.a.21178. [DOI] [PubMed] [Google Scholar]
- Bartoloni E, Ludovini V, Alunno A, et al. Increased levels of circulating DNA in patients with systemic autoimmune diseases: a possible marker of disease activity in Sjögren's syndrome. Lupus. 2011;20:928–35. doi: 10.1177/0961203311399606. [DOI] [PubMed] [Google Scholar]
- Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16:R122. doi: 10.1186/ar4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger J, Albers P, Müller SC, von Ruecker A, Bastian PJ. Circulating mitochondrial DNA in the serum of patients with testicular germ cell cancer as a novel noninvasive diagnostic biomarker. BJU Int. 2009;104:48–52. doi: 10.1111/j.1464-410X.2008.08289.x. [DOI] [PubMed] [Google Scholar]
- Ellinger J, Müller SC, Wernert N, von Ruecker A, Bastian PJ. Mitochondrial DNA in serum of patients with prostate cancer: a predictor of biochemical recurrence after prostatectomy. BJU Int. 2008;102:628–32. doi: 10.1111/j.1464-410X.2008.07613.x. [DOI] [PubMed] [Google Scholar]
- Nicklas JA, Buel E. Development of an Alu-based, real-time PCR method for quantitation of human DNA in forensic samples. J Forensic Sci. 2003;48:936–44. [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Stone JH, Hoffman GS, Merkel PA, et al. A Disease-Specific Activity Index for Wegener's granulomatosis modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Umetani N, Giuliano AE, Hiramatsu SH, et al. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol. 2006;24:4270–6. doi: 10.1200/JCO.2006.05.9493. [DOI] [PubMed] [Google Scholar]
- Wang BG, Huang H-Y, Chen Y-C, et al. Increased plasma DNA integrity in cancer patients. Cancer Res. 2003;63:3966–8. [PubMed] [Google Scholar]
- Ellinger J, Müller DC, Müller SC. Circulating mitochondrial DNA in serum: a universal diagnostic biomarker for patients with urological malignancies. Urol Oncol. 2012;30:509–15. doi: 10.1016/j.urolonc.2010.03.004. doi: 10.1016/j.urolonc.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–65. [PubMed] [Google Scholar]
- Harper L, Cockwell P, Adu D, Savage CO. Neutrophil priming and apoptosis in anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Kidney Int. 2001;59:1729–38. doi: 10.1046/j.1523-1755.2001.0590051729.x. [DOI] [PubMed] [Google Scholar]
- Abdgawad M, Pettersson Å, Gunnarsson L, et al. Decreased neutrophil apoptosis in quiescent ANCA-associated systemic vasculitis. PLOS ONE. 2012;7:e32439. doi: 10.1371/journal.pone.0032439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy DJ, Jarnicki AG, Au GG, et al. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care. 2014;29:1133.e1–5. doi: 10.1016/j.jcrc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Pilsczek FH, Salina D, Poon KKH, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–25. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- Yipp BG, Petri B, Salina D, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–93. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy DJ, Bigland M, White AE, Hardy BM, Lott N, Smith DW, et al. Cell necrosis-independent sustained mitochondrial and nuclear DNA release following trauma injury. J Trauma Acute Care Surg. 2015;78:282–8. doi: 10.1097/TA.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–71. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau LH, Duchez A-C, Cloutier N, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–83. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (a) Exemplary dissociation curve of real-time polymerase chain reaction (PCR) products. mtDNA79 coloured blue and mtDNA230 coloured red. (b) Exemplary dissociation curve of nDNA Alu repeats real-time PCR products.
Fig. S2. Neutrophil apoptosis in patients with granulomatosis with polyangiitis and healthy controls. Twelve patients with granulomatosis with polyangiitis (GPA) were enrolled into the study: 11 in inactive state of disease (remission) and one in active state. Control group consisted of 10 healthy volunteers. Neutrophil apoptosis was measured using flow cytometry and cells were stained with annexin V for early apoptosis and with 7-aminoactinomycin D (7AAD) for late apoptosis/necrosis. Results were presented at the 9th International Congress on Autoimmunity, 26–30 March, Nice, France 2014. Abstract available online at: http://autoimmunity.meetingxpert.net/AUTOIMMUNITY_475/poster_87589/program.aspx
Fig. S3. Neutrophil/platelet aggregates in patients with granulomatisis with polyangiitis and healthy controls. Neutrophils/platelet aggregates were analysed in fresh-sampled sodium citrate blood. Neutrophils were gated based on forward-/side-scatter and CD16-positive staining. Neutrophil-platelet aggregates index was calculated as a percentage of CD16/CD42a-positive cells to total number of neutrophils (CD16-positive). Results were presented at the 9th International Congress on Autoimmunity, 26–30 March, Nice, France 2014. Abstract available online at: http://autoimmunity.meetingxpert.net/AUTOIMMUNITY_475/poster_87588/program.aspx


