Abstract
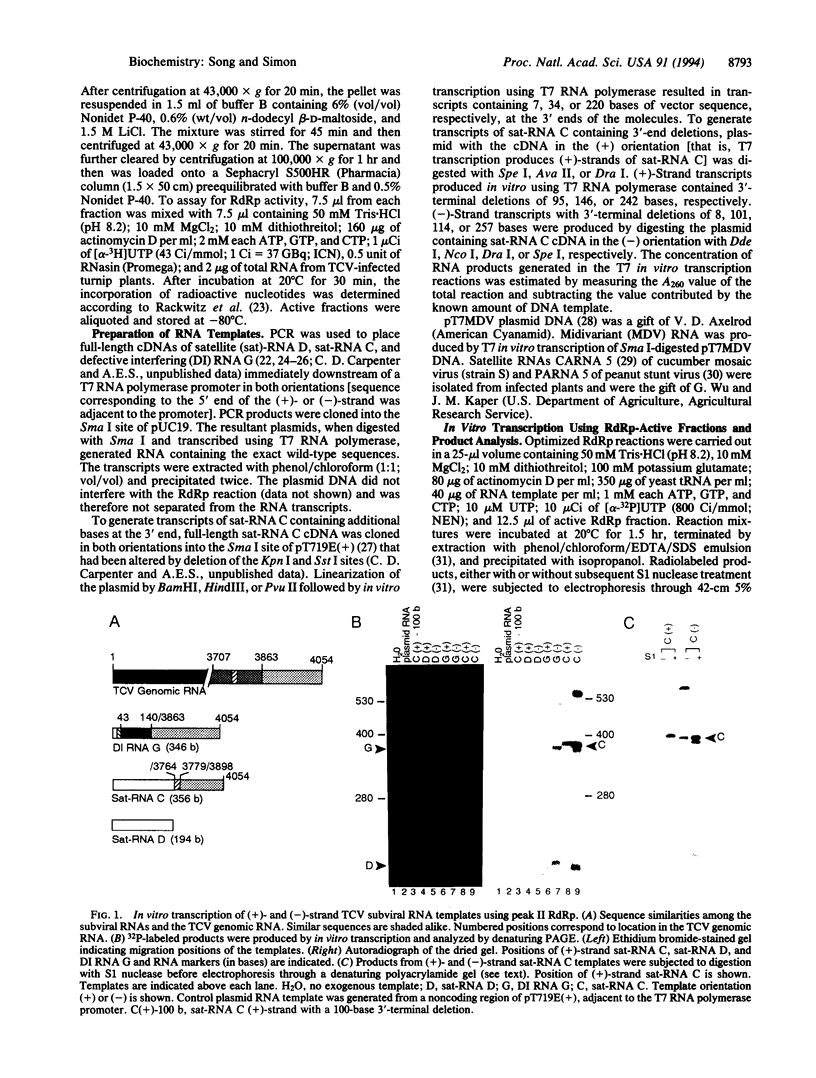
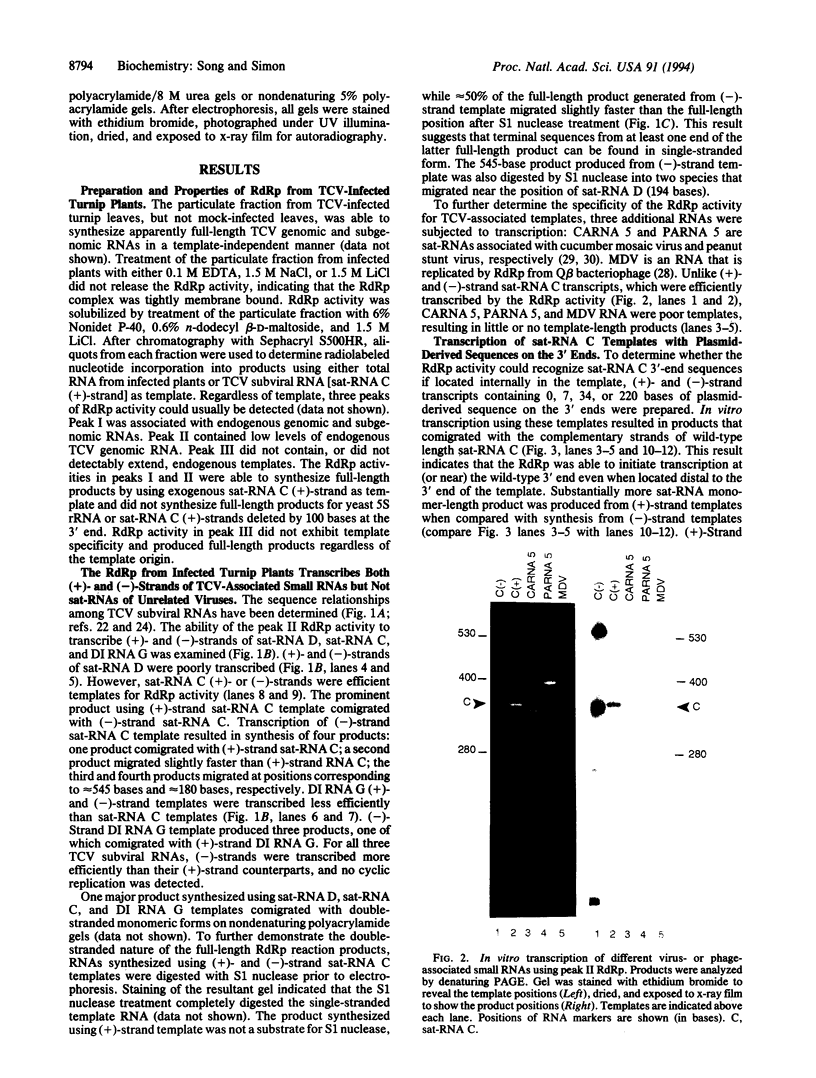
RNA-dependent RNA polymerase (RdRp) was solubilized from membranes of turnip infected with turnip crinkle virus (TCV), a single-stranded, monopartite RNA virus. The RdRp activity could be separated into three peaks by Sephacryl S500HR chromatography. RdRp from peak I, which contained substantial amounts of endogenous TCV genomic RNA, and peak II were template-specific, synthesizing full-length complementary strands of exogenous TCV subviral RNAs but not control RNA templates. Peak III RdRp was nonspecific, synthesizing full-sized products for all added RNA templates. Peak II RdRp transcribed several different TCV satellite (sat) and defective interfering RNA templates in both (+)- and (-)-sense orientations but did not transcribe (+)-strands of satellite RNAs associated with unrelated viruses. Monomeric-length sat-RNA C was synthesized from a template containing as many as 220 nonsatellite bases at the 3' ends of either (+)- or (-)-strands, indicating that the RdRp was able to recognize 3'-end sequences in an internal location. Deletion of 95-242 bases from the 3' end of (+)-strand sat-RNA C abolished the synthesis of template-length product. However, transcription of template-length products was not affected by the deletion of at least 257 bases from the 3' end of (-)-strand sat-RNA C template (leaving only the 100 5'-terminal residues), implying that different mechanisms exist for synthesis of (+)-and (-)-strand satellite RNA in vitro.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andino R., Rieckhof G. E., Achacoso P. L., Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5'-end of viral RNA. EMBO J. 1993 Sep;12(9):3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod V. D., Brown E., Priano C., Mills D. R. Coliphage Q beta RNA replication: RNA catalytic for single-strand release. Virology. 1991 Oct;184(2):595–608. doi: 10.1016/0042-6822(91)90430-j. [DOI] [PubMed] [Google Scholar]
- Barton D. J., Sawicki S. G., Sawicki D. L. Solubilization and immunoprecipitation of alphavirus replication complexes. J Virol. 1991 Mar;65(3):1496–1506. doi: 10.1128/jvi.65.3.1496-1506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Cascone P. J., Carpenter C. D., Li X. H., Simon A. E. Recombination between satellite RNAs of turnip crinkle virus. EMBO J. 1990 Jun;9(6):1709–1715. doi: 10.1002/j.1460-2075.1990.tb08294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone P. J., Haydar T. F., Simon A. E. Sequences and structures required for recombination between virus-associated RNAs. Science. 1993 May 7;260(5109):801–805. doi: 10.1126/science.8484119. [DOI] [PubMed] [Google Scholar]
- Collmer C. W., Hadidi A., Kaper J. M. Nucleotide sequence of the satellite of peanut stunt virus reveals structural homologies with viroids and certain nuclear and mitochondrial introns. Proc Natl Acad Sci U S A. 1985 May;82(10):3110–3114. doi: 10.1073/pnas.82.10.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C., Gargouri-Bouzid R., Haenni A. L. RNA replication of plant viruses containing an RNA genome. Prog Nucleic Acid Res Mol Biol. 1992;42:157–227. doi: 10.1016/s0079-6603(08)60576-0. [DOI] [PubMed] [Google Scholar]
- Dorssers L., van der Krol S., van der Meer J., van Kammen A., Zabel P. Purification of cowpea mosaic virus RNA replication complex: Identification of a virus-encoded 110,000-dalton polypeptide responsible for RNA chain elongation. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1951–1955. doi: 10.1073/pnas.81.7.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri-Bouzid R., David C., Haenni A. L. The 3' promoter region involved in RNA synthesis directed by the turnip yellow mosaic virus genome in vitro. FEBS Lett. 1991 Dec 2;294(1-2):56–58. doi: 10.1016/0014-5793(91)81342-6. [DOI] [PubMed] [Google Scholar]
- Hacker D. L., Petty I. T., Wei N., Morris T. J. Turnip crinkle virus genes required for RNA replication and virus movement. Virology. 1992 Jan;186(1):1–8. doi: 10.1016/0042-6822(92)90055-t. [DOI] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell. 1990 Oct 19;63(2):363–368. doi: 10.1016/0092-8674(90)90169-f. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y. Specific cessation of minus-strand RNA accumulation at an early stage of tobacco mosaic virus infection. J Virol. 1991 Feb;65(2):861–868. doi: 10.1128/jvi.65.2.861-868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Dolja V. V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28(5):375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Koonin E. V. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol. 1991 Sep;72(Pt 9):2197–2206. doi: 10.1099/0022-1317-72-9-2197. [DOI] [PubMed] [Google Scholar]
- Lemm J. A., Rice C. M. Roles of nonstructural polyproteins and cleavage products in regulating Sindbis virus RNA replication and transcription. J Virol. 1993 Apr;67(4):1916–1926. doi: 10.1128/jvi.67.4.1916-1926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H., Heaton L. A., Morris T. J., Simon A. E. Turnip crinkle virus defective interfering RNAs intensify viral symptoms and are generated de novo. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9173–9177. doi: 10.1073/pnas.86.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H., Simon A. E. In vivo accumulation of a turnip crinkle virus defective interfering RNA is affected by alterations in size and sequence. J Virol. 1991 Sep;65(9):4582–4590. doi: 10.1128/jvi.65.9.4582-4590.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Tran C. T., Robinson W. S. Hepatitis B virus particles of plasma and liver contain viral DNA-RNA hybrid molecules. Virology. 1984 Nov;139(1):53–63. doi: 10.1016/0042-6822(84)90329-5. [DOI] [PubMed] [Google Scholar]
- Molla A., Paul A. V., Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991 Dec 13;254(5038):1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- Petty I. T. A plasmid vector for cloning directly at the transcription initiation site of a bacteriophage T7 promoter. Nucleic Acids Res. 1988 Sep 12;16(17):8738–8738. doi: 10.1093/nar/16.17.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue G. P., Hall T. C. The requirement for a 5' stem-loop structure in brome mosaic virus replication supports a new model for viral positive-strand RNA initiation. J Virol. 1992 Feb;66(2):674–684. doi: 10.1128/jvi.66.2.674-684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackwitz H. R., Rohde W., Sänger H. L. DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature. 1981 May 28;291(5813):297–301. doi: 10.1038/291297a0. [DOI] [PubMed] [Google Scholar]
- Régnier P., Grunberg-Manago M. RNase III cleavages in non-coding leaders of Escherichia coli transcripts control mRNA stability and genetic expression. Biochimie. 1990 Nov;72(11):825–834. doi: 10.1016/0300-9084(90)90192-j. [DOI] [PubMed] [Google Scholar]
- Saunders K., Kaesberg P. Template-dependent RNA polymerase from black beetle virus-infected Drosophila melanogaster cells. Virology. 1985 Dec;147(2):373–381. doi: 10.1016/0042-6822(85)90139-4. [DOI] [PubMed] [Google Scholar]
- Schiebel W., Haas B., Marinković S., Klanner A., Sänger H. L. RNA-directed RNA polymerase from tomato leaves. I. Purification and physical properties. J Biol Chem. 1993 Jun 5;268(16):11851–11857. [PubMed] [Google Scholar]
- Schiebel W., Haas B., Marinković S., Klanner A., Sänger H. L. RNA-directed RNA polymerase from tomato leaves. II. Catalytic in vitro properties. J Biol Chem. 1993 Jun 5;268(16):11858–11867. [PubMed] [Google Scholar]
- Simon A. E., Howell S. H. Synthesis in vitro of infectious RNA copies of the virulent satellite of turnip crinkle virus. Virology. 1987 Jan;156(1):146–152. doi: 10.1016/0042-6822(87)90445-4. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Howell S. H. The virulent satellite RNA of turnip crinkle virus has a major domain homologous to the 3' end of the helper virus genome. EMBO J. 1986 Dec 20;5(13):3423–3428. doi: 10.1002/j.1460-2075.1986.tb04664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. X., Ahlquist P., Kaesberg P. Active complete in vitro replication of nodavirus RNA requires glycerophospholipid. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11136–11140. doi: 10.1073/pnas.89.23.11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. C., Tuschall D. M., Flanegan J. B. Poliovirus RNA-dependent RNA polymerase and host cell protein synthesize product RNA twice the size of poliovirion RNA in vitro. J Virol. 1985 May;54(2):256–264. doi: 10.1128/jvi.54.2.256-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen A. The replication of plant virus RNA. Microbiol Sci. 1985 Jun;2(6):170–174. [PubMed] [Google Scholar]