Abstract
Introduction
Roux-en-Y gastric bypass (RYGB) restricts food intake. Consequently, patients consume less calcium. In addition, food no longer passes through the duodenum, the main site of calcium absorption. Therefore, calcium absorption is significantly impaired. The goal of this study is to compare two common calcium supplements in gastric bypass patients.
Method
Nineteen patients were enrolled in a randomized, double-blinded, crossover study comparing the absorption of calcium from calcium carbonate and calcium citrate salts. Serum and urine calcium levels were assessed for peak values (Cmax) and cumulative calcium increment (area under the curve [AUC]). Serum PTH was assessed for minimum values (PTHmin) and cumulative PTH decrement (AUC). Statistical analysis was performed using a repeated analysis of variance model.
Results
Eighteen subjects completed the study. Calcium citrate resulted in a significantly higher serum Cmax (9.4+0.4 mg/dl vs. 9.2+0.3 mg/dl, p=0.02) and serum AUC (55+2 mg/dl vs. 54+2 mg/dl, p=0.02). Calcium citrate resulted in a significantly lower PTHmin (24+11 pg/ml vs. 30+13 pg/ml, p=0.01) and a higher AUC (−32+51 pg/ml vs. −3+56 pg/ml, p=0.04). There was a non-significant trend for higher urinary AUC in the calcium citrate group (76.13+36.39 mg/6 h vs. 66.04+40.82, p=0.17).
Conclusion
Calcium citrate has superior bioavailability than calcium carbonate in RYGB patients.
Keywords: Roux-en-Y gastric bypass, Obesity, Calcium citrate, Calcium carbonate
Introduction
The prevalence of obesity in the US is escalating [1]. Lifestyle interventions or pharmacological interventions remain inadequate in lowering the risk of comorbid conditions and mortality in this population [2–6]. Roux-en Y surgery has recently substituted the JI bypass procedure [7–9]. This procedure has been shown to be effective in sustaining weight loss [3, 10–14], diminishing multiple associated coexisting conditions, including hypertension, glucose intolerance, and dyslipidemia, and consequently improving the quality of life [3, 13–17]. However, several studies reported the increased risk of bone loss and kidney stones with this procedure [18–28]. Impaired intestinal calcium absorption from rapid intestinal transit time and derangement in vitamin D metabolism is likely to occur and may be responsible for secondary PTH stimulation, which ultimately increases the risk of bone loss [24, 25, 27–30]. Moreover, hyperoxaluria from intestinal fat malabsorption and hypocitrituria due to metabolic acidosis from diarrheal state may increase the risk of kidney stone formation [18, 20]. To date, the differential effects of two commonly used calcium supplements, calcium citrate and calcium carbonate, in the reversal of secondary hyperparathyroidism following gastric bypass has not been fully elucidated.
This study was undertaken to explore whether calcium supplementation may play a beneficial role in post-surgical RYGB patients.
Materials and Methods
Subjects
Eighteen healthy volunteers following RYGB surgery were enrolled into the study. The study sample included 17 Caucasian females and one Caucasian male. The mean age was 44.2 years (range, 30–61 years), and the mean BMI was 30.3 kg/m2 (range, 20–40 kg/m2). All the subjects underwent RYGB surgery (either open or laparoscopic) approximately 1 year prior to enrollment in the study (mean, 22.7 months; range, 11–35 months).
Men or women, aged 18–75 years, who had a gastric bypass operation at least 12 months prior were included in the study. Patients with a previous oophorectomy, liver disease, renal disease, hypercalcemia, hyperthyroidism or parathyroid disorders; use diuretics, bisphosphonates, calcitonin, corticosteroids, anabolic steroids, or anticonvulsants within 3 months of the study; and are heavy smokers (>10 cigarettes/day) or abusing alcohol (>70 ml/day) were excluded from the study. All participants were given a written informed consent for protocol that was approved by the University of Texas Southwestern Medical Center Institutional Review Board.
Study Design
This is a randomized, double-blind, crossover trial comparing the absorption of calcium carbonate to calcium citrate. Each subject participated in two phases of the study and was assigned a supplement order using a block randomization scheme. In the first phase, the participants ingested a single dose of 500 mg calcium citrate as Citracal® 250 mg + D (two tablets of Citracal, each containing 250 mg of elemental calcium and 62.5 IU of vitamin D, retailed by the Mission Pharmacal Company, San Antonio, TX, USA). In the second phase, subjects took a single dose of 500 mg calcium carbonate as Os-Cal®500+D (one tablet containing 500 mg elemental calcium and 125 IU of vitamin D, marketed by SmithKline Beecham Consumer Healthcare, Pittsburgh, PA, USA). For 1 week prior to each phase and between the first and second phases, participants were instructed to maintain dietary calcium (400 mg/day) and sodium (100 mEq/day) restrictions. None of the patients was on H2 receptor antagonists or proton pump inhibitor during the study.
The day prior to the test phase, each subject fasted after 6 p.m. and ingested 300 ml of distilled water at 8 p.m. and 11 p.m. The morning of the test day, subjects ingested 600 ml of distilled water at 6 a.m. and then 300 ml at 8 a.m., 10 a.m., and noon. At 8 a.m., one of the test medications was given with a standard breakfast (prepared by the GCRC dietician). The breakfast meal provided 403 kcal: 9.4 g of protein, 18.6 g of fat, 49.6 g of carbohydrate. The mineral composition was 97 mg calcium, 130 mg potassium, 19 mEq of sodium, 13 mEq of potassium, 40 mg of magnesium, 17 mEq of chloride, and 2 mg of oxalate. An intravenous heparin line was placed at 7:30 a.m., which was used to draw blood at 7:55 a.m. and 8 a.m., and then hourly from 8 a.m. to 2 p.m. A fasting urine sample was collected from 6 a.m. to 8 a.m. Urine was then collected in 2-h pools for 6 h after the calcium load (from 8 a.m. until 2 p.m.). The blood samples were evaluated for calcium and PTH levels. Urine was analyzed for calcium and creatinine.
Assays
Serum and urine calcium levels were measured by atomic absorption spectrophotometry, and urine creatinine was measured on an autoanalyzer using the Jaffe rate method. Serum intact PTH was determined using an immunoradiometric assay kit (ALPCO Diagnostics, Windham, NH, USA). Assays were performed without knowledge of treatment phase.
Statistical Analysis
Statistical analysis of the two-phase crossover trial was performed using mixed linear model and repeated measures analysis to assess order or carryover effects. The targeted sample size was 16 to 19 subjects, estimated to detect a difference between supplements of 0.80 mg/dl h for the serum calcium delta area under the curve. The estimated standard deviation was 1.0 mg/dl h for a desired power between 85% and 90% at the 0.05 level of significance. For the hourly timed measurements, repeated measures models were used to assess the effect of calcium supplement, time, and the interaction between supplement and time. Serum and urine calcium levels were assessed for peak values (Cmax) and area under the curve (AUC), cumulative excretion for urinary calcium. Serum parathyroid suppression was assessed with minimum PTH values (PTHmin) and PTH decrement (AUC). Time (tmax and tmin) to achieve serum and urinary Cmax and serum PTHmin were also derived from the hourly time course data. For these pharmacokinetic variables, the calcium supplements were compared with repeated measures models, which included factors to assess the effect of treatment order and the interaction between supplement and order. p values less than 0.05 were considered statistically significant. Results are expressed as mean and standard deviation. Analyses were performed using SAS® statistical software version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
Serum Calcium
At the baseline, serum calcium levels were similar (Table 1 and Fig. 1a). The mean serum calcium values for the calcium citrate and calcium carbonate phases were 9.1+0.4 and 9.0+0.3 mg/dl, respectively (p=0.22). The serum calcium levels for calcium carbonate were not significantly different throughout the experiment. For calcium citrate, the calcium levels increased considerably at hours 5 and 6. When the responses between phases were compared, serum calcium was notably higher with calcium citrate (ANOVA p<0.0001). In addition, the Cmax was significantly higher with calcium citrate compared to calcium carbonate (Table 1 and Fig. 1a) with 11 patients having a higher Cmax after calcium citrate than after calcium carbonate. Moreover, the length of time to achieve the Cmax was considerably longer for the calcium citrate group (3.60+2.30 h vs. 2.10+2.10 h, p=0.016). Calcium citrate also yielded a significantly greater AUC compared to calcium carbonate (p=0.02; Table 1 and Fig. 2b).
Table 1.
Calcium citrate vs. calcium carbonate–serum and urine changes
| n=18 | Calcium citrate | Calcium carbonate | p value |
|---|---|---|---|
| Serum calcium | |||
| Baseline mean mg/dl | 9.1±0.4 | 9.0±0.3 | 0.22 |
| AUC, mg/dl h | 55±2 | 54±2 | 0.02 |
| ΔAUC, mg/dl h | 0.40±1.03 | −0.04±0.02 | 0.24 |
| Cmax, mg/dl | 9.4±0.4 | 9.2±0.02 | 0.02 |
| Tmax, h | 3.6±2.3 | 2.1±0.02 | 0.02 |
| Median | 3.5 | 2 | |
| Peak-baseline, mg/dl | 0.30±0.22 | 0.19±0.11 | 0.11 |
| Serum PTH | |||
| Baseline mean pg/ml | 37±17 | 40±21 | 0.43 |
| AUC, pg/ml h | 191±73 | 235±94 | 0.001 |
| ΔAUC, pg/ml h | −32±51 | −3±0.01 | 0.04 |
| Cmin, pg/ml | 24±11 | 30±13 | 0.01 |
| Tmin, h | 2.3±1.7 (2.0) | 1.8±1.8 (1.0) | 0.32 |
| Nadir-baseline, pg/ml | −14±−14 | −11±0.22 | 0.22 |
| Urinary calcium | |||
| Baseline mean mg/2 h | 10.5±8.2 (8.7) | 10.1±7.6 (7.2) | 0.81 |
| AUC, mg/2 h h | 76±36 | 66±41 | 0.17 |
| ΔAUC, mg/2 h h | 13±30 | 5±33 | 0.23 |
| Cumulative increment | 51±25 | 42±25 | 0.07 |
| Urinary Ca/Cr | |||
| Baseline mean | 0.10±0.08 (0.08) | 0.10±0.07 (0.08) | 1.0 |
| AUC, mg/2 h h | 0.82±0.38 | 0.70±0.45 | 0.13 |
| ΔAUC, mg/2 h h | 0.21±0.27 | 0.09±0.21 | 0.05 |
| Cumulative increment | 0.53±0.25 | 0.45±0.28 | 0.06 |
Results are expressed mean±SD. Medians are presented in parentheses
AUC area under the curve, Cmax maximum concentration, Cmin minimum concentration, Tmax time at maximum concentration, Tmin time at minimum concentration
Fig. 1.
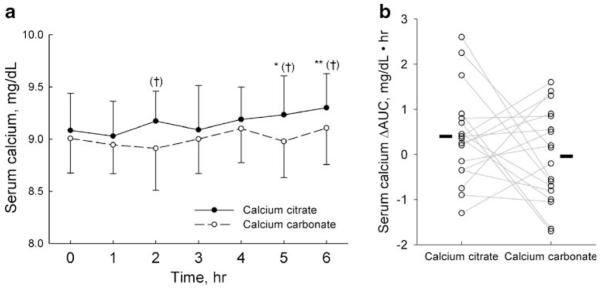
Serum calcium. a Data are presented as mean and standard deviation. The omnibus difference between the calcium supplements were statistically significant (p<0.0001, repeated measures analysis). Filled circles p<0.05, **p<0.01 vs. time 0; †p<0.001 vs. calcium carbonate. b Symbols and lines represent individual subjects. Solid bars indicate the mean ΔAUC of each phase
Fig. 2.
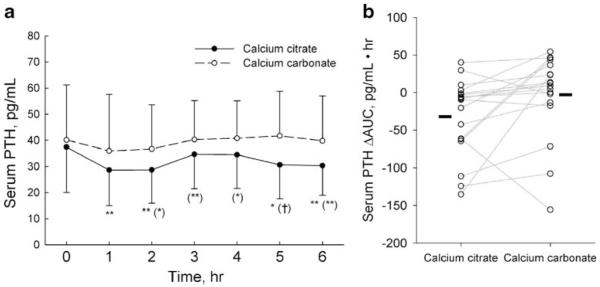
Serum PTH. a Data are presented as mean and standard deviation. The omnibus difference between the calcium supplements were statistically significant (p<0.0001, repeated measures analysis). *p<0.05, **p<0.01 vs. time 0; (*)p<0.05, (**)p<0.01, (†) p<0.001 vs. calcium carbonate. b Symbols and lines represent individual subjects. Solid bars indicate the mean ΔAUC of each phase
Serum PTH
At the baseline, serum PTH values were also similar between the two phases (Table 1 and Fig. 2a). However, during the study, the serum PTH levels dropped dramatically in the calcium citrate group. These values were lower at hours 1, 2, 5, and 6 when compared to the baseline. Serum PTH for the calcium carbonate phase showed no notable difference throughout the experiment. In a comparison between the two calcium supplement phases, serum PTH was significantly lower for calcium citrate (ANOVA, p<0.0001). As shown in Table 1, calcium citrate was associated with a considerably lower PTHmin (p=0.011) in which 12 of the 18 patients had a lower PTHmin after calcium citrate than after calcium carbonate. Calcium citrate was also associated with a lower ΔAUC (Table 1). The time required to achieve the PTHmin was not drastically different between the two phases (2.30+1.70 vs. 1.80+ 1.80, p=0.32), and these results were not affected by the order in which the calcium supplements were given.
Urinary Calcium
Urinary calcium values were similar at the baseline for both of the phases (Table 1). During the study, the urine calcium levels increased significantly following calcium citrate ingestion (p=0.002) but remained unchanged during the calcium carbonate phase (p=0.52). This differential response between the calcium supplements was statistically significant (p=0.03, phase by hour interaction; Fig. 3a and b).
Fig. 3.
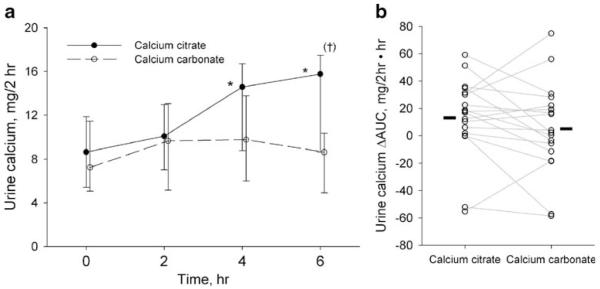
Urine calcium. a Data are presented as geometric mean and 95% confidence interval. *p=0.001 compared Time 0 within phase, (†)p=0.01 compared to calcium carbonate. The response differences over time between the calcium supplements were statistically significant (p=0.03, supplement by hour interaction). b Symbols and lines represent individual subjects. Solid bars indicate the mean ΔAUC of each phase
Urinary Calcium/Creatinine (Ca/Cr)
Urinary Ca/Cr values were similar at the baseline for each of the two phases (Table 1). During the study, the urine Ca/Cr levels increased significantly following ingestion of both calcium citrate (p<0.001) and calcium carbonate (p=0.02). However, over time, the responses between the phases showed a distinct difference (p=0.001, phase by hour interaction) with a larger increase in urinary Ca/Cr sustained at hour 6 during the calcium citrate phase (Fig. 4a and b).
Fig. 4.
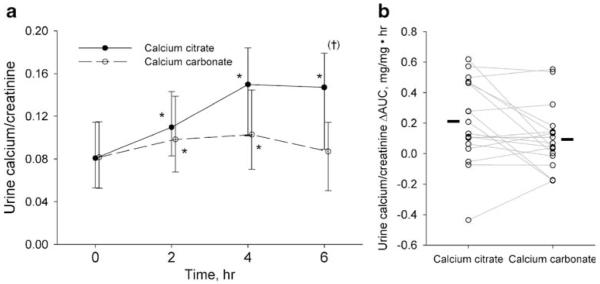
Urine calcium/creatinine. a Data are presented as geometric mean and 95% confidence interval. *p<0.05 vs. Time 0; (†)p=0.007 vs. calcium carbonate. The response differences over time between the calcium supplements were statistically significant (p=0.001, supplement by hour interaction). b Symbols and lines represent individual subjects. Solid bars indicate the mean ΔAUC of each phase
Discussion
Poor intestinal calcium absorption and disturbances in calciotropic hormone metabolism have been shown to play a major role in increasing the risk of bone loss and kidney stones following bariatric surgery [18–29]. This study for the first time explored the main differences in calcemic and calciuric responses between the two commonly used calcium supplements. In this study, the mean serum calcium concentration and peak basal variations in serum calcium were significantly higher for calcium citrate than calcium carbonate. Moreover, the cumulative increment in urinary calcium following the test load from baseline was significantly greater for calcium citrate than calcium carbonate (Table 1). It was also noteworthy that calcium citrate lowered the serum PTH concentration significantly as displayed by a greater cumulative fall in peak serum PTH concentrations. Thus, both the greater increment in serum calcium concentration and urinary calcium excretion in parallel with greater suppression of serum PTH suggest both the pharmacokinetic and pharmacodynamic superiority of calcium citrate.
Several studies have shown that the absorption of calcium carbonate is more dependent on gastric acid secretion than calcium citrate [31–36]. A previous study in healthy, postmenopausal women with varying degrees of gastric acid secretion reported a 2.5-fold higher intestinal calcium absorption following a single test load of calcium citrate than calcium carbonate [37]. Although it has been never tested, limited gastric acid secretion after RYGB could, in part, be a possible explanation for the similar findings in this study. In addition, other factors as a result of RYGB surgery, including diminished calcium absorption from both decreased intestinal absorptive capacity and from vitamin D derangements as a result of intestinal fat malabsorption, may also have influenced the findings [20, 39]. A recent year-long, prospective, longitudinal study showed that RYGB surgery is associated with high serum PTH, low serum 25-hydroxy vitamin D concentrations, and evidence of increased bone turnover with a rise in urinary N-telopeptide and serum osteocalcin [38]. A factor which influences the bioavailability of various calcium salts is gastric acid secretion. In contrast to the dependency of calcium carbonate solubility on an acidic environment, calcium citrate has even been shown to be partially soluble in water [32]. It is conceivable that this superior intestinal bioavailability of calcium citrate in the face of limited gastric acid secretion may compensate for both diminished intestinal absorptive capacity [40] and deranged vitamin D metabolism detected after RYGB [38].
However, there are some limitations to this study. We were unable to quantify gastrointestinal absorption of calcium isotopically since commercial calcium salts could not be labeled with calcium isotopes. Another limitation to this study was that we used only serum PTH levels as a surrogate marker of the patient’s response to calcium supplementation. However, a recent study showed a marked rise in urinary N-telopeptide commensurate with a significant rise in serum PTH immediately following RYGB [38]. It would have been ideal to simultaneously measure both urinary and serum markers of bone turnover in order to predict the magnitude of skeletal response to calcium citrate. Previous studies with calcium citrate alone [41] or comparing both the supplements showed a significant decrease in bone turnover markers with calcium citrate [42]. Our data suggest that in tablet formulation, calcium citrate is more bioavailable than calcium carbonate in lowering serum PTH levels and may prove to be superior in the reversal of secondary hyperparathyroidism, a condition which may accompany RYGB procedures. Long-term longitudinal studies to confirm the superiority of calcium citrate supplementation in patients following RYGB, preferably using different oral preparations, is needed to integrate these results to clinical practice.
Acknowledgments
This work was supported by the National Institute of Health (NIH) grants MO1-RR00633 and CTSA UL1-RR024982. The authors would like to acknowledge the editorial assistance of Ms. Hadley Armstrong.
Contributor Information
P. Tondapu, Department of Internal Medicine, Charles and Jane Pak Center for Mineral Metabolism, UT Southwestern Medical Center, Dallas 75390-8885 TX, USA
D. Provost, Provost Bariatrics, Denton, TX, USA
B. Adams-Huet, Division of Biostatistics, Department of Clinical Sciences, UT Southwestern Medical Center, Dallas, TX, USA
T. Sims, Department of Internal Medicine, Charles and Jane Pak Center for Mineral Metabolism, UT Southwestern Medical Center, Dallas 75390-8885 TX, USA
C. Chang, Citizens Bariatric Center, Victoria, TX, USA
K. Sakhaee, Department of Internal Medicine, Charles and Jane Pak Center for Mineral Metabolism, UT Southwestern Medical Center, Dallas 75390-8885 TX, USA
References
- 1.WHO information sheet and obesity and overweight. 2003.
- 2.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. National Institutes of Health. Obes Res. 1998;6(Supp 2):51S–209S. [PubMed] [Google Scholar]
- 3.Shah M, Simha V, Garg A. Long term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–31. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 4.North American Association for the study of obesity and the National Heart, Lung, and Blood Institute The practice guide: identification, evaluation, and treatment of overweight and obesity in adults. 2000 NIH publication 00-4084. [Google Scholar]
- 5.National Task Force on the Prevention and Treatment of Obesity Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 6.Eckel RH. Non surgical management of obesity. N Engl J Med. 2008;358:1941–50. doi: 10.1056/NEJMcp0801652. [DOI] [PubMed] [Google Scholar]
- 7.Steinbrook R. Surgery for severe obesity. NEJM. 2004;350:1075–9. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 8.Byrne TK. Complications of surgery for obesity. Surg Clin North Am. 2001;81:1181–93. doi: 10.1016/s0039-6109(05)70190-0. [DOI] [PubMed] [Google Scholar]
- 9.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–17. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 10.Waters GS, Pories WJ, Swanson MS, et al. Long term studies of mental health after the Greenville gastric bypass operation for morbid obesity. Am J Surg. 1991;161:154–7. doi: 10.1016/0002-9610(91)90377-p. [DOI] [PubMed] [Google Scholar]
- 11.Balsiger BM, Kennedy FP, Abu-Lebdeh HS, et al. Prospective evaluation of Roux-en-Y gastric bypass as primary operation for medically complicated obesity. Mayo Clin Proc. 2000;75:673–80. doi: 10.4065/75.7.673. [DOI] [PubMed] [Google Scholar]
- 12.Sjostorm L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 13.Sujerman HJ, Wolfe LG, Sica DA, et al. Diabetes and hypertension in severe obesity and effects of gastric bypass induced weight loss. Ann Surg. 2003;237:751–6. doi: 10.1097/01.SLA.0000071560.76194.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald KG, Jr, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg. 1997;1:213–20. doi: 10.1016/s1091-255x(97)80112-6. [DOI] [PubMed] [Google Scholar]
- 15.Flum DR, Dellinger EP. Impact of gastric bypass on survival: a population based analysis. J Am Coll Surg. 2004;199:543–51. doi: 10.1016/j.jamcollsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–23. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson J, Sjostrom L, Sullivan M. Swedish Obese Subjects (SOS): an intervention study of obesity: two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998;22:113–26. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 18.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565–9. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Nelson WK, Houghton SG, Milliner DS, et al. Enteric hyper-oxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481–5. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100–7. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 21.Patel BN, Passman CM, Fernandez A, et al. Prevalence of hyperoxaluria after modern bariatric surgery. Presented at 25th World Congress of Endurology & SWL; 2007.2007. [Google Scholar]
- 22.Prisco CD, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61. doi: 10.1097/00000441-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Goldner WS, O’Dorisio TM, Dillon JS, et al. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg. 2002;12:685–92. doi: 10.1381/096089202321019693. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JM, Maher JW, DeMaria EJ, et al. The long term effects of gastric bypass on Vitamin D metabolism. Ann Surg. 2006;243:701–5. doi: 10.1097/01.sla.0000216773.47825.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–5. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 26.Von Mach MA, Stoeckli R, Bilz S, et al. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53:918–21. doi: 10.1016/j.metabol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Goode LR, Brolin RE, Chowdhury HA, et al. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12:40–7. doi: 10.1038/oby.2004.7. [DOI] [PubMed] [Google Scholar]
- 28.Basha B, Rao S, Han ZH, et al. Osteomalacia due to vitamin D depletion: neglected consequence of intestinal malabsorption. Am J Med. 2000;108:296–300. doi: 10.1016/s0002-9343(99)00460-x. [DOI] [PubMed] [Google Scholar]
- 29.Slater GH, Ren CF, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. Gastrointest Surg. 2004;8:48–55. doi: 10.1016/j.gassur.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Cifuentes M, Riedt CS, Brolin RE, et al. Weight loss and calcium intake influence calcium absorption in overweight postmenopausal women. Am J Clin Nutr. 2004;80:123–30. doi: 10.1093/ajcn/80.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recker RR. Calcium absorption and achlorohydria. N Engl J Med. 1985;313:70–3. doi: 10.1056/NEJM198507113130202. [DOI] [PubMed] [Google Scholar]
- 32.Pak CYC, Poindexter J, Finlayson B. A model system for assessing physicochemical factors affecting calcium absorbability from the intestinal tract. J Bone Miner Res. 1989;4:119–27. doi: 10.1002/jbmr.5650040117. [DOI] [PubMed] [Google Scholar]
- 33.Pak CY, Avioli LV. Factors affecting absorbability of calcium from calcium salts and food. Calcif Tissue Int. 1988;43:55–60. doi: 10.1007/BF02555147. [DOI] [PubMed] [Google Scholar]
- 34.Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract. 2007;22:286–96. doi: 10.1177/0115426507022003286. [DOI] [PubMed] [Google Scholar]
- 35.Malone M. Recommended nutritional supplements for bariatric surgery patients. Ann Pharmacother. 2008;42(12):1851–1858. doi: 10.1345/aph.1L321. [DOI] [PubMed] [Google Scholar]
- 36.Hunt JN, Johnson C. Relation between gastric secretion of acid and urinary excretion of calcium after oral supplements of calcium. Dig Dis Sci. 1983;28:417–21. doi: 10.1007/BF02430530. [DOI] [PubMed] [Google Scholar]
- 37.Heller HJ, Stewart A, Haynes S, Pak CYC. Pharmacokinetics of calcium absorption from two commercial calcium supplements. Am J Clin Pharmacol. 1999;39:1151–4. [PubMed] [Google Scholar]
- 38.Fleischer J, Stein EM, Bessler M, et al. The decline in Hip Bone Density after Gastric Bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gagner M, Rogula T, Strain G, et al. Decreased Lipid malabsorption in both gastric bypass and biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. 2005;1(3):240–1. [Google Scholar]
- 40.Reidt CS, Brolin RE, Sherell RM, et al. True fractional calcium absorption is decreased after Roux-en-Y Gastric Bypass surgery. Obesity. 2006;11:1940–8. doi: 10.1038/oby.2006.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakhaee K, Maalouf NM, Abrams SA, et al. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2005;90:3528–33. doi: 10.1210/jc.2004-2451. [DOI] [PubMed] [Google Scholar]
- 42.Kenny AM, Prestwood KM, Biskup B, et al. Comparison of the effects of calcium loading with calcium citrate or calcium carbonate on bone turnover in postmenopausal women. Osteoporos Int. 2004;15:290–4. doi: 10.1007/s00198-003-1567-0. [DOI] [PubMed] [Google Scholar]


