Abstract
Cumulating evidence from epidemiologic studies implicates cardiovascular health and cerebrovascular function in several brain diseases in late life. We examined vascular risk factors with respect to a cerebrovascular measure of brain functioning in subjects in mid-life, which could represent a marker of brain changes in later life. Breath-hold functional MRI (fMRI) was performed in 541 women and men (mean age 50.4 years) from the Coronary Artery Risk Development in Young Adults (CARDIA) Brain MRI sub-study. Cerebrovascular reactivity (CVR) was quantified as percentage change in blood-oxygen level dependent (BOLD) signal in activated voxels, which was mapped to a common brain template and log-transformed. Mean CVR was calculated for anatomic regions underlying the default-mode network (DMN) - a network implicated in AD and other brain disorders - in addition to areas considered to be relatively spared in the disease (e.g. occipital lobe), which were utilized as reference regions. Mean CVR was significantly reduced in the posterior cingulate/precuneus (β = -0.063, 95% CI: - 0.106, -0.020), anterior cingulate (β = -0.055, 95% CI: -0.101, -0.010), and medial frontal lobe (β = -0.050, 95% CI: -0.092, -0.008) relative to mean CVR in the occipital lobe, after adjustment for age, sex, race, education, and smoking status, in subjects with pre-hypertension/hypertension compared to normotensive subjects. By contrast, mean CVR was lower, but not significantly, in the inferior parietal lobe (β = -0.024, 95% CI: -0.062, 0.014) and the hippocampus (β = -0.006, 95% CI: -0.062, 0.050) relative to mean CVR in the occipital lobe. Similar results were observed in subjects with diabetes and dyslipidemia compared to those without these conditions, though the differences were non-significant. Reduced CVR may represent diminished vascular functionality for the DMN for individuals with prehypertension/ hypertension in mid-life, and may serve as a preclinical marker for brain dysfunction in later life.
Keywords: Alzheimer's disease, neurophysiology, vascular risk factors
1. INTRODUCTION
Changes in brain functioning in mid-life, before structural changes in the brain, may represent an important marker of future brain health in later life (Alsop et al., 2010; Bendlin et al., 2010). Vascular risk factors (VRF) and vascular disease have been shown to be associated with reduced cerebrovascular function (e.g., cerebral blood flow) (Claus et al., 1998; Riecker et al., 2003; Wu et al., 2008; Jagust and D'Esposito, 2009). In addition, cerebrovascular disorders -- e.g., cerebral small vessel disease, white matter lesions, and reduced white matter integrity-- have been linked to reduced brain network functioning that include the default-mode network (DMN) and other networks involved in cognitive control (Damoiseaux and Greicius, 2009; Mayda et al., 2011; Papma et al., 2013). Evidence from epidemiologic studies of VRF in mid-life and risk of dementia in late-life (Launer et al., 1995; Launer et al., 2000; Kivipelto et al., 2001; Roberts et al., 2014), as well as cumulating evidence of neurovascular mechanisms of Alzheimer's disease (AD) (Benarroch, 2007; Novak, 2012) warrants investigation of VRF and brain changes that could represent vascular contributions to AD.
The DMN represents a resting state network that consists of a set of brain regions that co-activate when subjects are at rest, and deactivate together when subjects become engaged in external cognitive tasks (e.g., episodic learning) (Raichle et al., 2001; Daselaar et al., 2004; Sperling, 2007; Buckner et al., 2008; Miller et al., 2008). Studies of brain network dysfunction, using blood-oxygen level dependent functional MR imaging (BOLD fMRI), have implicated the DMN as one of the central representative networks in aging and AD pathophysiology (Greicius et al., 2004; Hedden et al., 2009; Brier et al., 2014). These studies have shown that DMN disconnectivity could represent a biomarker for preclinical AD (Greicius et al., 2004; Hedden et al., 2009; Sheline and Raichle, 2013; Brier et al., 2014) as well as a marker of AD progression (Damoiseaux et al., 2012). Evidence from previous studies has implicated vascular changes in DMN disruption (Damoiseaux and Greicius, 2009; Mayda et al., 2011; Papma et al., 2013). Additionally, reduced cerebrovascular function (e.g., cerebral blood flow) has been observed in brain regions that overlap DMN regions (e.g., parietal cortex) in individuals with AD (Alsop et al., 2000; Johnson et al., 2005; Dai et al., 2009; Jagust and D'Esposito, 2009; Schuff et al., 2009; Stefani et al., 2009) mild cognitive impairment (MCI) (Johnson et al., 2005; Duschek and Schandry, 2007) as well as cognitively healthy older adults(Claus et al., 1998; Wu et al., 2008; Jagust and D'Esposito, 2009).
Cerebrovascular reactivity (CVR) represents a measure of vascular responsiveness, based on vasodilation of cerebral vessels, given induced changes in the brain (e.g., increased CO2) that can occur during given physical states (e.g., breath-hold) (Kastrup et al., 1999; Kastrup et al., 2001; Handwerker et al., 2007). Typically, CVR has been used to evaluate global brain response (Last et al., 2007; Jagust and D'Esposito, 2009) as well as to measure effects on hemodynamic response in specific vascular territories (Vernieri et al., 1999; Silvestrini et al., 2000; Sorond et al., 2010). However, regional differences in CVR have been observed (Kastrup et al., 1999) and, additionally, CVR has been shown to vary regionally with respect to VRF (Novak, 2012). It is possible that reduced CVR —i.e., representative of lower cerebrovascular function associated with different VRF—may occur in brain regions that underlie the DMN and may affect DMN function.
We hypothesize that for adults in midlife reduced CVR, associated with VRF (e.g., hypertension, diabetes, dyslipidemia), occurs in regions of the brain that underlie the DMN, and could reflect reduced vascular functionality in the network. Furthermore, we hypothesize that reduced CVR occurs simultaneously in the hippocampus, which has a functional relationship with the DMN (i.e., the hippocampus activates while the DMN deactivates during learning tasks). Reduced CVR in these regions could represent changes in vascular brain function for individuals with VRF in mid-life, and could represent a preclinical marker of brain health in later life.
2. MATERIALS AND METHODS
2.1 Study Sample
Participants were enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, a longitudinal study to investigate the determinants and development of cardiovascular disease in young adults. Details of the recruitment of the study sample are available (Friedman et al., 1988). Of the 5,115 adults enrolled in the study, 3499 (72% of survivors) were evaluated at the 25-year follow-up exam. As part of this exam, a sub-sample was invited to participate in the CARDIA Brain sub-study. This study recruited subjects from 3 of the 4 CARDIA field centers, and was designed to investigate the morphology, pathology, physiology and function of the brain with magnetic resonance imaging (MRI) technology. Exclusion criteria at the time of sample selection, or at the MRI site, were a contraindication to MRI or a body size that was too large for the MRI scanner. Of those who were eligible for the sub-study, our target was scans in 700 individuals; we obtained 719 individuals, who received MRI scans.
All participants provided written informed consent at each exam, and institutional review boards from each field center and the coordinating center annually approved the CARDIA study. Separate participant written consent for participation in the CARDIA brain sub-study was obtained, and separate approval was given by the IRBs of the participating sites and the IRB covering Intramural Research at the National Institute on Aging (NIA).
2.2 MRI acquisition and processing
MRI scans were obtained for patients using 3-T MR scanners located proximal to each CARDIA clinical site. Details of the scanners used, training of MRI technologists at the different sites, implementation of study protocols, and quality assurance of scanner stability and performance are provided elsewhere (Launer et al., 2015). A 40-minute scan protocol was implemented and a prioritized order of brain sequences was obtained. For participant safety, scans were examined initially by the MR technician. Any detection of pathology or other medical abnormalities were reported to the site PI and radiologist immediately and appropriate steps were taken. Otherwise, each site followed standard operating procedures that involved reading of the scan within 48 hours.
Post-scan image processing was performed by the Section of Biomedical Image Analysis (BIA), Department of Radiology, University of Pennsylvania. An initial QC protocol identified any motion artifacts or any other quality issues. Subjects that failed this QC test were flagged for inspection. After this inspection, an automated pipeline was applied on the scans. Quality checks were performed on intermediate and final processing steps by visual inspection and by identification of outliers of calculated variable distributions.
2.3 MRI Measures
According to previously described methods (Goldszal et al., 1998; Shen and Davatzikos, 2002; Lao et al., 2008; Zacharaki et al., 2008), an automated computer algorithm was used to segment MRI structural images of supratentorial brain tissue into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF). GM and WM were further characterized as normal and abnormal tissue, and assigned as 92 anatomic regions of interest (ROIs) in each hemisphere. These 92 anatomic regions comprise the Jakob atlas (Kabani et al., 1998), which was used as a brain template to which the MRI brain measures were co-registered.
Based on previous reports (Buckner et al., 2008; Hedden et al., 2009), and the Jakob atlas available in the current study, a priori defined brain regions were selected to investigate the DMN. Regions, and corresponding subregions, included: posterior cingulate/precuneus (PCC); inferior parietal lobe (angular gyrus and supramarginal gyrus) (INF); anterior cingulate (ACC); and medial frontal lobe (MFL). In addition, although the hippocampus is not part of the DMN, it was selected as a region of interest given its functional relationship with the DMN (i.e., the hippocampus activates while the DMN deactivates during learning tasks) (Sperling, 2007; Miller et al., 2008; Jagust and D'Esposito, 2009). As a reference for comparison throughout the study, we selected the occipital lobe (i.e., occipital pole and superior, middle, and inferior occipital gyri) and sensorimotor cortex (i.e., precentral gyrus, postcentral gyrus), which are thought to be less vulnerable to disease (e.g., AD) (Thompson et al., 2001; Resnick et al., 2003; Yakushev et al., 2008). In addition, other cortical brain regions (i.e., excluding those representative of the DMN, hippocampus, occipital and sensorimotor cortex) were used to represent Non-DMN regions.
2.4 Cerebrovascular Reactivity (CVR)
2.4.1 CVR Acquisition
Each participant performed a breath-hold task during acquisition of BOLD fMRI. We used a block design with two interleaved conditions. Subjects received a visual instruction, while in the scanner, to breathe normally for 30 seconds; then hold their breath after expiration for 16 seconds; then resume normal breathing. This procedure was repeated 4 times in succession, and the recorded measurements were averaged for the 4 repetitions. The BOLD scans were corrected for motion and smoothed. This step was followed by a general linear model (GLM) analysis, for each subject, where the time course in BOLD signal at each voxel was fit with: 1) a regressor representative of the interleaved block-design, or block-model; and 2) a 9 second delay in the block-model to account for the lag in the BOLD signal. The analysis generated a voxel-wise statistical parametric map of t-scores, which was transformed to a z-score map. A threshold was applied to the z-scores (Z ≥ 2.3), with cluster correction at p=0.05, to identify contiguous clusters of voxels which activated in response to the breath-hold task. A percent signal change map, based on these clusters, was generated and registered to the Jakob atlas (See Appendix for details).
2.4.2 CVR Scan Inclusion Criteria
Of the 719 subjects in the Brain MRI sub-study, 680 subjects had fMRI scans. Of these, 668 subjects had images that passed quality checks for further processing (Fig 1). To ensure that subjects had a valid CVR hypercapnic stimulus from a compliant breath-hold, we identified subjects with scans that showed a global response to the breath-hold task as reflected by activation of the superior sagittal sinus (SSS) (Bandettini and Wong, 1997, Pillai and Milkulis, 2015). Presence of this signal was determined by using the threshold map above masked to a predefined region of interest (ROI) in the SSS. In addition, thirty subjects with multiple regions with little or no activation, specifically 30 individuals who did not activate voxels in DMN regions and, of these, 28 subjects who did not activate voxels in Non-DMN portions of the brain, were excluded from the analysis. In the 542 subjects that remained, each subject had CVR measures, based on the % mean change in BOLD signal, for different brain regions.
Figure 1.
Flowchart of subjects with cerebrovascular reactivity (CVR) measure included in the analysis.
2.4.3 Mean CVR Region-of-Interest (ROI) Calculation
Prior to combining CVR measures from different brain regions and calculating mean CVR for each ROI of interest in the study (i.e., different ROI comprising the DMN and other brain regions), we examined the distributions of the measures (i.e. between measure variability of adjacent brain regions and left and right brain hemispheres) (data not shown). Mean CVR (mean % BOLD-fMRI change) for each ROI was calculated based on the following:
for jth side (i.e., left, right hemisphere) and kth subregion (e.g., angular gyrus, supramarginal gyrus). Measures of mean CVR were calculated similarly for overall, composite measures of the DMN and Non-DMN, respectively, the hippocampus, occipital, and sensorimotor cortex using the activated voxels of regions in those areas.
Given the distribution of CVR was skewed, the measure was log-transformed for analysis. One subject with a high CVR reading across different brain regions (> 3 SD relative to the group mean) was excluded from the analysis.
2.5 Vascular Risk Factors (VRF)
Three measures of cardiovascular disease risk were investigated in the study. Variables for VRF groups were coded based on American Heart Association (AHA) 2012 criteria. Prehypertension/ hypertension was defined as 0=no, 1=yes, based on one or more of the following: systolic blood pressure>=130 mmHg; diastolic blood pressure >=85 mmHg; or blood pressure medication use (Chobanian et al., 2003). Diabetes was defined as 0=no, 1=yes, based on one or more of the following criteria: fasting glucose >=126 mg/dL where the duration of the fast was >=480 minutes; 2-hour glucose tolerance test >=200 mg/dL; glycosylated hemoglobin (HbA1c) >=6.5%; or diabetes medication use (American Diabetes Association, 2011). For 29 subjects who were missing either the 2-hour glucose tolerance test or the HbA1C measurement, diabetes status was imputed and set to 0 given the values of the remaining variables (e.g., fasting glucose < 126 mg/dL). Dyslipidemia was defined 0=no, 1=yes using the following criteria: for males, triglyceride levels >=150 mg/dL or HDL levels < 40 mg/dL; for females, triglyceride levels >=150 mg/dL or HDL levels< 50 mg/dL; or cholesterol-lowering medication use (Miller et al., 2011).
2.6 Resting Cerebral Blood Flow (CBF)
2.6.1 CBF Acquisition
Arterial spin labeling (ASL) was utilized to measure participants’ resting cerebral blood flow, at two of the three MRI sites in the Brain sub-study, and has been used widely in studies of brain aging and disease (Wang et al., 2008). The scan consists of an interleaved sequence of control and tag volumes from which the mean perfusion volume (ml/100g/min) at each brain voxel can be quantified. The mean CBF at the locations of these voxels was obtained and co-registered with MRI regions from the Jakob brain atlas, which allowed for examination of mean CBF at the level of the same anatomical ROIs as for CVR.
2.6.2 Mean CBF ROI Calculation
Similarly to CVR, descriptive analyses and calculations were conducted (i.e., weighed average of mean CBF and CBF voxels of different subregions) to derive mean CBF for the different ROIs in the analysis (i.e., different ROIs comprising the DMN).
2.7 Covariates
Covariates included age (years), defined at the time of the 25-year visit, sex, education (<16 years/≥ 16 years), race (black/white), ApoE4 genotype status (0=no E4 allele, 1= ≥ 1 E4 alleles), and smoking status at the 25-year exam, which was recorded 0=never, 1=former, 2=current. In addition, occurrence of lung disease (chronic obstructive pulmonary disease, emphysema, asthma, chronic bronchitis: 0=no, 1=yes) was considered as a potential co-factor. Following an analysis of the whole study group (see below), we repeated the analysis in subjects with no reported lung disease.
2.8 Analysis
2.8.1 Overview
A cross-sectional analysis, based on the Year 25 exam of the CARDIA study, was used to assess the associations of VRF and CVR in regions of the brain correspondent with the DMN. Specifically, in groups with normal and abnormal VRF, the analysis quantified the relative difference in mean CVR in brain regions defined a priori in the DMN, and the hippocampus, with respect to mean CVR in the occipital lobe (i.e., mean CVRDMN regions – mean CVROccipital lobe), which is thought to be less susceptible to disease (Ishii et al., 2007; Resnick et al., 2003; Thompson et al., 2001).
These regional comparisons were followed by an analysis that quantified the relative differences in mean CVR for the DMN (i.e., DMN regions combined as one ROI), mean CVR for the hippocampus, mean CVR in the non-DMN cortical regions (i.e., Non-DMN cortical regions combined as one ROI), and mean CVR in the occipital lobe. The Non-DMN cortical regions excluded the occipital lobe and sensorimotor cortex which represented reference regions in the analysis. The rationale for investigation of these differences was to determine whether effects of interest might extend to other brain regions beyond the DMN.
For these two preceding sets of analyses (i.e., regional DMN and DMN/Non-DMN), sensitivity analyses were performed that substituted the occipital lobe with sensorimotor cortex (i.e., precentral gyrus, postcentral gyrus), as a reference region, given that sensorimotor cortex has been found to be less susceptible to AD-related changes (Thompson et al., 2001; Yakushev et al., 2008).
Lastly, given that CVR signal represents a change in cerebral blood flow, we examined VRF with respect to resting CBF in subgroup analyses in participants for whom the resting CBF measure was available.
2.8.2 Statistical Analysis
Mean difference in CVR was assessed using mixed models with respect to each VRF risk group, where data from multiple regions available for each subject represented repeated measures. Models were used to assess mean difference in CVR that could potentially vary by region for the VRF group levels. For example, the following model was used to examine the possible interaction of VRF group i (e.g., i represents pre-hypertension/hypertension yes/no) by region, where the occipital lobe represents the reference region:
Coefficients based on this model represent the following: β0 represents the mean CVR for the occipital lobe in subjects with no VRF for the ith group; β1 represents the mean difference in CVR, in the occipital lobe, for subjects with a VRFi compared to subjects with no VRFi; β2j represents the mean differences in CVR, for each region j (i.e., 5 regions comprising the DMN and the hippocampus), compared to the occipital lobe in subjects with no VRFi; β3ij represents the relative differences in mean CVR for each region j, compared to the occipital lobe, in subjects with VRFi compared to subjects without VRFi.
Given this multilevel modeling approach, covariates were included at the overall level and at the regional level--e.g., age and smoking status might differentially affect CVR levels in specific regions underlying the DMN. Estimation of the model coefficients and standard errors accounted for within-subject (i.e., region-to-region) variability (Littell et al., 1996).
Analyses were conducted with SAS version 9.3 and R version 3.0.1.
3. RESULTS
Subjects were mean age 50 years and included more women than men (52% vs 48%) (Table 1). The sample included 35% African-Americans, and was well-educated with 53% completing college. Subjects’ smoking history included 60% never, 26% former, and 14% current smokers. Most subjects had no underlying lung disease, although 14% of subjects reported asthma and/or taking medication for asthma. Prevalence of VRF investigated in the study were approximately as follows: prehypertension/ hypertension (32%); diabetes (8%); and dyslipidemia (35%). Excluded subjects had similar age and sex characteristics compared to included subjects, but included more African-Americans (57%) and participants with a higher prevalence of VRF: prehypertension/hypertension (50%); diabetes (14%); and dyslipidemia (46%).
Table 1.
Sample characteristicsa
| Includedb | Excludedb | |
|---|---|---|
| N | 536 | 183 |
| Age, years | 50.4 (3.5) | 49.8 (3.6) |
| Sex: male | 47.8 | 47.0 |
| Race: black | 34.9 | 56.8 |
| Education: ≥ 16 years | 52.9 | 36.3 |
| Smoking Status | ||
| - Never | 60.3 | 58.0 |
| - Former | 25.6 | 17.7 |
| - Current | 14.1 | 24.3 |
| ApoE4 Status: ≥1allele | 28.7c | 34.8c |
| Chronic Bronchitis | 3.2 | 2.8 |
| Emphysema | 0.6 | 1.6 |
| COPD | 0.9 | 1.0 |
| Asthma | 14.0 | 13.3 |
| Prehypertension/Hypertension | 31.8 | 50.0 |
| Diabetes | 8.5 | 13.5 |
| Dyslipidemia | 35.0 | 45.6 |
| CVR (Overall) | 1.36[0.46,2.82] d | NA |
| Log CVR (Overall) | 0.28(0.30) | NA |
| Regions | ||
| - Posterior Cingulate/Precuneus | 0.20(0.35) | NA |
| - Inferior Parietal | 0.14(0.32) | NA |
| - Anterior cingulate | 0.03(0.32) | NA |
| - Medial frontal lobe | 0.30(0.34) | NA |
| - Occipital lobe | 0.24(0.35) | NA |
| - Sensorimotor cortex | 0.16(0.32) | NA |
| - Hippocampus | -0.08(0.38) | NA |
| Grouped Regions | ||
| - DMN | 0.19(0.31) | NA |
| - Non-DMN | 0.33(0.31) | NA |
ApoE4, apolipoprotein e4 allele; CVR, Cerebrovascular reactivity; COPD, chronic obstructive pulmonary disease; DMN, default-mode network.
Distributions reported as Mean (SD) and %.
Range of missing values 0-6 (included participants) and 0-5 (excluded participants) for different covariates, except for ApoE4 status.
ApoE4 status based on subset of 495 subjects (included participants) and 164 subjects (excluded participants).
Distribution reported as median and range.
Analyses that examined mean CVR by region for the different VRF groups are presented in Table 2. Comparisons in those without VRF across regions (i.e., region main effect coefficients) showed that mean CVR was lower, relative to the mean CVR in the occipital lobe, in the different brain regions examined with exception of the medial frontal region where the mean CVR was higher. The model that compared differences in prehypertensive/hypertensive vs. normotensive subjects (i.e., region x VRF group terms in Table 2, left column) indicated an additional decrease in mean CVR in multiple regions of the DMN (PCC: -0.072 (95% CI: -0.115, -0.029); ACC: -0.059 (95% CI: -0.104,-0.014); MFL: -0.039 (95% CI: -0.080, 0.003)), with the PCC and ACC reaching significance. These findings remained significant but were attenuated for the posterior cingulate/precuneus and anterior cingulate, and were stronger and significant for the difference observed for the medial frontal lobe after adjustment for covariates (Table 3): PCC: -0.063 (95% CI: -0.106, -0.020); ACC: -0.055 (95% CI: -0.101,-0.010); MFL: -0.050 (95% CI: -0.092, -0.008). Figure 2 includes a graphical presentation of the adjusted results.
Table 2.
Regional analysis of unadjusted mean differences in cerebrovascular reactivity for DMN regions and hippocampus compared to occipital lobe by VRF
| Prehypertension/Hypertension | Diabetes | Dyslipidemia | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | |
| Intercepta | 0.217 | 0.182, 0.253 | 0.227 | 0.196, 0.258 | 0.215 | 0.178, 0.251 |
| PCCb | −0.012 | −0.037, 0.012 | −0.032 | −0.053, −0.010 | −0.026 | −0.051, −0.001 |
| Inferior Parietal | −0.086 | −0.108, −0.065 | −0.094 | −0.112, −0.075 | −0.090 | −0.111, −0.068 |
| ACC | −0.188 | −0.214, −0.163 | −0.202 | −0.224, −0.180 | −0.205 | −0.231, −0.178 |
| Medial Frontal | 0.072 | 0.048, 0.095 | 0.065 | 0.045, 0.085 | 0.067 | 0.043, 0.091 |
| Hippocampus | −0.310 | −0.341, −0.279 | −0.315 | −0.342, −0.288 | −0.307 | −0.339, −0.275 |
| VRF Groupc | 0.063 | 0.000, 0.126 | 0.137 | 0.031, 0.243 | 0.068 | 0.006, 0.129 |
| PCC × VRF Groupd | −0.072 | −0.115, −0.029 | −0.043 | −0.116, 0.030 | −0.028 | −0.070, 0.014 |
| Inferior Parietal × VRF Group | −0.029 | −0.067, 0.009 | −0.011 | −0.075, 0.052 | −0.015 | −0.052, 0.022 |
| ACC × VRF Group | −0.059 | −0.104, −0.014 | −0.054 | −0.130, 0.022 | −0.008 | −0.052, 0.036 |
| Medial Frontal × VRF Group | −0.039 | −0.080, 0.003 | −0.060 | −0.129, 0.010 | −0.023 | −0.064, 0.017 |
| Hippocampus × VRF Group | −0.022 | −0.077, 0.034 | −0.040 | −0.132, 0.053 | −0.028 | −0.082, 0.026 |
CVR, cerebrovascular reactivity; CI, confidence interval; DMN, default-mode network; PCC posterior cingulate/precuneus; ACC anterior cingulate; VRF, vascular risk factors.
CVR units represent log-transformed % mean change in Bold-fMRI
Intercept represents mean CVR for the occipital lobe for those with no VRF (i.e., no risk factor) under the different conditions listed.
Region coefficients represent comparisons between mean CVR for each region and the mean CVR for the occipital lobe (i.e., mean difference in CVR of each region relative to the occipital lobe), in subjects with no VRF.
VRF Group coefficient represents the difference in mean CVR for the occipital lobe for those with a VRF compared to those with no VRF for the different conditions listed.
Region × VRF Group coefficients represent comparisons between mean CVR for each region and mean CVR for the occipital lobe (i.e., mean difference in CVR of each region relative to the occipital lobe) in subjects with a VRF compared to those with no VRF for the different conditions listed.
Table 3.
Regional analyses of adjusted mean differences in cerebrovascular reactivity for DMN regions and hippocampus relative to occipital lobe in subjects with prehypertension/hypertension compared with normotensive subjectsa
| Coefficient | 95% CI | |
|---|---|---|
| Intercept b | 0.201 | 0.148, 0.254 |
| PCC c | −0.002 | −0.036, 0.032 |
| Inferior Parietal | −0.098 | −0.128, −0.067 |
| ACC | −0.214 | −0.250, −0.178 |
| Medial Frontal | 0.028 | −0.006, 0.061 |
| Hippocampus | −0.311 | −0.356, −0.266 |
| VRF Groupd | 0.056 | −0.010, 0.122 |
| PCC × VRF Groupe | −0.063 | −0.106, −0.020 |
| Inferior Parietal × VRF Group | −0.024 | −0.062, 0.014 |
| ACC × VRF Group | −0.055 | −0.101, −0.010 |
| Medial Frontal × VRF Group | −0.050 | −0.092, −0.008 |
| Hippocampus × VRF Group | −0.006 | −0.062, 0.050 |
CVR, cerebrovascular reactivity; CI, confidence interval; DMN, default-mode network; PCC posterior cingulate/precuneus; ACC anterior cingulate.
CVR units represent log-transformed % mean change in Bold-fMRI
Model adjusted for age, sex, race, education, and smoking status.
Intercept represents mean CVR for the occipital lobe for normotensive subjects.
Region coefficients represent comparisons between mean CVR for each region and mean CVR for the occipital lobe (i.e., mean difference in CVR in each region relative to the occipital lobe), in normotensive subjects.
VRF Group coefficient represents the difference in mean CVR for the occipital lobe for those subjects with prehypertension/hypertension compared to normotensive subjects.
Region × VRF Group coefficients represent comparisons between mean CVR for each region and mean CVR for the occipital lobe (i.e., mean difference in CVR of each region relative to the occipital lobe), in subjects with prehypertension/hypertension compared to normotensive subjects.
Figure 2.
Relative adjusted mean differences in CVR (95% CI) for different brain regions relative to occipital lobe in subjects with prehypertension/ hypertension compared to normotensive subjects. The reduction in CVR was significantly lower for the posterior cingulate/precuneus (PCC), anterior cingulate (ACC), and medial frontal lobe (MFL), relative to the occipital lobe, in subjects with prehypertension/hypertension than for the same region comparison in normotensive subjects.
Similarly, analyses suggested lower mean CVR by region, relative to mean CVR in the occipital lobe, in subjects with diabetes, and separately for subjects with dyslipidemia, compared to subjects without these conditions (Table 2). However, given that the findings for these VRF groups were non-significant, no further analyses that adjusted for covariates were carried out.
To determine if lower mean CVR observed in prehypertensive/hypertensive subjects was specific to the DMN, we examined a model which compared CVR for DMN and Non-DMN regions, as overall composite measures, to the occipital lobe (Table 4). Findings based on this model indicated that mean CVR was lower for both regions, but to a greater and significant extent in the DMN (-0.043, 95% CI: -0.076, -0.009) than the Non-DMN (-0.013, 95% CI: -0.042, 0.017) after adjustment. While the additional lower mean CVR in the prehypertensive/ hypertensive group compared to the normotensives was larger for the DMN compared to the Non-DMN (-0.043 vs -0.013), the result was not significantly different (p< 0.15 ).
Table 4.
Analysis of mean differences in cerebrovascular reactivity for DMN and Non-DMN regions relative to occipital lobe in prehypertensive/hypertensive subjects compared with normotensive subjectsa
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| Coefficient | 95% CI | Coefficient | 95% CI | |
| Interceptb | 0.217 | 0.182, 0.253 | 0.188 | 0.121, 0.255 |
| DMNc | −0.036 | −0.055, −0.017 | −0.057 | −0.084, −0.030 |
| Non-DMN | 0.091 | 0.074, 0.107 | 0.067 | 0.044, 0.091 |
| Hippocampus | −0.310 | −0.341, −0.279 | −0.311 | −0.356, −0.266 |
| VRF Groupd | 0.063 | 0.000, 0.126 | 0.057 | −0.010, 0.123 |
| DMN × VRF Groupe | −0.043 | −0.077, −0.010 | −0.043 | −0.076, −0.009 |
| Non-DMN × VRF Group | −0.010 | −0.038, 0.019 | −0.013 | −0.042, 0.017 |
| Hippocampus × VRF Group | −0.022 | −0.077, 0.034 | −0.006 | −0.062, 0.050 |
CVR, vascular reactivity; DMN, default-mode network
CVR units represent log-transformed % mean change in Bold-fMRI
Model adjusted for age, sex, race, education, and smoking status.
Intercept represents mean CVR for the occipital lobe for normotensive subjects.
Region coefficients represent comparisons between mean CVR for each region and mean CVR for the occipital lobe (i.e., mean difference in CVR of each region relative to the occipital lobe), in normotensive subjects.
VRF Group coefficient represents the difference in mean CVR for the occipital lobe for those subjects with prehypertension/hypertension compared to normotensive subjects.
Region × VRF Group coefficients represent comparisons between mean CVR for each region and mean CVR for the occipital lobe (i.e., mean difference in CVR of each region relative to the occipital lobe), in subjects with prehypertension/hypertension compared to normotensive subjects.
This analysis evaluated differences in CVR in the hippocampus for VRF groups as well. While mean CVR was lower for the hippocampus (Table 2 prehypertension/hypertension: -0.310, 95% CI: -0.341, -0.279; diabetes: -0.315, 95% CI: -0.342, -0.288; dyslipidemia: -0.307, 95% CI: -0.339, -0.275) compared with the occipital lobe, there was no evidence to suggest additional reduction in mean CVR in subjects with VRF. In those with prehypertension/hypertension compared to normotensive subjects, for example, mean CVR was reduced but not significantly in unadjusted (Table 2 -0.022, 95% CI:-0.077, 0.034) and adjusted analyses (Table 3, -0.006, 95% CI: -0.062, 0.050).
Results based on the sensitivity analysis that compared different regions with respect to sensorimotor cortex, which was substituted as a reference region, varied in some respects from those based on the occipital lobe. Differences in mean CVR between these regions and motor cortex were smaller (data not shown); however, results suggested a similar pattern of reduced mean CVR for subjects with VRF compared to those without VRF. In particular, CVR was reduced in multiple DMN regions in subjects with prehypertension/hypertension compared to normotensive subjects after adjustment (PCC: -0.061 95% CI: -0.101, -0.021; ACC: -0.055 95% CI: -0.089, -0.021; MFL: -0.047 95% CI: -0.080, -0.013). Similarly, reductions in mean CVR were observed for overall DMN and Non-DMN regions (DMN: -0.041 95% CI: -0.066, -0.016 Non-DMN: -0.009 95% CI: -0.038, 0.020), that were comparable to those observed based on analyses with the occipital lobe included as the reference region. In contrast, no reduction in mean CVR was observed for these regions when compared with motor cortex, in subjects with diabetes and dyslipidemia compared to subjects without these conditions, respectively.
In subgroup analyses that examined VRF and regional variation in CBF, CBF was reduced in the different DMN regions relative to the occipital lobe, with the exception of the PCC where mean CBF was higher (Supplementary Table 1). However, by contrast with the CVR analysis, mean CBF was not significantly lower for different DMN regions relative to the occipital lobe, notably those with prehypertension/hypertension, compared to those without. In a subset of participants with both CBF and CVR measures (n=412), significantly lower mean differences in CBF were observed for different DMN regions, in those with prehypertension/hypertension compared to those without (Supplementary Table 2, left column); however, these differences were attenuated and no longer significant for most of the DMN regions after adjustment for covariates (Supplementary Table 3). Lastly, as part of the subgroup analyses (n=412), regional variation in CVR, as the outcome measure, was examined as before, and CBF was included as a covariate in the models (Supplementary Table 4). A different pattern of results was observed for this subgroup compared to the CVR sample (n=536) examined previously (Table 2), because of sampling variability (data not shown); however, the findings with CBF as a covariate did not affect the results substantially (Supplementary Table 4).
4. DISCUSSION
Prior to morphologic changes, changes in brain function may signify brain abnormalities that represent early markers of brain disease. While several studies have examined the association of VRF and vascular pathology on brain function in different brain regions (Claus et al., 1998; Jennings et al., 2005; Glodzik et al., 2011; Beason-Held et al., 2012), this is the first to examine the effects of VRF on CVR with respect to the DMN in a large sample of relatively young subjects. Disruption of the DMN, which represents a network of major interest in aging and AD, and has been shown in different patient groups, including those with mild cognitive impairment (MCI) and normal older controls (Sperling, 2007;Dunn et al., 2014), may in part be related to reduced cerebrovascular function.
Given evidence of the association of mid-life VRF and dementia in late-life (Launer et al., 1995; Launer et al., 2000; Kivipelto et al., 2001; Roberts et al., 2014), potential neurovascular mechanisms of AD (Benarroch, 2007; Novak, 2012), as well as studies suggesting reduced cerebrovascular function in brain regions overlapping the DMN (Claus et al., 1998; Johnson et al., 2005; Dai et al., 2009; Jagust and D'Esposito, 2009), we hypothesized that mean CVR would be lower for regions underlying the DMN, relative to reference brain regions, in those subjects with VRF as opposed to those without. Mean CVR was reduced significantly in different regions of the DMN in subjects with prehypertension/ hypertension when compared with normotensive subjects. In particular, mean CVR was lowest for the posterior cingulate/precuneus, a region which has been shown consistently to have reduced connectivity in early AD (Greicius et al., 2004; Hedden et al., 2009; Sheline and Raichle, 2013; Brier et al., 2014). Similar but non-significant reductions were observed for subjects with, compared to those without diabetes and dyslipidemia. Studies have shown that individuals with VRF, including diabetes and hypertension, have reduced blood flow and vascular reactivity across different cortical regions (Claus et al., 1998; Jennings et al., 2005; Dai et al., 2008; Glodzik et al., 2011). Our findings, together with this previous evidence, suggest that VRF may underlie the observed differences in cerebrovascular function related to the DMN.
We assessed whether the associations that were observed for CVR and prehypertension/ hypertension, with respect to the DMN, extended to non-DMN regions. A test of the mean difference of lower mean CVR for the DMN vs. the Non-DMN (Table 4: -0.043 vs -0.013) was not significant. There are different possible explanations for this result. Adjacent regions of the DMN and Non-DMN may share “vascular space”; therefore, reduction in CVR would have been similar for adjacent areas which may have attenuated any differences observed based on non-adjacent regions. Another possibility may be a result of pooling different regions of the DMN, where different associations between CVR and prehypertension/hypertension were observed (Tables 2 and 3). Similarly, the Non-DMN may include a heterogeneous mix of brain tissue including tissue that underlies other networks, that may be more susceptible to vascular disease, as well as tissue that is less so. Therefore, the low mean difference in CVR between DMN and Non-DMN could be explained partly by the reduced sensitivity based on the comparison of overall summary measures of these larger regions.
Although the hippocampus does not constitute part of the DMN, it functions in conjunction with it by activating while the DMN deactivates during cognitive tasks (Sperling, 2007; Miller et al., 2008). Our findings did not include an expected association between VRF and lower CVR in this region. Previous studies report that the hippocampus functions even as other parts of the brain may be compromised. For example, in subjects with MCI and cognitively normal subjects at genetic risk for AD, the hippocampus has been shown to activate and may help to compensate for reduced brain function in other regions until later stages in AD (Bookheimer et al., 2000; Bondi et al., 2005; Dickerson et al., 2005; Celone et al., 2006). Hippocampal atrophy, related to aging and neurodegenerative processes, may occur later relative to our sample of subjects in mid-life (Hedden and Gabrieli, 2004; Jack et al., 2010; Jack et al., 2013). Reduced cerebrovascular function, which has been shown to be associated with VRF in this region (Convit et al., 2003; Wu et al., 2008) may occur later as well in conjunction with these deleterious processes. In a previous study which measured CVR response to hypercapnia, cardiovascular risk factors were more inversely associated with hippocampal CVR in MCI patients compared with healthy controls (Glodzik et al., 2011).
In addition to CVR, we examined resting CBF as another measure of cerebrovascular function given its relationship with CVR. While lower CBF was observed for DMN regions compared to the occipital lobe, as was the case for CVR, the results were less conclusive (i.e., non-significant). This pattern was observed in both a large sample of subjects (n=537), all of whom had the CBF measure, and a subgroup of subjects with CBF and CVR; therefore, the differences in results observed for CBF and CVR are not based on sample size alone. It is possible that in this particular study group, CVR may be a more sensitive measure of changes in function than CBF. CVR was recorded as subjects underwent a breath-hold challenge, which could aid in identifying underlying pathophysiological abnormalities. By contrast, CBF was recorded in subjects ‘at rest’ which may not provide as sensitive a measure of subjects’ underlying pathophysiology until it advances to a more progressed state.
The study includes some limitations that should be considered when evaluating these results. The findings are based on cross-sectional data, for which we cannot infer causal direction. However, there is evidence from previous studies to suggest that VRF impair vasodilation and other components involved in hemodynamic response (Convit et al., 2003; Last et al., 2007; Wu et al., 2008; Muller et al., 2012). Different methodological aspects of the study may represent important factors with respect to interpretation of the results. To assess CVR activation based on BOLD signal images, CVR response was modeled as a boxcar function, which included a 9s delay to account for variability in CVR response –i.e., CO2 accumulation during breath-hold and drop in CO2 with normal breathing. However, these predictors may not have provided the best fit of individuals’ data, which may have been more closely fit using a different model. Similarly, while BOLD scans were corrected for motion and smoothed, motion covariates were not included as factors in the activation models. Furthermore, anatomical regions used to define the DMN were based on a standard brain template (i.e., Jakob atlas). It is possible that the functional regions underlying the DMN for different subjects did not align completely with the template regions.
Respiratory belt data were collected by MRI technicians during the fMRI scan and were used to assess participants’ compliance during the breath-hold task. While subject compliance during the task was recorded, the tracings of the respiratory data were not saved with the exception of a limited number of subjects from one of the MRI sites. However, our inclusion criteria did not depend on recorded subject compliance, but was based instead on subjects’ activation of the superior sagittal sinus (SSS), which provides a direct, objective measure of a global response to the breath hold task (Bandettini and Wong, 1997, Pillai and Milkulis, 2015).
We examined other factors that may have affected the findings. Lung disease, with the exception of asthma, was rare in this study group and elimination of subjects with lung disease had little effect on the findings. When we restricted the analysis to those subjects free of reported lung disease, our main findings were attenuated somewhat. Specifically, based on model results which compared mean difference in CVR for DMN regions relative to CVR in the occipital lobe, by pre-hypertension/hypertension status, a lower CVR was still observed in the unadjusted results, and after adjustment for covariates. ApoE4 status was available for a subsample of the study group (n=495). Inclusion of this covariate in the analysis attenuated the results somewhat, but did not alter the significance of the findings. Prevalence of cerebrovascular co-factors—e.g., brain atrophy, cortical brain lesions, carotid artery disease, and white matter lesions, while not high (data not shown), may have been associated with CVR independently in the brain regions of interest. However, we hypothesized that these factors represent pathology secondary to alterations in cerebrovascular function already present in subjects (e.g., morphologic changes), or causal intermediates, between VRF and CVR, therefore, we did not adjust for them.
As a preliminary step in data processing of the CVR measure, a voxel-level threshold of Z ≥ 2.3 was used in conjunction with a cluster threshold to define contiguous clusters of activated brain voxels. In addition, we selected individuals with SSS region signal to reduce potential noise in the CVR measure. Moreover, we selected those participants who activated all regions included in the DMN, the hippocampus, and most of the regions included in the Non-DMN. By doing so, we were able to compare CVR for different brain regions simultaneously. While these steps provided the necessary data on individuals with detectable CVR signal to conduct our analysis, it is possible that we removed individuals with low CVR signal that was related to underlying vascular disease. As a result, our findings may represent a bias in terms of an underestimation of the effects of vascular disease on CVR in the study.
There are several areas of investigation that arise from our findings. CVR is a measure of vasodilatory response to breath-hold. As such, it represents a vascular parameter rather than a measure of vascular response to neural activity, or a measure of neural function. Therefore, the ramifications of the results with respect actual neural activity and DMN disconnectivity are unclear and require further investigation. Other compensatory mechanisms may be involved (Rossini et al., 2004), that would allow the DMN to function normally, although the occurrence of such a mechanism may represent a marker of dysfunction in and of itself. Separately, while CVR represents a purely vascular parameter for which the neural implications are not clear, its reduction in DMN regions in subjects with VRF compared to those without raises important questions for studies that investigate resting state (i.e., DMN) connectivity that utilize BOLD fMRI. Given the dependence of BOLD fMRI on cerebrovascular function (Gazzaley and D'Esposito, 2005; Jagust and D'Esposito, 2009), future studies should consider the potential influence of VRF on BOLD signal. Other studies have highlighted the importance of vascular measures as cofactors, particularly in relationship to other brain parameters in the elderly, who represent the majority of participants in studies of AD (Riecker et al., 2003: Dai, 2009 #56).
The study has several strengths and advances our understanding of the relationship of VRF, regional differences in brain vasculature, and the potential implications these have for brain health in late-life. The study was conducted in a large population-based sample in mid-life for whom we could observe early brain pathophysiologic effects of VRF. Studies have found associations of VRF in mid-life and brain and dementia risk in late-life (Launer et al., 1995; Launer et al., 2000; Kivipelto et al., 2001; Roberts et al., 2014). This study represents one of the first to assess brain changes with VRF early in the disease process. Secondly, the study utilized multiple neuroimaging measures which were collected simultaneously for individuals, which allowed for mapping of physiologic brain measures to anatomic regions correspondent with the DMN. Thirdly, analytical methods were applied which allowed for examination of the regional variability of CVR with respect to VRF in brain ROIs simultaneously, as a direct test of our hypothesis of reduced CVR in areas known to be susceptible to AD, like the DMN, compared to those regions that are known to be relatively spared in the disease.
5. CONCLUSION
In sum, in a population-based sample of subjects at mid-life, a functional measure of cerebrovascular reactivity was found to be reduced in brain regions underlying the DMN in subjects with prehypertension/hypertension compared with normotensive subjects. Earlier treatment of this vascular risk factor may mitigate brain pathophysiological processes that can contribute to important brain disorders represented in late-life.
Supplementary Material
Highlights.
Cerebrovascular reactivity (CVR) was examined in relation to vascular risk factors (VRF).
CVR was compared in brain regions underlying the default-mode network vs non-DMN.
CVR was lower for DMN regions relative to non-DMN regions in those with VRF.
CVR may represent an important preclinical marker for brain health in later life.
ACKNOWLEDGEMENTS
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005).
In addition, the authors would like to acknowledge Dr. John Detre for his helpful comments with regard to the manuscript, and Meng-Kang Hsieh for providing graphical analysis.
APPENDIX
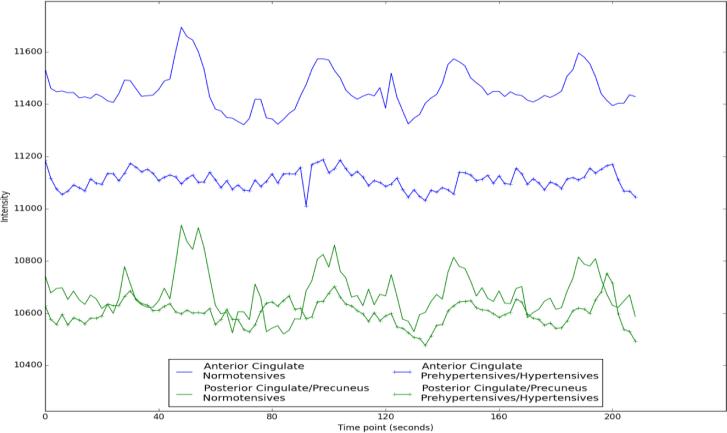
APPENDIX A - Representative time series of mean CVR response in two DMN regions. The blue lines represent CVR response in the anterior cingulate in a normotensive (solid) and hypertensive subject (dashed). Similarly, the green lines represent CVR response in the posterior cingulate/precuneus in the same subjects.
APPENDIX B - Cerebrovascular Reactivity (CVR) Acquisition and Analysis
Each participant performed a breath-hold task during acquisition of BOLD fMRI. Breath-hold tasks induce hypercapnia, and the brain's blood vessels are assumed to respond to the increased level of CO2, resulting in increased cerebral blood flow and volume. In turn, these vascular effects influence the BOLD signal in the acquired fMRI scans. We used a design with two interleaved conditions: 30s normal breathing, and 16s breath-hold after expiration, the latter of which should cause an immediate BOLD signal increase.
To model the breath-hold response, we used a block regressor that incorporated the regressor's temporal derivative, allowing for a time shift between the regressor and the BOLD signal. We also included a 9s delay in the block model to account for the lag in hemodynamic response. It has been previously observed that including both a delay and the regressor's temporal derivative results in a statistically significant increase in the average variance explained by the model (Murphy et al., 2011).
We used FEAT, part of the FSL software package for the BOLD analysis (FSL, 2013). Each scan was corrected for motion by realignment to the median volume, then smoothed using a FWHM = 6mm 3D isotropic Gaussian kernel. A first- level general linear model (GLM) analysis was carried out for each scan to determine how well our block model ‘fit’ the time series at each voxel. The resulting Z-statistical map was thresholded to identify contiguous clusters of voxels which activated in response to the breathhold task (z ≥ 2.3, p≤0.05). In a successful breath-hold activation, we expect to see a whole-brain response.
Using FEAT's statistical output, the percent signal change between normal and breath-hold states was calculated at each voxel to create a percent signal change map. This was thresholded using the Z-score map – i.e. if the voxel's Z-score was less than 2.3, its percent signal change was set to zero (i.e. excluded from the map). This prevents the mean ROI measurements from being contaminated by noise from voxels whose response did not follow the breath-hold paradigm. This map was registered to the Jakob atlas, and mean percentage signal change in BOLD signal was calculated for those brain regions with activated voxels.
In addition, we also reported CVR within a specific ROI exclusively defined for the BOLD images around the posterior superior sagittal sinus (SSS). The SSS allows blood to drain from the anterior cerebral hemispheres. As such, it could be used as a measure of whole-brain response. In the atlas space, the SSS ROI is defined as a 3x3x3 voxel cube. The voxel with the median Z-score within the ROI was identified, and the percentage signal change is calculated and reported for that specific voxel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- Alsop DC, Dai W, Grossman M, Detre JA. Arterial spin labeling blood flow MRI: its role in theearly characterization of Alzheimer's disease. J Alzheimers Dis. 2010;20:871–880. doi: 10.3233/JAD-2010-091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed. 1997;10:197–203. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Thambisetty M, Deib G, Sojkova J, Landman BA, Zonderman AB, Ferrucci L, Kraut MA, Resnick SM. Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke. 2012;43:1542–1547. doi: 10.1161/STROKEAHA.111.638437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Neurovascular unit dysfunction: a vascular component of Alzheimer disease? Neurology. 2007;68:1730–1732. doi: 10.1212/01.wnl.0000264502.92649.ab. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Carlsson CM, Gleason CE, Johnson SC, Sodhi A, Gallagher CL, Puglielli L, Engelman CD, Ries ML, Xu G, Wharton W, Asthana S. Midlife predictors of Alzheimer's disease. Maturitas. 2010;65:131–137. doi: 10.1016/j.maturitas.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Ances BM. Network dysfunction in Alzheimer's disease: refining the disconnection hypothesis. Brain Connect. 2014;4:299–311. doi: 10.1089/brain.2014.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Claus JJ, Breteler MM, Hasan D, Krenning EP, Bots ML, Grobbee DE, Van Swieten JC, Van Harskamp F, Hofman A. Regional cerebral blood flow and cerebrovascular risk factors in the elderly population. Neurobiol Aging. 1998;19:57–64. doi: 10.1016/s0197-4580(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci U S A. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–354. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–866. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol Aging. 2012;33:828 e819–830. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CJ, Duffy SL, Hickie IB, Lagopoulos J, Lewis SJ, Naismith SL, Shine JM. Deficits in episodic memory retrieval reveal impaired default mode network connectivity in amnestic mild cognitive impairment. Neuroimage Clin. 2014;4:473–480. doi: 10.1016/j.nicl.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res. 2007;17:69–76. doi: 10.1007/s10286-006-0379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr., Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- FSL [2013 June 27 2013];FSL Software for BOLD Analysis. 2013 Available from: http://www.fmrib.ox.ac.uk/fsl/
- Gazzaley A, D'Esposito M. BOLD fMRI and cognitive aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. Oxford University Press; New York: 2005. pp. 107–131. [Google Scholar]
- Glodzik L, Rusinek H, Brys M, Tsui WH, Switalski R, Mosconi L, Mistur R, Pirraglia E, de Santi S, Li Y, Goldowsky A, de Leon MJ. Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J Cereb Blood Flow Metab. 2011;31:671–679. doi: 10.1038/jcbfm.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–837. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Gazzaley A, Inglis BA, D'Esposito M. Reducing vascular variability of fMRI data across aging populations using a breathholding task. Human brain mapping. 2007;28:846–59. doi: 10.1002/hbm.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk K, Becker A, Mehta A, Sperling R, Johnson K, Buckner RL. Disruption of Functional Connectivity in Clinically Normal Older Adults Harboring Amyloid Burden. Journal of Neuroscience. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Soma T, Kono AK, Sofue K, Miyamoto N, Yoshikawa T, Mori E, Murase K. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer's disease. J Nucl Med. 2007;48:704–711. doi: 10.2967/jnumed.106.035691. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, D'Esposito M, editors. Imaging the Aging Brain. Oxford University Press; New York.: 2009. [Google Scholar]
- Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno- Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabani NJ, Collins DL, Evans AC. A 3D neuroanatomical atlas. Fourth International Conference on Functional Mapping of the Human Brain. 1998 [Google Scholar]
- Kastrup A, Kruger G, Glover GH, Neumann-Haefelin T, Moseley ME. Regional variability of cerebral blood oxygenation response to hypercapnia. Neuroimage. 1999;10:675–681. doi: 10.1006/nimg.1999.0505. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Kruger G, Neumann-Haefelin T, Moseley ME. Assessment of cerebrovascular reactivity with functional magnetic resonance imaging: comparison of CO2 and breath holding. Magn Reson Imaging. 2001;19:13–20. doi: 10.1016/s0730-725x(01)00227-2. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- Lao Z, Shen D, Liu D, Jawad AF, Melhem ER, Launer LJ, Bryan RN, Davatzikos C. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Lewis CE, Schreiner PJ, Sidney S, Battapady H, Jacobs DR, Lim KO, D`Esposito M, Zhang Q, Reis J, Davatzikos C, Bryan RN. Vascular factors and multiple measures of early brain health: CARDIA Brain MRI Study. PlosOne. 2015;10(3):e0122138. doi: 10.1371/journal.pone.0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute; Cary, NC.: 1996. [Google Scholar]
- Mayda AB, Westphal A, Carter CS, DeCarli C. Late life cognitive control deficits are accentuated by white matter disease burden. Brain. 2011;134:1673–1683. doi: 10.1093/brain/awr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S, American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism, Council on Arteriosclerosis, Thrombosis, and Vascular Biology, Council on Cardiovascular Nursing,and Council on the Kidney in Cardiovascular Disease Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI, Group SS. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol. 2012;71:825–833. doi: 10.1002/ana.23554. [DOI] [PubMed] [Google Scholar]
- Murphy K, Harris AD, Wise RG. Robustly measuring vascular reactivity differences with breath-hold: normalising stimulus-evoked and resting state BOLD fMRI data. Neuroimage. 2011;54:369–379. doi: 10.1016/j.neuroimage.2010.07.059. [DOI] [PubMed] [Google Scholar]
- Novak V. Cognition and Hemodynamics. Curr Cardiovasc Risk Rep. 2012;6:380–396. doi: 10.1007/s12170-012-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papma J, den Heijer T, de Koning I, Mattace-Raso F, van der Lugt A, van der Lijn F, van Swieten J, Koudstaal P, Smits M, Prins N. The influence of cerebral small vessel disease on default mode network deactivation in mild cognitive impairment. Neuroimage: Clinical. 2013;2:33–42. doi: 10.1016/j.nicl.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JJ, Milkulis DJ. Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. AJNR. 2015;36:7–13. doi: 10.3174/ajnr.A3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Grodd W, Klose U, Schulz JB, Groschel K, Erb M, Ackermann H, Kastrup A. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab. 2003;23:565–573. doi: 10.1097/01.WCB.0000056063.25434.04. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Przybelski SA, Mielke MM, Kantarci K, Preboske GM, Senjem ML, Pankratz VS, Geda YE, Boeve BF, Ivnik RJ, Rocca WA, Petersen RC, Jack CR., Jr. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 2014;82:1132–1141. doi: 10.1212/WNL.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Altamura C, Ferretti A, Vernieri F, Zappasodi F, Caulo M, Pizzella V, Del Gratta C, Romani GL, Tecchio F. Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain. 2004;127:99–110. doi: 10.1093/brain/awh012. [DOI] [PubMed] [Google Scholar]
- Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, Miller BL, Kramer JH, Jagust WJ, Chui HC, Weiner MW. Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5:454–462. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer's disease. Biol Psychiatry. 2013;74:340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Caltagirone C. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–2127. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- Sorond FA, Galica A, Serrador JM, Kiely DK, Iloputaife I, Cupples LA, Lipsitz LA. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston Study. Neurology. 2010;74:1627–1633. doi: 10.1212/WNL.0b013e3181df0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Stefani A, Sancesario G, Pierantozzi M, Leone G, Galati S, Hainsworth AH, Diomedi M. CSF biomarkers, impairment of cerebral hemodynamics and degree of cognitive decline in Alzheimer's and mixed dementia. J Neurol Sci. 2009;283:109–115. doi: 10.1016/j.jns.2009.02.343. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Mega MS, Woods RP, Zoumalan CI, Lindshield CJ, Blanton RE, Moussai J, Holmes CJ, Cummings JL, Toga AW. Cortical change in Alzheimer's disease detected with a disease-specific population-based brain atlas. Cereb Cortex. 2001;11:1–16. doi: 10.1093/cercor/11.1.1. [DOI] [PubMed] [Google Scholar]
- Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke. 1999;30:593–598. doi: 10.1161/01.str.30.3.593. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aguirre G, Rao H, Wang J, Fernández-Seara M, Childress A, Detre J. Empirical optimization of of ASL data analysis using an ASL data processing toolbox: ALS tbx. Magnetic Resonance Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, Brown T, DeCarli C, Barnes CA, Mayeux R, Vannucci SJ, Small SA. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol. 2008;64:698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushev I, Landvogt C, Buchholz HG, Fellgiebel A, Hammers A, Scheurich A, Schmidtmann I, Gerhard A, Schreckenberger M, Bartenstein P. Choice of reference area in studies of Alzheimer's disease using positron emission tomography with fluorodeoxyglucose-F18. Psychiatry Res. 2008;164:143–153. doi: 10.1016/j.pscychresns.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv. 2008;11:620–627. doi: 10.1007/978-3-540-85988-8_74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.