SUMMARY
Tropical Pulmonary Eosinophilia (TPE) is a severe form of allergic asthma caused by the host inflammatory response to filarial helminths in the lung microvasculature, and is characterized by pulmonary eosinophilia, increased filarial-specific IgG and IgE antibodies, and airway hyperresponsiveness. The current study examined the effect of IL-12 on pulmonary eosinophilia, deposition of eosinophil major basic protein and airway hyperresponsiveness in mice inoculated i.v. with Brugia malayi microfilariae. Injection of recombinant murine IL-12 modulated the T helper (Th) response in the lungs from Th2- to Th1-like, with elevated IFN-γ, and decreased IL-4 and IL-5 production. Consistent with this shift in cytokine response, antigen-specific IgG2a was elevated, and IgG1 and total serum IgE were decreased. In addition, eosinophils in BAL fluid from IL-12 treated mice were reduced from 56% to 11%, and there was no detectable MBP on respiratory epithelial cells. Importantly, IL-12 suppressed airway hyperresponsiveness compared with saline-injected control animals. Taken together, these data clearly demonstrate that by modulating Th associated cytokine production, IL-12 down-regulates filaria-induced lung immunopathology.
Keywords: Brugia malayi, IL-12, eosinophil, major basic protein, lung, airway hyperresponsiveness
INTRODUCTION
Interleukin 12 has a profound effect on the development of cytokine response to a number of infectious agents, primarily by inducing production of IFN-γ by T cells and natural killer cells (Trinchieri 1995). IL-12 modulates the T helper (Th) associated cytokine response to helminth parasites such as Nippostrongylus brasiliensis and Schistosoma mansoni from a phenotype associated with Th2 cells (IL-4 and IL-5 > IFN-γ) to a predominant Th1 phenotype, with elevated IFN-γ and reduced IL-4 and IL-5 (Finkelman et al. 1994, Oswald et al. 1994). In addition, IL-12 enhances the protective efficacy of either irradiated S. mansoni cercariae or soluble larval antigens (Mountford et al. 1996, Wynn et al 1995a). However, the role of IL-12 in modulating helminth-induced immunopathology is less consistent. Wynn and coworkers demonstrated that IL-12 suppresses lung granuloma formation induced by eggs of S. mansoni, and significantly reduced liver fibrosis, the major cause of pathology in this disease (Wynn et al. 1994, 1995a). In contrast, studies from our laboratory showed that IL-12 exacerbates corneal inflammation induced by Onchocerca volvulus antigens despite modulating the Th associated cytokine response (Pearlman et al. 1997).
Tropical Pulmonary Eosinophilia (TPE) is a severe form of allergic asthma caused by the host inflammatory response to filarial helminths in the lung microvasculature, and is characterized by pulmonary eosinophilia, increased filarial-specific IgG and IgE antibodies, and airway hyperresponsiveness (Ottesen et al. 1992). The immunopathological manifestations can be induced in sensitized mice by i.v. inoculation of B. malayi microfilariae (Egwang et al. 1990), and are dependent on IL-5 and eosinophils (Hall et al. 1998). In the current study, we utilized this murine model to examine the effect of rIL-12 on pulmonary eosinophilia, deposition of eosinophil major basic protein in the airways, and development of airway hyperresponsiveness.
MATERIALS AND METHODS
Parasites and antigen
B. malayi microfilariae were obtained by peritoneal lavage from male jirds (Meriones unguiculatis) which had been previously infected with third stage larvae (NIH contract 73262). Soluble parasite extract for use in ELISA and in vitro stimulation assays was prepared as previously described (Pearlman et al. 1993a,c). Briefly, parasites were separated from jird cells by centrifugation through Ficoll (600 × g), and sonicated on ice until no intact worms remained. The sonicate was centrifuged 15 min at 10 000 × g and supernatant was passaged through a 0·2 μm filter. Protein concentration of the soluble parasite antigens was determined using a Bradford assay (Bio-Rad Labs., Hercules, CA).
Immunization and IL-12 treatment
Female C57BL/6 mice (4–6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA). Mice were immunized by three weekly s.c. injections of 100 000 killed (frozen) microfilarae in 0·2 ml saline. One week after the final immunization, animals received a tail vein injection of 200 000 live microfilariae.
Murine rIL-12 was a kind gift of Dr Stanley Wolf at Genetics Institute (Cambridge, MA, USA), and was stored at −70°C. Animals were given IL-12 by i.p. injection during the week of first immunization as follows: 0·5 μg in 0·5 ml saline on days 0 and 1, and 0·25 μg of IL-12 on days 3, 5, and 7. This protocol has previously been shown to skew the cytokine response to filarial antigens (Pearlman et al. 1995a, 1997). Control animals received i.p. injections of saline (HBSS) on the same schedule.
Spleen cell preparation
Spleen cell suspensions were prepared as previously described (Pearlman et al. 1993a,c). Briefly, red cells were lysed with 0·01 M Tris, pH 7·2, containing 0·75% ammonium chloride and splenocytes were suspended in complete medium [RPMI-1640 (BioWhittaker, Walkersville, MD, USA) containing 1 mM sodium pyruvate, 2 mM L-glutamine, 20 mM HEPES, 200 U/ml penicillin, 200 μg/ml streptomycin, 0·5 μg/ml fungizone, and 10% heat-inactivated fetal calf serum (FCS; Life Technologies, Inc., New City, NY, USA)]. Duplicate wells containing 5 × 105 cells were stimulated for 72 h with either 10 μg/ml soluble microfilarial Ag, or 2 μg/ml soluble anti-murine CD3 Ab (2C11; kindly provided by Dr Thomas Forsthuber, Department of Pathology, Case Western Reserve University, Cleveland, OH, USA) in a final volume of 200 μl and incubated at 37°C in 5% CO2.
Cytokine gene expression in lungs
Lungs were homogenized in RNA STAT-60 (Tel-Test ‘B’, Inc., Friendswood, TX, USA), and total RNA was extracted, reverse transcribed, and the resulting cDNA was prepared for PCR using primers and conditions described elsewhere (Pearlman et al. 1995b, 1996). The housekeeping gene hypoxanthine phosphoribosyl-transferase (HPRT) was used to determine equal levels of cDNA, and positive (cDNA from anti-CD3-stimulated splenocytes of naive mice) and negative (distilled H2O) controls were included in each set of reactions. PCR products were visualized by ethidium bromide after agaraose gel electrophoresis, and transferred to positively charged nylon membrane (‘Hybond-N+’, Amersham, Arlington Heights, IL, USA) for Southern blotting as described (Pearlman et al. 1995b). Products were hybridized with FITC-deoxyuridine triphosphate labelled cytokine-specific probes (Enhanced Chemiluminescence, Amersham, Corp., Piscataway, NJ, USA), and exposed to NEF-496 autoradiography film (DuPont, Wilmington, DE, USA). Densitometric analysis was performed using a Sci-Scan densitometer (US Biochemical Corp., Cleveland, OH, USA), and the expression of each cytokine was calculated as a ratio of the cytokine band intensity to the intensity of the corresponding HPRT band (OD ratio).
Cytokine assays
Measurements of IL-4, IL-5, and IFN-γ in culture supernatatants of in vitro stimulated splenocytes were performed by two-site ELISA using the following MoAbs: for IL-4, BVD-6 and BVD-4; for IL-5, TRFK-5 and TRFK-4, and for IFN-γ, R4-6A2 and XMG-1.2 (PharMingen, San Diego, CA, USA). Recombinant murine cytokines (PharMingen or Genzyme, Cambridge, MA, USA) were used to generate standard curves.
IgG and IgE measurements
Microfilariae Ag-specific serum IgG1 and IgG2a were measured by ELISA using biotinylated rabbit antibodies (Zymed Lab., Inc., San Francisco, CA, USA). Immulon 4 plates (Dynatech Lab., Inc., Chantilly, VA, USA) were coated with 10 μg/ml soluble B. malayi Ag, incubated overnight at 37°C, and washed extensively with PBS containing 0·05% Tween 20. Sera were diluted in PBS and incubated for two h at 37°C. After addition of biotinylated Ab, reactivity was detected using hydrogen peroxide and o-phenylene diamine substrate (Cirex, Warrington, PA, USA). Total serum IgE was measured by two-site ELISA using MoAbs EM-95 and BF-8, as previously described (Pearlman et al. 1993a,c).
Bronchoalveolar lavage and differential leucocyte counts
BAL fluid was obtained by cannulating the trachea through a small incision and performing lavage twice with 0·5 ml PBS (Sigma, St. Louis, MA, USA). Total leucocyte counts in BAL fluids were determined using a haemocytometer.
For performing differential counts, cytocentrifuge preparations from BAL fluids were stained with modified Wright-Giemsa stain (Diff-Quik, Dade Diagnostics, Aguada, PR, USA), and 400 cells were counted from two slides for each animal.
Immunohistochemistry
For detection of eosinophils and extracellular major basic protein (MBP) in tissue, we utilized rabbit polyclonal antisera to murine MBP which was prepared by Dr Kirsten Larsen (Lee et al. 1997), and kindly provided by Dr Gerald Gleich, Mayo Clinic, Rochester, MN, USA). Anti-MBP was diluted 1:1000 in 1% FCS in 0·05 M TBS, and incubated with 5 μm paraffin sections at room temperature for two h. Biotinylated goat anti-rabbit immunoglobulin (DAKO, Carpenteria, CA, USA), diluted 1:200 in 1% FCS in 0·05 M TBS, was then added for 30 min, and alkaline phosphatase-conjugated streptavidin (BioGenex, San Ramon, CA, USA) was added for a further 30 min. Vector Red Substrate containing 12 mg Levamisole (Sigma) was added, and sections were counterstained using modified Harris’ haematoxylin (Richard-Allen, Kalamazoo, MI, USA).
For the immunohistochemical phenotyping of mononuclear cells in BAL fluid, cytocentrifuge preparations were incubated with the following rat anti-mouse MoAbs (Phar-Mingen) at a concentration of 10 μg/ml: for T cells, anti-CD4 (L3T4) and anti-CD8a (Ly-2); for B cells, anti-CD45R (B220), and for macrophages anti-MAC-3.
Isometric measurement of tracheal smooth muscle response to acetylcholine
Tracheal reactivity was determined using the method described by Garssen et al. (Garssen et al. 1990) using tracheas from animals that were not subject to alveolar lavage. Tracheas were kept at 4°C in modified Krebs-Henseleit solution (118·2 mM NaCl, 25 mM Na2HCO3, 4·6 mM KCl, 1·2 mM KH2PO4, 1·2 mM MgSO4, 2·5 mM CaCl2 and 10 mM dextrose, pH 7·4) which was continuously gassed with a mixture containing 95% O2 and 5% CO2. After removal of adventitia and fat tissue, a 3·0 mm cylindrical section was cut from the mid-portion of the tissue. Tracheal cylinders were suspended between a glass rod and a force displacement transducer (FT03, Grass Instr., Quincy, MA, USA) connected to an amplifier. Tissues were equilibrated in an organ bath (Radnoti Glass Tech., Inc., Monrovia, CA, USA) filled with 8·0 ml Krebs-Henseleit solution and aerated for 40–45 min before stimulation. The bath medium was changed every 15 min, and the temperature maintained at 37°C by a constant-temperature circulating unit. The optimal length at which maximal isometric force was developed was identified by electric field submaximal stimulation (EFS, 5 V AC applied through platinum electrodes, 250 mA/cm2 for 10 s duration). Starting preload was 1 g followed by 0·1 g adjustments after each stimulation until the reproducible maximal response was obtained. Concentration response curves to acetylcholine (Ach) were performed in each animal, and isometric force (g) generated by smooth muscle was monitored and recorded on a rectilinear four-channel chart recorder (Gould, Cleveland, OH, USA).
Statistics
Statistical analysis was performed using a Student’s t-test. P < 0·05 was considered significant.
RESULTS
Filaria-induced cytokine responses in the lungs and spleen are modulated by rIL-12
Previous studies demonstrated that repeated immunization with B. malayi antigens is required for development of an antigen-specific response, and induction of a Th2 response (Pearlman et al. 1993a,b,c). To determine the effect of IL-12 on cytokine production in the lungs, animals were injected i.p. with IL-12 at the time of initial s.c. immunization with parasite Ag. Ten days after i.v. injection of 100 000 live microfilariae, animals were sacrificed and cytokine production was determined by RT-PCR. As shown in Figure 1a, lungs of control animals given i.p. saline rather than IL-12 produce more transcripts for IL-4 and IL-5 relative to IFN-γ, consistent with a predominant Th2 response at this site. In contrast, lungs from IL-12 treated mice had a four-fold elevated IFN-γ expression, whereas IL-5 expression was decreased 2·3-fold. Similar modulation of cytokine responses were observed after in vitro stimulation of spleen cells with soluble parasite antigen (Figure 1b). Animals injected with IL-12 had 25-fold elevated IFN-γ, whereas IL-5 production was decreased 13·6-fold. IL-4 levels were also reduced in lungs and spleens of IL-12 treated mice, although to a lesser extent than IL-5. A similar effect of IL-12 on cytokines was noted on animals sacrificed on days 1, 4 and 7 after i.v. parasite inoculation (data not shown). Together, these data show that IL-12 treatment modulates the cytokine response from Th2- to Th1-like both systemically in the spleen, and locally at the site of inflammation in the lungs. Naive mice or naive mice given IL-12 had no Ag-specific cytokine response (data not shown).
Figure 1.
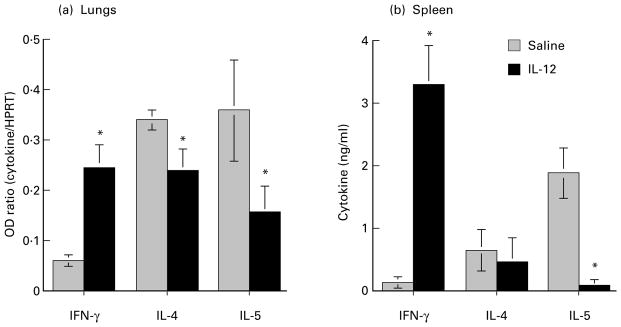
IL-12 modulation of cytokine production in lungs and spleen. C57Bl/6 mice were immunized × 3 s.c. with 100 000 killed B. malayi larvae (microfilariae) and injected intravenously with 200 000 live parasites. One group of animals received either saline or IL-12 i.p. during the week of initial immunization with parasite antigens. Ten days after intravenous injection of parasite larvae, lungs were processed for RT-PCR, and splenic lymphoid cells were stimulated with parasite antigens. (a): Densitometric representation of cytokine gene expression in the lungs of control and IL-12 treated animals after RT-PCR and Southern transfer. The expression of each cytokine was calculated as a ratio of the cytokine band intensity to the intensity of the housekeeping gene HPRT (OD ratio) (b): Spleen cells were incubated with parasite antigen (10 μg/ml) for 72 h, and cytokines released into the supernatant were measured by two-site ELISA. Note the increased IFN-γ and decreased IL-4 and IL-5 production in both tissues of IL-12 treated animals. Data are mean ± SD of five animals per group, and are representative of four repeat experiments. Asterisks represent statistical significance between control and IL-12 treated animals (P > 0·05).
Since IL-4 induces an isotype switch to IgG1 and IgE production, and IFN-γ favours IgG2a production (Snapper et al. 1988a,b), we determined if the IL-12-mediated shift in Th-associated cytokine production also induced a shift in serum Ig isotype production. As shown in Figure 2, IgG2a was significantly elevated in IL-12 treated mice, whereas IgG1 and total serum IgE were reduced, indicating that IL-12 skews the helminth-induced isotype profile from Th2- to Th1-like. In naive animals, the parasite-specific IgG1 was OD 0·34, IgG2a was OD 0·20, and IgE was undetectable.
Figure 2.
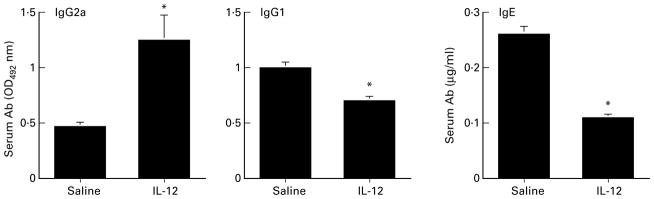
IL-12 modulation of Ab isotype production. Animals were immunized s.c. as described in Figure 1, and serum Ab levels were assessed by ELISA 10 days after i.v. challenge. Parasite Ag-specific IgG2a and IgG1 is expressed as OD492, whereas total serum IgE is shown in μg/ml. Data represent mean ± SD of five mice in each group. Asterisks represent statistical significance between control and IL-12 treated animals (P < 0·05).
IL-12 inhibits filaria-induced pulmonary eosinophilia, but enhances recruitment of mononuclear cells
To determine if the modulating effect of IL-12 on cytokine production in the lungs was associated with a shift in inflammatory cell recruitment, cells recovered in BAL were identified after staining with modified Wright-Giemsa, or immunostaining for CD4+ and CD8+ T cells, B cells (B220) and macrophages (MAC-3). The total number of cells in BAL increased over time to reach a maximum at day 10 after challenge. There was no significant difference in total cell numbers recovered in BAL fluids from control (given i.p. saline) and IL-12 treated animals at any time point examined (Data from day 10 are shown in Figure 3). However, eosinophils increased over time in control mice from 3·6% on day 1 to 56·3% of the total cells on day 10 compared with IL-12 treated animals which increased from 1·6% on day 1 to 11·5% on day 10. Figure 3 illustrates the difference in total number of eosinophils between the two groups on day 10, and demonstrates that the difference in BAL cell numbers in IL-12 treated animals was compensated by an increase in mononuclear cells. (Neutrophils comprised < 4% of total cells in both groups.) BAL recovered from naive mice contained 10-fold fewer cells (5·14 ± 1·3 × 104 cells/ml), and comprised > 90% mononuclear cells. Eosinophils were not detected, and there was no effect of IL-12 on BAL cell recovery from naive animals. Immunostaining of the BAL mononuclear cell population from immunized mice showed that CD4+ cells and CD8+ cells were elevated in IL-12 treated animals (41·2 ± 10·7%, vs 2·6 ± 3·05%, and 8·5 ± 3·5% vs 0·88 ± 1·1%, respectively). B cells (B220 +ve) and macrophages (MAC3 +ve) were also increased in IL-12 treated mice, although to a lesser extent (Figure 4).
Figure 3.
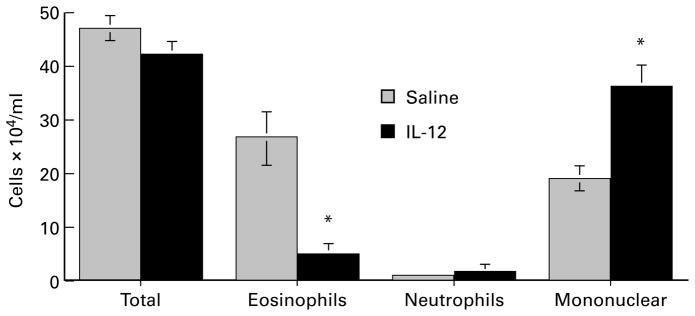
Effect of IL-12 on total and differential cell numbers in BAL fluids of mice after intravenous injection of B. malayi larvae. Animals were immunized and injected i.v. with microfilariae as described in the legend to Figure 1. Ten days after i.v. injection, lungs were lavaged with PBS, and cytocentrifuge preparations from BAL fluids were stained with modified Wright-Giemsa stain. Data represent number of cells per animal (mean ± SD of five mice in each group). Asterisks represent statistical significance between control and IL-12 treated animals (P < 0·05).
Figure 4.
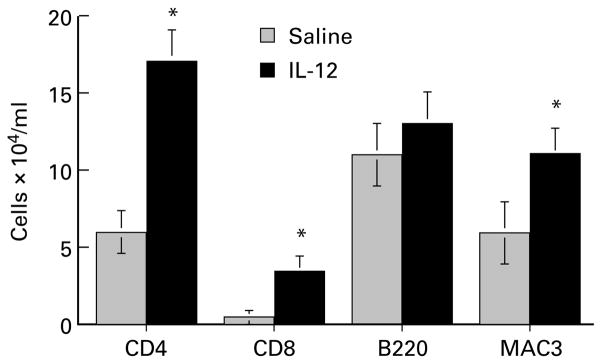
Effect of IL-12 on mononuclear cell phenotypes in BAL fluid. Cytocentrifuge preparations from BAL fluids described for Figure 3 were immunostained with MoAbs to T cells, B cells and macrophages as described in Methods. Positively stained cells were enumerated by counting a total of 400 cells from two slides for each animal, and the data represent the number of cells per animal (mean ± SD of five mice in each group). Asterisks indicate statistical significance between control and IL-12 treated animals (P < 0·05).
Absence of eosinophil major basic protein on respiratory epithelial cells of IL-12 treated animals
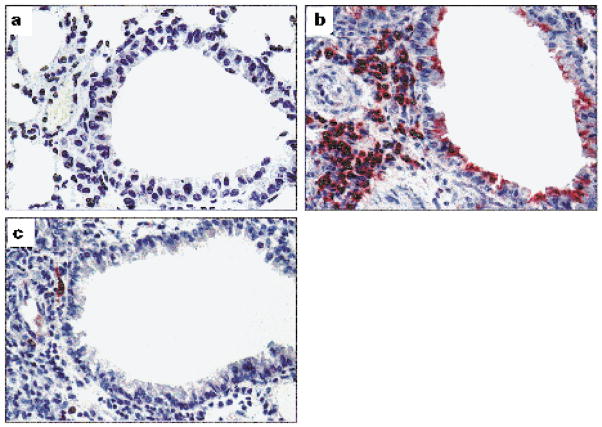
As airway deposition of eosinophil major basic protein is closely associated with the pathogenesis of allergic asthma (Frigas et al. 1981, Filley et al. 1982, Lefort et al. 1996), we examined the effect of IL-12 on MBP deposition. Lung sections from immunized animals given saline or IL-12 were obtained 1 or 10 days after i.v. injection of microfilariae and compared with naive mice. Lungs from naive mice had normal architecture with no perivascular or peribronchial inflammation, and did not react with Ab to MBP (Figure 5a). In contrast, lungs from immunized, challenged animals had numerous peribronchial eosinophils, and extracellular MBP was prominent on the apical surface of airway epithelium both on day 1 (not shown) and on day 10 (Figure 5b). Lungs from immunized, challenged animals given IL-12 had a predominantly mononuclear cell infiltrate, with rare eosinophils and no extracellular MBP on the respiratory epithelium at any time after parasite inoculation (Figure 5c). IL-12 given to naive mice had no effect on histopathology (data not shown).
Figure 5.
Absence of eosinophil major basic protein in the airways of IL-12-treated animals. Immunohistochemical staining of lung sections from naive mice (a), or from immunized, challenged mice injected with either saline (b) or IL-12 (c). Mice were sacrificed 10 days after challenge, and 5 μm lung sections were incubated with anti-MBP. Note the presence of numerous eosinophils in the parenchyma, and MBP staining apical surfaces of respiratory epithelial cells in the bronchiole of lung of saline injected animal. In contrast, significantly fewer eosinophils were present in IL-12-treated animals, and no MBP was detected in the airways. (Original magnification is × 400). The figure is representative of two experiments, with five animals per group.
IL-12 suppresses filaria-induced AHR
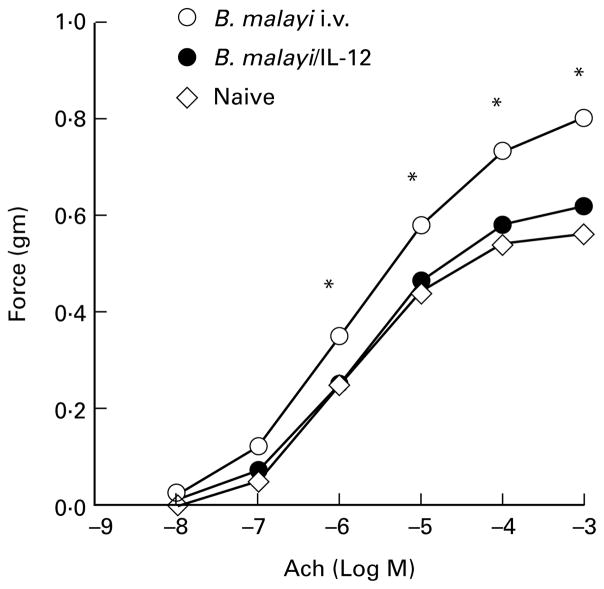
Allergic hypersensitivity in response to microfilariae was determined by measuring the contractile response of tracheal smooth muscle to the cholinergic agonist acetylcholine. As shown in Figure 6, tracheal smooth muscle from naive mice responded to acetylcholine in a dose-dependent manner, and peak smooth muscle contraction (0·56 g force) was observed at 3 × 10−4 M acetylcholine. IL-12 treatment of naive mice had no effect on AHR in naive mice (not shown). However, tracheas from sensitized and challenged mice sacrificed 1 day after challenge were significantly more responsive at each concentration of acetylcholine, and reached a higher peak level of reactivity (0·9 g force). In contrast, tracheas from immunized, challenged animals given IL-12 had diminished reactivity compared to control mice at acetylcholine concentrations ranging from 10−8 to 10−3 M (Figure 6). Reactivity to acetylcholine in the IL-12 group was not significantly different from that observed for tracheas from naive mice, indicating that IL-12 suppresses filaria-induced airway hyperresponsiveness. Similar results were detected at later time points, up to 10 days after i.v. inoculation (data not shown).
Figure 6.
Inhibition of AHR in IL-12-treated animals. Tracheal smooth muscle response to acetylcholine in immunized, challenged mice injected i.p. with either saline (control) or IL-12 was measured as described in the Methods. Each data point represents mean value of contraction force generated by isolated tracheas from eight mice in each group. Hyperresponsiveness to acetylcholine (Ach) was detected in immunized, challenged mice given saline rather than IL-12, whereas mice injected with IL-12 had no greater response than naive mice. Asterisks represent statistical significance between control and IL-12 treated animals (P < 0·05).
DISCUSSION
Previous studies from this laboratory demonstrated that injection of B. malayi microfilariae or soluble microfilarial antigens selectively induces a Th2-associated cytokine response (Pearlman et al. 1993a,b,c). Although this response could be modulated by IL-12, there was no effect on clearance of blood microfilaremia after i.v. injection (Pearlman et al. 1995a). The current study demonstrates that IL-12 significantly down-modulates the physiological and immunological changes in the lung that are induced by B. malayi larvae, including airway eosinophilia, deposition of eosinophil granule proteins and tracheal hyperreactivity. Thus, the outcome of IL-12 treatment more closely resembles the down-regulatory effect of IL-12 on S. mansoni lung granuloma formation (Wynn et al. 1994) than the exacerbative effect of IL-12 on O. volvulus-mediated corneal inflammation (Pearlman et al. 1997).
Differences in resident cell population between the lungs and cornea likely contribute to the differences in outcome between IL-12 treatment on immunopathology in these tissues. Although O. volvulus-mediated corneal inflammation is associated with selective induction of Th2 response and eosinophil infiltration into the corneal stroma (Pearlman et al. 1995b, 1996), IL-12 exacerbates the inflammatory response by augmenting chemokine expression and eosinophil infiltration (Pearlman et al. 1997). The difference in the effect of IL-12 in these models of filarial-mediated immunopathology may also relate to differences in expression of chemokines and vascular cell adhesion molecules at these sites. Consistent with the importance of tissue microenvironment are the observations that IL-12 exacerbates hapten-mediated contact dermatitis by elevating IFN-γ production by CD4 cells, and enhancing inflammatory cell recruitment (Dilulio et al. 1996). We also observed that dermatitis induced by O. volvulus antigens is exacerbated by IL-12 (unpublished observations). However, the resident cell population is not the only factor influencing the modulatory activity of rIL-12. Local cytokine environment, especially IFN-γ is essential for IL-12 activity in many of these studies, as Ab neutralization of IFN-γ abrogated the effect of IL-12 (Oswald et al. 1994, Wynn et al. 1994). The inhibitory role of IFN-γ in lung immunopathology was also demonstrated in mice expressing the transgene for IFN-γ in the lungs, as these animals have diminished pulmonary eosinophilia and decreased airway hyperresponsiveness (Li et al. 1996). Conversely, IFN-γ gene knockout mice given IL-12 treatment prior to i.v. injection of S. mansoni eggs had greatly enlarged lung granulomas, consistent with elevated Th2 associated cytokine production (Wynn et al. 1995b). The role of IFN-γ in filaria-induced immunopathology has yet to be determined.
IL-12 has also been reported to abrogate pulmonary eosinophilia and airway hyperresponsiveness in response to sensitization and aerosol challenge with aeroallergens such as sheep red blood cells (Gavett et al. 1995), ovalbumin (Kips et al. 1996) or ragweed (Sur et al. 1996), and prevents antigen-induced eosinophil infiltration into the mouse trachea in a dose-dependent manner (Iwamoto et al. 1996). The sequence of events leading to helminth-mediated airway hyperresponsiveness is therefore likely to be similar to that proposed for environmental allergens (Drazen et al. 1996). Repeated subcutaneous immunization with parasite antigens selectively induces expression of IL-4 and IL-5, which stimulate production of IgE and eosinophils, respectively. Entrapment of microfilariae in the lungs after intravenous inoculation may then up-regulate expression of adhesion molecules and chemokines by resident vascular endothelial cells which mediate eosinophil exocytosis. Once in the airways, release of MBP and other granule proteins may be mediated by cross-linking of IgE or IgG receptors on the eosinophil membrane. In the current study, the effect of IL-12 in modulating lung immunopathology appears to be at the level of induction of the Th2 associated cytokine response, especially in inhibiting IL-5 and eosinophil production. Our observations that IL-5 gene knockout mice do not develop pulmonary eosinophilia or airway hyperresponsiveness are consistent with an essential role for IL-5 in this model (Hall et al. 1998). As a consequence of IL-12 modulation, the inflammatory cell response in the lungs shifts from predominantly eosinophilic to mononuclear, especially CD4+ and CD8+ T cells. Although we did not measure IFN-γ in a cell-specific manner, it is possible that IL-12 primes local CD4+ and CD8+ T cells for the increased production of IFN-γ, which may then have a modulatory effect in the lungs. Further understanding of the mechanisms by which these cytokines regulate inflammatory cell recruitment to the lungs may identify targets for immunotherapy in this and related diseases.
Acknowledgments
The authors gratefully acknowledge receipt of IL-12 from Dr S.F. Wolf, and receipt of rabbit anti-MBP from Drs K.A. Larson and G.J. Gleich. We also thank F.E. Hazlett, E. Diaconu and J.L. Albright for their excellent technical assistance and Drs W.H. Boom, F.P. Heinzel, C.L. King and W.K. Kroeze for critical reading of the manuscript.
This work was supported by a Burroughs Wellcome New Investigator Award no. 0720 (E.P.) and National Institutes of Health grant HL50527 (M.A.H.).
References
- Dilulio NA, Xu H, Fairchild RL. Diversion of CD4+ T cell development from regulatory T helper to effector T helper cells alters the contact hypersensitivity response. European Journal of Immunology. 1996;26:2606–2612. doi: 10.1002/eji.1830261111. [DOI] [PubMed] [Google Scholar]
- Drazen J, Arm J, Austen K. Sorting out the cytokines of asthma [comment] Journal of Experimental Medicine. 1996;183:1–5. doi: 10.1084/jem.183.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egwang TG, Kazura JW. The BALB/c mouse as a model for immunological studies of microfilariae-induced pulmonary eosinophilia. American Journal of Tropical Medicine and Hygiene. 1990;43:61–66. doi: 10.4269/ajtmh.1990.43.61. [DOI] [PubMed] [Google Scholar]
- Filley W, Holley K, Kephart G, Gleich G. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet. 1982;2:11–16. doi: 10.1016/s0140-6736(82)91152-7. [DOI] [PubMed] [Google Scholar]
- Finkelman F, Madden K, Cheever A, et al. Effect of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. Journal of Experimental Medicine. 1994;179:1563–1572. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigas E, Loegering D, Solley G, Farrow G, Gleich G. Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clinic Proceedings. 1981;56:345–353. [PubMed] [Google Scholar]
- Garssen J, Van LH, Van DVH, Nijkamp F. An isometric method to study respiratory smooth muscle responses in mice. Journal of Pharmacological Methods. 1990;24:209–217. doi: 10.1016/0160-5402(90)90031-f. [DOI] [PubMed] [Google Scholar]
- Gavett S, O’Hearn D, Li X, Huang S, Finkelman F, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. Journal of Experimental Medicine. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LR, Mehlotra RK, Higgins AW, Haxhiu MA, Pearlman E. An essential role for IL-5 and eosinophils in helminth-induced airway hyperresponsiveness. Infection and Immunity. 1998 doi: 10.1128/iai.66.9.4425-4430.1998. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto I, Kumano K, Kasai M, Kurasawa K, Nakao A. Interleukin-12 prevents antigen-induced eosinophil recruitment into mouse airways. American Journal of Respiratory Critical Care Medicine. 1996;154:1257–1260. doi: 10.1164/ajrccm.154.5.8912732. [DOI] [PubMed] [Google Scholar]
- Kips JC, Brusselle GJ, Joos GF, et al. Interleukin-12 inhibits antigen-induced airway hyperresponsiveness in mice. American Journal of Respiratory Critical Care Medicine. 1996;153:535–539. doi: 10.1164/ajrccm.153.2.8564093. [DOI] [PubMed] [Google Scholar]
- Lee J, McGarry M, Farmer S, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. Journal of Experimental Medicine. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort J, Nahori MA, Ruffie C, Vargaftig BB, Pretolani M. In vivo neutralization of eosinophil derived major basic protein inhibits antigen induced bronchial hyperreactivity in sensitized guinea pigs. Journal of Clinical Investigation. 1996;97:1117–1121. doi: 10.1172/JCI118505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XM, Chopra RK, Chou TY, Schofield CH, Wills-Karp M, Huang SK. Mucosal IFN-γ gene transfer inhibits pulmonary allergic responses in mice. Journal of Immunology. 1996;157:3216–3219. [PubMed] [Google Scholar]
- Mountford A, Anderson S, Wilson R. Induction of Th1 cell-mediated protective immunity to Schistosoma mansoni by co-administration of larval antigens and IL-12 as an adjuvant. Journal of Immunology. 1996;156:4739–4745. [PubMed] [Google Scholar]
- Oswald IP, Caspar P, Jankovic D, Wynn TA, Pearce EJ, Sher A. IL-12 inhibits Th2 cytokine responses induced by eggs of Schistosoma mansoni. Journal of Immunology. 1994;153:1707–1713. [PubMed] [Google Scholar]
- Ottesen EA, Nutman TB. Tropical pulmonary eosinophilia. Annual Review of Medicine. 1992;43:417–424. doi: 10.1146/annurev.me.43.020192.002221. [DOI] [PubMed] [Google Scholar]
- Pearlman E, Hazlett FE, Boom WH, Kazura JW. Induction of murine T-helper-cell responses to the filarial nematode Brugia malayi. Infection and Immunity. 1993a;61:1105–1112. doi: 10.1128/iai.61.3.1105-1112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E, Heinzel FP, Hazlett FE, Kazura JW. IL-12 modulation of T helper responses to the filarial helminth, Brugia malayi. Journal of Immunology. 1995a;154:4658–4664. [PubMed] [Google Scholar]
- Pearlman E, Kazura JW, Hazlett FE, Boom WH. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. Journal of Immunology. 1993b;151:4857–4864. [PubMed] [Google Scholar]
- Pearlman E, Kroeze WK, Hazlett FE, et al. Brugia malayi: acquired resistance to microfilariae in BALB/c mice correlates with local Th2 responses. Experimental Parasitology. 1993c;79:200–208. doi: 10.1006/expr.1993.1023. [DOI] [PubMed] [Google Scholar]
- Pearlman E, Lass JH, Bardenstein DS, et al. Onchocera volvulus-mediated keratitis: cytokine production by IL-4-deficient mice. Experimental Parasitology. 1996;84:274–281. doi: 10.1006/expr.1996.0113. [DOI] [PubMed] [Google Scholar]
- Pearlman E, Lass JH, Bardenstein DS, et al. IL-12 exacerbates helminth-mediated corneal pathology by augmenting cell recruitment and chemokine expression. Journal of Immunology. 1997;158:827–833. [PubMed] [Google Scholar]
- Pearlman E, Lass JH, Bardenstein DS, et al. Interleukin 4 and T helper type 2 cells are required for development of experimental onchocercal keratitis (river blindness) Journal of Experimental Medicine. 1995b;182:931–940. doi: 10.1084/jem.182.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper CM, Finkelman FD, Paul WE. Differential regulation of IgG1 and IgE synthesis by interleukin 4. Journal of Experimental Medicine. 1988a;167:183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper CM, Peschel C, Paul WE. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. Journal of Immunology. 1988b;140:2121–2127. [PubMed] [Google Scholar]
- Sur S, Lam J, Bouchard P, et al. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. Journal of Immunology. 1996;157:4173–4180. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annual Reviews of Immunology. 1995;13:215–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Eltoum I, Oswald I, Cheever A, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. Journal of Experimental Medicine. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Cheever AW, Jankovic D, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995a;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Jankovic D, Hieny S, et al. IL-12 exacerbates rather than suppresses T helper 2-dependent pathology in the absence of endogenous IFN-gamma. Journal of Immunology. 1995b;154:3999–4009. [PubMed] [Google Scholar]