Abstract
The present study aimed to determine different peripheral blood neutrophil functions in 18 morbidly obese subjects with body mass index (BMI) ranging between 35 and 69 kg/m2 in parallel with age- and gender-matched lean controls. Peripheral blood neutrophil functions of obese subjects and matched lean controls were determined. Neutrophils of obese subjects showed significant elevation of the release of basal superoxides (P < 0·0001), formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated superoxides (P < 0·0001) and opsonized zymosan (OZ)-stimulated superoxides (P < 0·045) compared with lean controls. Interestingly, there were no differences in phorbol myristate acetate (PMA)-stimulated superoxide production by neutrophils of the obese subjects and controls. There was also a significant elevation of chemotactic (P < 0·0003) and random (P < 0·0001) migration of neutrophils from obese subjects compared with lean controls. Phagocytosis, CD11b surface expression and adherence of neutrophils from obese subjects were not significantly different from those of the lean controls. The elevated superoxide production and chemotactic activity, together with the normal phagocytosis and adherence, suggest that neutrophils from obese subjects are primed and have the capability to combat infections. However, neutrophils in the priming state may participate in the pathogenesis of obesity-related diseases.
Keywords: cell activation, chemotaxis, neutrophils, phagocytosis
Introduction
Obesity is increasingly accepted as a condition characterized by low-grade chronic inflammation 1. Systemically, this is evidenced by elevated levels of various inflammatory markers, including C-reactive protein, tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 2. Obesity has been identified as a risk factor for infection and poor wound healing following surgical procedures and burns 3,4. Morbidly obese individuals coincided with low neutrophil bactericidal capacity 5,6. Adipose tissue in obesity has been shown to be infiltrated by macrophages, at least some of which are of bone marrow origin 7,8. The close relationship between adipocyte size and the abundance of macrophages in adipose tissue suggests that the influence of adipocyte size on adipocyte function may be conveyed through paracrine pathway involving adipose tissue macrophages 9. Currently, most myeloid cell types have been implicated in the process, including B cells, various T cell classes and even eosinophils and mast cells 10,11. In our previous studies 12,13 we demonstrated a significant body weight gain and infiltration of neutrophils to the parenchyma of intra-abdominal adipose tissue early (3 and 7 days) after initiating high-fat feeding of C57BL/6J mice. This early appearance of neutrophils in adipose tissue, which was recently confirmed 14, suggests that adipose tissue inflammation in obesity largely follows the classical inflammation paradigms of acute versus chronic inflammatory cell infiltrates. Neutrophils are the body's first line of defence against microorganisms and a critical effector cell in both innate and humoral immunity 15. There are conflicting reports about their activation state in obese subjects 5,6,16. Given their importance for host defence and the potential for increased susceptibility of obese subjects to infections, the present study aimed to determine different neutrophil functions in highly obese subjects.
Methods and procedures
Subjects
Eighteen morbidly obese subjects [body mass index (BMI) 35–68 kg/m2] scheduled to elective laparoscopic surgery (gastric banding) and age- and gender-matched lean healthy controls were enrolled into the study. In some cases, two patients were studied in parallel to one matched lean control. All the subjects were healthy. Diabetic patients were excluded from the study, as glucose has been shown to activate superoxide production by neutrophils 17. Eight obese people were receiving statin treatment and were analysed in comparison to statin-treated lean controls. The study was approved by the Soroka University Medical Center ethical committee. All subjects signed an informed consent form. Heparinized blood was drawn from the subjects at the time blood tests were performed, as well as from healthy donor lean controls, and were transferred at room temperature for evaluation within 1 h.
Materials
Fericytochrome c, formyl-methionyl-leucyl-phenylalanine (fMLP), Ficoll-Hypaque and zymosan were purchased from Sigma (St Louis, MO, USA). Percoll was obtained from Pharmacia (Uppsala, Sweden). Sodium dodecyl sulphate (SDS) was purchased from Bio-Rad Laboratories (Hercules, CA, USA). Cell-culture media and sera were purchased from Biological Industries (Beit Haemek, Israel).
Preparation of granulocytes
Granulocytes at 95% purity were obtained by Ficoll-Hypaque centrifugation, dextran sedimentation and hypotonic lysis of erythrocytes, as described 18, within 2 h of blood drawn. Cells were counted and their viability was determined by trypan blue exclusion.
Superoxide generation
The production of the superoxide anion by intact monocytes or granulocytes was measured as the superoxide dismutase inhibitable reduction of ferricytochrome c by the microtitre plate technique, as described previously 19. Cells (2·5 × 105/well) were suspended in 100 μl Hanks's balanced salts solution (HBSS) containing ferricytochrome c (150 mM). Superoxide production by the cells was stimulated with 1 mg/ml opsonized zymosan (OZ), 5 × 10−7 M formyl-methionyl-leucyl-phenylalanine (fMLP) or 50 ng/ml phorbol 12-myristate 13-acetate (PMA). The reduction of acetyl ferricytochrome c was followed by the change of absorbance at 550 nm at 2-min intervals on a Thermomax Microplate Reader (Molecular Devices, Menlo Park, CA, USA). The maximal rates of superoxide generation were determined and expressed as nmol O2−/106 cells/min using the extinction coefficient E550 = 21 mM/cm1.
Chemotaxis
Cell migration was assessed as described earlier 20. Agarose was dissolved in sterile, distilled boiling water for 10 min. After cooling to 48°C in a water bath, the agarose was mixed with an equal volume of prewarmed × 2 minimal essential medium (MEM) with 10% heat-inactivated fetal calf serum (FCS) and 7·5% (w/v) sodium bicarbonate. Five ml of the agarose medium was delivered to 60 × 15 mm tissue culture dishes and allowed to harden. Six series of three wells, 2·4 mm in diameter and spaced 2·4 mm apart, were plucked out. The centre well of each three-well series received a 10 μL aliquot cell suspension containing 2·5 × 105 purified cells in MEM. The outer well received 10 μl of fMLP (10−8 M) and the inner well received 10 μl of MEM. The dishes were incubated subsequently at 37°C in a humidified atmosphere containing 5% CO2 in air. Dishes containing granulocytes were incubated for 2 h. The plates were fixed by addition of 3 ml methanol at 4°C overnight or for 30 min at room temperature. After the methanol was poured off, the plates were placed in glutaraldehyde (2·5%) for 30 min at room temperature. The agarose gel was removed intact after fixation and the plates stained by Giemsa and air-dried. Chemotaxis was defined as the ratio between the linear migration towards fMLP and the random migration towards control medium (MEM).
Phagocytosis
Cells (5 × 105) were suspended in RPMI-1640 containing 10% heat-inactivated FCS and incubated at 37°C for 1 h with 5 μl zymosan (1 mg/ml), opsonized by pooled human serum. Subsequently, the cells were smeared and stained with differential Wright–Giemsa. Phagocytosis was determined under the microscope in at least 100 cells, and defined as the percentage of cells containing more than two phagocytized particles of OZ.
Surface expression of CD11b
Resting or stimulated neutrophils were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CD11b antibody or with a control FITC-conjugated IgG2b antibody for 40 min in 4°C in HBSS. After two washes with the same buffer the cells were fixed with 2·5% formaldehyde, and surface CD11b was detected by fluorescence activated cell sorter (FACS) analysis (Becton Dickinson, Mountain View, CA, USA).
Adherence of neutrophils to endothelial cells
Neutrophils were labelled with 1 mCi Cr51/106 cells at 37°C during 1 h of gentle shaking 21. Following two washes with cold phosphate-buffered saline (PBS) the neutrophils were resuspended in HBSS2+ or HBSS2− at 7·5 × 105 cells/ml. Cr51 radiolabelled neutrophils were pretreated for 3 min with 50 ng/ml PMA or 5 × 10−7M fMLP added onto 12-well plates of confluent ECV304 cell line and allowed to adhere for 30 min in a 5% CO2 incubator (37°C). Following the adherence period, cells were washed twice thoroughly with PBS, and the remaining adhered prelabelled phagocytic cells, together with the ECV304 cells, were lyzed in 500 μl lysis buffer containing 1% Triton-X 100, 50 mM Tris-HCl (pH 7·5), 1 mM ethylenediamine tetraacetic acid (EDTA) and 1 mM ethylene glycol tetraacetic acid (EGTA). Radioactivity was then measured in the lysates by γ counter (Diagnostic Products Corporation, Biermann, Germany), along with a sample of prelabelled phagocytic cells to calculate the 100% count, based on which the proportional radioactivity in the adherence assay was calculated as the relative percentage.
Statistics
Data are presented as the mean ± standard error of the mean (s.e.m.). Statistical significance for between-group comparisons was determined using Student's paired two-tailed t-test or analysis of variance (anova) followed by Bonferroni correction. Correlations were analysed using Pearson's χ2 tests.
Results
Obese subjects
The characteristics of morbidly obese subjects enrolled into the study are presented in Table1. There was a significant (P < 0·001) elevation in BMI (kg/m2) and of serum triglycerides (P < 0·01) in the obese group compared with the lean control group. All other parameters, including age, white blood cell (WBC) count, neutrophil count and cholesterol were similar in the obese and lean subjects.
Table 1.
Characteristics of the subjects enrolled into the study
| Lean | Obese > 35 | |
|---|---|---|
| Gender F/M | 8/6 | 13/5 |
| Age (years) | ||
| Mean | 40·2 ± 2·3 | 39·7 ± 1·7 |
| Range | (24–49) | (21–50) |
| BMI (kg/m2) | ||
| Mean | 22·1 ± 0·5 | 45·7 ± 2* |
| Range | (19–24) | (38–69) |
| WBC (*103/μl) | ||
| Mean | 6·1 ± 0·5 | 6·9 ± 0·3 |
| range | (4·1–9·1) | (4·5–8·7) |
| Neutrophils (*103/μl) | ||
| Mean | 4·1 ± 0·3 | 4·5 ± 2·4 |
| Range | (2·9–6·7) | (3·1–6·7) |
| Cholesterol (mg/dl) | ||
| Mean | 184·6 ± 11·3 | 208·4 ± 7·3 |
| Range | (125–247) | (157–288) |
| Triglycerides (mg/ml) | ||
| Mean | 96·2 ± 10·9 | 178·6 ± 16·9† |
| Range | (46–179) | (65–659) |
P < 0·001 compared to the lean controls
P < 0·01 compared to the lean controls. WBC = white blood cells; BMI = body mass index; F/M = female/male.
Neutrophil functions of obese versus lean donors
Neutrophil functions were studied in obese subjects in parallel with matched lean control subjects. Figure 1 presents superoxide production from unstimulated and stimulated neutrophils with two physiological agonists (OZ or fMLP) and a non-physiological agonist, PMA. As shown in Fig. 1, the levels of basal superoxide production by unstimulated neutrophils from all obese subjects were higher than that of the matched controls with no overlap between the groups. The mean ± s.e.m. was significantly higher (P < 0·0001) in the obese individuals than in the controls, 15·33 ± 0·34 and 6·72 ± 0·72 nmol/106 cells/min, respectively. Moreover, there was a correlation (r = 0·78) between basal superoxide production and BMI. Similarly, a significant (P < 0·0001) elevated superoxide release was obtained in neutrophils of obese people stimulated with fMLP (mean ± s.e.m. of 32·06 ± 2·10 nmol/106 cells/min compared with 1732 ± 094 nmol/106 cells/min in the controls). Stimulation of neutrophils with OZ also resulted in increased superoxide production by neutrophils of the obese subjects compared with the controls, but with lower significance (P < 0·045). The mean ± s.e.m. was 22·92 ± 1·50 and 18·22 ± 1·25 nmol/106 cells/min in the obese and control subjects, respectively. In contrast, there was no significant difference (P = n.s.) in the release of superoxides between both groups when neutrophils were stimulated with PMA; 35·17 ± 3·78 and 31·66 ± 2·54 nmol/106 cells/min in the obese and control subjects, respectively. A lower concentration of PMA (5 ng/ml) studied did not show a difference between both groups (not shown).
Figure 1.
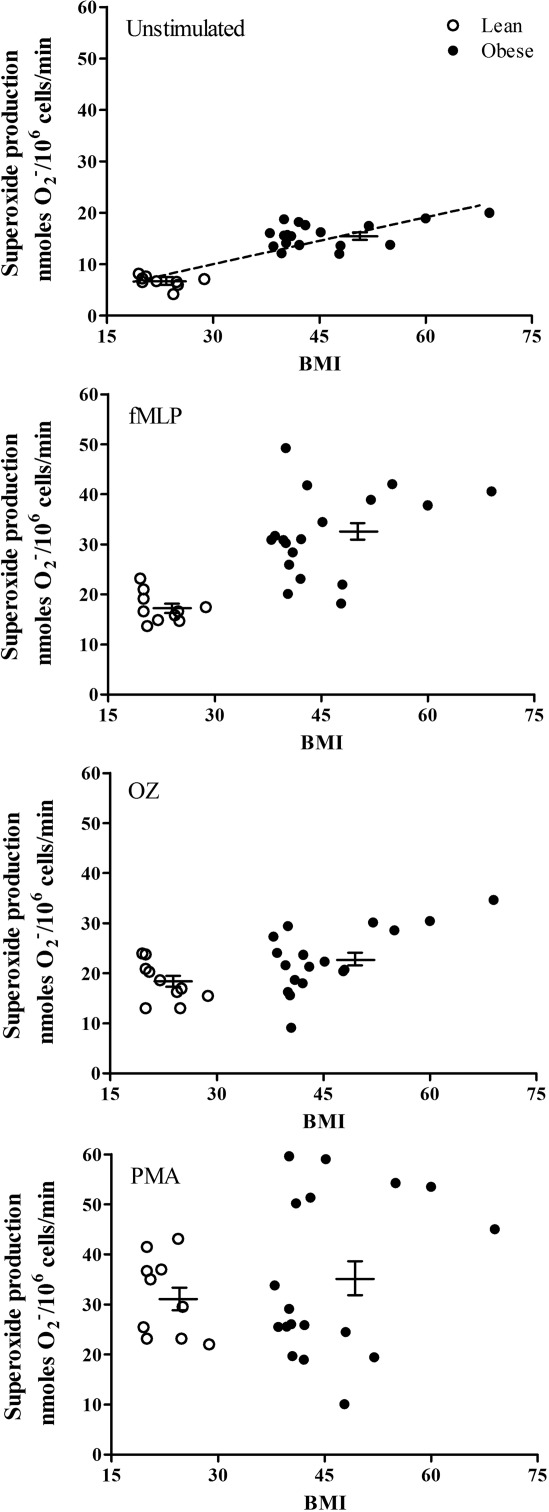
Superoxide production was measured in neutrophils from obese subjects or from healthy donors unstimulated or stimulated with either 5 × 10−7 M formyl-methionyl-leucyl-phenylalanine (fMLP), 1 mg/ml opsonized zymosan (OZ) or 50 ng/ml phorbol myristate acetate (PMA). Each value is a mean of two independent tests from each subject. The mean ± standard error of the mean are presented by the horizontal lines. There is a correlation (r = 0·78) between basal superoxide production secreted from unstimulated neutrophils and the body mass index.
Chemotactic migration and random migration of neutrophils from obese subjects were also elevated. As shown in Fig. 2, the obese subjects showed elevated neutrophil chemotactic migration and random migration compared to the lean controls. The mean ± s.e.m. of the chemotactic migration was 35·54 ± 1·48 and 24·93 ± 1·45 nm (P < 0·0003) of the random migration was 27·52 ± 0·83 and 17·36 ± 1·03 nm (P < 0·0001) for obese subjects and lean controls, respectively.
Figure 2.
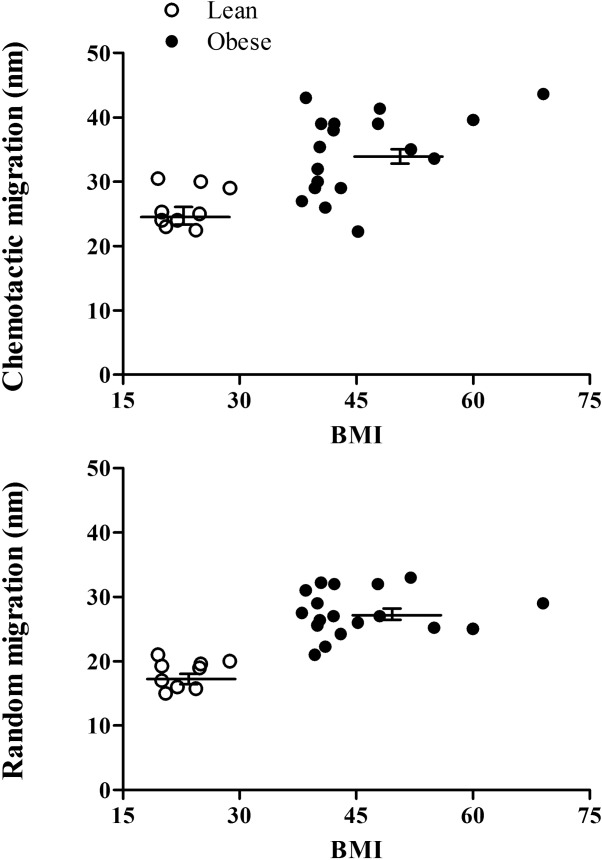
Chemotactic and random migration of neutrophils towards formyl-methionyl-leucyl-phenylalanine (fMLP). Chemotactic migration towards 10−8 M fMLP and random migration were studied in neutrophils from obese patients and healthy controls. Each value is a mean of two independent tests from each subject. The mean ± standard error of the mean are presented by the horizontal lines.
Phagocytosis activity was similar in neutrophils of the obese and lean subjects, with mean ± s.e.m. of 80·4 ± 1·5% and 72·2 ± 1·8%, respectively (Fig. 3).
Figure 3.
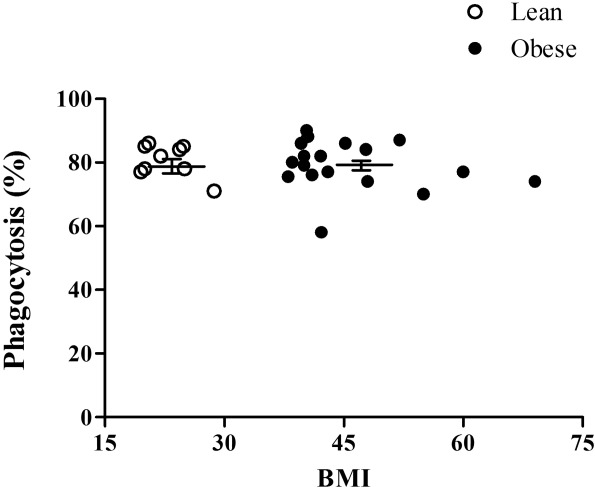
Phagocytosis of opsonized zymosan (OZ) by neutrophils of obese subjects. Each value is a mean of two independent tests from each subject. The mean ± standard error of the mean are presented by the horizontal lines.
Neutrophil adherence
CD11b surface expression determined by FACS analysis was similar to unstimulated and stimulated neutrophils of the obese patients and the matched controls (Table2). Adherence of unstimulaed and stimulated neutrophils to endothelial cells was assayed. As shown in Fig. 4, the adherence of unstimulated neutrophils or stimulated neutrophils with either fMLP or PMA was similar in obese and lean subjects.
Table 2.
Neutrophils’ CD11b surface expression
| Lean (median) | Obese > 35 (median) | |
|---|---|---|
| Unstimulated | 76 ± 4·5 | 78 ± 6·1 |
| Stimulated with PMA | 265 ± 5·8 | 278 ± 9·1 |
| Stimulated with fMLP | 158 ± 3·1 | 150 ± 6·2 |
Surface CD11b on unstimulated neutrophils and neutrophils stimulated with 50 ng/ml phorbol myristate acetate (PMA) or 5 × 10−7M formyl-methionyl-leucyl-phenylalanine (fMLP). The means ± standard error of the mean of median fluorescence of 10 000 cells of all subjects in each group are presented.
Figure 4.
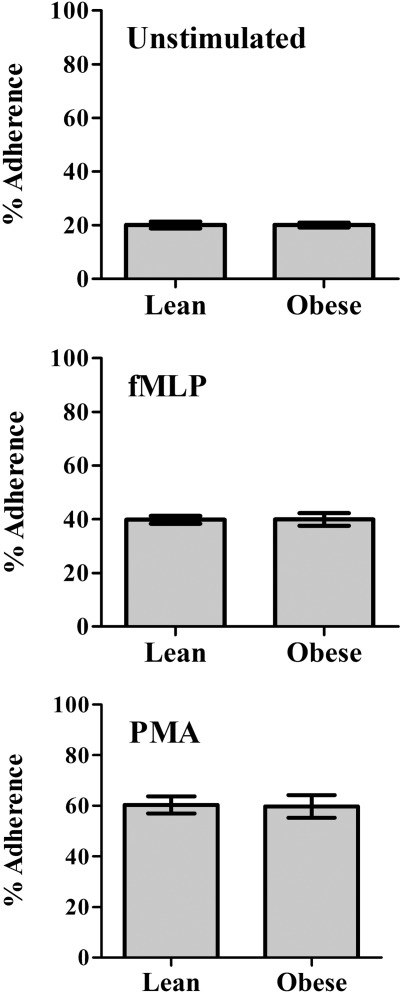
Neutrophil adherence to endothelial cells was determined in unstimulated neutrophils or stimulated neutrophils by 5 × 10−7 M formyl-methionyl-leucyl-phenylalanine (fMLP) or 50 ng/ml phorbol myristate acetate (PMA). Results are mean ± standard error of the mean of % adhered neutrophils of the subject studies.
Discussion
The present study shows that superoxide production by neutrophils of the obese people with BMI ranging between 38 and 69 kg/m2 was elevated significantly. The major alteration was the elevated basal superoxide production by unstimulated neutrophils of obese people that was more than twofold higher than that of lean controls, suggesting that the neutrophils were at a primed state. Moreover, there was a correlation between basal superoxide production and BMI. Stimulation with the physiological stimuli fMLP or OZ also caused a significant increase in superoxide production, further supporting their primed state. Stimulation with the non-physiological stimuli PMA [that does not bind to specific receptor on the plasma membranes, but activates protein kinase C (PKC) directly] caused a non-significant elevation of superoxide production. The phagocytosis process of OZ particles, number of circulating neutrophils, surface expression of CD11b and adhesion of neutrophils to endothelial cells were not affected by obesity and were similar to those of the lean controls. All functions were not altered by age and gender (not shown), in accordance with an earlier study 22 of neutrophil function in obese people. Similar to our results, that study 22 showed an increased release of basal superoxides from unstimulated neutrophils, although the elevation was much lower, only 59·6%. They have reported a low reduction in total superoxide production by neutrophils of obese people stimulated by 0·1 μmol/l PMA and a low reduction in the rate of superoxide production stimulated with 0·01 μmol/ml. They did not analyse superoxide production stimulated by physiological stimuli. Similar to our results, they 22 reported elevated migration and normal phagocytosis. The normal surface expression of CD11b in neutrophils of obese people, consistent with others’ results 23, may explain the normal adherence to endothelial cells, as this integrin is the major one facilitating the adherence process 12. Although obesity is a well-known risk factor for several morbid conditions and infections were reported to be more common in obese people than in those with normal weight 24, our results suggest the neutrophils’ capability to combat infections in normal individuals, as none of the neutrophil functions studied was shown to be defective. Moreover, stimulated superoxide production by physiological stimuli and chemotactic activity were significantly higher in neutrophils of obese subjects in comparison to those of lean controls. Thus, the increased risk of infections in obese subject is probably not attributing to neutrophil dysfunction.
The elevated basal superoxide production from unstimulated neutrophils of obese subjects is in line with studies demonstrating that obesity is associated with oxidative stress 25–30. The basal elevated superoxide release from neutrophils indicates a low grade of inflammation, in accordance with the proinflammatory state of circulating mononuclear cells of obese subjects 31. The elevated basal superoxide production may be a result of neutrophil activation by high levels of triglycerides in their blood (Table1), as an immediate activation of circulating neutrophils, release of superoxides and increased expression of nicotinamide adenine dinucleotide phosphate oxidase (NOX2-NADPH) were demonstrated in healthy human subjects exposed to a high-fat diet 32–37. It has been reported that long-chain saturated free fatty acids induced insulin resistance in cell cultures and in vivo by affecting several steps in signal transduction, such as insulin receptor substrate 2 (IRS-2) tyrosine phosphorylation, IRS-2-associated phosphoinositide (PI) 3-kinase activity and phosphorylation of protein kinase B (Akt), p70 S6 kinase, glycogen synthase kinase 3 (GSK-3) and forkhead box protein O1A (FOXO1A) 38–40. Although neutrophils are usually considered to be a part of the innate immune system and the first line of defence against infection, accumulating evidence indicates a role for neutrophils in the pathogenesis of non-infectious inflammatory diseases. For example, there was a significant increased infiltration of neutrophils into systemic vasculature of pre-eclamptic women that was associated with inflammatory markers 41. A number of studies have reported that women with pre-eclampsia are at an increased risk of developing cardiovascular disease late in life 42,43. Similarly, obese women have significant vasculature infiltration of neutrophils, which may put them at risk of cardiovascular disease 44. Our earlier study, as well as others 13,45,46, demonstrated that non-stimulated peripheral blood neutrophils from mice on a 3-day high-fat diet (3dHFD) with elevated body weight released significantly higher levels of basal superoxides, and their infiltration to adipose tissues was responsible for the development of insulin resistance in the liver. It was reported recently that overproduction of oxidants from NADPH oxidase and mitochondrial sources caused subsequent enhanced engagement of stress-activated kinases, associated with diminished insulin-stimulated insulin signalling and reduced glucose transport activity in obese subjects 47–49, as well as in mutant mouse models 50.
In conclusion, our results show that superoxide production from unstimulated or stimulated neutrophils with the physiological stimuli and neutrophil migration were elevated in obese subjects while all other functions were normal, indicating the capability of neutrophils to combat infections. The high basal superoxide production from unstimulated neutrophils is consistent with oxidative stress associated with obesity.
Acknowledgments
All co-authors have reviewed the manuscript and have contributed substantially and intellectually to the work described.
Disclosure
The authors declare that they have no financial and commercial conflicts of interest.
References
- Shoelson S, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Bruun J, Lihn A, Madan A, et al. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286:E8–13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- Fasol R, Schindler M, Schumacher B, et al. The influence of obesity on perioperative morbidity: retrospective study of 502 aortocoronary bypass operations. Thorac Cardiovasc Surg. 1992;40:126–9. doi: 10.1055/s-2007-1020129. [DOI] [PubMed] [Google Scholar]
- Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99–108. [PubMed] [Google Scholar]
- Palmblad J, Hallberg D, Engstedt L. Polymorphonuclear (PMN) function after small intestinal shunt operation for morbid obesity. Br J Haematol. 1980;44:101–8. doi: 10.1111/j.1365-2141.1980.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Palmblad J, Hallberg D. Rossner S. Obesity, plasma lipids and polymorphonuclear (PMN) granulocyte functions. Scand J Haematol. 1977;19:293–303. doi: 10.1111/j.1600-0609.1977.tb02109.x. [DOI] [PubMed] [Google Scholar]
- Weisberg S, McCann D, Desai M, Rosenbaum M, Leibel R, Ferrante AJ. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancello R, Tordjman J, Poitou C, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–61. doi: 10.2337/db06-0133. . [CrossRef] [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani P, Leclercq I. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2009;298:G107–16. doi: 10.1152/ajpgi.00391.2009. [DOI] [PubMed] [Google Scholar]
- Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- Hadad N, Burgazliev O, Elgazar-Carmon V, et al. Induction of cytosolic phospholipase a2alpha is required for adipose neutrophil infiltration and hepatic insulin resistance early in the course of high-fat feeding. Diabetes. 2013;62:3053–63. doi: 10.2337/db12-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Oh D, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Nehlsen-Cannarella SI, Henson DA, et al. Immune response to obesity and moderate weight loss. Int J Obes Relat Metab Disord. 1996;20:353–60. [PubMed] [Google Scholar]
- Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–3. doi: 10.1210/jcem.85.8.6854. . [CrossRef] [DOI] [PubMed] [Google Scholar]
- Boyum A. Isolation of lymphocytes, granulocytes and macrophages. 1976;Suppl5:9–15. Scand J Immunol. [PubMed] [Google Scholar]
- Levy R, Malech HL, Rotrosen D. Production of myeloid cell cytosols functionally and immunochemically deficient in the 47 kDa or 67 kDa NADPH oxidase cytosolic factors. Biochem Biophys Res Commun. 1990;170:1114–20. doi: 10.1016/0006-291x(90)90508-k. [DOI] [PubMed] [Google Scholar]
- Liel Y, Rudich A, Nagauker Shriker O, Yermiyahu T, Levy R. Monocyte dysfunction in patients with Gaucher disease: evidence for interference of glucocerebroside with superoxide generation. Blood. 1994;83:2646–53. [PubMed] [Google Scholar]
- Kuijpers TW, Hakkert BC, van Mourik JA, Roos D. Distinct adhesive properties of granulocytes and monocytes to endothelial cells under static and stirred conditions. J Immunol. 1990;145:2588–94. [PubMed] [Google Scholar]
- Trottier MD, Naaz A, Kacynski K, Yenumula PR, Fraker PJ. Functional capacity of neutrophils from class III obese patients. Obesity (Silver Spring) 2012;20:1057–65. doi: 10.1038/oby.2011.354. [DOI] [PubMed] [Google Scholar]
- Cottam DR, Schaefer PA, Fahmy D, Shaftan GW, Angus LD. The effect of obesity on neutrophil Fc receptors and adhesion molecules (CD16, CD11b, CD62L) Obes Surg. 2002;12:230–5. doi: 10.1381/096089202762552674. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–46. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- Couillard C, Ruel G, Archer WR, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005;90:6454–9. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
- Hansel B, Giral P, Nobecourt E, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89:4963–71. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11. . [CrossRef] [DOI] [PubMed] [Google Scholar]
- Mohn A, Catino M, Capanna R, Giannini C, Marcovecchio M, Chiarelli F. Increased oxidative stress in prepubertal severely obese children: effect of a dietary restriction-weight loss program. J Clin Endocrinol Metab. 2005;90:2653–8. doi: 10.1210/jc.2004-2178. [DOI] [PubMed] [Google Scholar]
- Urakawa H, Katsuki A, Sumida Y, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–6. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- Weinbrenner T, Schroder H, Escurriol V, et al. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr. 2006;83:30–5. doi: 10.1093/ajcn/83.1.30. [DOI] [PubMed] [Google Scholar]
- Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–71. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- Bae J, Bassenge E, Kim K, et al. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. 2001;155:517–23. doi: 10.1016/s0021-9150(00)00601-8. [DOI] [PubMed] [Google Scholar]
- Alipour A, Oostrom AV, Izraeljan A, et al. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:792–7. doi: 10.1161/ATVBAHA.107.159749. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr. 2002;75:767–72. doi: 10.1093/ajcn/75.4.767. [DOI] [PubMed] [Google Scholar]
- Aljada A, Mohanty P, Ghanim H, et al. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr. 2004;79:682–90. doi: 10.1093/ajcn/79.4.682. [DOI] [PubMed] [Google Scholar]
- Ghanim H, Abuaysheh S, Sia C, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–7. doi: 10.2337/dc09-0979. . [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deopurkar R, Ghanim H, Friedman J, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33:991–7. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordier S, Iynedjian PB. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem Biophys Res Commun. 2007;362:206–11. doi: 10.1016/j.bbrc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Ruddock MW, Stein A, Landaker E, et al. Saturated fatty acids inhibit hepatic insulin action by modulating insulin receptor expression and post-receptor signalling. J Biochem. 2008;144:599–607. doi: 10.1093/jb/mvn105. [DOI] [PubMed] [Google Scholar]
- Shah T, Walsh S. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196:48.e1–8. doi: 10.1016/j.ajog.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Paradisi G, Biaggi A, Savone R, et al. Cardiovascular risk factors in healthy women with previous gestational hypertension. J Clin Endocrinol Metab. 2006;91:1233–8. doi: 10.1210/jc.2005-1337. [DOI] [PubMed] [Google Scholar]
- Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–6. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- Shah TJ. Obese women have significant vascular infiltration of neutrophils which may put them at risk for cardiovascular diseases. J Soc Gynecol Invest. 2006;13:194A–5A. [Google Scholar]
- Tagzirt M, Corseaux D, Pasquesoone L, et al. Alterations in neutrophil production and function at an early stage in the high-fructose rat model of metabolic syndrome. Am J Hypertens. 2014;27:1096–104. doi: 10.1093/ajh/hpu021. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Oh da Y, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdolo G, Piroddi M, Luchetti F, et al. Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie. 2013;95:585–94. doi: 10.1016/j.biochi.2012.12.014. . [CrossRef] [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–9. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styskal J, Van Remmen H, Richardson A, Salmon AB. Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 2012;52:46–58. doi: 10.1016/j.freeradbiomed.2011.10.441. [DOI] [PMC free article] [PubMed] [Google Scholar]


