Abstract
Nasal application of native cholera toxin (nCT) as a mucosal adjuvant has potential toxicity for the CNS through binding to GM1 gangliosides in the olfactory nerves. Although mutants of cholera toxin (mCTs) have been developed that show mucosal adjuvant activity without toxicity, it still remains unclear whether these mCTs will induce CNS damage. To help overcome these concerns, in this study we created new double mutant CTs (dmCTs) that have two amino acid substitutions in the ADP-ribosyltransferase active center (E112K) and COOH-terminal KDEL (E112K/KDEV or E112K/KDGL). Confocal microscopic analysis showed that intracellular localization of dmCTs differed from that of mCTs and nCTs in intestinal epithelial T84 cells. Furthermore, both dmCTs exhibited very low toxicity in the Y1 cell assay and mouse ileal loop tests. When mucosal adjuvanticity was examined, both dmCTs induced enhanced OVA-specific immune responses in both mucosal and systemic lymphoid tissues. Interestingly, although both dmCT E112K/KDEV and dmCT E112K/KDGL showed high Th2-type and significant Th1-type cytokine responses by OVA-specific CD4+ T cells, dmCT E112K/KDEV exhibited significantly lower Th1-type cytokine responses than did nCT and dmCT E112K/KDGL. These results show that newly developed dmCTs retain strong biological adjuvant activity without CNS toxicity.
An important aspect of immune responses at mucosal surfaces is the production of polymeric IgA Abs, as well as their transport across the epithelium and release as secretory IgA (S-IgA).3 Because this S-IgA Ab response represents the first major line of defense against invasion by viral and bacterial pathogens (1), recent efforts have been focused on the development of vaccines that are capable of inducing effective immune responses in mucosal tissues. However, most protein Ags are rather weak immunogens when given by a mucosal route. If the full potential of the new generation of mucosal vaccines is to be realized, effective and reliable mucosal adjuvants must be developed.
Our recent study (2) showed that nasal vaccines for nasopharyngeal-associated lymphoreticular tissue (NALT)-based mucosal immunity could make a significant contribution to protecting the elderly. Furthermore, these nasal and oral vaccines would be easier to administer than parenteral ones. Mucosal vaccines would also carry less risk of transmitting infections like hepatitis B and HIV, which are still associated with the use of injectable vaccines in several parts of the world. Despite these many attractive features, it has often proved difficult in practice to stimulate strong mucosal S-IgA Ab responses with subsequent protection by the use of mucosal administration of vaccines, and the results to date for mucosal vaccinations using soluble protein Ags have been, with a few notable exceptions, rather disappointing (3).
Native cholera toxin (nCT) produced by Vibrio cholerae is structurally similar to the native heat-labile enterotoxin (nLT) of enterotoxigenic Escherichia coli. Both toxins act as adjuvants for the enhancement of mucosal and systemic Ab responses to coadministered protein Ags given by either oral or nasal routes (4–7) and, consequently, are the most widely used experimental mucosal adjuvants in animal models. Furthermore, both act as mucosal adjuvants by inducing CD4+ Th2 cells secreting IL-4, IL-5, IL-6, and IL-10, which provide help for Ag-specific S-IgA as well as plasma IgG1, IgA, and IgE Ab responses (8, 9). Although they are potent mucosal adjuvants, both nCT and nLT are also toxic and, thus, are not suitable for use with mucosal vaccines in humans. Therefore, a number of nontoxic mutant derivatives of cholera toxin (CT) or heat-labile enterotoxin (LT) have been constructed.
Our own group has contributed to the efforts of constructing nontoxic mutant derivatives; we generated two mutant CTs (mCTs), mCT S61F and mCT E112K, by substituting a single amino acid in the ADP-ribosyltransferase active center of the A subunit (10, 11). Although these originally created forms of mCT did not induce ADP-ribosylation and cAMP formation, they still served as mucosal adjuvants by inducing CD4+ Th2 cells, thereby providing effective help for Ag-specific, mucosal S-IgA, as well as plasma IgG and IgA Ab responses.
It is clearly too dangerous to use an enterotoxin as an adjuvant for mucosal vaccines in humans. Our previous studies have shown that nasally administered nCT accumulates in the olfactory nerves and epithelium (ON/E) and olfactory bulbs (OBs) of mice after binding to GM1 gangliosides (12). Furthermore, nCT as a mucosal adjuvant redirects coadministered protein Ags into these neuronal tissues (12). This finding has provoked some concern about the potential role for ganglioside GM1-binding molecules that target neuronal tissues, including the CNS, in nasal immunization. Although deposition of nCT via the ON/E and OBs did not lead to obvious pathologic changes in brain tissue after nasal administration (13), it has been reported that a human vaccine containing inactivated influenza and nLT as an adjuvant resulted in a very high incidence of Bell's palsy (14, 15). These results strongly indicate that it is essential to develop a safer and more effective nasal adjuvant for human use.
Both nCT and nLT consist of a ring of five B subunits and a single A subunit that is cleaved into A1 and A2 chains. Previous studies showed that the carboxyl terminus of the A2 subunit of nCT contains the ER retention signal tetrapeptide KDEL (RDEL in LT). These results show that the toxin is transported via the vesicles from the plasma membrane to the Golgi compartment with subsequent separation of the A and B subunits of nCT (designated throughout as CT-A and CT-B, respectively). The CT-A subunit is redirected to the plasma membrane by retrograde transport via the ER, whereas the CT-B subunit persists in the Golgi compartment. The intracellular target of toxin is Gs, the stimulatory regulatory component of adenyl cyclase (16, 17). Thus, mutations in KDEL influence the movement of CT from the Golgi apparatus to the ER (18–21). It has previously been shown that mutation in K(R)DEL of both CT and LT delayed the time course of toxin-induced Cl−secretion. Furthermore, consistent with a slower rate of signal transduction, KDEL mutants trafficked more slowly to the basolateral membrane than did nCT (18, 19).
However, a recent report indicated that mutant LT RDEL retained ADP-ribosyltransferase activity and induced morphological changes in Chinese hamster ovary (CHO) cell cultures (19). In contrast, mCT E112K has proven to be a safe and stable adjuvant (10, 11, 13, 22). The mCT E112K was constructed to be devoid of toxicity while retaining its adjuvant activity. Our previous studies showed that mCT E112K was effective in the murine system. Nasal immunization of OVA, the pneumococcal surface protein A of Streptococcus pneumoniae, or diphtheria toxoid plus mCT E112K elicited both Ag-specific IgA and IgG Ab responses in mucosal and systemic immune compartments (10, 11, 13). Although our recent studies showed that mCT E112K did not elicit any neuronal damage based upon nerve growth factor-β1 production in the CNS as well as Ag redirection (23, 24), these studies did not provide any direct information as to whether mCT E112K migrates into the CNS after nasal application. In this regard, we have developed double mutants of CT (dmCTs) by introducing a potent mutation in the ADP-ribosylation activity center and KDEL (E112K/KDEV or E112K/KDGL). Therefore, it is now essential for us to determine whether potentially less trafficking of these new dmCTs, when compared with mCT E112K, will occur in their distribution into the CNS. In this study, we have examined the mucosal adjuvanticity and toxicity of these two dmCTs for our continuing efforts to develop safe and effective nasal adjuvants for mucosal vaccines.
Materials and Methods
Preparation of recombinant mCTs
The plasmids containing nCT or mCT E112K genes were constructed as described previously (10). These plasmids were cloned into pUC119 of a 3.1-kb EcoRI/PstI DNA fragment including nCT or mCT E112K genes (10). The KDGL and KDEV mutants were constructed by site-directed mutagenesis in which amino acid substitutions were introduced on a plasmid of nCT using PCR. The following oligonucleotides were used to create genes encoding the mutant toxins KDEV and KDGL: 5′-TAA GGA TGA AGT ATG ATT AAA TTA A-3′ and 5′-TAG AAT TAA GGA TGG ATT ATG ATT A-3′ (mutant codons underlined). To construct dmCT E112K/KDEV and E112K/KDGL, the BspEI/HincII fragments including KDEV or KDGL mutations were ligated to a plasmid of mCT E112K. After the DNA sequences were confirmed, pUC119 harboring the mutated CT genes at the EcoRI/PstI site were transformed into E. coli DH5-α. The E. coli strains containing the plasmids for the dmCT genes were grown in Luria-Bertani medium (10 g of NaCl, 10 g of tryptone, and 5 g of yeast extract per liter) with 100 μg/ml ampicillin, and dmCTs were purified according to the method described previously (25). Briefly, the bacteria were harvested and lysed with a sonicator (Insonator 201M; Kubota). The crude lysate was then applied to an immobilized D-galactose column (Pierce) and eluted with galactose. The purified recombinant dmCTs contained <0.05 endotoxin U/μg protein.
Intracellular tracking
Human intestinal epithelial T84 cells were incubated with 10 μg/ml Alexa Fluor 488-conjugated nCT, mCT E112K, dmCT E112K/KDEV, or dmCT E112K/EDGL. To identify their intracellular destination, we used boron dipyrromethane Texas Red ceramide (Invitrogen Life Technologies) as a marker for the Golgi apparatus (26) and ER-Tracker (Invitrogen Life Technologies) Blue-White p-xylene-bis-pyridinium bromide as a marker for the ER (27). Boron dipyromethane Texas Red-ceramide was added to individual cultures at a concentration of 5 μM for 10 min before termination of the cultures. Cells were washed once before being further cultured in medium containing 2 μM ER-Tracker for 30 min. After incubation, cells were washed three times with PBS and then fixed with 3.7% formaldehyde in PBS for 10 min at room temperature. After fixation, a Leica TCS SP2-AOBS model confocal microscope or a LMS510 model confocal microscope (Zeiss) was used to visualize the cells. The merged color (yellow) area of each Golgi or ER staining image was picked up using Adobe Photoshop software and converted into a black and white picture. The black area in this black and white picture is based upon the original yellow color and was measured using ImageJ (National Institutes of Health). The condition for picking up the yellow area was saved and applied to all pictures. A monolayer of T84 cells covered most of the dish surface; however, for accuracy, we measured the total pixel area covered with cells in the identical picture using ImageJ software. Thus, each picture covers 12.96 mm2 of culture surface, which is equal to 262144 pixels. The results were shown as the percentage of yellow color area pixels per the total area pixels covered with cells.
Bioassay and toxicity analysis
We next studied the ability of newly created dmCTs and nCT (List Biological Laboratories) to induce toxic effects on cultured mouse Y-1 adrenal tumor cells, following a previously developed procedure (28). Briefly, serial dilutions of dmCT E112K/KDEV, dmCT E112K/KDGL, mCT E112K, or nCT were added to cultures of Y-1 cells at a density of 5 × 104 cells/well in 0.1 ml of F-10 medium (Invitrogen Life Technologies) containing 15% horse serum and 2.5% FCS and incubated at 37°C with 5% CO2 for 24 h. Light microscopy was then used to examine cells for morphological changes such as the common “rounding” of cells. The toxin concentration required to initiate the rounding of Y-1 cells was determined. For the cAMP assay, 1.5 × 104 CHO cells in F-10 medium containing 1% FCS were cultured with 100 ng/ml dmCT E112K/KDEV, dmCT E112K/KDGL, mCT E112K, or nCT at 37°C with 5% CO2 for 18 h. Intracellular cAMP measurement was done with an enzyme immunoassay kit (Amersham Biosciences). The protein amount for some samples was determined with a Coomassie protein assay reagent (Pierce), and the levels of cAMP were expressed as picomoles of cAMP per milligram of protein (10). The mouse ileal loop test was conducted essentially as described previously (29, 30). Briefly, the jejenum was ligated with a piece of cotton thread at a distance of ~3 cm from the pylorus. Immediately after ligation, each loop was injected with 0.1 ml of toxin or PBS (as a control). After 3 h, each loop was hung on a fixed clip and stretched by placing another clip weighing 2 g on the other end of the loop. Then, the length and weight of each loop were measured. The weight/length ratio (mg/cm) was used to express the intensity of the reaction.
Mice
C57BL/6 mice were obtained from the Frederick Cancer Research Facility (National Cancer Institute, Frederick, MD) as well as Japan SLC at 8–12 wk of age. Upon arrival, all mice were immediately transferred to mi-croisolators, maintained in horizontal laminar flow cabinets, and provided sterile food and water ad libitum. The health of the mice was tested semi-annually, and all mice used in experiments were determined to be free of bacterial and viral pathogens.
CNS trafficking
We investigated the distribution of acridinium-labeled toxins in the ON/E and OBs after nasal immunization. In these experiments, we used acridinium-labeled toxins because acridinium is a chemiluminescent molecule that can be triggered with sodium hydroxide and hydrogen peroxide in a luminometer to emit light at 430 nm. Because of its high sensitivity, acridinium can be detected at levels as low as femtograms. nCT, mCT E112K, dmCT E112K/KDEV, and dmCT E112K/KDGL were labeled with acridinium as described elsewhere (31). Five or 0.5 μg of acridinium-labeled nCT, mCT E112K, dmCT E112K/KDEV, or dmCT E112K/KDGL was given nasally, and its distribution in CNS tissues was then checked after 24 h. In some experiments, mice were given nasal OVA plus 0.5 μg of acridinium-labeled nCT, mCT E112K, dmCT E112K/KDEV, or dmCT E112K/KDGL three times at weekly intervals. Seven days after the last immunization, residual amounts of enterotoxin in the CNS tissues were detected. For isolation of CNS tissues, we examined both ON/E and OBs as previously described (12). The levels of acridinium present were determined by triggering with sodium hydroxide and hydrogen peroxide in a luminometer emitting light at 430 nm. The CNS tissues of mice given nasal PBS were examined for their background levels of luminescence. These control values are subtracted from each experimental value.
Nasal immunization and sample collection
C57BL/6 mice were immunized three times at weekly intervals with a nasal dose of 100 μg of OVA (Fraction V; Sigma-Aldrich) and 0.5 μg of nCT, mCT E112K, dmCT E112K/KDEV, or dmCT E112K/KDGL in PBS (11, 22, 32). Plasma and mucosal secretions (nasal washes, saliva, and fecal extracts) were collected on day 21. Saliva was obtained from mice following i.p. injection of 100 μg of sterile pilocarpine hydrochloride (Sigma-Aldrich) (33). Fecal pellets (100 mg) were suspended in 1 ml of PBS containing 0.1% sodium azide and then extracted by vortexing for 5 min. The samples were spun at 10,000 × g for 5 min, and the supernatants were collected as fecal extracts (11, 22, 33). The nasal washes were obtained by injecting 1 ml of PBS containing 1% BSA on three occasions into the posterior opening of the nasopharynx with a hypodermic needle (34).
Ab assays
Ab titers in plasma and external secretions were determined by an ELISA (10, 11, 22, 35). Falcon microtest assay plates (BD Biosciences) were coated with an optimal concentration of OVA (100 μl of 1 mg/ml) in PBS overnight at 4°C. Two-fold serial dilutions of samples were added after blocking with PBS containing 1% BSA. To detect Ag-specific Ab levels, HRP-conjugated, goat anti-mouse μ, γ, or α H chain-specific Abs were used (Southern Biotechnology Associates). For IgG Ab subclass determinations, biotinylated mAbs specific for IgG1, IgG2a, IgG2b, and IgG3 (BD Pharmingen) and peroxidase-conjugated goat anti-biotin Ab were used. End point titers were expressed as the last dilution yielding an OD414 nm of >0.1 U above negative control values after 15 min of incubation.
ELISA for OVA-specific IgE Ab responses
OVA-specific IgE Abs were determined by an ELISA (32). Plasma samples were collected 2 wk after the initial nasal immunization, because our previous studies showed that peak Ag-specific IgE Ab responses were seen at this time point, when mice were immunized with OVA and either nCT or mCT E112K as mucosal adjuvants (11). For detection of OVA-specific plasma IgE Ab levels, 96-well immunoplates (Nunc) were coated with rat anti-mouse IgE mAb (R35-72; BD Pharmingen) and incubated overnight at 4°C. After blocking with 3% BSA in PBS, serial dilutions of plasma samples were added and incubated overnight at 4°C. Following extensive washing, biotinylated OVA was added and the plates were incubated overnight at 4°C. Next, HRP-labeled goat anti-biotin Ab (Vector Laboratories) was added, and the color reaction was developed with 2, 2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma). End point titers were expressed as the last dilution yielding an OD414 nm of >0.1 U above negative control values after 15 min incubation.
Enumeration of Ab-forming cells (AFCs)
The spleen and cervical lymph nodes (CLNs) were removed aseptically, and single-cell suspensions were prepared as described elsewhere (11, 22, 32, 35). The submandibular glands (SMGs) and PBS-perfused lungs were removed aseptically and minced into small fragments. Mononuclear cells were isolated by a combination of an enzymatic dissociation procedure with collagenase type IV (0.5 mg/ml; Sigma-Aldrich) followed by discontinuous Percoll (Amersham Biosciences) gradient centrifugation (33). For isolation of mononuclear cells from NALT and nasal passages (NPs), a modified dissociation method was used based upon a previously described protocol (36–38). Individual NALTs were carefully removed using micro-surgical tweezers under a stereoscopic microscope. Following removal of the NALT, the NP tissues were also removed from the nasal cavity. Cells from individual tissues were prepared by gently teasing them through sterile stainless steel screens, followed by enzymatic dissociation using collagenase type IV to obtain single-cell preparations (36–38). Mononuclear cells were purified by discontinuous Percoll gradients (Amersham Biosciences). Mononuclear cells in the interface between the 40 and 75% layers were removed, washed, and resuspended in RPMI 1640 (Mediatech) supplemented with HEPES buffer (15 mM), L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% FCS (complete medium). Cells were then subjected to an OVA-specific ELISPOT assay to detect cells producing IgM, IgG, and IgA AFCs (11, 22, 32, 35). Ninety-six-well nitrocellulose plates (Millipore) were coated with 1 mg/ml OVA for analysis of anti-OVA specific AFCs (11, 32, 33).
OVA-specific T cell responses
CD4+ T cells were purified by the magnetic-activated cell sorter system (Miltenyi Biotec) as described previously (11, 35). Briefly, cells from the spleen and CLNs were incubated in a nylon wool column (Polysciences) to remove B cells and macrophages. Enriched T cell fractions were then incubated with anti-mouse CD4 (GK 1.5) microbeads (Miltenyi Biotec) and passed through a magnetized column. The purified T cell fractions were >97% CD4+ and >99% viable. Cells were resuspended in complete medium, and the purified CD4+ T cells (4 × 106 cells/ml) were cultured with or without 1 mg/ml OVA in the presence of T cell-dependent, mitomycin C-treated splenic APCs. These APCs were derived from naive mice and were placed in 96-well or 24-well tissue culture plates (Corning Glass) for 5 days at 37°C in a moist atmosphere of 5% CO2 in air. In some experiments, purified CD4+ T cells from CLNs and spleen of naive mice were incubated with anti-CD3 mAb (10 μg/ml)- and anti-CD28 mAb (10 μg/ml)-coated wells for 2 days at 37°C in a moist atmosphere of 5% CO2 in air.
Proliferation of CD4+ T cells was assessed using a cell proliferation ELISA (Roche Diagnostic Systems). Briefly, 10 μmol of BrdU was added for the final 18 h of incubation. The plates were centrifuged at 300 × g for 10 min before drying. The plates were incubated with FixDenat solution (Roche) for 30 min at room temperature. Once the solution had been removed, the cells were labeled with a peroxidase-conjugated anti-BrdU mAb for 90 min at room temperature. The plates were washed with PBS-Tween 20 and then developed using a tetramethyl benzidine substrate solution. Incubations were terminated by addition of 1 M H2SO4. The absorbance of samples was measured by an ELISA reader at 450 nm. In some experiments, culture supernatants were harvested after 2 or 5 days of incubation and were then subjected to cytokine-specific ELISA.
Cytokine-specific ELISA
Levels of cytokines in culture supernatants were measured by ELISA. The details of the ELISA for IFN-γ, IL-2, IL-4, IL-5, IL-6, and IL-10 have been described previously (10, 39–41). For coating and detection, the following mAbs were used: for anti-IFN-γ, R4-6A2 and XMG1.2 mAbs; for anti-IL-2, JSE6-1A12 and JES-5H4 mAbs; for anti-IL-4, BVD4-1D11 and BVD6-24G2 mAbs; for anti-IL-5, TRFK-5 and TRFK-4 mAbs; for anti-IL-6, MP5-20F3 and MP5-32C11 mAbs; and for anti-IL-10, JES5-2A5 and JES5-16E3 mAbs. The levels of Ag-specific cytokine production were calculated by subtracting the results of control cultures (e.g., without OVA stimulation) from those of OVA-stimulated T cell cultures. This ELISA was capable of detecting 0.10 ng/ml IFN-γ, 30.5 pg/ml IL-2, 23.4 pg/ml IL-4, 6.1 pg/ml IL-5, 78.1 pg/ml IL-6, and 24.4 ng/ml IL-10.
Statistics
The data are expressed as the mean ± SEM. Each mouse group that received dmCT was compared with the mice given nCT or mCT E112K as nasal adjuvants using a Mann-Whitney U test with StatView II (Abacus Concepts) designed for Macintosh computers. A p value of <0.05 or <0.01 was considered significant.
Results
Double mutant CTs fail to track from the Golgi into the ER
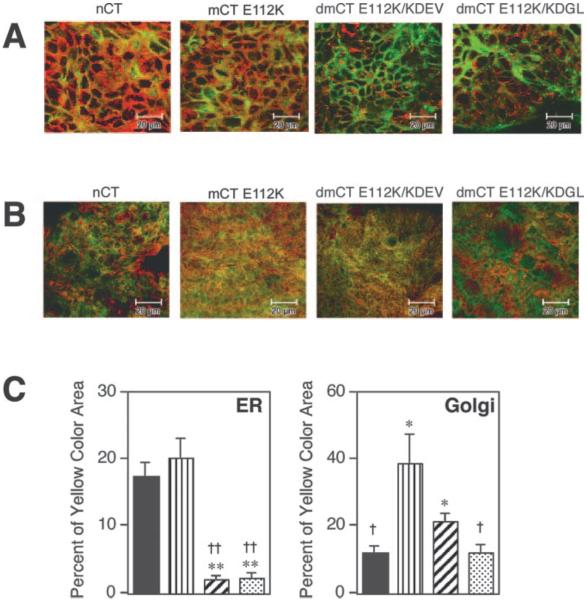
Because it has been shown that the COOH terminus of the CT-A subunit KDEL is essential for intracellular movement of CT from the Golgi to the ER, we initially examined intracellular trafficking of our newly developed second generation dmCTs in the T84 human intestinal cell line using confocal microscopic analysis (Fig. 1). After 4 h of incubation, nCT and mCT E112K were detected in the ER of T84 cells (Fig. 1, A and C, yellow staining); however, dmCT E112K/KDEV and dmCT E112K/KDGL were not detected there (segregated green and red staining; Fig. 1A). Thus, the area of yellow staining with both dmCTs was significantly lower than that of nCT and mCT E112K (Fig. 1C). Both mCT E112K and dmCT E112K/KDEV were seen in the Golgi apparatus (Fig. 1B), and the large area of yellow staining was noted (Fig. 1C). Interestingly, the distribution pattern of dmCT E112K/KDEV and dmCT E112K/KDGL differed with the former by being retained longer in the Golgi apparatus than the latter (Fig. 1B). Thus, dmCT E112K/KDGL resulted in segregated green and red staining with only a small area of yellow staining (Fig. 1, B and C). Because a small area of nCT was noted in the Golgi apparatus (Fig. 1C), it is possible that nCT moved quickly into the ER or the surface membrane. In contrast, dmCT E112K/KDGL is most likely redistributed to the surface membrane, because a significantly lower retention signal was found in the ER (Fig. 1C). As these results show, because these dmCTs are either retained in the Golgi apparatus (dmCT E112K/KDEV) or redistributed to the surface membrane (dmCT E112K/KDGL), they do not undergo retrograde transport into the ER. In contrast, both nCT and mCT E112K reached the ER within 4 h of incubation.
FIGURE 1.
Localization of nCT, dmCT E112K/KDEV, dmCT E112K/KDGL, and mCT E112K in T84 cells after incubation. Cells were fixed and analyzed 4 h after the start of Alexa Fluor 488-conjugated nCT, dmCT E112K/KDEV, dmCT E112K/KDGL, and mCT E112K uptake. To identify the intracellular destination, colocalization with ER-Tracker Blue-White p-xylene-bispyridinium bromide, a marker for the ER, is shown in quasi-red (A), whereas BODIPY TR-cer-amide, a marker for the Golgi apparatus, is shown in red (B). The merged signals appear yellow. Typical image pictures are presented for each group. The yellow color area of nCT (■), mCT E112K (▥), dmCT E112K/KDEV (▨), and dmCT E112K/KDGL ( ) were quantified with ImageJ software (C). The values represent the mean ± 1 SEM for six pictures from two different experiments. Each picture covers 12.96 mm2 of culture surface, which was equal to 262,144 pixels. *, p < 0.05; **, p < 0.01, when compared with nCT; †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
) were quantified with ImageJ software (C). The values represent the mean ± 1 SEM for six pictures from two different experiments. Each picture covers 12.96 mm2 of culture surface, which was equal to 262,144 pixels. *, p < 0.05; **, p < 0.01, when compared with nCT; †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
Enzymatic activity and toxicity of mCTs
The biologic properties and toxicity of dmCTs were examined and compared with those of mCT E112K and nCT (Table I). Essentially no cAMP induction was seen when CHO cells were incubated with dmCTs or mCT E112K. To further examine the toxicity of dmCTs, the morphological changes in Y-1 cells were assessed. Both dmCT E112K/KDEV and E112K/KDGL showed significantly lower toxicity than nCT (1/2083 and 1/2778 of nCT, respectively). In addition, when the mouse ileal loop test was performed to measure toxic manifestations, both dmCTs were found to induce significantly lower levels of fluid accumulation than nCT.
Table I.
Comparison of biologic and toxic activity of mCTs
| Nasal Adjuvant | cAMP Inductiona (pmol/mg protein) | Y1-Cell Assayb (pg/ml) | Heal Loop Testc (W:L ratio) |
|---|---|---|---|
| nCT | 104.4 ± 2.3†† | 0.06 ± 0.01†† (1)d | 164.3 ± 4.0†† |
| E112K/KDEV | 5.3 ± 0.3** | 125.0 ± 20.8** (1/2083) | 47.7 ± 9.7** |
| E112K/KDGL | 6.5 ± 0.2** | 166.7 ± 9.7** (1/2778) | 41.7 ± 3.4** |
| E112K | 7.0 ± 1.0** | 234.1 ± 44.3* (1/3902) | 45.5 ± 0.4** |
| PBS | 7.4 ± 0.5** | 37.6 ± 4.4** |
CHO cells (1.5 × 104 cells/well) were cultured in F-10 medium containing 1 % FCS with 100 ng/ml of each toxin for 18 h, and the cAMP induction was assessed using an enzyme immunoassay. Values represent the mean ± SEM of nine culture wells in each group. **, p < 0.01, when compared with nCT. ††, p < 0.01, when compared with mCT E112K.
Y-1 cells were cultured with F-12 medium containing 15% horse serum and 2.5% FCS and serial dilution of each toxin for 24 h. The enterotoxin concentration required to initiate rounding was determined. Values represent the mean ± SEM of of 12 culture wells in each group. *, p < 0.05; **, p < 0.01, compared with nCT. ††, p < 0.01, when compared with mCT E112K.
The ratio of the weight in milligrams to length in centimeters (W:L ratio) after injection of dmCTs into the loop. Each loop was injected with 0.1 ml of 1 /xg of dmCTs or nCT. The weight and length of each loop were measured after 3 h values. Values represent the mean ± SEM of six loops in each group. **, p < 0.01, when compared with nCT. ††, p < 0.01, when compared with mCT E112K.
The values in parenthesis is the ratio of the toxicity of dmCTs to the toxicity of nCT.
dmCTs do not accumulate in the CNS
To determine whether dmCTs undergo retrograde transport into the CNS, acridinium-labeled dmCT E112K/KDEV, dmCT E112K/KDGL, mCT E112K, or nCT were given by the nasal route. Twenty-four hours after nasal administration of 0.5 μg of nCT, mCT E112K, dmCT E112K/KDEV, and dmCT E112K/KDGL, similar levels of all administered enterotoxins could be detected in the ON/E, but no significant accumulation of the enterotoxins was seen in the OBs (Table II). Accumulation in the ON/E appeared to be dose dependent. Thus, 2- to 4-fold higher quantities of nCT and dmCTs were found in the ON/E after nasal delivery of a 5-μg dose (Table II). At a 5-μg nasal dose, dmCTs accumulated more in the ON/E of mice than did nCT or mCT E112K, but nCT accumulated in the OBs whereas dmCTs did not (Table II). Furthermore, some mCT E112K accumulation was seen in the OBs, although the levels were lower than that of nCT (Table II). The residual levels of dmCT-acridinium compounds in ON/E and OBs were also examined 7 days after the last immunization. Mice were given nasal OVA and acridinium-conjugated nCT, mCT E112K, or dmCTs three times at weekly intervals. Interestingly, no accumulation of either dmCT E112K/KDEV or dmCT E112K/KDGL was detected in the ON/E or the OBs; however, significant amounts of nCT were seen in the OBs (Table II). Furthermore, small amounts of mCT E112K were also detected in the OBs (Table II). Taken together, our studies show that although dmCTs bind and accumulate in the ON/E, these dmCTs do not enter the OBs, whereas nCT quickly traffics into the OBs and remains for at least 7 days after immunization. Furthermore, our findings show that novel dmCTs may have an improved safety profile as nasal adjuvants when compared with mCT E112K, because mCT E112K shows some accumulation in the ON/E and OBs, although the levels were significantly lower than those seen with nCT.
Table II.
Comparison of the distribution of dmCTs and nCT in olfactory tissues
| CNS Tissues |
||||||
|---|---|---|---|---|---|---|
| ON/Ea |
OBsa |
ON/Eb |
OBsb |
|||
| Nasal Adjuvants | 5 μg | 0.5 μg | 5 μg | 0.5 μg | 5 μg | 5 μg |
| nCT | 9.61 ± 0.49†c | 4.03 ± 0.41 | 1.84 ± 0.55 | 0.26 ± 0.09 | 2.23 ± 0.07 | 1.36 ± 0.02 |
| mCT E112K | 3.97 ± 0.37* | 4.01 ± 0.65 | 0.83 ± 0.16 | 0.12 ± 0.07 | 1.83 ± 0.19 | 0.19 ± 0.01** |
| E112K/KDEV | 17.92 ± 1.59*† | 4.51 ± 0.58 | <0.1**† | <0.1 | 0.46 ± 0.14**† | <0.1** |
| E112K/KDGL | 14.79 ± 1.07*† | 4.73 ± 0.37 | 0.25 ± 0.03**† | <0.1 | 0.39 ± 0.01**† | <0.1** |
Distribution of acridinium-labeled enterotoxins into the OBs and ON/E was determined 24 h after nasal application of 5 or 0.5 fig of acridinium-labeled nCT, mCT E112K, dmCT E112K/KDEV, or dmCT E112K/KDGL. The OB and ON/E were collected and analyzed for the presence of acridinium-labeled enterotoxin.
Groups of mice were nasally immunized with 100 fig of OVA plus 5 fig of acridinium-labeled nCT, mCT E112K, dmCT E112K/KDEV, or dmCT E112K/KDGL three times at weekly intervals. The distribution of acridinium-labeled enterotoxins in the OBs and ON/E were determined 7 days after the last nasal application. The OBs and ON/E were collected and analyzed for the presence of acridinium-labeled enterotoxin.
Data are expressed as nanograms per 10 mg of tissue ± SEM for nine mice in each experimental group. The CNS tissues of nine mice given nasal PBS were examined for background levels of luminescence. These control values were subtracted from each experimental value. Difference from the value of nCT was statistically significant. (*, p < 0.05; **, p < 0.01). †, P < 0.05, when compared with mCT E112K.
Mucosal adjuvant activity of dmCTs
To assess the mucosal adjuvant properties of dmCTs, the mice were nasally immunized with 100 μg of OVA plus 0.5 μg of dmCT E112K/KDEV, dmCT E112K/KDGL, mCT E112K, or nCT three times at weekly intervals. Plasma and mucosal external secretions (nasal washes, fecal extracts, and saliva) were collected 7 days after the last immunization. Significant levels of OVA-specific S-IgA Ab responses were seen in all external secretions of mice nasally immunized with OVA plus dmCT E112K/KDEV or E112K/KDGL. However, mCT E112K exhibited significantly lower OVA-specific IgA Ab responses in mucosal external secretions than did nCT (Fig. 2). Elevated levels of OVA-specific IgG Ab responses were seen in mice given nasal dmCTs as mucosal adjuvants. OVA-specific IgG Ab responses in mice immunized with OVA plus dmCT E112K/KDEV or dmCT E112K/KDGL were identical with those seen in mice given nasal OVA plus nCT (Fig. 3). In contrast, mice given nasal mCT E112K as a mucosal adjuvant showed significant but lower OVA-specific IgG Ab responses than did mice immunized with OVA plus dmCTs or nCT (Fig. 3).
FIGURE 2.
Comparison of OVA-specific IgA Ab responses in nasal washes, fecal extracts, and saliva of mice immunized with OVA plus nCT, mCT, or dmCTs. Each mouse group was nasally immunized once a week for three consecutive weeks with 100 μg of OVA plus 0.5 μg of nCT (■), dmCT E112K/KDEV (▨), dmCT E112K/KDGL ( ), mCT E112K (▥), or PBS (□) as mucosal adjuvant. Seven days after the last immunization, the IgA levels in nasal washes and saliva were determined by an OVA-specific ELISA. The values shown are the mean ± 1 SEM for 30 mice in each experimental group. ND indicates that the titer was not detectable. *, p < 0.05; **, p < 0.01, when compared with nCT; †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
), mCT E112K (▥), or PBS (□) as mucosal adjuvant. Seven days after the last immunization, the IgA levels in nasal washes and saliva were determined by an OVA-specific ELISA. The values shown are the mean ± 1 SEM for 30 mice in each experimental group. ND indicates that the titer was not detectable. *, p < 0.05; **, p < 0.01, when compared with nCT; †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
FIGURE 3.
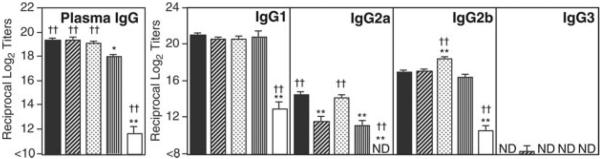
Comparison of OVA-specific plasma IgG and IgG subclass Ab responses of C57BL/6 mice nasally immunized with OVA plus nCT, mCT, or dmCTs. Each mouse group was nasally immunized once a week for three consecutive weeks with 100 μg of OVA plus 0.5 μg of nCT (■), dmCT E112K/KDEV (▨), dmCT E112K/KDGL ( ), mCT E112K (▥), or PBS (□) as mucosal adjuvant. Seven days after the last immunization, plasma IgG and IgG subclass Ab levels were determined by OVA-specific ELISA. The values shown are the mean ± 1 SEM for 30 mice in each experimental group. ND, not detected. *, p < 0.05; **, p < 0.01, when compared with nCT. †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
), mCT E112K (▥), or PBS (□) as mucosal adjuvant. Seven days after the last immunization, plasma IgG and IgG subclass Ab levels were determined by OVA-specific ELISA. The values shown are the mean ± 1 SEM for 30 mice in each experimental group. ND, not detected. *, p < 0.05; **, p < 0.01, when compared with nCT. †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
Furthermore, plasma OVA-specific IgG subclass Ab responses were also examined. High levels of OVA-specific-IgG1 and IgG2b Ab responses were seen in mice nasally immunized with OVA plus dmCT E112K/KDEV or E112K/KDGL, as well as in mice given nCT. Although OVA-specific IgG2a Ab responses were relatively lower than those of the IgG1 and IgG2b subclasses, anti-OVA IgG2a titers were significant in mice given nasal nCT or dmCT E112K/KDGL but reduced in those given dmCT E112K/KDEV or mCT E112K. An OVA-specific IgE ELISA revealed elevated levels of OVA-specific IgE Ab responses in plasma of mice given OVA plus nCT (Table III). Most interestingly, significantly lower levels of OVA-specific IgE Abs were noted in mice given nasal OVA plus dmCTs as well as mCT as a nasal adjuvant (Table III).
Table III.
Comparison of OVA-specific IgE Ab responses induced by nCT, mCT, or dmCTs
| Treatment Groupa | Ag-Specific IgEb (reciprocal log2 titer) |
|---|---|
| nCT | 7.5 ± 0.3††c |
| E112K/KDEV | 3.3 ± 0.4** |
| E112K/KDGL | 3.3 ± 0.3** |
| E112K | 2.3 ± 0.2** |
| PBS | <2 |
Each mouse group was nasally immunized once a week for two consecutive weeks with 100 μg of OVA plus 0.5 μg of nCT, mCT E112K/KDEV, mCT E112K/KDGL, mCT E112K, or PBS.
Seven days after the last immunization, OVA-specific IgE levels in plasma were determined by ELISA.
The values shown are the mean ± SEM for 6–8 mice in each experimental group. **, p < 0.01, when compared with nCT. ††, p < 0.01, when compared with mCT E112K.
OVA-specific AFC responses to mCTs
The results of OVA-specific Ab responses were further confirmed at the B cell level by using an OVA-specific ELISPOT assay (Fig. 4). Mononuclear cells from spleens, CLNs, lung, NPs, SMGs, and NALT of mice nasally immunized with OVA plus nCT, mCT E112K, dmCT E112K/KDEV, or dmCT E112K/KDGL were subjected to an OVA-specific ELISPOT assay to determine the numbers and isotypes of AFCs present. In the spleen, CLNs, NPs, and lungs of mice given nasal OVA plus dmCT E112K/KDEV or E112K/KDGL, OVA-specific IgG AFC were elevated to levels comparable to those seen in mice given nCT as nasal adjuvant. In contrast, nasal administration of mCT E112K resulted in levels of anti-OVA IgG AFCs in spleen, CLNs, and lungs that were lower than those seen with dmCTs and nCT. Furthermore, both dmCT E112K/KDEV and dmCT E112K/KDGL induced high numbers of OVA-specific IgA AFCs in NALT, SMGs, and NPs. These results clearly show that the newly developed second generation of dmCT E112K/KDEV and dmCT E112K/KDGL induces Ag-specific Ab responses as effectively as nCT in both systemic and mucosal lymphoid tissues.
FIGURE 4.
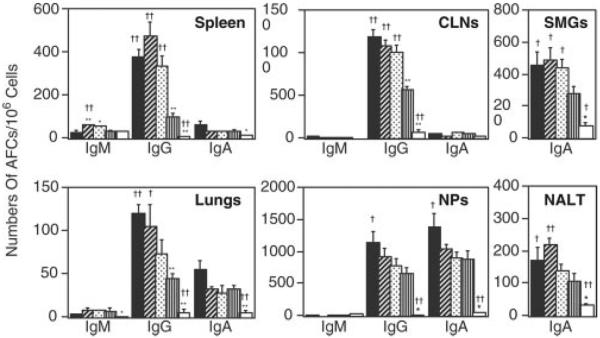
Analysis of OVA-specific AFCs in mice immunized nasally with OVA and nCT, mCT, or dmCTs. Each mouse group was nasally immunized once a week for three consecutive weeks with 100 μg of OVA plus 0.5 μg of nCT (■), dmCT E112K/KDEV (▨), dmCT E112K/KDGL ( ), mCT E112K (▥), or PBS (□). Seven days after the last immunization, mononuclear cells isolated from the NPs, SMGs, CLNs, lungs, and spleen were examined using an OVA-specific ELISPOT assay to determine the numbers of IgM, IgG, and IgA AFCs. The results represent the mean values ± 1 SEM for 20 mice in each experimental group. *, p < 0.05; **, p < 0.01, when compared with nCT. †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
), mCT E112K (▥), or PBS (□). Seven days after the last immunization, mononuclear cells isolated from the NPs, SMGs, CLNs, lungs, and spleen were examined using an OVA-specific ELISPOT assay to determine the numbers of IgM, IgG, and IgA AFCs. The results represent the mean values ± 1 SEM for 20 mice in each experimental group. *, p < 0.05; **, p < 0.01, when compared with nCT. †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
OVA-specific CD4+ T cell proliferative and cytokine responses
Because nasal immunization with OVA plus either dmCT E112K/KDEV or dmCT E112K/KDGL induced OVA-specific Ab responses in both systemic and mucosal compartments, it was important to examine the nature of OVA-specific CD4+ Th cell responses. We initially assessed OVA-specific CD4+ T cell proliferative responses in spleen and CLNs of mice nasally immunized with OVA plus dmCTs, nCTs, or mCT E112K. Significantly higher OVA-specific CD4+ T cell proliferative responses were seen in the spleen and CLNs of mice immunized with both dmCT E112K/KDEV and dmCT E112K/KDGL than in mice immunized with mCT E112K (Fig. 5). These OVA-specific CD4+ T cell proliferative responses were comparable to those seen in mice given nCT.
FIGURE 5.
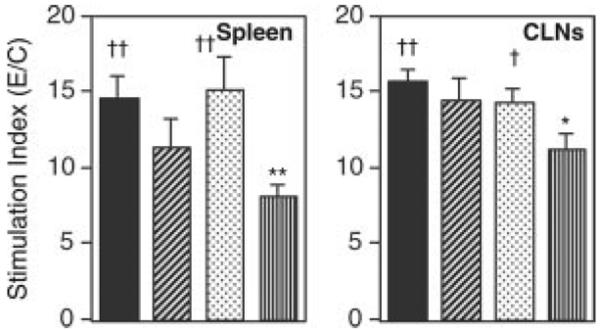
Analysis of Ag-specific CD4+ T cell proliferative responses induced by nasal immunization with OVA plus CT derivatives. Each mouse group was nasally immunized once a week for three consecutive weeks with 100 μg of OVA plus 0.5 μg of nCT (■), dmCT E112K/KDEV (▨), dmCT E112K/KDGL ( ), or mCT E112K (□). The splenic and CLN CD4+ T cells were isolated 7 days after the last immunization and cultured with or without OVA in the presence of APCs. The stimulation index was determined as A450 nm of wells with OVA divided by wells without OVA (control). The results represent the individual values from three separate experiments. *, p < 0.05; **, p < 0.01, when compared with nCT; †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
), or mCT E112K (□). The splenic and CLN CD4+ T cells were isolated 7 days after the last immunization and cultured with or without OVA in the presence of APCs. The stimulation index was determined as A450 nm of wells with OVA divided by wells without OVA (control). The results represent the individual values from three separate experiments. *, p < 0.05; **, p < 0.01, when compared with nCT; †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
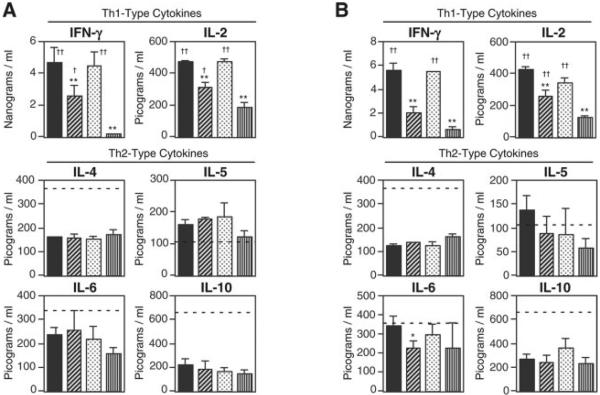
We next examined CD4+ Th1- and Th2-type cytokine responses by Ag-specific CD4+ T cells from the spleen and CLNs of mice nasally immunized with OVA plus dmCTs (Fig. 6). Both dmCT E112K/KDEV and dmCT E112K/KDGL induced high levels of Th2-type cytokines (IL-4, IL-5, IL-6, and IL-10) in OVA-stimulated CD4+ T cell cultures. The levels of these cytokines were almost comparable to those seen in OVA-stimulated CD4+ T cells from mice given nCT as nasal adjuvant. Of interest, these Th2-type cytokine responses were comparable to those induced by polyclonal stimulation (solid phase anti-CD3 and anti-CD28 mAb; Fig. 6, dotted lines). In contrast, OVA-stimulated CD4+ T cells from the spleen and CLNs of mice given nasal OVA plus nCT or dmCT E112K/KDGL exhibited similar levels of Th1-type cytokines (IFN-γ and IL-2) (Fig. 6). Although these Th1-type cytokine responses were significantly higher than those responses induced by mCT E112K or dmCT E112K/KDEV as nasal adjuvants, relative Th1-type cytokine production was markedly lower than that induced by anti-CD3 and anti-CD28 mAb stimulation.
FIGURE 6.
OVA-induced CD4+ Th1-type and Th2-type cytokine responses in mice given nasal OVA plus nCT, mCT, or dmCTs. Each mouse group was nasally immunized once a week for three consecutive weeks with 100 μg of OVA plus 0.5 μg of nCT (■f), dmCT E112K/KDEV (▨), dmCT E112K/KDGL ( ), or mCT E112K (□). A, The splenic CD4+ T cells (4 × 106 cells/ml) from each mouse group were cultured with 1 mg/ml OVA in the presence of APCs (8 × 106 cells/ml). B, The CLN CD4+ T cells (4 × 106 cells/ml) from each mouse group were cultured with 1 mg/ml OVA in the presence of APCs (8 × 106 cells/ml). Culture supernatants were harvested after 5 days of incubation (or after 2 days for IL-2) and analyzed by the respective cytokine-specific ELISA. The dotted lines indicate levels of each cytokine in the wells of anti-CD3 mAb- and anti-CD28 mAb-stimulated CD4+ T cells from spleen or CLNs of naive mice. Th1-type cytokine production by anti-CD3 and anti-CD28 mAb treatments were IFN-γ at ~80 ng/ml and IL-2 at ~10 ng/ml, respectively. The values shown are the mean ± SEM of 15 mice in each group. *, p < 0.05; **, p < 0.01, when compared with nCT; †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
), or mCT E112K (□). A, The splenic CD4+ T cells (4 × 106 cells/ml) from each mouse group were cultured with 1 mg/ml OVA in the presence of APCs (8 × 106 cells/ml). B, The CLN CD4+ T cells (4 × 106 cells/ml) from each mouse group were cultured with 1 mg/ml OVA in the presence of APCs (8 × 106 cells/ml). Culture supernatants were harvested after 5 days of incubation (or after 2 days for IL-2) and analyzed by the respective cytokine-specific ELISA. The dotted lines indicate levels of each cytokine in the wells of anti-CD3 mAb- and anti-CD28 mAb-stimulated CD4+ T cells from spleen or CLNs of naive mice. Th1-type cytokine production by anti-CD3 and anti-CD28 mAb treatments were IFN-γ at ~80 ng/ml and IL-2 at ~10 ng/ml, respectively. The values shown are the mean ± SEM of 15 mice in each group. *, p < 0.05; **, p < 0.01, when compared with nCT; †, p < 0.05; ††, p < 0.01, when compared with mCT E112K.
Discussion
It is well known that nCT and nLT are effective adjuvants that are capable of enhancing both mucosal S-IgA and systemic IgG Ab responses to coadministered protein Ags in mice and other experimental models. However, both enterotoxins are unsuitable for use in humans due to their toxicity, causing severe diarrhea if given orally and CNS toxicity if administered nasally (14, 15, 42–44). To overcome these obstacles to clinical practicability, several groups, including ours, have focused on developing nontoxic derivatives of CT or LT (10, 16, 17, 45–50). However, although these studies have been successful in producing nontoxic mutants, they did so at times by sacrificing the mucosal adjuvanticity associated with nCT (10, 16, 17, 45–50).
This study shows that a newly developed second generation of dmCTs, which have two amino acid substitutions in the ADP-ribosyltransferase active center (E112K) and COOH-terminal KDEL (dmCT E112K/KDEV or dmCT E112K/KDGL), offer real advantages as nasal adjuvants over the previously developed mCT E112K. When used as nasal adjuvants, even small doses (0.5 μg) of the two dmCTs induced levels of Ag-specific Ab responses in both mucosal and systemic lymphoid tissues that were comparable to those induced by nCT. Unlike nCT, however, that high degree of mucosal adjuvanticity was not accompanied by toxicity. Indeed, both dmCT E112K/KDEV and dmCT E112K/KDGL lacked ADP-ribosyltransferase activity and proved unable to move from the Golgi to the ER. Both dmCTs were thus unable to induce increases in intracellular cAMP. In addition dmCTs did not elicit fluid accumulation in mouse-ligated ileal loops. Furthermore, confocal microscopic analysis showed that the majority of the dmCTs were localized between the surface membranes and the Golgi apparatus in the T84 cell line and in dendritic cells, and this localization differed from that of mCTs and nCT. Although dmCTs accumulate in the ON/E for a short period, this accumulation did not last and the dmCTs were not transferred into the OBs. Furthermore, nasal application of OVA, together with dmCT E112K/DEV and dmCT E112K/KDGL as adjuvants, resulted in only minimal induction of IgE Ab responses. Collectively, these results indicate that these newly created dmCTs are potent nontoxic mucosal adjuvants suitable for the induction of both mucosal and systemic immune responses in humans.
Although the advantages of nasal immunization have made it the route of choice for the administration of enterotoxins, one area of continuing concern has been the possibility that nasal vaccines could enter the CNS because of the anatomical proximity of the ON/E and OBs to the brain. This potential for neurotoxicity could, of course, rule out the use of enterotoxin-based mucosal adjuvants in humans. Indeed, it was reported that nLT and detoxified LT mutants also targeted the CNS of mice following nasal application (51). In addition, our previous study showed the potential toxicity of CT for the CNS (12). Once CT-B or another nasal enterotoxin vaccine has been associated with neurons via GM1 binding, it cannot be efficiently cleared, and it accumulates in neuronal tissues associated with the olfactory tract tissues (12).
Although our past studies provided evidence that the nCT that accumulates in the olfactory tissue after nasal administration did not lead to obvious pathologic changes in brain tissue (52), our more recent study showed that nasal application of nCT induced nerve growth factor-β, an indicator of nerve cell damage in the olfactory tissues of rhesus macaques (23). We still do not understand the full biological and pathogenic consequences of enterotoxin deposition in the CNS mediated by ganglioside GM1 binding of olfactory tissues; however, it has been shown that a human nasal influenza vaccine with nLT as mucosal adjuvant resulted in the side effect of Bell's palsy (14, 15). It is likely that the nLT was the active component in this vaccine leading to facial paralysis.
In this regard, we have created a second generation of mCTs that avoid transport to the CNS tissues. Our current study clearly shows that dmCTs were not transferred into the OBs, although they were bound and began to accumulate in the ON/E within 24 h. More importantly, the dmCTs that accumulated in the ON/E cleared after 24 h and were not seen in the OBs 7 days after the last immunization even though they were given three times in three consecutive administrations at weekly intervals. Furthermore, because the second generation of dmCTs was designed to avoid cAMP activation by failing to traffic to the ADP-ribosylation activity center, dmCTs did not induce any inflammatory responses despite their early accumulation in the ON/E. Indeed, nasal washes of mice given nasal dmCTs as mucosal adjuvants resulted in essentially no TNF-α production (data not shown). These findings strongly support the safety of newly developed second generation E112K/KDEV and E112K/KDGL dmCTs.
In addition to our current approach, which inhibits intracellular trafficking of the enterotoxin A subunit, others have developed a nCT-based safe and effective adjuvant which targets the Ig receptor on both naive and memory B cells to avoid GM1 ganglioside binding (53). Thus, the enzymatically active A1 subunit of nCT was combined with a dimer of an Ig binding element (DD) from Staphylococcus aureus protein A (CT-A1-DD) and coadministered with Ag. This CT-Al-DD adjuvant induced significant Ab and T cell responses without CNS toxicity (53). Furthermore, the same study showed that mCT-A1 E112K-DD failed to elicit adjuvanticity (53). In contrast, others as well as our studies have clearly shown that mCT E112K and dmCTs (E112K/KDEV and E112K/KDGL) retained their nasal adjuvant activities (10, 11, 22, 23). It is possible that the different outcomes between these studies is due to the GM1 ganglioside binding ability of the adjuvants that we have used (10, 13, 17). Thus, mCT and dmCTs target all nucleated cells including professional APCs such as dendritic cells and macrophages, whereas mCT-A1 E112K-DD is limited to binding to B cells. The precise cellular and molecular mechanisms of these new nasal adjuvants needs to be elucidated in future studies. Both dmCTs and CT-A1-DD adjuvants are potential candidates for the development of safe mucosal vaccines.
Past studies have shown that nCT induces marked increases in Ag-specific IgE Ab responses after nasal immunization (10). To develop a mucosal adjuvant that is both safe and effective, the potential for IgE Ab production must be reduced without sacrificing the ability to generate Ag-specific mucosal S-IgA and plasma IgG Ab responses. Our previous studies singled out mCT E112K as the safest and most effective CT-derived adjuvant because it supported Ag-specific S-IgA Ab responses with lower levels of total and anti-CT-B IgE Ab responses than those observed with other enterotoxin-derived adjuvants after nasal immunization (13). Furthermore, when used as a nasal adjuvant, the mCT-A E112K/LT-B chimera, a mCT E112K derivative, induced significantly lower levels of total and Ag-specific IgE Ab responses in the plasma of mice (32). In the current study, we have assessed whether newly created dmCTs induce Ag-specific IgE Ab responses. Our results show that nasal administration of dmCT E112K/KDEV or E112K/KDGL does not enhance IgE Ab responses. Thus, levels of OVA-specific IgE Ab responses were comparable to those elicited by mCT E112K. These results indicate that these dmCT E112K/KDEV and dmCT E112K/KDGL molecules likely avoid allergic reactions to the coadministered Ag and, thus, are promising candidates for being potent nontoxic mucosal adjuvants for human use.
In an attempt to circumvent the toxicity of enterotoxin adjuvants, we developed double mutants of nCT that both lacked the ADP-ribosyltransferase activity and expressed altered ER retention signals (dmCT E112K/KDEV and dmCT E112K/KDGL). To examine the efficacy of these dmCTs for the induction of Ag-specific mucosal S-IgA and systemic IgG Ab responses, we initially used a 5-μg dose of dmCT as nasal adjuvant. We chose this dosage because our previous studies showed that the induction of Ag-specific immune responses in both mucosal and systemic tissues of mice and nonhuman primates required a 10-fold higher dose of mCT E112K/S61F or mCT-A E112K/LT-B than of nCT (2, 10, 11, 23). Because our initial experiments showed dmCTs to be effective adjuvants at the 5-μg dose, we next tested whether 0.5 μg of the newly developed dmCTs could also elicit nasal adjuvanticity. Our results showed both dmCTs to be potent mucosal adjuvants, even when administered at a 10-fold lower rate. In contrast, at a dosage of 0.5 μg, mCT E112K failed to induce the high titers of Ag-specific immune responses seen with nCT.
Although we cannot currently offer a precise explanation for this outcome, it seems clear that the additional point mutation in mCT E112K improves nasal adjuvanticity. Because the structure of the CT molecule is thought to be key to its biological activity (13), there has been concern that the introduction of a point mutation into CT might change or destabilize its own structure and cause diminished mucosal adjuvanticity (13). However, our finding that second generation dmCTs retained nasal adjuvanticity even at lower doses suggests that the molecular structures of dmCTs may be unchanged or stable even after the introduction of double mutations. To explore that possibility, we are currently testing the crystal structures of these dmCTs.
The newly developed dmCTs showed their adjuvant activities through Th2-type cytokine production, especially via an IL-4-dependent mechanism. Thus, nasal immunization with OVA plus dmCT E112K/KDEV or dmCT E112K/KDGL failed to induce OVA-specific Ab responses in IL-4-deficient mice (data not shown). Interestingly, analyses of Th1- and Th2-type cytokine production by CD4+ Th cells revealed that dmCT E112K/KDGL exhibited similar characteristics, especially in the responses of Th1-type cytokine synthesis. Thus, nasal administration of either nCT or dmCT E112K/KDGL resulted in higher levels of IFN-γ and IL-2 production than did that of mCT E112K or dmCT E112K/KDEV, although the levels were relatively lower than the IFN-γ and IL-2 production induced by anti-CD3 and -CD28 mAb treatment. In further support of the similar adjuvant properties shared by nCT and dmCT E112K/KDGL, their OVA-specific plasma IgG2a Ab responses, which are known to be associated with Th1-type cytokines, were significantly higher than those seen in mice given nasal mCT E112K or dmCT E112K/KDEV. We propose that these differences in cytokine profile and OVA-specific IgG subclass Ab responses are due to a difference in the intracellular trafficking of dmCT E112K/KDEV and dmCT E112K/KDGL. These findings indicate that dmCT E112K/KDGL may retain the prototype adjuvanticity of nCT, including the induction of cell-mediated immunity. Because nCT as a nasal adjuvant successfully induced CTL activity by CD8+ T cells, we are currently testing cytokine production and CTL activity by CD8+ T cells from mice given nasal dmCT E112K/KDGL as a mucosal adjuvant.
In summary, a newly developed second generation of both dmCT E112K/KDEV and dmCT E112K/KDGL retained adjuvant activity and elicited mucosal and systemic immunity to nasally coadministered Ags without exhibiting ADP-ribosyltransferase activity or participating in normal intracellular trafficking. Interestingly, like mCT E112K, dmCT E112K/KDEV showed dominant Th2-type cytokine responses. In contrast, like nCT, dmCT E112K/KDGL elicited not only Th2-type cytokine responses but also significantly higher Th1-type cytokine responses than did dmCT E112K/KDEV and mCT E112K. Although different in their immunobiological characteristics, both of our newly developed second-generation dmCTs show promise as effective and safe nasal adjuvants in mice. Future studies will determine whether these adjuvants are safe and effective in both nonhuman primates and humans.
Acknowledgments
We thank Dr. Kimberly K. McGhee for her editorial advice on the manuscript. We also thank Sheila Turner for the final preparation of this manuscript.
Footnotes
This research was supported by National Institutes of Health Grants DC 04976, DE 12242, AI 18958, and AI 43197 and Grants-in-Aid from the Ministry of Health and Labor, Japan, the Ministry of Education, Science and Sports, Japan, and CREST of Japan Science and Technology Corporation.
Abbreviations used in this paper: S-IgA, secretory IgA; AFC, Ab forming cell; CHO, Chinese hamster ovary; CLN, cervical lymph node; CT, cholera toxin; nCT, native CT; CT-A, A subunit of nCT; CT-B, B subunit of nCT; dmCT, double mutant CT; mCT, mutant CT; DD, dimer of an Ig binding element; ER, endoplasmic reticulum; LT, heat-labile enterotoxin; nLT, native LT; NALT, nasopharyngeal-associated lymphoreticular tissue; NP, nasal passage; OB, olfactory bulb; ON/E, olfactory nerves and epithelium; SMG, submandibular gland.
Disclosures The authors have no financial conflict of interest.
References
- 1.Mestecky J, Blumberg RS, Kiyono H, McGhee JR. The mucosal immune system. In: Paul WE, editor. Fundamental Immunology. Lippincott Williams & Wilkins, Philadelphia; 2003. pp. 965–1020. [Google Scholar]
- 2.Hagiwara Y, McGhee JR, Fujihashi K, Kobayashi R, Yoshino N, Kataoka K, Etani Y, Kweon MN, Tamura S, Kurata T, et al. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J. Immunol. 2003;170:1754–1762. doi: 10.4049/jimmunol.170.4.1754. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003;21:89–95. doi: 10.1016/s0264-410x(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 4.Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 5.Katz JM, Lu X, Young SA, Galphin JC. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J. Infect. Dis. 1997;175:352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- 6.Elson CO, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J. Immunol. 1984;133:2892–2897. [PubMed] [Google Scholar]
- 7.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 8.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 9.Xu-Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, Burrows PD, Elson CO, Pillai S, McGhee JR. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Yamamoto M, Fujihashi K, Noda M, Kiyono H, McGhee JR. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 1997;185:1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, van Ginkel FW, Noda M, Takeda Y, McGhee JR. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 2000;165:4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 13.Hagiwara Y, Komase K, Chen Z, Matsuo K, Suzuki Y, Aizawa C, Kurata T, Tamura S. Mutants of cholera toxin as an effective and safe adjuvant for nasal influenza vaccine. Vaccine. 1999;17:2918–2926. doi: 10.1016/s0264-410x(99)00135-8. [DOI] [PubMed] [Google Scholar]
- 14.Gluck R, Mischler R, Durrer P, Furer E, Lang AB, Herzog C, Cryz SJ., Jr Safety and immunogenicity of intranasally administered inactivated trivalent virosome-formulated influenza vaccine containing Escherichia coli heat-labile toxin as a mucosal adjuvant. J. Infect. Dis. 2000;181:1129–1132. doi: 10.1086/315337. [DOI] [PubMed] [Google Scholar]
- 15.Durrer P, Gluck U, Spyr C, Lang AB, Zurbriggen R, Herzog C, Gluck R. Mucosal antibody response induced with a nasal virosome-based influenza vaccine. Vaccine. 2003;21:4328–4334. doi: 10.1016/s0264-410x(03)00457-2. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc. Nat. Acad. Sci. USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lencer WI, Constable C, Moe S, Jobling MG, Webb HM, Ruston S, Madara JL, Hirst TR, Holmes RK. Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J. Cell Biol. 1995;131:951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cieplak W, Jr., Messer RJ, Konkel ME, Grant CC. Role of a potential endoplasmic reticulum retention sequence (RDEL) and the Golgi complex in the cytotonic activity of Escherichia coli heat-labile enterotoxin. Mol. Microbiol. 1995;16:789–800. doi: 10.1111/j.1365-2958.1995.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 20.Majoul IV, Bastiaens PI, Soling HD. Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells. J. Cell Biol. 1996;133:777–789. doi: 10.1083/jcb.133.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majoul I, Sohn K, Wieland FT, Pepperkok R, Pizza M, Hillemann J, Soling HD. KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J. Cell Biol. 1998;143:601–612. doi: 10.1083/jcb.143.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto M, Briles DE, Yamamoto S, Ohmura M, Kiyono H, McGhee JR. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J. Immunol. 1998;161:4115–4121. [PubMed] [Google Scholar]
- 23.Yoshino N, Lu FX, Fujihashi K, Hagiwara Y, Kataoka K, Lu D, Hirst L, Honda M, van Ginkel FW, Takeda Y, et al. A novel adjuvant for mucosal immunity to HIV-1 gp120 in nonhuman primates. J. Immunol. 2004;173:6850–6857. doi: 10.4049/jimmunol.173.11.6850. [DOI] [PubMed] [Google Scholar]
- 24.van Ginkel FW, Jackson RJ, Yoshino N, Hagiwara Y, Metzger DJ, Connell TD, Lan Vu H, Martin M, Fujihashi K, McGhee JR. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect. Immun. 2005;73:1–11. doi: 10.1128/IAI.73.10.6892-6902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uesaka Y, Otsuka Y, Lin Z, Yamasaki S, Yamaoka J, Kurazono H, Takeda Y. Simple method of purification of Escherichia coli heat-labile enterotoxin and cholera toxin using immobilized galactose. Microb. Pathog. 1994;16:71–76. doi: 10.1006/mpat.1994.1007. [DOI] [PubMed] [Google Scholar]
- 26.Thieblemont N, Wright SD. Transport of bacterial lipopolysaccha-ride to the Golgi apparatus. J. Exp. Med. 1999;190:523–534. doi: 10.1084/jem.190.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole L, Davies D, Hyde GJ, Ashford AE. ER-Tracker dye and BODIPY-brefeldin A differentiate the endoplasmic reticulum and Golgi bodies from the tubular-vacuole system in living hyphae of Pisolithus tinctorius. J. Microsc. 2000;19:239–249. doi: 10.1046/j.1365-2818.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- 28.Donta ST, Moon HW, Whipp SC. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974;183:334–336. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto K, Ohishi I, Sakaguchi G. Fluid accumulation in mouse ligated intestine inoculated with Clostridium perfringens enterotoxin. Appl. Environ. Microbiol. 1979;37:181–186. doi: 10.1128/aem.37.2.181-186.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda Y, Takeda T, Yano T, Yamamoto K, Miwatani T. Purification and partial characterization of heat-stable enterotoxin of enterotoxigenic Escherichia coli. Infect. Immun. 1979;25:978–985. doi: 10.1128/iai.25.3.978-985.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller SA, Morton MS, Turkes A. Chemiluminescence immunoassay for progesterone in plasma incorporating acridinium ester labelled antigen. Ann. Clin. Biochem. 1988;25:27–34. doi: 10.1177/000456328802500103. [DOI] [PubMed] [Google Scholar]
- 32.Kweon MN, Yamamoto M, Watanabe F, Tamura S, Van Ginkel FW, Miyauchi A, Takagi H, Takeda Y, Hamabata T, Fujihashi K, et al. A nontoxic chimeric enterotoxin adjuvant induces protective immunity in both mucosal and systemic compartments with reduced IgE antibodies. J. Infect. Dis. 2002;186:1261–1269. doi: 10.1086/344526. [DOI] [PubMed] [Google Scholar]
- 33.Fujihashi K, McGhee JR, Kweon MN, Cooper MD, Tonegawa S, Takahashi I, Hiroi T, Mestecky J, Kiyono H. γ/δ T cell-deficient mice have impaired mucosal immunoglobulin A responses. J. Exp. Med. 1996;183:1929–1935. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura S, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J. Immunol. 1996;156:3892–3900. [PubMed] [Google Scholar]
- 35.Koga T, McGhee JR, Kato H, Kato R, Kiyono H, Fujihashi K. Evidence for early aging in the mucosal immune system. J. Immunol. 2000;165:5352–5359. doi: 10.4049/jimmunol.165.9.5352. [DOI] [PubMed] [Google Scholar]
- 36.Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, Kiyono H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur. J. Immunol. 1998;28:3346–3353. doi: 10.1002/(SICI)1521-4141(199810)28:10<3346::AID-IMMU3346>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 37.Asanuma H, Inaba Y, Aizawa C, Kurata T, Tamura S. Characterization of mouse nasal lymphocytes isolated by enzymatic extraction with collagenase. J. Immunol. Methods. 1995;187:41–51. doi: 10.1016/0022-1759(95)00165-7. [DOI] [PubMed] [Google Scholar]
- 38.Wu HY, Nikolova EB, Beagley KW, Russell MW. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers DC. Immunological principles and emerging strategies of vaccination for the elderly. J. Am. Geriatr. Soc. 1992;40:81–94. doi: 10.1111/j.1532-5415.1992.tb01835.x. [DOI] [PubMed] [Google Scholar]
- 40.Schmucker DL, Heyworth MF, Owen RL, Daniels CK. Impact of aging on gastrointestinal mucosal immunity. Dig. Dis. Sci. 1996;41:1183–1193. doi: 10.1007/BF02088236. [DOI] [PubMed] [Google Scholar]
- 41.Yanagita M, Hiroi T, Kitagaki N, Hamada S, Ito HO, Shimauchi H, Murakami S, Okada H, Kiyono H. Nasopharyngeal-associated lymphoreticular tissue (NALT) immunity: fimbriae-specific Th1 and Th2 cell-regulated IgA responses for the inhibition of bacterial attachment to epithelial cells and subsequent inflammatory cytokine production. J. Immunol. 1999;162:3559–3565. [PubMed] [Google Scholar]
- 42.Gorbach SL, Khurana CM. Toxigenic Escherichia coli in infantile diarrhea in Chicago. J. Lab. Clin. Med. 1971;78:981–982. [PubMed] [Google Scholar]
- 43.Rowe B, Taylor J, Bettelheim KA. An investigation of traveller's diarrhoea. Lancet. 1970;1:1–5. doi: 10.1016/s0140-6736(70)90520-9. [DOI] [PubMed] [Google Scholar]
- 44.Sack RB, Gorbach SL, Banwell JG, Jacobs B, Chatterjee BD, Mitra RC. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J. Infect. Dis. 1971;123:378–385. doi: 10.1093/infdis/123.4.378. [DOI] [PubMed] [Google Scholar]
- 45.de Haan L, Verweij WR, Feil IK, Lijnema TH, Hol WG, Agsteribbe E, Wilschut J. Mutants of the Escherichia coli heat-labile enterotoxin with reduced ADP-ribosylation activity or no activity retain the immunogenic properties of the native holotoxin. Infect. Immun. 1996;64:5413–5416. doi: 10.1128/iai.64.12.5413-5416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fontana MR, Manetti R, Giannelli V, Magagnoli C, Marchini A, Olivieri R, Domenighini M, Rappuoli R, Pizza M. Construction of nontoxic derivatives of cholera toxin and characterization of the immunological response against the A subunit. Infect. Immun. 1995;63:2356–2360. doi: 10.1128/iai.63.6.2356-2360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giuliani MM, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 49.Pizza M, Fontana MR, Giuliani MM, Domenighini M, Magagnoli C, Giannelli V, Nucci D, Hol W, Manetti R, Rappuoli R. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. J. Exp. Med. 1994;180:2147–2153. doi: 10.1084/jem.180.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris MT. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect. Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourguignon P, Henderickx V, Friede M, Lobet Y, Francotte M. Molecular Approaches to Vaccine Design, December 2–5. Cold Spring Harbor Lab. Press, Cold Spring Harbor; NY: 1999. Reactogenicity in the nose and the brain of enterotoxins administered intranasally to mice; p. 23. [Google Scholar]
- 52.Hagiwara Y, Iwasaki T, Asanuma H, Sato Y, Sata T, Aizawa C, Kurata T, Tamura S. Effects of intranasal administration of cholera toxin (or Escherichia coli heat-labile enterotoxin) B subunits supplemented with a trace amount of the holotoxin on the brain. Vaccine. 2001;19:1652–1660. doi: 10.1016/s0264-410x(00)00412-6. [DOI] [PubMed] [Google Scholar]
- 53.Eriksson AN, Schön KM, Lycke NY. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. J. Immunol. 2004;173:3310–3319. doi: 10.4049/jimmunol.173.5.3310. [DOI] [PubMed] [Google Scholar]