Abstract
Aims
To evaluate trends in use of stop-smoking medications (SSMs) before and after varenicline (Chantix™) was introduced to the market-place in the United States, and to determine whether varenicline reached segments of the population unlikely to use other SSMs.
Design
Cohort survey.
Setting
United States.
Participants
A nationally representative sample of adult smokers in the United States interviewed as part of the International Tobacco Control Four Country Survey between 2004 and 2011. Primary analyses used cross-sectional data from 1737 smokers who attempted to quit (~450 per wave).
Measurements
Reporting an attempt to quit smoking; use of each of the following types of SSMs for the purpose of quitting smoking: nicotine gum, nicotine patch, other nicotine replacement therapy, bupropion and varenicline.
Findings
There was a significant increase in the rate of use of any SSM among quit attempters across the study period [odds ratio (OR) = 1.15, 95% confidence interval (CI) = 1.10–1.21 per year]. This increase was largest after varenicline was introduced (OR = 1.16, 95% CI = 1.07–1.26 per year); however, there was a decline in nicotine patch use during this time (OR = 0.87, 95% CI = 0.76–0.99 per year). Varenicline users were generally similar to users of other SSMs but differed from those who did not use any SSMs, in that they tended to be older (OR = 5.46, P = 0.024), to be white (OR = 2.33, P = 0.002), to have high incomes (OR = 1.85, P = 0.005), to have high nicotine dependence prior to quitting (OR = 2.40, P = 0.001) and to have used medication in the past (OR = 3.29, P < 0.001).
Conclusions
The introduction of varenicline in the United States coincided with a net increase in attempts to quit smoking and, among these, a net increase in use of stop-smoking medications. The demographic profile of varenicline users is similar to the profile of those who use other stop-smoking medications and different from the profile of those who attempt to quit without any medication.
Keywords: Bupropion, cessation, nicotine replacement therapy, stop-smoking medication, trends, varenicline
INTRODUCTION
Varenicline is a prescription smoking cessation medication sold in the United States under the trade name Chantix™ (in other parts of the world varenicline is sold under the trade name Champix™). It is a partial α4β2 nicotine acetylcholine receptor agonist believed to work by stimulating the release of dopamine to reduce nicotine withdrawal while also blocking the binding of nicotine [1]. Clinical trials have shown varenicline to be efficacious in increasing smoking cessation rates between two-and threefold compared to placebo [2].
The US Food and Drug Administration (FDA) approved varenicline for use as a prescription-only stop smoking aid in May 2006 [3]. In August 2006, varenicline was made available in the US market-place, joining nicotine replacement therapy (NRT; see [4] for review of NRT efficacy) and bupropion (i.e. Zyban™ and Welbutrin™; see [5] for review of bupropion efficacy) as other FDA-approved stop smoking medications (SSMs). However, following the release of varenicline into the market-place, post-marketing surveillance reports in both Europe and the United States began to suggest that use of varenicline was associated with an increased risk of heart attack and various neuropsy-chiatric symptoms.
In February 2008, the FDA issued an alert warning consumers and prescribing physicians of possible side effects associated with the use of varenicline [6]. Even though the adverse effects of varenicline were considered rare, in July 2009 the FDA required that a ‘black box’ warning on possible adverse side effects be added to the drug insert for varenicline [7]. The warning advises doctors and people who use varenicline to look out for signs of behavioral or mood changes such as lasting or worsening depression and suicidal thoughts. Also, because there have been reports of drowsiness, the FDA advises people not to drive cars or operate heavy machinery if they do not know how varenicline will affect them. Despite these precautions, the majority of studies, including meta-analyses and reviews independent of Pfizer, have found varenicline to be safe (e.g. [8–11]).
The broad public health impact of varenicline in the United States will depend upon the prevalence of its use, including the extent to which it may substitute for, or add to, overall use of SSMs. Previous large-scale studies have aimed to evaluate the impact of the introduction of varenicline on use of SSMs in the United Kingdom (e.g. [12–13]), where varenicline became available in December 2006. Data from the ‘Smoking Toolkit Study’, a cross-sectional national survey of smokers in England conducted every 3 months, indicated that the introduction of varenicline did not result in its use substituting for use of other prescription SSMs; however, there was a decrease in the use of over-the-counter (OTC) NRT as varenicline use increased [12]. The UK survey also indicated that the proportion of smokers attempting to quit decreased as varenicline use increased, suggesting that the introduction of varenicline did not increase the total number of UK smokers who tried to quit.
A second UK study evaluated trends in rates of prescribing varenicline, bupropion and NRT using data from primary care records, and concluded that the introduction of varenicline was not associated with an increase in overall rates of prescribing SSMs [13]. However, this study evaluated rates among all patients in the primary care records, as opposed to evaluating rates among smokers who attempted to quit, which underestimates prescription rates inasmuch as there were declines in the proportion of smokers who attempted to quit.
Although early data from the International Tobacco Control Four Country Survey showed an initial spike in use of SSMs in the United States following the introduction of varenicline to the market [14], there have been no large-scale, US-specific studies evaluating trends in use of SSMs in multiple years preceding and following the introduction of varenicline. The purpose of this study was: (i) to evaluate trends in use of SSMs by smokers attempting to quit before and after the introduction of varenicline to the United States, and to evaluate trends in varenicline use following the various public reports about possible adverse side effects associated with its use; and (ii) to evaluate the characteristics of varenicline users in contrast to users of other SSMs, and in contrast to smokers attempting to quit without medication.
METHODS
Participants
Participants were a nationally representative sample of adult smokers from the United States who were interviewed as part of the International Tobacco Control Four Country Survey (ITC-4) between 2004 and 2011. The ITC-4 is a prospective cohort survey that uses randomdigit dialling to recruit current smokers (i.e. those who smoked at least 100 cigarettes during their life-times and at least once in the past 30 days) from the United States, Canada, the United Kingdom and Australia. Participants are re-contacted approximately annually to complete follow-up surveys, and new smokers are recruited each year to offset those lost to follow-up (~25% in the United States [15]). Response rates in the United States ranged from 21% (2007) to 35% (2004) and previous analyses have indicated that responders to this survey were demographically similar to responders to national benchmark surveys [15,16]. Detailed information about the survey design, procedures and limitations can be found elsewhere [15–18].
We used data collected from US respondents between 2004 and 2011. Cross-sectional analyses of medication use prevalence included previous year smokers who reported making a quit attempt (n = 1737), and estimates of making a quit attempt included all previous year smokers (n = 3087, i.e. the only criterion for exclusion from our overall sample was having already quit smoking at baseline survey). Following the introduction of varenicline to the US market-place in 2006, paired-repeat longitudinal analyses were used to evaluate the characteristics of varenicline users (n = 1220). The study protocol was approved by the institutional review boards/ research ethics boards within the United States (Roswell Park Cancer Institute) and separately within other ITC countries (data not presented here).
Measures
Quit attempts
At each survey wave, participants who were smokers during the previous wave were asked: ‘Have you made any attempts to stop smoking since we last talked with you?’.Those who had made any attempts were asked how many quit attempts they had made.
Use of stop-smoking medications
During each interview, participants were asked if they used any stop-smoking medications during the last year/ since last survey date. Prior to wave 5, respondents who reported having used any medication were asked what type(s)of medication they used (types were categorized as nicotine gum, nicotine patch, nicotine lozenges, nicotine tablets, nicotine inhaler, nicotine nasal spray, bupropion or other medication). For each type of medication indicated, respondents were asked a series of questions, including: ‘What was the main reason you used [medication]?’. Response options were: to stop smoking completely, to reduce the amount you smoke, to cope with times when you could not or were not allowed to smoke, or other reason. Beginning in wave 5, respondents who reported having used any medication were first asked their reason(s) for using medication (same response options as above). For each reason indicated, respondents were asked what type(s) of medication they used, which were categorized in the same way as above, and additionally included varenicline. During the wave 8 interview, respondents were asked specifically about medication used during the last quit attempt in particular, and later asked about the last time medication was used (since the last survey) if it was not during the last quit attempt in particular. Data from both these series of items were combined to produce assessments of medication use that were most comparable to the assessments used during previous waves. Medication use data from wave 1 were not included in this study, because survey items from that wave did not allow for the identification of reasons for use of the specific medications indicated; medication use data from wave 2 were not included in this study because the time-frame within which respondents were asked to recall their medication use was 6 months, as opposed to 12 months.
A separate medication use variable was created for each of five types of medication used for the purpose of quitting smoking (i.e. use of SSM for non-cessation purposes was counted as non-use): nicotine gum, nicotine patch, other NRT (which included nicotine lozenges, nicotine tablets, nicotine inhaler and nicotine nasal spray), bupropion (i.e. Zyban™/Wellbutrin™) and varenicline (waves 5–8 only). Any respondent who reported using more than one type of medication to quit smoking since the last survey was classified as a user of each type indicated (respondents who reported using multiple types of medication since the last survey generally used them on different quit attempts; fewer than 3% of quit attempters reported concurrent use of NRT patch plus another form of NRT). Non-users of each type of medication were those who did not use the given type of medication.
Ever use of any stop-smoking medication was identified at baseline survey among those who responded affirmatively to the following item: ‘Have you ever used any stop-smoking medication?’. Among those who had never used medication when interviewed at baseline, ever use was re-evaluated at each subsequent survey wave when respondents were asked if they had used any medication since the last survey.
Demographic and smoking-related variables
The following characteristics were evaluated as predictors of medication use: sex, age group (i.e. 18–24, 25–39, 40–54, and 55+ years), race/ethnicity (i.e. non-Hispanic white versus other), level of education [i.e. ‘low’ if completed high school or less, ‘moderate’ if completed community college/trade/technical school/some university (no degree) or ‘high’ if completed university or postgraduate education], annual household income [i.e. ‘low’ if less than $30 000, ‘moderate’ if $30 000–59 999 or ‘high’ if $60 000 or more; those who did not provide this information (~5%) were included in adjusted analyses as a valid unknown group] and nicotine dependence [measured with the heaviness of smoking index (HSI), a short form of the Fagerström Tolerance Questionnaire [19]], which was assessed at the wave preceding the wave in which medication use was evaluated. The specific wording of all items used in the ITC questionnaires can be found at: http://www.itcproject.org [20].
Analyses
The proportion of all prior wave smokers who reported making at least one quit attempt since the last survey was determined at each survey wave, and among those who made at least one quit attempt, the proportion of participants who had made two or more quit attempts was determined. Also, among those who had made at least one quit attempt, the proportion who used each of the five types of medication and the proportion who used any type of stop-smoking medication was determined at each survey wave.
Generalized estimating equations (GEEs [21,22]) were used to evaluate trends in quit attempts and medication use during three time-periods: full study period, prevarenicline time-period (i.e. beginning of study period (2004) to wave 5 (2006–07) since responders to wave 5 were interviewed between October 2006 and February 2007, leaving them little time to obtain a prescription and start using varenicline prior to the wave 5 interview) and post-varenicline time-period [i.e. wave 5 to end of study period (2010–11)]. That is, time was regressed on each outcome (i.e. quit attempts and medication use) for each time-period. Thus, odds ratios (OR) indicate the average increase/decrease in likelihood of each outcome per unit increase in time (i.e. ~1 year), within each time-period. Next, the post-varenicline time-period was deconstructed into the following three subtime-periods: introduction of varenicline to pre-FDA alert, pre-FDA alert to pre-black box warning, pre-black box warning to end of study period and the trend in use of varenicline during these three subtime-periods was evaluated using GEEs. Each GEE model included a specification for the binomial distribution of the dichotomous dependent variable, a specification for the unstructured within-person correlation matrix, and all confidence intervals were computed using a robust variance estimator.
Secondly, logistic regression analyses via GEEs were used to evaluate the characteristics of: (i) varenicline users compared to bupropion users, (ii) varenicline users compared to those who used any stop-smoking medication and (iii) varenicline users compared to those who did not use any medication when attempting to quit. These analyses were limited to the waves in which varenicline was used (i.e. waves 6–8, or 2007–08 to 2010–11), included the same model specifications as above and were adjusted for sex, age group, race/ethnicity, education, income, HSI (evaluated at preceding survey wave) and survey wave. All analyses were conducted using Stata version 11 [23].
RESULTS
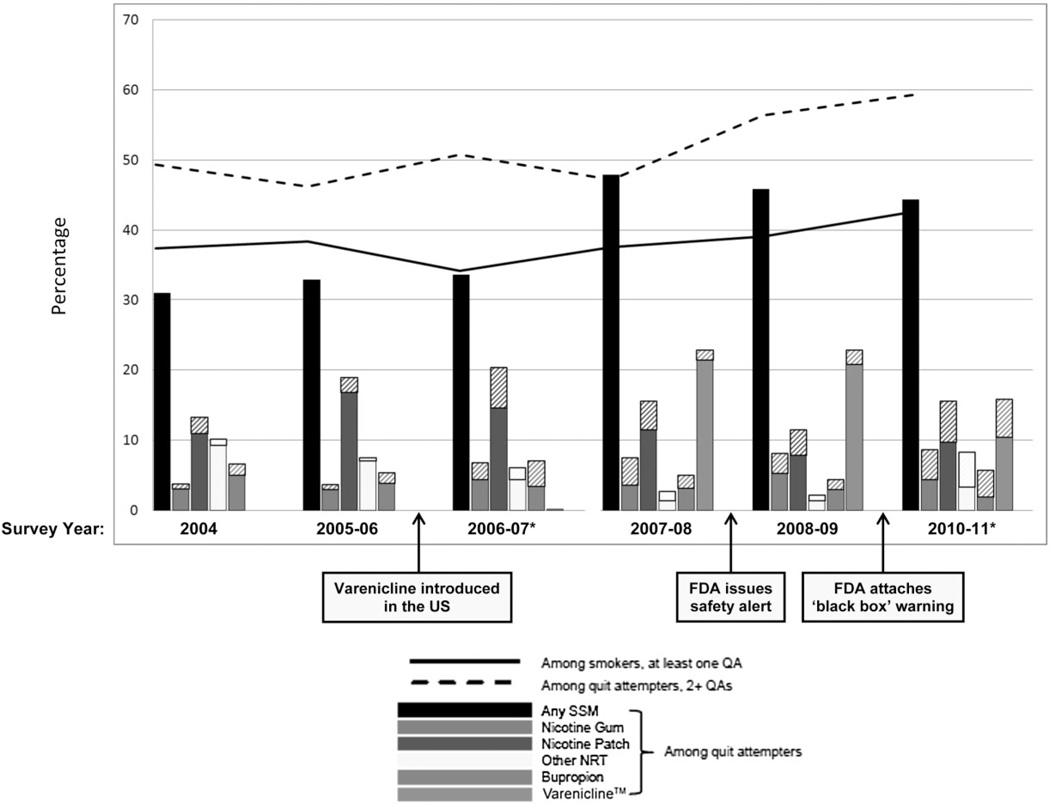
The demographic characteristics of our sample are presented in Table 1, both among individual participants and among total observations (as an individual participant could contribute multiple observations during the course of the study period). Trends in quit attempts and use of SSMs across the study period are displayed visually in Fig. 1, and results from the associated trend analyses are shown in Table 2.
Table 1.
Sample characteristics among all participants and among all observations.
| Individual participants (n=3087) |
Total observations (n=6775) |
|||
|---|---|---|---|---|
| Sample characteristics | na | %b | n | % |
| Time-point | ||||
| Wave 3 (June 2004–December 2004) | 1135 | 36.77 | 1135 | 16.75 |
| Wave 4 (October 2005–January 2006) | 1164 | 37.71 | 1164 | 17.18 |
| Wave 5 (October 2006–February 2007) | 1138 | 36.86 | 1138 | 16.80 |
| Wave 6 (September 2007–February 2008) | 1130 | 36.61 | 1130 | 16.68 |
| Wave 7 (October 2008–February 2009) | 1242 | 40.23 | 1242 | 18.33 |
| Wave 8 (July 2010–January 2011) | 966 | 31.29 | 966 | 14.26 |
| Total number of waves participated | ||||
| 2 | 959 | 31.07 | 959 | 14.15 |
| 3 | 844 | 27.34 | 1379 | 20.35 |
| 4 | 491 | 15.91 | 1257 | 18.55 |
| 5 | 311 | 10.07 | 1015 | 14.98 |
| 6 | 212 | 6.87 | 869 | 12.83 |
| 7 | 122 | 3.95 | 553 | 8.16 |
| 8 | 148 | 4.79 | 743 | 10.97 |
| Sex | ||||
| Female | 1764 | 57.14 | 3973 | 58.64 |
| Male | 1323 | 42.86 | 2802 | 41.36 |
| Baseline age group (years) | ||||
| 18–24 | 200 | 6.48 | 349 | 5.15 |
| 25–39 | 621 | 20.12 | 1229 | 18.14 |
| 40–54 | 1242 | 40.23 | 2854 | 42.13 |
| 55+ | 1024 | 33.17 | 2343 | 34.58 |
| Race/ethnicity | ||||
| Non-Hispanic white | 2579 | 83.54 | 5760 | 85.02 |
| Other | 500 | 16.20 | 987 | 14.57 |
| Unknown | 8 | 0.26 | 28 | 0.41 |
| Education | ||||
| Low | 1259 | 40.78 | 2680 | 39.56 |
| Moderate | 1244 | 40.30 | 2750 | 40.59 |
| High | 607 | 19.66 | 1331 | 19.65 |
| Unknown | 6 | 0.19 | 14 | 0.21 |
| Income | ||||
| Low | 1182 | 38.29 | 2382 | 35.16 |
| Moderate | 1150 | 37.25 | 2312 | 34.13 |
| High | 832 | 26.95 | 1708 | 25.21 |
| Unknown | 217 | 7.03 | 373 | 5.51 |
| HSI | ||||
| 0–1 | 861 | 27.89 | 1426 | 21.05 |
| 2–3 | 1752 | 56.75 | 3131 | 46.21 |
| 4–6 | 1172 | 37.97 | 2056 | 30.35 |
| Unknown | 27 | 0.87 | 162 | 2.39 |
Number of individuals in each category of each variable. Sum of ns exceeds total number of participants inasmuch as individual participants are present in more than one category of a variable at different time-points.
Percentage is given out of total number of participants, some of whom contributed more than one observation. HSI = heaviness of smoking index.
Figure 1.
Prevalence of use of any stop-smoking medications (SSM) (black bars) and of each SSM (coloured bars) among those making a quit attempt (QA) (n=1737), across the study period. Diagonal patterned sections of bars indicate more than one type of medication was used during a particular survey wave (i.e. the bars overlap); n= 3087 for prevalence of at least one QA among all smokers (black solid line); n= 1706 for prevalence of 2+ QAs among quit attempters (black dashed line). *Battery of items used to assess SSM use during this survey wave differed somewhat from battery used during preceding wave.
Table 2.
Trends in quit attempts and use of stop-smoking medications (SSMs) during the pre-varenicline period, the post-varenicline period and across the full study period.
| Pre-varenicline period (2004 to 2006–07) |
Post-varenicline period (2006–07 to 2010–11) |
Full study period (2004 to 2010–11) |
|||||
|---|---|---|---|---|---|---|---|
| Sample | Outcome | OR | 95% CI | OR | 95% CI | OR | 95%, CI |
| Smokers | Any quit attempt since LSD | n = 2036 | n = 2225 | n = 3087a | |||
| 0.98 | 0.91–1.06 | 1.12 | 1.07–1.18 | 1.05 | 1.02–1.08 | ||
| Quit attempters | 2+ quit attempts since LSD | n = 979 | n = 1208 | n = 1706a | |||
| 1.05 | 0.92–1.19 | 1.12 | 1.03–1.21 | 1.09 | 1.04–1.14 | ||
| Quit attempters | Dse of medication since LSD | n = 1012 | n = 1220 | n = 1737a | |||
| Any SSM | 1.01 | 0.89–1.16 | 1.16 | 1.07–1.26 | 1.15 | 1.10–1.21 | |
| NRT gum | 1.31 | 0.92–1.85 | 1.11 | 0.95–1.30 | 1.21 | 1.09–1.35 | |
| NRT patch | 1.23 | 1.04–1.47 | 0.87 | 0.76–0.99 | 0.96 | 0.90–1.03 | |
| Other NRT | 0.76 | 0.58–1.00 | 1.12 | 0.86–1.45 | 0.84 | 0.74–0.94 | |
| Any NRT | 1.08 | 0.94–1.25 | 0.95 | 0.85–1.05 | 0.96 | 0.91–1.01 | |
| Bupropion | 0.86 | 0.60–1.22 | 1.01 | 0.74–1.39 | 0.93 | 0.79–1.09 | |
| Varenicline | – | – | 1.40 | 1.26–1.56 | – | – | |
|
Introduction (0%) to pre-FDA alert (23%) 2006–07 to 2007–08 |
Pre-FDA alert (23%,) to pre-black box warning (23%) 2007–08 to 2008–09 |
Pre-black box warning (23%,) to end of study (16%,) (2008–09 to 2010–11 |
|||||
| Sample | Outcome | OR | 95% CI | OR | 95%, CI | OR | 95%, CI |
| Quit attempters | Dse of varenicline since LSD | n = 717 | n = 789 | n = 745 | |||
| 120.79 | 16.81–868.13 | 1.04 | 0.79–1.36 | 0.69 | 0.51–0.93 | ||
Odds ratios (OR) indicate the average increase/decrease in likelihood of each outcome per unit increase in time (i.e. ~1 year), within each time-period; generalized estimating equation (GEE) modelling was used to account for repeated observations of the same individuals over time; ns indicate number of unique individuals within each time-period.
Full study ns do not equal sum of ns from the two separate time-periods because some individuals participated in both time-periods.
FDA = Food and Drug Administration; NRT = nicotine replacement therapy; CI = confidence interval; LSD = last survey date.
Among all smokers, there was a significant increase in making at least one quit attempt across the study period (OR = 1.05, 95% CI = 1.02–1.08; i.e. on average, there was a 5% increase in the odds of making a quit attempt per year across the entire study period), and this increase was greatest during the post-varenicline time-period (OR = 1.12, 95% CI = 1.07–1.18). Among those who made at least one quit attempt, there was a significant increase in the odds of making two or more quit attempts across the study period (OR = 1.09, 95% CI = 1.04– 1.14), and this increase was also strongest during the post-varenicline time-period (OR = 1.12, 95% CI = 1.03–1.21).
Among those who had made at least one quit attempt, use of any SSM increased between the beginning of the study period (31%) and the end of the study period (44%, OR = 1.15, 95% CI = 1.10–1.21) and, again, the largest increase occurred during the post-varenicline time-period (OR = 1.16, 95% CI = 1.07–1.26). A noteworthy spike in use of any SSM occurred in 2007–08 (48%), the year following the introduction of varenicline, when 23% of quit attempters reported use of it. The trend in use of any NRT across the entire study period was flat (OR = 0.96, 95% CI = 0.91–1.01), and was generally flat during both the pre-varenicline time-period (OR = 1.08, 95% CI = 0.94–1.25) and the post-varenicline time-period (OR = 0.95, 95% CI = 0.85–1.05). However, looking specifically at NRT patch use, there was a significant increase in its use during the pre-varenicline time-period (OR = 1.23, 95% CI = 1.04–1.47) and a significant decrease in its use during the post-varenicline time-period (OR = 0.87, 95% CI = 0.76–0.99).
When deconstructing the post-varenicline time-period into its 3 component years, there was a > 100-fold increase in use of varenicline after its introduction and prior to the FDA issuing a safety alert (OR = 120.79, 95% CI = 16.81–868.13), a flattening-out of the trend after the safety alert and prior to the FDA adding a black box warning to the drug (OR = 1.04, 95% CI = 0.79–1.36) and a decline in the trend after the black box warning was added (OR = 0.69, 95% CI = 0.51–0.93; Table 1).
Table 3 shows characteristics of varenicline users compared to those who used bupropion (model A—among users of prescription medication), compared to those who used any stop-smoking medication other than varenicline (model B—among users of any SSM), and compared to those who did not use medication when attempting to quit smoking (model C—varenicline use versus no use of any SSM). Among those who used prescription medication, females and those with higher incomes were more likely than their counterparts to use varenicline as opposed to bupropion (model A). Among those who used any SSM, those with higher incomes were more likely than their low-income counterparts to use varenicline as opposed to another type of SSM (model B). When compared to those who attempted to quit without medication, those who used varenicline were more likely to be older, white, to have higher incomes, to have higher HSI scores prior to quitting and to have used medication in the past (model C).
Table 3.
Correlates of using varenicline versus using bupropion/using any stop-smoking medications (SSM)/using no SSM among smokers attempting to quit.
| Distribution among all quit attempters |
Model A—among Rx SSM users |
Model B—among SSM users |
Model G—varenicline versus no SSM use |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n= 274 (82% used varenicline) | n = 481 (39% used varenicline) | n = 779 (28% used varenicline) | |||||||||||
| Correlates | % | n | % varenicline | OR | P | n | % varenicline | OR | P | n | %varenicline | OR | P |
| Sex | |||||||||||||
| Female | 59 | 163 | 85 | Referent | 297 | 40 | Referent | 450 | 30 | Referent | |||
| Male | 41 | 111 | 78 | 0.51 | 0.047 | 184 | 38 | 0.84 | 0.369 | 329 | 25 | 0.80 | 0.189 |
| Age group (years) | |||||||||||||
| 18–24 | 4 | 3 | 75 | Referent | 10 | 19 | Referent | 35 | 8 | Referent | |||
| 25–39 | 16 | 48 | 77 | 1.43 | 0.805 | 70 | 44 | 3.03 | 0.179 | 124 | 26 | 5.61 | 0.025 |
| 40–54 | 41 | 112 | 82 | 1.64 | 0.731 | 214 | 36 | 2.02 | 0.386 | 314 | 28 | 5.01 | 0.032 |
| 55+ | 39 | 111 | 84 | 2.96 | 0.454 | 187 | 42 | 3.34 | 0.138 | 306 | 30 | 5.46 | 0.024 |
| Race/ethnicity | |||||||||||||
| Other | 17 | 25 | 80 | Referent | 50 | 32 | Referent | 131 | 15 | Referent | |||
| Non-Hispanic white | 83 | 249 | 82 | 1.22 | 0.683 | 431 | 40 | 1.59 | 0.140 | 648 | 30 | 2.33 | 0.002 |
| Education | |||||||||||||
| Low | 33 | 88 | 82 | Referent | 185 | 35 | Referent | 325 | 22 | Referent | |||
| Moderate | 36 | 123 | 80 | 0.89 | 0.747 | 204 | 39 | 1.19 | 0.407 | 286 | 32 | 1.76 | 0.002 |
| High | 30 | 64 | 87 | 1.59 | 0.312 | 95 | 46 | 1.59 | 0.076 | 171 | 31 | 1.50 | 0.071 |
| Income | |||||||||||||
| Low | 42 | 70 | 75 | Referent | 153 | 29 | Referent | 245 | 21 | Referent | |||
| Moderate | 36 | 98 | 84 | 1.93 | 0.082 | 166 | 42 | 1.57 | 0.040 | 260 | 31 | 1.54 | 0.034 |
| High | 22 | 80 | 87 | 2.67 | 0.025 | 130 | 46 | 2.01 | 0.006 | 218 | 31 | 1.85 | 0.005 |
| HSIa | |||||||||||||
| 0–1 | 27 | 51 | 78 | Referent | 100 | 35 | Referent | 226 | 16 | Referent | |||
| 2–3 | 47 | 138 | 80 | 1.22 | 0.616 | 256 | 37 | 1.09 | 0.729 | 393 | 28 | 1.67 | 0.015 |
| 4–6 | 25 | 102 | 87 | 2.36 | 0.054 | 167 | 44 | 1.66 | 0.050 | 226 | 39 | 2.40 | 0.001 |
| Ever used any SSMb | |||||||||||||
| No | 43 | 64 | 81 | Referent | 119 | 39 | Referent | 344 | 13 | Referent | |||
| Yes | 57 | 221 | 82 | 0.97 | 0.943 | 382 | 39 | 1.07 | 0.755 | 452 | 38 | 3.29 | <0.001 |
Analyses included data from waves 6℃8 and were adjusted for sex, age group, race/ethnicity, education, income, heavy smoking index (HSI) and survey wave; generalized estimating equation (GEE) modelling was used to account for repeated observations of the same respondents over time; ns indicate number of unique individuals within cells; percentages consider multiple observations per individual.
Assessed at wave preceding evaluation of medication use;
Assessed prior to evaluation of medication use by combining life-time use with annual use. OR = odds ratio.
DISCUSSION
The key finding from this analysis is that the introduction of varenicline to the US market-place appears to have added to the total use of cessation medications among smokers who attempted to quit, similar to findings from the United Kingdom [12]. Additionally, Kotz and colleagues observed a decline in use of OTC NRT following the introduction of varenicline to the United Kingdom, and we observed a post-varenicline decline in NRT patch use in the United States. Although we observed non-significant increases in use of other NRT as NRT patch use decreased, it is plausible that at least some varenicline use substituted for NRT patch use.
Importantly, after the initial spike in varenicline use, we observed a decline in its use, which coincided with FDA communications about possible safety concerns [6,7]. Although several studies have found varenicline to be safe [8–11], the publicity surrounding the FDA communications may have left smokers less inclined to use varenicline, although it is also possible that there were natural declines in its use over time as the novelty of the drug wore off.
Of note, the majority of varenicline users at a given wave in our study reported prior SSM use (~80%, which was similar to the proportion of other SSM users who reported prior SSM use), indicating that medication use begets medication use, and making it more probable that some smokers who used NRT patches at one wave would try a new medication at a subsequent wave. Indeed, we found that the demographic profiles of those who used varenicline were similar to the profiles of those who used other SSMs, but were different from those who did not use any medication, suggesting that varenicline did not add variability to who uses SSMs. In particular, younger smokers, racial/ethnic minorities and those with lower incomes were less likely to use medication than their counterparts. In light of the strong clinical trial evidence demonstrating the efficacy of varenicline in increasing cessation rates [2], along with population-based studies that have shown varenicline to be effective in the ‘real world’ [24,25], these demographic differences in varenicline users illustrate disparities in opportunities to quit smoking. Given the higher rates of smoking among the uninsured/underinsured segments of the population [26], especially those with mental illness, these results suggest that there continue to be barriers to the delivery of evidence-based stop smoking treatments for smokers in need of assistance.
Finally, unlike in the United Kingdom [12], we observed increases in the proportion of US smokers who make quit attempts, and increases in the proportion of quit attempters making multiple attempts. We cannot attribute these increases to varenicline availability itself. Policy changes that have occurred during this time-period, including increases in cigarette taxes and smoke-free laws, have undoubtedly contributed to the upward trend in quit attempts [27]. Also, and importantly, increases in the number of quit attempts among quit attempters may have contributed to the observed increases in medication use over time. That is, prior to 2007–08, about half of quit attempters reported making two or more quit attempts, but by the end of the study period (2010–11) nearly 60% of quit attempters made two or more quit attempts. As we cannot connect each specific use of medication to each specific quit attempt that occurred over the course of an entire year, we cannot determine the extent to which increases in number of quit attempts may have accounted for increases in medication use. Further research using a study design with more frequent follow-up is needed to address this question.
Among our study limitations is the low survey response rate, ranging from 21 to 35%. However, non-response is unlikely to have biased our findings because the characteristics of respondents to our survey correspond well to the demographic profiles of respondents to several national benchmark surveys [16]. Secondly, our survey relies upon recall of quit attempts and medication use that occurred since the last survey (approximately 1 year ago), and as recall diminishes as time passes this may result in underestimation of quit attempts and overestimation of medication use among quit attempters [28]. Thirdly, there was some variability in the amount of time between waves, meaning that some participants had slightly more time during which to make a quit attempt than others. We were also unable to evaluate trends in medication use in the years preceding or following our study period. Finally, electronic cigarettes have been touted as cessation devices, but they first entered the tobacco landscape at the tail end of our study period, leaving us without the follow-up data needed to evaluate what impact they may have on smokers’ use of varenicline and other FDA-approved cessation medications.
CONCLUSIONS
The introduction of varenicline in the United States appears to have added to the total use of SSMs among smokers attempting to quit, although NRT patch use declined alongside the increase in use of varenicline. The demographic profile of varenicline users was similar to the profile of other SSM users; however, compared to those who attempted to quit without any medication, varenicline users tended to be older, white, had higher incomes, had higher nicotine dependence and were more likely to have used medication in the past.
Acknowledgements
The ITC Four Country Survey has been funded by the US National Cancer Institute (P50 CA111326, P01 CA138389, R01 CA100362, R01 CA125116), Canadian Institutes of Health Research (57897, 79551 and 115016), National Health and Medical Research Council of Australia (265903, 450110 and 1005922), Cancer Research UK (C312/A3726, C312/A6465 and C312/A11039), Robert Wood Johnson Foundation (045734) and Canadian Tobacco Control Research Initiative (014578). Geoffrey T.Fong is supported by a Senior Investigator Award from the Ontario Institute for Cancer Research and a Prevention Scientist Award from the Canadian Cancer Society Research Institute. None of the sponsors played any direct role in the design or conduct of the study, the collection, management, analysis or interpretation of the data, the preparation of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Declaration of interests
K. Michael Cummings has served as a paid consultant on smoking cessation to Pfizer and Novartis, has received payment from Pfizer and GlaxoSmithKline for lectures on smoking cessation to health professionals and has served as a paid expert witness in litigation against the tobacco industry. Within the past 3 years, Andrew Hyland has received funding from Pfizer to purchase varenicline to conduct a pilot study to examine the feasibility of administering varenicline in a quitline setting. All other authors declare no conflicts of interest.
References
- 1.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4-beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008;1:006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. [accessed 21 April 2014];NME Drug and New Biologic Approvals in 2006: Varenicline. 2006 Available at: http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/drugandbiologicapprovalreports/ucm081673.htm.
- 4.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1:000146. doi: 10.1002/14651858.CD000146.pub3. pub3. [DOI] [PubMed] [Google Scholar]
- 5.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. [accessed 21 April 2014];Information for healthcare professionals: varenicline (marketed as Chantix) 2008 FDA ALERT [2/1/2008]. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124818.htm.
- 7.US Food and Drug Administration. [accessed 21 April 2014];Information for healthcare professionals: varenicline (marketed as Chantix) and bupropion (marketed as Zyban, Wellbutrin, and generics) 2009 FDA ALERT [7/1/2009]. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm169986.htm.
- 8.Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin Ther. 2009;31:463–491. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons RD, Mann JJ. Varenicline, smoking cessation, and neuropsychiatric adverse events. Am J Psychiatry. 2013;170:1460–1467. doi: 10.1176/appi.ajp.2013.12121599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiménez-Ruiz C, Berlin I, Hering T. Varenicline: a novel pharmacotherapy for smoking cessation. Drugs. 2009;69:1319–1338. doi: 10.2165/00003495-200969100-00003. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:2856. doi: 10.1136/bmj.e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotz D, Fidler JA, West R. Did the introduction of varenicline in England substitute for or add to the use of other smoking cessation medications? Nicotine Tob Res. 2011;13:793–799. doi: 10.1093/ntr/ntr075. [DOI] [PubMed] [Google Scholar]
- 13.Langley TE, Huang Y, McNeill A, Coleman T, Szatkowski L, Lewis S. Prescribing of smoking cessation medication in England since the introduction of varenicline. Addiction. 2011;106:1319–1324. doi: 10.1111/j.1360-0443.2011.03426.x. [DOI] [PubMed] [Google Scholar]
- 14.Fix BV, Hyland A, Rivard C, McNeill A, Fong GT, Borland R, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: findings from the 2006–2008 International Tobacco Control (ITC) Four Country Survey. Int J Environ Res Public Health. 2011;8:222–233. doi: 10.3390/ijerph8010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong GT, Thompson M, Hammond D, Boudreau C, Driezen P. [accessed 17 February 2012];International Tobacco Control Policy Evaluation Survey (ITC), Four Country Project, Waves 2–8 Technical Report. 2011 Available at: http://www.itcproject.org/documents/keyfindings/4cw28techreportmay2011_2_pdf (Archived by WebCite® at http://www.webcitation.org/65WmeqN1E).
- 16.Hammond D, Fong GT, Thompson ME, Driezen P. [accessed 17 February 2012];International Tobacco Control Policy Evaluation Survey (ITC), Four Country Project, Wave 1 Technical Report. 2004 Available at: http://www.itcproject.org/documents/keyfindings/technicalreports/itcw1techreportfinalpdf (Archived by WebCite® at http://www.webcitation.org/65WlnsWWy). [Google Scholar]
- 17.Fong GT, Cummings KM, Borland R, Hastings G, Hyland AJ, Giovino GA, et al. The conceptual framework of the International Tobacco Control (ITC) Policy Evaluation Project. Tob Control. 2006;15:3–11. doi: 10.1136/tc.2005.015438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson ME, Fong GT, Hammond D, Boudreau C, Driezen P, Hyland A, et al. Methods of the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15:12–18. doi: 10.1136/tc.2005.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84:791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 20.International Tobacco Control Policy Evaluation Project. [accessed 17 February2012];4-Country Survey Questionnaires. 2009–2011 Available at: http://www.itcproject.org/ (Archived by WebCite® at http://www.webcitation.org/65Wm59nLc).
- 21.Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton, FL: Chapman & Hall/CRC; 2003. [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 23.StataCorp. Stata Statistical Software, Version 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 24.Kotz D, Brown J, West R. ‘Real-world’ effectiveness of smoking cessation treatments: a population study. Addiction. 2013;109:491–499. doi: 10.1111/add.12429. [DOI] [PubMed] [Google Scholar]
- 25.Kasza KA, Hyland AJ, Borland R, McNeill A, Bansal-Travers M, Fix BV, et al. Effectiveness of stop-smoking medications: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2013;108:193–202. doi: 10.1111/j.1360-0443.2012.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gfroerer J, Dube SR, King BA, Garrett BE, Babb S, McAfee T. Vital signs: current cigarette smoking among adults aged >18 years with mental illness—United States, 2009–2011. Morb Mortal Wkly Rep. 2013;62:81–87. [PMC free article] [PubMed] [Google Scholar]
- 27.International Tobacco Control (ITC) Project. ITC United States National Report. Findings from the Wave 1 to 8 Surveys (2002–2011) University of Waterloo, Waterloo, Ontario, Canada and Medical University of South, Carolina, Charleston, South Carolina, United States; 2014. Feb, [Google Scholar]
- 28.Borland R, Partos TR, Cummings KM. Systematic biases in cross-sectional community studies may underestimate the effectiveness of stop-smoking medications. Nicotine Tob Res. 2012;14:1483–1487. doi: 10.1093/ntr/nts002. [DOI] [PMC free article] [PubMed] [Google Scholar]