Summary
Aging is accompanied by involuntary loss of skeletal muscle mass, strength and function, called sarcopenia. The mechanisms underlying the development of sarcopenia are not completely understood and most likely multi-factorial, but significant progress has been made over the past few years to identify some of the major contributors.
Besides life style-related factors, as diet and physical activity, sarcopenia seems to be also determined by hormonal dysregulation, chronic inflammatory status, ectopic adipose tissue accumulation, neurological and vascular changes associated with aging.
The present mini-review focused on the basic factors that primarily impact muscle homeostasis in older subjects.
A better understanding of cellular mechanism leading to sarcopenia is required to establish evidence-based intervention in order to prevent onset of symptoms associated with sarcopenia and to extend the time free from disability in older adults.
Keywords: sarcopenia, aging, chronic inflammation, muscle lipotoxicity
Introduction
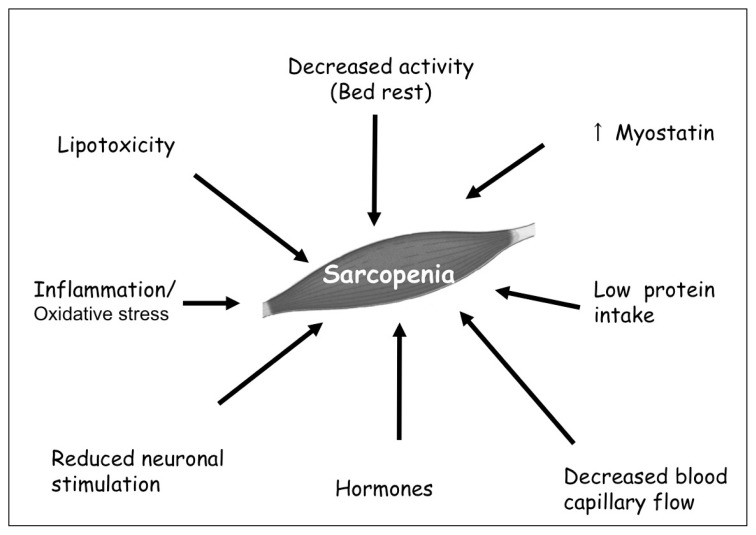
Numerous clinical studies proved the presence of an involuntary loss of skeletal muscle mass and strength loss with aging, starting in the fifth decade of life (1). This progressive age-related muscle wasting process, called sarcopenia, is associated with an increased risk of falls and fractures (2), disability, loss of functionality and independence in older individuals (3), leading to increased morbidity and all-cause mortality (4). As the population over 60 years of age is expected to triple in the next fifty years (5), there is an increased interest on the necessity of healthy ageing, not only to prevent many chronic diseases, but also to maintain elderly subjects free from disability. Although the term “sarcopenia” has been used over the last two decades, is in 2010 that the European working group on sarcopenia (EWGSOP) defined it as a syndrome characterised by progressive and generalized loss of muscle mass and strength with the risk of adverse outcome, such as physical disability, poor quality of life and death (6). Thus, the working definition requires the presence of both low muscle mass plus low muscle function (strength or physical performance) (Table 1). From a histopatological muscle-specific point of view, sarcopenia is characterised by the atrophy of type II fibers, necrosis and reduction of cross-bridging elements between fibers, smaller and fewer mithocondria (7). Even though there are still few data on the specific mechanism of human muscle aging, significant progress has been made over the past few years to identify some of the major contributors to this multifactorial process (Figure 1). A better understanding of the cellular mechanism driving these changes is required in order to establish evidence-based interventions to prevent the onset of symptoms associated with sarcopenia and to promote healthy ageing. Hence, this review focuses on the basic factors that primarily influence muscle homeostasis in older individuals.
Table 1.
EWGSOP criteria. Diagnosis is based on documentation of criterion 1 plus criterion 2 or criterion 3 (adapted from Cruz-Jentoft AJ and Morley JE, Wiley-Blackwell Ed. 2012).
| Criteria for the diagnosis of SARCOPENIA |
|---|
|
Figure 1.
Main mechanisms involved in sarcopenia. The etiology of sarcopenia is multifactorial; the control of overall muscle mass is practically due to an imbalance between the rates of muscle protein synthesis and breakdown.
Inactivity and bed rest
Older adults are more likely to have prolonged periods of inactivity or bed rest, in particular related with hospitalization. Several studies showed that bed rest (with or without hospitalization) may contribute to functional impairment in older patients, as inactivity could compromise muscle metabolic homeostasis (8–10). Kortebein et al. reported that healthy older volunteers lost almost 1 kg of lean leg mass after only 10 days of bed rest, with an associated 16% decline in isokinetic knee extensor strength and a significant decrease in protein synthesis (8). This marked loss of muscle mass was greater than that observed in young healthy individuals after 28 days of bed rest, whereas the decline in protein synthesis and strength was similar to that of younger participants evaluated after 14 day of inactivity (11). Moreover, in another study, the same group found that 10 days of bed rest resulted in a substantial loss of lower extremity strength, power and aerobic capacity with a reduction in physical activity, with no effect on physical performance (12). Additionally, Drummond et al. demonstrated a significant bed rest-induced reduction in total lean mass in a group of 6 healthy volunteers (mean age 67.2 years) after 7 days of bed rest (10). Moreover, this study links this result to the impaired response to amino-acids supplementation, as bed rest seemed to attenuated the amino acid-induced increase in muscle protein synthesis with a mechanism involving reduced mTORC1 signalling and amino acid transporter protein content. Nevertheless, it has been demonstrated that short-term bed rest in older overweight volunteers determined a significant decrease in hepatic and peripheral insulin sensitivity, which could further negatively influence muscular homeostasis (9). Additionally, muscle levels of TLR4 protein expression and IL6, nuclear factor-kB1, IL10 and IL15 mRNA expression were increased after short-time bed rest in healthy older adults, while serum samples of IFN-gamma and macrophage inflammatory protein – 1 beta (MIP-1β) were elevated (13). This increased expression of local pro-inflammatory mediators, may be an important factor predisposing to muscle catabolism response triggered by concomitant acute disease and bed rest inactivity in older adults. In fact, in a recent study, Puthucheary et al. (14) observed a significant muscle mass loss among 63 critically ill patients (mean age 55 years) admitted to an intensive care unit. This wasting process was early and rapid during the first week of critical illness and was more severe among subjects with multi-organ failure and higher inflammatory status. Additionally, the net leg protein balance was catabolic, despite the administration of enteral nutrition (14).
Nutrition
Older people need to make up for age-related changes in protein metabolism, such as high splanchnic extraction and declining anabolic response to ingested proteins. Additionally, aging is characterized by the reduction of nutrients intake, known as “anorexia of aging”, representing a physiological feature of old age associated with decreased energy expenditure and loss of muscle mass (15). They also need more protein in order to counteract inflammatory and catabolic conditions associated with chronic and acute diseases. All these aspects contribute to the development of sarcopenia, as observed in the Health ABC study population. In this study, the participants that had the highest intake of protein presented approximately 40% less lean mass waste after the 3 years of observation. For this reason, the PROT-AGE study group recommended average daily intake in the range of 1.0 to 1.2 g protein/kg body weight/day, and higher protein intake (i.e., ≥ 1.2 g/kg body weight/day) was advised for subjects with high physical activity level (16). Moreover, protein quality, timing of ingestion, and intake of other nutritional supplements may be relevant, but evidence is not yet sufficient to support specific recommendations. To this date, there are a few studies regarding the effect of protein intake on muscle mass and function, most of them using a combined intervention, including protein supplementation and resistance exercise. So far, evidence from long-term intervention studies shows no clear benefit of dietary protein supplementation on skeletal muscle mass in elderly people. Yet, Tieland et al. showed that 24 weeks of protein supplementation in frail older adults improved physical performance, that might be attributable to improvements in neuromuscular action or muscle quality (17). On the other hand, Daly et al. (18) showed that 1.3 g protein/kg body weight/day supplementation was effective in enhancing the effects of resistance exercise training on muscle mass and strength in 100 elderly women (60 to 90 years). Other studies investigated the role of essential amino acids (EAA) supplementation on muscle homeostasis, combined or not with exercise training. Dillon et al. (19) proved that EAA improved lean body mass and basal muscle protein synthesis in older individuals, while Kim et al. (20) suggested that EAA and exercise together may be effective in enhancing not only muscle strength, but also physical performance as evaluated by and walking speed in a population of sarcopenic women. Further studies are needed in order to evaluate long-term benefits determined by long term protein supplementation.
Chronic inflammation
It has been demonstrated that inflammation, along with oxidative stress, increase with aging both are considered significant contributors to age-related muscle wasting process (21, 22). Hence, some studies reported that high IL6 an C-Reactive Protein (CRP) levels are associated with increased risk of muscle mass and strength loss (23, 24). Nevertheless, only few studies evaluated loss of muscle strength in relation with inflammatory status in hospitalized elderly patients (25, 26). For example, in a study with 620 patients (mean age 56.4 years), Norman et al. (25) showed that CRP, as an indicator of acute inflammation, was an independent predictor of grip strength even after adjustment for relevant confounders, such as age, gender and body composition. Moreover, Bautmans et al. (26) proved that geriatric hospitalized patients with inflammation showed significantly worse muscle function, which did not improved during hospitalization despite adequate treatment of the primary disease. Reduced strength and fatigue resistance were significantly related with circulating CRP, IL6 and fibrinogen levels. Moreover, some studies proved that elevated levels of tumour necrosis factor-alpha (TNF-alpha) can increase muscle catabolism by suppressing the Akt/mTOR pathway (28). In addition, it seems that cytokines may antagonize the anabolic effect of Insulin Growth Factor-1 (IGF-1), because of the development of growth hormone resistance, which decreases both circulating and muscle IGF-I (27, 28). The relation between inflammation, muscle strength and muscle mass seems to have a pathogenetic explanation based on the effect of inflammation on the balance between protein synthesis and protein catabolism, at the muscle level associated with the presence of CD68+ macrophage infiltration. Moreover, skeletal muscle aging is associated with myofibrosis and myosteatosis and to gradual fibrous replacement in the muscle (29). In addition, recent histological studies have shown that high values of serum CRP seem to be related with reduced protein synthesis and increased protein catabolism (30).
Lipotoxicity
With aging fat mass increase is associated with ectopic adipose tissue deposition and lipid deposition is modified with increased ectopic adipose tissue deposition, including muscle (31). This age-associated fat depots seems to act synergically with sarcopenia and the ageing process should be considered as a physiological degenerative process potentially accelerated by concomitant lipotoxic insults (32). Some studies proved that the increased accumulation of ectopic fat inside the muscle in older persons is independently associated with metabolic abnormalities as insulin resistance, and with reduced strength and performance (33). Moreover, Bollheimer et al. (34) showed a strong negative correlation between muscle volume and myosteatosis with proves of a blunted muscular protein biosynthesis in old high-fat diet fed rats. In addition, in a recent in vivo study, Tardif et al. (35) registered lipid redistribution and ectopic lipid accumulation into the muscle of diet-induced obese old rats. The same study showed that insulin resistance observed in these animals was independently related to intramuscular fat accumulation, and that it could be induced by high ceramide synthesis, stimulated by adipocyte-derived cytokines, including TNF-alpha and that decreases the ability to upregulate muscle insulin pathway, in old high-fat diet fed rats. Furthermore, Zhou et al. (36) showed that high fat fed mice presented increased muscle protein catabolism and this was associated with an increase in plasma free fatty acid and a decrease of adiponectin plasma levels. In fact, free fatty acids increased protein degradation of C2C12 skeletal muscle cells and this process was blocked by adiponectin over-secretion, mainly by attenuating the E3 ubiquitin ligase activation by increasing both insulin receptor substrate 1 tyrosine phosphorilation. Hence, this study suggested that the balance between free fatty acids and adiponectin impacts muscle proteolysis in insulin-resistant condition. Additionally, ectopic fat accumulation into the muscle determines mitochondrial changes determining a reduction of fatty acids oxidation capacity. Free fatty acids negatively influence protein anabolism, which becomes less sensitive to nutritional state in old high-fat fed animals (35).
Other mechanism involved in sarcopenia
Nevertheless, muscle homoeostasis relies also on the status of motor units, neuromuscular junctions, circulation factors and hormones.
It has been shown that the loss of muscle strength is associated with age-related changes in motor units, mainly due to the age-dependent reduction in motor axon conduction velocity and the number of myelinated axons (37). Moreover, aging is associated with a reduction in motor unit reinnervation after denervation, specially in type II muscular fibers, and when denervation outpaces reinnervation, it triggers muscle loss by an increase in apoptotic potential of myocites due to the loss of trophic factors. In particular, Kulakowski et al. (38) showed, using an animal model, that TrkB might play a role in synaptic stabilization and synaptic potentiating of the neuromuscular junction. Thus, reduced TrkB expression resulted in precocious neuromuscular aging of the predominately slow-twitch soleus muscle, while methods to maintain TrkB-mediated signaling may be a potential treatment for sarcopenia.
Aging determines changes in microcirculation and ultrastructure of the vascular endothelial function, mainly due to an age-related reduction in vasodilatory capacity and capillarization (39) that leads to a reduction in oxygen, energy sources and metabolites exchanges in muscle. This may explain the decline in exercise induced blood flow observed in elderly patients (39). Recently, several studies highlight the potential contribution of inflammation and oxidative stress to an impaired endothelial responsiveness (40). TNF-alpha, in particular may reduce endothelial-dependent dilatation by disrupting intercellular communication (41).
Hormones and growth factors represent others important contributors of the maintenance of proper cellular functions, in particular, they are associated with the trophic state of myocites. It has been demonstrated that aging is associated with a decline in sex hormones, as androgen and oestrogen, in both male and female (42). Nevertheless, to this date, there are controversial results regarding the beneficial result of hormonal replacement therapy in preventing muscle mass loss (43). A recent meta-analisis showed that estrogen-based hormonal therapy was beneficial to skeletal muscle strength in post-menopause women, as women on hormonal treatment were 5% stronger than those without treatment (44). Considering that usually, woman lose strength at a rate of 1% a year after menopause, this relatively small effect might be clinically meaningful. Additionally, the levels of growth hormone (GH) and their relevant regulators (Insulin-like Growth Factor-IGF family) are usually lower in the elderly subjects, and these situation could further explain skeletal muscle mass wasting. In a recent in vivo study, Briosche et al. (45) proved that GH supplementation in old mice determined an increase in lean body mass, with an elevated synthesis rate of skeletal muscle protein and mitochondrial biogenesis pathways. Moreover, they registered a lowering of age-associated oxidative damage, an increase of plasma and hepatic IGF-1 levels, and an induction of antioxidant enzymes in the skeletal muscle of the treated animals. Further clinical studies are needed in order to confirm this preliminary results.
During aging, it has been registered a decline in 25(OH)vitamin D3 serum levels associated with reduced muscle mass, strength and performance in older adults (46, 47). Moreover, a recent skeletal muscle gene expression study carried out in older adults (68–79 years) showed that lower expression of vitamin D receptor (VDR) was associated with higher lean mass (48). Additionally, Redzic et al. demonstrated that vitamin D status might be involved in intramyocellular lipid accumulation independent of body mass index and physical activity level (49). Furthermore, a recent meta-analysis, considering randomized controlled trials, reported a positive association between vitamin D supplementation and muscle strength, but further studies are needed to define optimal treatment modalities (50). Moreover it seems that vitamin D supplementation increases intramyonuclear VDR concentration by 30% and increases muscle fiber size by 10% in older, mobility-limited women (46). Further studies are needed in order to clarify the role of vitamin D in affecting muscle metabolism and function.
Recently, great interest has been shown towards a specific myokine, called myostatin, that seems to be a potential target to prevent sarcopenia development. Myostatin negatively regulates skeletal mass and might also be implicated in regulating hepatic production of IGF-1 (51). Moreover, myostatin gene expression is up-regulated in elderly compared to young subjects, and the age-related elevation of myostatin serum levels correlated with muscle mass (52). Recently, McKay et al. showed that there is an age-related impairment of muscle stem cell function that might be explained by the co-localization with myostatin in older subjects (53). Other studies need to be made but myostatin could potentially prevent muscular mass wasting.
Conclusions
This review aimed to summarised the current data regarding the physiopathology of sarcopenia in older subjects. Lifestyle factors as diet and physical activity, together with age-related changes in cytokines and hormones levels are important risk factors for muscular impairment and each of them contribute in a different manner. To this date, epidemiological and clinical studies showed that muscle mass and strength are differently influenced by various interventional treatments, but long-term supplementation studies are still needed in order to further investigate the role of healthy lifestyle habits in the prevention and treatment of sarcopenia. Moreover, recent data showed that high fat diet is an important risk factor for ectopic fat depots, in particular intramyocellular adipose tissue accumulation. This phenomenon has a direct negative influence on muscular homeostasis and function with unfavourable consequences on the development of sarcopenia.
For all these reasons, sarcopenia should have a multidimensional approach in order to understand its pathophysiology and to define the molecular targets for intervention and future successful treatment.
References
- 1.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 years. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 2.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatric Med. 2011;27(3):387–399. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Van Kan GA. Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13(8):708–712. doi: 10.1007/s12603-009-0201-z. [DOI] [PubMed] [Google Scholar]
- 4.Bunout D, de la Maza MP, Barrera G, et al. Association between sarcopenia and mortality in healthy older people. Australian J Ageing. 2011;30(2):89–92. doi: 10.1111/j.1741-6612.2010.00448.x. [DOI] [PubMed] [Google Scholar]
- 5.OECD Factbook. Econimic, Environmental and Social Statistics. 2009. http://dx.doi.org/10.1787/factbook-2009-en.
- 6.Cruz-Jentolf Aj, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition an diagnosis: Report of the European Working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention and assessment. Osteoporosis Int. 2010;21:543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kortebein P, Ferrando A, Lombeida J, et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1772–3. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 9.Coker RH, Hays NP, Williams RH, et al. Bed rest worsens impairments in fat and glucose metabolism in older, overweight adults. J Gerontol a Biol Sci Med Sci. 2014;69(3):363–370. doi: 10.1093/gerona/glt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MJ, Dickinson JM, Fry CS, et al. Bed rest impairs skeletal muscle amino acid trasporter expression, mTORC1 signalling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:e1113–22. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paddon Jones D, Sheffield-Moore M, Cree MG, et al. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–41. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- 12.Kortebein P, Brock Symons T, Ferrando A, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol. 2008;63A(10):1076–81. doi: 10.1093/gerona/63.10.1076. [DOI] [PubMed] [Google Scholar]
- 13.Drummonds MJ, Timmerman KL, Markofski MM, et al. Short-term bed rest increases TLR4 and IL6 expression in skeletal muscle in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;305:R216–R223. doi: 10.1152/ajpregu.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 15.Morley JE, Silver AJ. Anorexia of the elderly. Neurobiol Aging. 1988;9:9–16. doi: 10.1016/s0197-4580(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 16.Bauer J, Cederholm BG, Cesari M, et al. Evidence-based recommendation for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. 2013;14(8):542–59. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Tieland M, van de Rest O, Dirks ML, et al. Protein supplementation improves physical performance in frail elderly people; a randomized, double-blind, placebo-controlled trial. JAMDA. 2012;13:720–6. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Daly RM, O’Connell SL, Mundell NL, et al. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL6 concentration in elderly women: a cluster randomized controlled trial. Am J Clin Nutr. 2014;99:899–910. doi: 10.3945/ajcn.113.064154. [DOI] [PubMed] [Google Scholar]
- 19.Dillon EL, Sheffield-Moore M, Paddon-Jones D, et al. Amino acid supplementation increases lean body mass, basal muscle protein synthesis and insulin-like growth factor-1 expression in older women. J Clin Endocrinol Metab. 2009;94(5):1630–1637. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KK, Suzuki T, Saito K, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly japanese sarcopenic women: a randomized controlled trial. JAGS. 2012;60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 21.Toth MJ, Ades PA, Tischler MD, Tracy RP, LeWinter MM. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. Int J Cardiol. 2006;109(2):179–87. doi: 10.1016/j.ijcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC study. J Gerontol. 2002;57A(5):M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 23.Schaap L, Pluijim SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. American J Medicine. 2006;119(6):526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 24.Schaap L, Pluijim SMF, Deeg DJH, et al. Higher inflammatory markers levels in older persons: association with 5-year change in muscle mass and strength. J Gerontol A BioSci Med Sci. 2009;64A(11):1183–9. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norman K, Stobaus N, Kulka K, Schulzke J. Effect of inflammation on handgrip strength in the non-critically ill is independent from age, gender and body composition. European J Clin Nutrition. 2014;68:155–158. doi: 10.1038/ejcn.2013.261. [DOI] [PubMed] [Google Scholar]
- 26.Bautmans I, Njemini R, Lambert M, Demanet C, Mets T. Circulating acute phase mediators and skeletal muscle performance in hospitalized geriatric patients. J Gerontol. 2005;60A(3):361–7. doi: 10.1093/gerona/60.3.361. [DOI] [PubMed] [Google Scholar]
- 27.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:e453–9. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 28.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signalling determining muscle mass. J Appl Physiol. 2007;103(1):378–87. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 29.Puthucheary ZA, Jaikitry R, McPhail M, Connoly B, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 30.Zoico E, Corzato F, Bambace C, et al. Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatrics. 2013;57(3):411–416. doi: 10.1016/j.archger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamboni M, Mazzali G, Fantin F, et al. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18(5):388–95. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 2006;5(2):144–64. doi: 10.1016/j.arr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Zoico E, Rossi AP, di Francesco V, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: a relationship with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65(3):295–9. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bollheimer LC, Buettner R, Pongratz G, et al. Sarcopenia in the aging high-fat fed rat: a pilot study for modelling sarcopenic obesity in rodents. Biogerontology. 2012;13:609–620. doi: 10.1007/s10522-012-9405-4. [DOI] [PubMed] [Google Scholar]
- 35.Tardif N, Salles J, Guillet C, et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2alpha activation. Aging Cell. 2014;13:1001–11. doi: 10.1111/acel.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Q, Du J, Hu Z, et al. Evidence for adipose muscle cross talk: opposing regulation of muscle proteolysis by adiponectin and fatty acids. Endocrinol. 2007;148(12):5696–5705. doi: 10.1210/en.2007-0183. [DOI] [PubMed] [Google Scholar]
- 37.Kwan P. Sarcopenia, a neurogenic syndrome? J Aging Res. 2013 doi: 10.1155/2013/791679. id791679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulakowski SA, Parker SD, Personius KE. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission and muscle function. J Appl Physiol. 2013;111:845–52. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- 39.Degens H. Age-related changes in the microcirculation of skeletal muscle. Advances Exp Med Biol. 1998;454:343–8. doi: 10.1007/978-1-4615-4863-8_40. [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Jentoft A, Morley JE. Sarcopenia. Ed. Wiley-Blackwell; 2012. [Google Scholar]
- 41.Csiszar A, Labinskyy N, Smith K, et al. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–98. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26(5):524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Rolland Y, Czerwinski S, Van Kan GA, et al. Sarcopenia: its assessment, etiology, pathogenesisi, consequences and future perspectives. J Nutr Health Aging. 2008;12(7):433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol. 2009;64A(10):1071–1081. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brioche T, Kireev RA, Cuesta S, et al. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: improvement of protein balance and of antioxidant defences. J Gerontology. 2014;69(10):1186–98. doi: 10.1093/gerona/glt187. [DOI] [PubMed] [Google Scholar]
- 46.Ceglia L, Niramitmahapanya S, da Silva Morais M, et al. A randomized study on the effect of vitamin D3 supplementation on the skeletal muscle morphology and vitamin d receptor concentration in older women. J Clin Endocrinol Metab. 2013;98:e1927–35. doi: 10.1210/jc.2013-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy EK, Kiely M. Vitamin D and muscle strength throughout the life course: a review of epidemiological and intervention studies. J Hum Nutr Diet. 2014 doi: 10.1111/jhn.12268. [DOI] [PubMed] [Google Scholar]
- 48.Patel HP, Al-Shanti N, Davies LC, et al. Lean mass, muscle strength and gene expression in community dwelling older men : findings from the Hertfordshire Sarcopenia Study (HSS) Calcif Tissue Int. 2014;95(4):308–16. doi: 10.1007/s00223-014-9894-z. [DOI] [PubMed] [Google Scholar]
- 49.Redzic M, Powell DK, Thomas DT. Vitamin D is related to intramyocellular lipid in older adults. Endocrine. 2014;47(3):854–61. doi: 10.1007/s12020-014-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beaudart C, Buckinx F, Rabenda V, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 51.Jackson MF, Luong D, Vang DD, et al. The aging myostatin null phenotype: reduced adiposity, cardiac hypertrophy, enhaced cardiac stress response, and sexual dimorphism. J Endocrinol. 2012;213:263–75. doi: 10.1530/JOE-11-0455. [DOI] [PubMed] [Google Scholar]
- 52.Yarasheski KE, Bhasin S, Sinha-Hikim I, et al. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle waste. J Nutr Health Aging. 2002;6:343–8. [PubMed] [Google Scholar]
- 53.McKay BR, Ogborn DI, Bellamy LM, et al. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012;26:2509–21. doi: 10.1096/fj.11-198663. [DOI] [PubMed] [Google Scholar]