Abstract
Regular physical exercise is beneficial for both physical and mental health. By contrast, stress is associated with deleterious effects on health and there is growing evidence that regular physical exercise counteracts some of the effects of stress. However, most previous studies have suggested that prior exercise does not alter the acute hypothalamic pituitary adrenal (HPA) axis responses to stress. The present series of studies provides evidence that in rats, 6 weeks (but not 1 or 3 weeks) of voluntary wheel running reduces the HPA axis responses to lower-intensity stressors such as an i.p. saline injection, exposure to a novel environment or exposure to moderate intensity noise, but not to more intense stressors such as predator odour exposure or restraint. Daily exercise does not appear to be necessary for the reduction in HPA axis responses, with intermittent access (24 h out of each 72-h period) to a running wheel for 6 weeks, resulting in similar decrements in adrenocorticotrophic hormone and corticosterone release in response to 85 dBA noise exposure. Data from in situ hybridisation for c-fos mRNA are consistent with the hypothesis that voluntary exercise results in a decrease in HPA axis responsiveness to a low-intensity stressor at a central level, with no changes in primary sensory processing. Together, these data suggest that 6 weeks of daily or intermittent exercise constrains the HPA axis response to mild, but not more intense stressors, and that this regulation may be mediated at a central level beyond the primary sensory input.
Keywords: stress, HPA axis, exercise, corticosterone, adrenocorticotrophic hormone (ACTH), c-fos
It is well established that regular physical exercise is beneficial for both physical and mental health. Conversely, stress has been associated with numerous deleterious effects on health and there is a growing body of empirical data indicating that regular physical exercise can prevent or reduce some of the physiological consequences of stressor exposure [1–5]. One of the hallmark characteristics of a stress response is activation of the hypothalamic pituitary adrenal (HPA) axis, which leads to release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland, and subsequent synthesis and release of cortisol (or corticosterone in rats) from the adrenal cortex into the general circulation.
Although able to reduce some consequences of stressor exposure, most previous studies have suggested that prior exercise does not reduce the HPA axis response to acute stressors. Studies using 6 weeks of treadmill training have even found an exacerbation of ACTH release after immobilisation [6] or footshock [7]. In addition, most studies using animals with continuous access to a running wheel for 4–12 weeks have not demonstrated a reduction in acute HPA axis responses to various stressors, including foot-shock [8, 9], tailshock [10], forced swim [11, 12], restraint [12–14] (but see also [15]), or loud (98 dBA) noise [16]. However, 4 weeks of treadmill or swim training resulted in a decreased ACTH response to cage switch stress in rats [17], whereas 4 weeks of voluntary wheel running in rats [11] or mice [12, 13] decreased the corticosterone (but not ACTH) response to a novel environment exposure [11].
It has been suggested that the reduction in corticosterone response after exposure to a novel environment in exercised rodents may be a result of the psychological nature of the novel stressor. This compares with a lack of effect of exercise on the HPA axis response to footshock, tailshock, forced swim or restraint, which all have a physical component. However, voluntary wheel running did not reduce the HPA axis response to a 98 dBA loud noise [16], which, like novelty, is considered to be a psychological stressor. This led us to hypothesise that the critical factor in determining whether a history of voluntary exercise reduces the HPA axis response to a stressor is the intensity of the stressor. That is, the HPA axis response to lower-intensity stressors, such as novelty, an i.p. saline injection, or a more moderate intensity noise (85 dBA) would be reduced by a history of voluntary exercise in male rats, whereas the HPA axis response to higher-intensity stressors, such as ferret odour or restraint, would not. We additionally hypothesised that the reduction in HPA axis response to a low-intensity stressor would be dependent on the duration of wheel running, as has been observed for learned helplessness behaviour [18]. In addition, given that most humans do not exercise on a daily basis, we tested the hypothesis that the reduction in HPA axis response to a low-intensity stressor would not require daily exercise, which, to our knowledge, has not been tested previously. The present series of experiments provide data in support of these hypotheses. By contrast to some previous studies using a novel environment exposure [11, 13], we generally observed a reduction in both the ACTH and corticosterone responses to low-intensity stressors in exercised rats, suggesting that the decrease in HPA axis activation after stressor exposure may be mediated, at least partially, at a central level. This possibility was explored by semi-quantitative in situ hybridisation for c-fos mRNA expression in the brain of exercised and sedentary rats after low-intensity stressor exposure.
Together, the data presented in this series of experiments suggest that one way in which regular exercise may counteract the deleterious effects of stress is by constraining, at a central level, the HPA axis response to low-intensity stressors that may be encountered on a daily basis. Preliminary data have been reported previously in abstract form [19, 20].
Materials and methods
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA), aged 2 months at the start of the experiment, were used. Animals were initially group-housed, four to five per cage, under stable conditions of temperature and humidity and with free access to standard laboratory chow and water. Animals were maintained under a 12 : 12 h light/dark cycle (lights on 07.00 h). All rats were weighed weekly, on the same day each week, within the colony room. Apart from the weekly weight check, and the changing of cages, animals were not handled. All procedures were approved by the University of Colorado at Boulder Institutional Animal Care and Use Committee, in accordance with NIH guidelines.
Wheel running
After 7–10 days of acclimation to the colony conditions, animals were single-housed in polycarbonate cages (14 × 9.5 × 7.5 inches; sedentary; SED) or in equivalent cages with access to a running wheel (RUN; Nalge Nunc Int., Rochester, NY, USA). Running distances were tracked with Vital View Software (Mini-Mitter/Respironics, Bend, OR, USA).
Tissue collection
In experiments where animals were killed, trunk blood was collected into chilled Vacutainers containing ethylenediaminetetraacetic acid (EDTA) for analysis of ACTH and corticosterone. Brains were removed and frozen in isopentane chilled to −20 °C to −30 °C, and stored at −80 °C for in situ hybridisation for c-fos mRNA for select experiments. With the exception of Experiments 1B, 2 and 7, adrenal glands were removed bilaterally, defatted and weighed, and the thymi removed and weighed, as potential indices of long-term elevated corticosterone levels [21]
Experiment 1: Effect of 6 weeks of wheel running on HPA axis responses to a novel environment
In this experiment, the effect of 6 weeks of SED or RUN conditions on the HPA axis response to a low-intensity stressor, a novel environment, was determined in rats. Baseline plasma levels, and the time course of the response to a novel environment, were determined for plasma corticosterone in Experiment 1B.
-
(A)
Rats were housed under SED (n = 9) or RUN (n = 9) conditions for 6 weeks. After this time, and 2–4 h after lights on, rats were placed, individually, in a white circular 5-gallon household plastic bucket (base diameter 25 cm, height 36 cm), which represented a novel environment, for 30 min. Animals were then killed by decapitation and tissue collected as described above. In situ hybridisation was not performed for this experiment.
-
(B)
In a different experiment, rats were housed under SED (n = 24) or RUN (n = 24) conditions for 6 weeks. At the end of the 6-week period, and 2–4 h after lights on, rats were placed individually in a white bucket, as described above. Animals remained in this environment for 15, 30 or 60 min (n = 6 per group). Additional animals (n = 6) remained in their home cages. After the appropriate length of time, animals were removed from the bucket (or home cage), gently restrained in a towel, and a blood sample was taken from a lateral tail vein by puncturing with the corner of a razor blade. Blood (300–400 µl) was collected with heparinised capillary tubes and placed into tubes containing 20 µl of EDTA tetrasodium salt (20 mg/ml) on ice. The procedure lasted less than 3 min. Plasma was collected and stored at −20 °C for analysis of corticosterone. The ACTH radioimmunoassay could not be performed on this plasma because of the interference of heparin with the assay. Animals were not killed at the end of this procedure and therefore tissue was not collected in this experiment.
Experiment 2: effect of 6 weeks of wheel running on HPA axis responses to a saline injection
This experiment investigated the effect of a second low-intensity stressor, an i.p. saline injection, on the HPA axis response in previously unhandled rats, after 6 weeks under SED or RUN conditions. Rats were housed under SED (n = 6) or RUN (n = 6) conditions for 6 weeks. After 6 weeks, and 2–4 h after lights on, animals were removed from their home cage, and given an i.p. injection of 0.9% sterile saline (1 ml/kg) and replaced in their home cage. RUN animals had access to the running wheel after the injection. Animals were killed by rapid decapitation 30 min after the injection, and tissue collected as described above, except that adrenals and thymi were not harvested.
Experiment 3: Effect of 6 weeks of wheel running on the HPA axis responses to 85 dBA noise
In this experiment, the effect of 6 weeks of SED or RUN conditions on the time course of the HPA axis response to a third low-intensity stressor (85 dBA white noise) was determined in rats. Baseline measures for ACTH and corticosterone were included.
Rats (n = 50) were housed under SED (n = 26) or RUN (n = 24) conditions for 6 weeks. For 1 week prior to testing for each group (after 5 weeks under SED or RUN conditions), animals were habituated daily to transport from the colony room to the behavioural testing suite in another part of the wing. Animals were left in an adjacent room for up to 4 h before exposure to one of eight identical acoustic isolation chambers for 30 min. The acoustic isolation chambers and noise exposure procedures have been described previously [22]. On the test day, animals were transported to the behavioural suite as previously experienced during the habituation phase. Rats were placed individually in the acoustic isolation chambers within their home cages and exposed to either background noise conditions (approximately 60 dBA), or 85 dBA white noise as follows. Rats in the background noise/60 dBA groups were placed in the acoustic isolation chambers without additional noise for 30 min, then removed and killed by rapid decapitation. Rats in the 15-min groups were first placed in the chambers without noise for 15 min, then exposed to 85 dBA white noise for 15 min and then removed from the chamber and killed. Rats in the 30-min groups were exposed to 85 dBA noise for 30 min and removed from the chamber and killed. Rats in the 60-min groups were placed in the chamber and exposed to 85 dBA noise for 30 min, then removed to an adjacent room (in their home cages) for a further 30 min, before being killed (60 min after the start of the 30 min of noise exposure). For each group, n = 6, except for the 15- and 30-min SED groups, where n = 7. Rats in the RUN groups had access to the wheel at all times, including during the noise exposure. An intensity of 85 dBA was chosen because this was the lowest intensity that led to a significant increase in ACTH and corticosterone levels, and that produced a submaximal hormonal response [23].
Experiment 4: Effect of 1, 3 or 6 weeks of wheel running on the HPA axis responses to 85 dBA noise
In Experiments 1–3, RUN rats had access to the running wheel for 6 weeks. However, it was not clear whether that length of time was necessary for HPA axis response attenuation to mild stress. Therefore, in this experiment, RUN rats had access to the running wheel for 1, 3 or 6 weeks, and the effect on the HPA axis response to a low-intensity stressor (85 dBA noise) was determined.
Rats (n = 48) were housed under SED (n = 24) or RUN (n = 24) conditions for 1, 3 or 6 weeks (n = 8 per condition). All animals were given access to running wheels at the same time, at the same age (2 months) and weight, and were tested at different intervals (1, 3 or 6 weeks) after the start of SED or RUN conditions. For 1 week prior to testing, animals were habituated daily to the acoustic isolation chambers, as for Experiment 3. On the test day (after 1, 3 or 6 weeks of SED or RUN conditions), animals were transported to the behavioural suite and placed in the acoustic isolation chambers within their home cages. Animals were then exposed to background noise conditions (60 dBA) or 85 dBA noise for 30 min. Rats in the RUN group had access to the wheel at all times, including during the noise exposure. After 30 min, animals were killed by rapid decapitation and tissue collected as described above. Brains were not processed for in situ hybridisation in this study.
Experiment 5: Effect of 6 weeks of wheel running, and locking the wheel during noise exposure, on the HPA axis response to 85 dBA noise
In Experiments 2–4, rats had access to the running wheel during stressor exposure. This experiment was designed to determine whether running during noise stress exposure is necessary for HPA axis response attenuation.
Rats (n = 42) were housed under SED (n = 18) or RUN (n = 24) conditions for 6 weeks. For 1 week prior to testing for each group, animals were habituated daily to transport from the colony room to the behavioural testing suite in another part of the wing, and exposure to the acoustic isolation chambers for 30 min. During exposure to the chambers, one group of RUN rats (n = 8) had the wheel ‘locked’ by insertion of a metal rod between the wheel rungs to prevent movement (RUN-Lock). On the test day, animals were transported to the behavioural suite and placed in their home cage in the isolation chamber, as for the habituation. The RUN-Lock group of rats had the wheels ‘locked’ during noise exposure, whereas the wheels for the other two RUN groups remained available. Animals were exposed to background (60 dBA; SED or RUN) or 85 dBA white noise (SED, RUN or RUN-Lock) for 30 min and then immediately decapitated and tissue collected as described above. A 60 dBA RUN-Lock group was not included in this experiment because we have previously shown that there is no difference in plasma levels of ACTH and corticosterone between SED and RUN-Lock rats under 60-dB noise conditions [16]. In this experiment, brains were not processed for c-fos mRNA.
Experiment 6: Effect of 6 weeks of continuous versus intermittent access to the running wheel on the HPA axis responses to 85 dBA noise
In the previous experiments, RUN rats had continuous access to the running wheel. This experiment was designed to determine if intermittent access to a running wheel (24 h out of each 72-h period) would be sufficient to attenuate the HPA axis response to low-intensity stressor (85 dBA noise).
Rats (n = 40; n = 8 per group) were housed under SED (n = 16), RUN (n = 16) or Intermittent RUN (n = 8) conditions for 6 weeks. For the Intermittent RUN group, rats had access to the running wheel for only 24 out of each 72-h period over 6 weeks. For the remaining 48 h, the wheel was ‘locked’ by insertion of a metal rod between the rungs of the wheel, to prevent turning. The wheel was unlocked or locked approximately 6 h after lights on. For 1 week prior to testing for each group, animals were habituated daily to transport from the colony room to the behavioural testing suite in another part of the wing, and exposure to the acoustic isolation chambers for 30 min. On the test day (after 6 weeks of SED, RUN or Intermittent RUN housing conditions), and the morning after all Intermittent RUN rats had had access to the running wheel during the dark phase, animals were transported to the behavioural suite and placed, within their home cages, in the acoustic isolation chambers. Animals were then exposed to background (60 dBA) or 85 dBA noise for 30 min. During noise presentation, all the RUN and Intermittent RUN animals had access to the running wheel. After this time, animals were killed by rapid decapitation and tissue collected for processing as described above.
Experiment 7: Effect of 6 weeks of wheel running on the HPA axis response to restraint or ferret (predator) odour
Experiments 1–6 all utilised low-intensity stressors. This experiment was designed to determine whether RUN and SED rats would have a similar HPA axis response to higher-intensity stressors, (ferret odour or restraint) as has been reported previously [6–14, 16].
Rats (n = 40) were housed under SED (n = 20) or RUN (n = 20) conditions for 6 weeks. For 1 week prior to testing for each group, animals were habituated daily to transport from the colony room to the behavioural testing suite in another part of the wing, and half of each group of animals were also habituated to exposure to the acoustic isolation chambers for 30 min. On the test day, animals were transported to the behavioural suite. Half of the rats in the SED and RUN groups (n = 10 per group) were then subjected to 30 min of restraint stress. The other rats (n = 10 per group) were exposed to ferret (predator) odour for 30 min, which has been shown to be a powerful stressor in terms of both physiological and behavioural responses [24, 25]. Restraint stress consisted of placing each animal in a clear Plexiglas tube (length 23.5 cm, diameter 7 cm) with tails protruding. The size of the tube restricted movement in all directions, but did not interfere with breathing. For ferret (predator) odour stress, a bath towel was used as bedding for undescented adult ferrets for approximately 1 month (courtesy of the Mile High Ferret Club; http://www.milehighferretclub.org. The towel was cut into 5 × 5 cm squares, sealed in a plastic bag, and stored at −80 °C until used. The towels were transported to the experimental rooms immediately before testing inside sealed glass bell jars. Four pieces of towel with ferret odour were carefully placed at each corner of the home cage without disturbing the rats by hooking the towels to the wire cage lid with paper clips, so that the towels hung inside the cage. Each rat was placed, within its home cage, within an acoustic isolation chamber, as described above. For both restraint and ferret odour stress, blood was sampled 30 min after the start of the stressor, via tail bleed. Rats were removed from the restraint tube or home cage, gently restrained in a towel, and a blood sample taken from a lateral tail vein by puncturing with the corner of a razor blade. To obtain plasma for the corticosterone ELISA assay, blood was collected with heparinised capillary tubes (100–150 µl) and placed in an empty microfuge tube on ice. To obtain plasma for the ACTH immunoradiometric assay (IRMA), blood was collected with plain capillary tubes (400–500 µl) and placed into microfuge tubes containing 20 µl of EDTA tetrasodium salt (20 mg/ml) on ice. The procedure lasted less than 3 min. Plasma was stored at −80 °C. Animals were not killed at the end of this procedure and therefore tissue was not collected in this experiment.
ACTH IRMA
Plasma (200 µl) was assayed for levels of ACTH using an Immunoradiometric Assay kit (Diasorin, Stillwater, MN, USA). Briefly, the plasma was incubated overnight with a 125I-labelled monoclonal antibody specific for ACTH 1–17, a goat polyclonal antibody specific for ACTH 26–39, and a polystyrene bead coated with a mouse anti-goat antibody. Only ACTH 1–39 in the sample bound both antibodies to form an antibody complex. Beads were washed to remove unbound radioactivity, counted with a gamma counter, and the concentrations of ACTH determined by comparison with a standard curve generated concurrently. All samples from a particular experiment were run in the same assay.
Corticosterone ELISA
Levels of corticosterone in plasma were determined by a commercially available Enzyme Linked ImmunoSorbent Assay (ELISA) kit (Assay Designs, Ann Arbor, MI, USA). Briefly, plasma or corticosterone standard was incubated in a 96-well plate coated with donkey anti-sheep antibody, together with a sheep polyclonal antibody specific for corticosterone, and corticosterone that was covalently attached to an alkaline phosphatase molecule. After a 2-h incubation period, unbound reagents were washed from the plate and substrate added. After a 1-h incubation period, the reaction was stopped and the intensity of the yellow colour generated read with a microplate reader (Biotek EL808; Winooski, VT, USA) at 405 nm. The concentration of corticosterone in the samples was calculated by comparison with a standard curve generated concurrently. Kit directions were followed, except with an adaptation for using a smaller volume of plasma as follows. The steroid displacement reagent (provided with the kit) was added to the assay buffer at a concentration of 0.5 µl/ml. Plasma (typically 10 µl) was diluted 1 : 50 with the amended assay buffer. The diluted plasma sample (100 µl) was then processed as described in the kit directions. This method used equivalent final concentrations of steroid displacement reagent, and was found to result in assayed corticosterone levels equivalent to the standard method, in which 2.5 µl of steroid displacement reagent is added to 97.5 µl of plasma, and the plasma diluted with standard assay buffer (data not shown). All samples from a particular experiment were run in the same assay.
In situ hybridisation
The method for in situ hybridisation has been described previously [26]. Briefly, 10 µm sections were cut on a cryostat (Leica model 1850; Leica Microsystems, Wetzlar, Germany), thaw-mounted onto polylysine-coated slides and stored at −80 °C. A [35S]-UTP-labelled riboprobe against c-fos mRNA (680 mer; courtesy of Dr T. Curran St Jude Children’s Hospital, Memphis, TN, USA) was generated using standard transcription methods. Sections were fixed in 4% paraformaldehyde (1 h), acetylated in 0.1 m tri-ethanolamine with 0.25% acetic anhydride (10 min) and dehydrated through graded alcohols. Sections were hybridised overnight at 55 °C with a [35S]-UTP-labelled riboprobe diluted in hybridisation buffer containing 50% formamide, 10% dextran sulphate, 3x saline sodium citrate, 50 m m sodium phosphate buffer, pH 7.4, 1x Denhardt’s solution, and 0.2 mg/m yeast tRNA. The next day, sections were treated with RNase A, 200 µg/m at 37 °C (1 h), and washed to a final stringency of 0.1 x saline sodium citrate at 65 °C (1 h). Dehydrated sections were exposed to X-ray film (BioMax MR; Eastman Kodak, Rochester, NY, USA) for up to 1 week and the films analysed as described below.
Semi-quantitative X-ray film analysis
Levels of c-fos mRNA were analysed by computer-assisted optical densitometry. Analysis was performed blind to the treatment conditions. Anatomical landmarks were based on the white matter distribution of the unstained tissue section, according to a standard rat brain atlas [27]. Brain section images were captured digitally (CCD camera, model XC-77; Sony, Tokyo, Japan), and the relative optical density of the X-ray film was determined using Scion Image, version 4.0 for PC (Scion Corp., Frederick, MD, USA). A macro was written (Dr S. Campeau) that enabled signal above background to be determined automatically. For each section, a background sample was taken over an area of white matter, and the signal threshold was calculated as mean grey value of background + 3.5 SD. The section was automatically density sliced at this value, so that only pixels with grey values above these criteria were included in the analysis. It should be noted that, although background criteria were relatively stringent, this thresholding method still results in a few pixels above background in areas off the brain section, indicating that even a weak-intensity mRNA signal on the tissue section is detected. A template was used for each brain region to ensure that the same area was included in the analysis for each region. For the majority of brain regions, left and right sides were analysed through the rostral-cauda extent of each nucleus. For the dorsal raphe (DR), a midline structure, a single value was obtained for each section analysed. The number of pixels above background was multiplied by the signal above background, to give an integrated density value. This method has been shown to reflect both the number of cells expressing mRNA and the expression level per cell, as determined by cell and grain counts of emulsion dipped slides [22]. Depending on the brain area, the mean of the highest three to 12 integrated density values for each animal was calculated, giving a single value for each animal representing the peak of c-fos mRNA expression.
Statistical analysis
Data were analysed by anova and, with the exception of the c-fos mRNA analysis, P < 0.05 was considered statistically significant. For c-fos mRNA analysis, the significance of the anova was set at P < 0.01 to reduce the possibility of type I errors. Fisher’s least significant difference (LSD) was used for post-hoc analysis, with P < 0.05 considered statistically significant Repeated measures anova was used to analyse body weight and running data. Additional information for specific experiments and measures is provided with the relevant data where appropiate.
Results
Body weights
Body weights for each experiment are given in Table 1. The pattern of body weight gain was similar between experiments, and an example of statistical analysis of the body weight data is given for Experiment 1B. Repeated measures anova revealed that there was a significant effect of time on body weight (F6,276 = 1553.9; P < 0.001), a significant time by group interaction (F6,276 = 78.57; P < 0.001) and a significant effect of group on body weight (F1,46 = 81.65; P < 0.001). The weight of the rats was not significantly different between SED (231.3 ± 2.0 g; n = 24) and RUN (230.2 ± 1.2 g; n = 24) groups at the start of wheel access (F1,46 = 0.221; P = 0.640). After as little as 1 week of access to the running wheel, rats in the RUN group weighed significantly less than the SED group (RUN: 250.9 ± 2.0 g; SED: 272.7 ± 1.9 g; F1,46 = 60.24; P < 0.001). This significant differential in weight was maintained across the course of the study. After 6 weeks under SED or RUN conditions, SED rats weighed 371.0 ± 4.2 g and RUN rats weighed 316.3 ± 3.6 g (F1,46 = 98.06; P < 0.001). A very similar general pattern of body weight gain was seen for all other experiments and, for brevity, are not described in detail. However, it should be noted that, for Experiment 6, there was a significant effect of group on body weight (F2,37 = 8.00; P = 0.001). Post-hoc analysis (Fisher’s LSD) revealed that there were significant differences between both SED and RUN groups (P < 0.001) and SED and Intermittent RUN groups (P = 0.017) but no significant difference between RUN and Intermittent RUN groups (P = 0.522) for body weights.
Table 1.
Mean ± SEM Body Weights for Experiments 1–7.
| Experiment | Group | n | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 |
|---|---|---|---|---|---|---|---|---|---|
| 1A | SED | 9 | 212.4 ± 2.5 | 254.2 ± 2.1 | 292.1 ± 1.6 | 312.0 ± 1.8 | 332.0 ± 2.5 | 349.4 ± 3.1 | 363.7 ± 2.9 |
| RUN | 9 | 211.6 ± 3.1 | 236.4 ± 4.0 | 271.7 ± 5.5 | 286.4 ± 5.8 | 302.9 ± 5.7 | 316.4 ± 6.1 | 325.6 ± 6.6 | |
| 1B | SED | 24 | 231.3 ± 2.0 | 272.7 ± 1.9 | 299.6 ± 2.3 | 321.3 ± 3.0 | 343.9 ± 3.3 | 353.0 ± 3.7 | 371.0 ± 4.2 |
| RUN | 24 | 230.2 ± 1.2 | 250.9 ± 2.0 | 270.7 ± 2.6 | 287.4 ± 2.9 | 301.4 ± 3.1 | 304.8 ± 3.2 | 316.3 ± 3.6 | |
| 2 | SED | 6 | 231.0 ± 3.7 | 254.2 ± 3.1 | 279.2 ± 5.7 | 301.0 ± 7.8 | 313.0 ± 9.1 | 324.8 ± 8.8 | 336.7 ± 10.0 |
| RUN | 6 | 228.8 ± 2.9 | 242.8 ± 5.6 | 267.7 ± 5.4 | 287.0 ± 4.9 | 302.0 ± 5.3 | 305.8 ± 5.9 | 315.8 ± 6.1 | |
| 3 | SED | 26 | 245.7 ± 1.7 | 288.2 ± 1.6 | 325.4 ± 1.9 | 349.9 ± 2.2 | 368.2 ± 2.5 | 383.1 ± 2.7 | 396.9 ± 2.8 |
| RUN | 24 | 245.1 ± 1.6 | 268.5 ± 2.2 | 300.9 ± 2.2 | 321.7 ± 2.8 | 332.0 ± 3.1 | 341.6 ± 3.9 | 350.8 ± 4.5 | |
| 4 | SED | 8–24 | 244.0 ± 2.4 | 267.3 ± 3.0 | 296.3 ± 4.7 | 308.8 ± 5.0 | 327.1 ± 8.5 | 345.1 ± 10.0 | 357.8 ±11.1 |
| RUN | 8–24 | 243.8 ± 1.9 | 247.5 ± 2.2 | 279.9 ± 2.9 | 288.4 ± 3.9 | 300.6 ± 8.7 | 314.0 ± 10.2 | 326.4 ± 11.4 | |
| 5 | SED | 18 | 224.5 ± 1.8 | 259.3 ± 2.5 | 289.1 ± 3.7 | 304.6 ± 4.6 | 315.9 ± 5.3 | 331.8 ± 6.2 | 344.7 ± 5.9 |
| RUN | 24 | 224.8 ± 1.4 | 244.2 ± 2.1 | 273.1 ± 2.9 | 286.3 ± 3.4 | 291.4 ± 3.4 | 302.3 ± 3.7 | 312.3 ± 3.9 | |
| 6 | SED | 16 | 222.8 ± 1.5 | 260.4 ± 2.0 | 286.0 ± 3.3 | 306.8 ± 3.7 | 322.4 ± 4.0 | 338.6 ± 4.4 | 347.8 ± 5.1 |
| RUN | 16 | 223.8 ± 1.5 | 247.2 ± 2.7 | 273.4 ± 3.4 | 290.1 ± 4.7 | 297.7 ± 4.7 | 305.7 ± 4.6 | 316.4 ± 4.7 | |
| RUN-I | 8 | 221.0 ± 1.8 | 243.5 ± 3.7 | 272.0 ± 5.5 | 293.1 ± 6.0 | 306.6 ± 7.0 | 317.9 ± 7.4 | 326.9 ± 7.8 | |
| 7 | SED | 20 | 219.2 ± 2.4 | 255.9 ± 3.3 | 283.2 ± 4.2 | 304.7 ± 4.6 | 323.4 ± 7.0 | 340.5 ± 6.6 | 345.9 ± 6.9 |
| RUN | 20 | 219.2 ± 2.4 | 235.5 ± 4.6 | 261.3 ± 5.6 | 283.1 ± 6.9 | 298.1 ± 7.9 | 309.6 ± 9.0 | 312.0 ± 10.1 |
For each experiment, there was a significant effect of time on body weight (P < 0.001). For all but Experiment 2, there was a significant time by group interaction (P < 0.001), and a significant effect of group (P < 0.001) on body weight. Body weight differences were observed after as little as 1 week of running (P < 0.001 for Experiments 1, 3–7), and were maintained across the 6-week period. For Experiment 4, in which animals were killed after 1, 3 or 6 weeks under SED or RUN conditions, the data reflect all available animals. Hence, n = 24 for weeks 0 and 1; n = 16 for weeks 2 and 3; n = 8 for weeks 4, 5 and 6. In Experiment 6, there was a significant difference in body weight between SED and Intermittent RUN (RUN-I) conditions (P < 0.05) but no significant difference between RUN and Intermittent RUN groups.
Adrenal and thymus weights
Both absolute values and gland weight relative to body weight values are provided in Table 2. Adrenal gland weight was sometimes higher in RUN compared to SED groups, although this was not always the case. In Experiments 1A and 6, there was no significant difference between SED and RUN or Intermittent-RUN groups for either absolute values, or corrected for body weight. By contrast, in Experiments 3 and 5, although there were no significant differences in absolute adrenal weights between SED and RUN groups, the adrenal to body weight ratio was significantly higher in RUN compared to SED rats (Table 2). In Experiment 4, in which animals ran for 1, 3 or 6 weeks, univariate anova, with time and exercise status as factors, revealed a significant effect of time on absolute adrenal weights (F2,41 = 12.37; P < 0.001) and adrenal: body weight ratios (F2,41 = 4.50; P = 0.017). There was a significant effect of exercise on absolute adrenal weights (F1,41 = 4.97; P = 0.031) but not on relative adrenal weights (F1,41 = 0.11; P = 0.742). There was no time by exercise interaction for either measure.
Table 2.
Adrenal and Thymus Gland Weights.
| Experiment | Group | Adrenals (mg) | Adrenal: body weight ratio (mg/kg) | Thymus (mg) | Thymus : body weight ratio (mg/kg) | n |
|---|---|---|---|---|---|---|
| 1A | SED | 47.7 ± 0.8 | 137.5 ± 2.3 | 418.0 ± 20.2 | 1204.6 ± 58.2 | 9 |
| RUN | 45.2 ± 2.0 | 130.2 ± 5.7 | 321.2 ± 16.2** | 925.6 ± 46.8** | 9 | |
| 3 | SED | 52.1 ± 1.0 | 128.1 ± 2.5 | 532.4 ± 19.4 | 1310.8 ± 50.5 | 26 |
| RUN | 52.3 ± 0.9 | 145.6 ± 2.9*** | 404.3 ± 21.4*** | 1116.7 ± 51.2** | 24 | |
| 4 | SED 1 week | 38.6 ± 1.7 | 145.0 ± 7.1 | 428.3 ± 12.1 | 1607.2 ± 43.1 | 8 |
| SED 3 week | 44.0 ± 1.7 | 142.9 ± 3.9 | 435.1 ± 30.6 | 1700.1 ± 77.4 | 8 | |
| SED 6 week | 43.6 ± 1.4 | 122.0 ± 1.9 | 372.8 ± 23.4 | 1404.6 ± 74.5 | 8 | |
| RUN 1 week | 39.2 ± 2.4 | 158.9 ± 8.7 | 419.3 ± 22.9 | 1408 ± 71.8 | 8 | |
| RUN 3 week | 44.7 ± 1.9 | 155.6 ± 6.7 | 404.0 ± 18.0 | 1046.8 ± 68.0 | 8 | |
| RUN 6 week | 40.8 ± 1.8 | 127.8 ± 3.9 | 325.0 ± 25.6 | 988.9 ± 37.5 | 8 (7) | |
| 5 | SED | 47.9 ± 1.1 | 138.0 ± 5.2 | 396.6 ± 18.0 | 1149.7 ± 45.0 | 18 (15) |
| RUN | 49.5 ± 2.0 | 160.0 ± 4.6** | 303.8 ± 10.2*** | 972.2 ± 28.4*** | 24 (22) | |
| 6 | SED | 52.0 ± 1.4 | 150.2 ± 4.8 | 383.7 ± 13.6 | 1103.7 ± 38.6 | 16 (15) |
| RUN | 47.6 ± 1.6 | 150.1 ± 3.6 | 301.6 ± 9.9*** | 954.1 ± 29.1** | 16 | |
| RUN-I | 50.3 ± 1.8 | 154.7 ± 6.8 | 341.9 ± 16.3* | 1051.3 ± 60.2 | 8 |
P < 0.001;
P < 0.01;
P < 0.05 with respect to appropriate SED group. There were no significant differences between the RUN and Intermittent RUN (RUN-I) groups. The number in each group (n) is given in the final column. In some cases, an adrenal gland was damaged and the data were excluded. A second value for n in parentheses reflects the number of cases for the adrenal glands, if different from the rest of the experiment. RUN-I, intermittent RUN group.
In several experiments (1A, 3, 5 and 6), there was evidence of thymus involution in RUN rats, with absolute and relative thymus weights significantly lower for this group (Table 2). For Experiment 6, post-hoc analysis revealed that the absolute thymus weight was decreased in both RUN (P < 0.001) and Intermittent RUN (P = 0.048) versus SED groups but, when corrected for body weight, relative thymus weights were only different between SED and RUN groups (P = 0.006) but not between SED and Intermittent RUN groups (P = 0.405). In Experiment 4, univariate anova, with time and exercise status as factors, revealed a significant effect of time on both absolute thymus weight (F2,41 = 47.45; P < 0.001) and thymus weight : body weight ratios (F2,41 = 6.649; P = 0.003). However, there was no effect of exercise status or a time by exercise interaction for either measure in this experiment.
Distance run
Consistent between experiments, repeated measures anova revealed that there was a significant effect of time on the mean distance run per rat per night over the 6-week period in each of Experiments 1–7. The distance run peaked in weeks 4–5 (Table 3).
Table 3.
Mean ± SEM Distance run (km) per Rat per 24-h Period of Wheel Access for Experiments 1–7.
| Experiment | Group | n | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 |
|---|---|---|---|---|---|---|---|---|
| 1A | RUN | 9 | 1.16 ± 0.13 | 3.24 ± 0.55 | 5.00 ± 0.74 | 6.08 ± 0.88 | 5.68 ± 0.96 | 4.79 ± 0.82 |
| 1B | RUN | 24 | 0.98 ± 0.10 | 2.72 ± 0.35 | 4.51 ± 0.44 | 5.72 ± 0.57 | 5.51 ± 0.58 | 4.81 ± 0.51 |
| 2 | RUN | 6 | 0.96 ± 0.06 | 2.65 ± 0.48 | 4.22 ± 1.02 | 5.07 ± 1.42 | 4.83 ± 1.49 | 4.96 ± 1.70 |
| 3 | RUN | 24 | 1.16 ± 0.11 | 2.55 ± 0.30 | 3.63 ± 0.42 | 4.60 ± 0.60 | 5.05 ± 0.59 | 4.56 ± 0.61 |
| 4 | RUN | 8–24 | 1.13 ± 0.08 | 3.26 ± 0.23 | 4.92 ± 0.49 | 6.31 ± 1.07 | 6.75 ± 0.97 | 4.68 ± 0.87 |
| 5 | RUN | 24 | 1.22 ± 0.10 | 3.03 ± 0.34 | 4.40 ± 0.43 | 5.98 ± 0.53 | 6.22 ± 0.59 | 5.10 ± 0.54 |
| 6 | RUN | 16 | 1.18 ± 0.11 | 3.02 ± 0.39 | 5.43 ± 0.78 | 6.43 ± 0.87 | 7.02 ± 1.01 | 5.84 ± 0.90 |
| RUN-I | 8 | 1.03 ± 0.07 | 1.93 ± 0.30 | 3.18 ± 0.63 | 3.94 ± 0.97 | 3.31 ± 0.74 | 2.25 ± 0.61 | |
| 7 | RUN | 20 | 0.82 ± 0.09 | 2.46 ± 0.34 | 3.95 ± 0.58 | 4.58 ± 0.76 | 3.94 ± 0.67 | 3.53 ± 0.43 |
A repeated measures anova revealed that there was a significant effect of time on the mean distance run over the 6 week period in each of Experiments 1–7 (P = 0.001 for each experiment). The distance run peaked in weeks 4–5. For Experiment 4, in which animals were killed after 1, 3 or 6 weeks under RUN conditions, the data reflect all available animals. Hence, n = 24 for week 1; n = 16 for weeks 2 and 3; n = 8 for weeks 4, 5 and 6. RUN-I refers to the Intermittent Run group, which had access to the running wheel for only 24 h out of each 72-h period. The values reported for this group reflect the distance run during times of wheel access only.
For Experiment 6, running distances for the RUN and Intermittent RUN groups were initially compared by calculating the average distance run per 24-h period for which wheel access was available, for weeks 1–6 (The time that the wheel was locked for the Intermittent RUN group was not a factor in this comparison). There was a significant within-subjects effect of time (F1,22 = 21.74; P < 0.001) and a time by group (RUN or Intermittent RUN) interaction (F1,22 = 3.982; P = 0.002). The groups ran a similar distance per 24-h wheel availability in the first week of wheel access and the pattern of activity was broadly similar, peaking between 4–5 weeks of wheel access, and tapering off during weeks 5–6. However, on average, rats in the Intermittent RUN group ran a significantly smaller distance over a 24-h period of wheel access than rats in the RUN group (F1,22 = 5.289; P = 0.031). The approximate time spent running by each rat in a 24-h period of wheel access was also calculated, by totaling the number of 10-min periods in which the total number of wheel turns was greater than 10. The approximate time spent running per 24-h wheel access was fairly stable across the 6-week period: RUN group: week 1: 4.9 ± 0.2 h; week 2: 5.9 ± 0.2 h; week 3: 6.6 ± 0.3 h; week 4: 6.7 ± 0.4 h; week 5: 6.8 ± 0.4 h; week 6: 6.0 ± 0.4 h; Intermittent RUN group: week 1: 4.8 ± 0.3 h; week 2: 5.6 ± 0.4 h; week 3: 6.6 ± 0.3 h; week 4: 6.2 ± 0.4 h; week 5: 5.5 ± 0.4 h; week 6: 4.7 ± 0.3 h. There was no difference between the RUN and Intermittent RUN groups in the average time spent running per 24-h period of wheel access (F1, 22 = 1.580; P = 0.222). However, it should be reiterated that comparisons for both distance run and time spent running took into account only the time that the wheel was available for the Intermittent RUN group. Therefore, over the 6-week period, the Intermittent RUN group ran for approximately one-third the amount of time that the RUN group spent running, because the wheel was locked for 2 of 3 days. Similarly, the total distance run by the Intermittent RUN group over the 6-week period was less than 20% that of the RUN group (RUN group: 202.2 ± 26.1 km; RUN-I group: 36.4 ± 7.5 km; P < 0.001).
HPA axis
Experiment 1: Effect of 6 weeks of wheel running on HPA axis responses to a novel environment
Experiment 1A
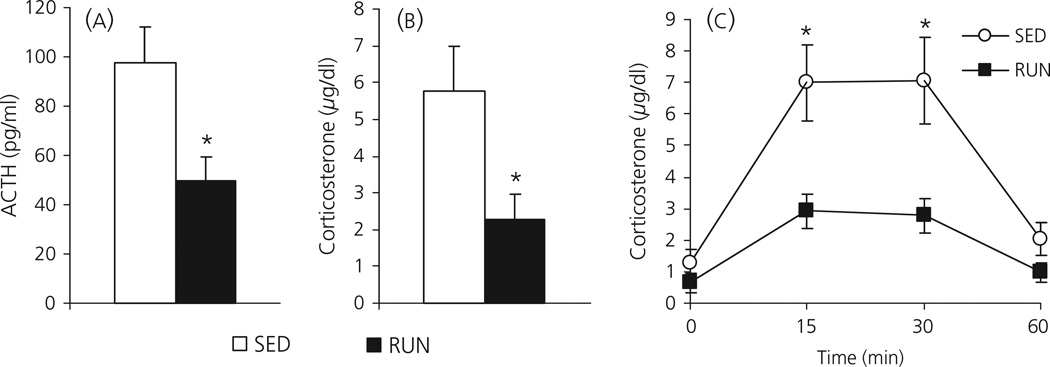
In Experiment 1A, SED rats exposed to a novel environment for 30 min had significantly higher levels of plasma ACTH (97.6 ± 14.8 pg/ml) compared to RUN rats (49.5 ± 9.6 pg/ml; F1, 16 = 7.445; P = 0.015; Fig. 1a). SED rats also had higher levels of corticosterone (5.8 ± 1.2 µg/dl) compared to RUN rats (2.3 ± 0.7 µg/dl; F1,16 = 6.698; P = 0.020) 30 min after exposure to the novel environment (Fig. 1b).
Fig. 1.
(a, b) Experiment 1a: Graphs to show the effect of a 30-min exposure to a novel environment on plasma levels of adrenocorticotrophic hormone (ACTH) (1a) or corticosterone (1b) in rats that had continuous access to a running wheel in their home cage for 6 weeks (RUN) or rats housed under sedentary conditions for 6 weeks, in similar cages but without a running wheel (SED). (c) Experiment 1B: Graph to show the levels of plasma corticosterone in response to different lengths of time in a novel environment, in different groups of SED and RUN rats. Rats either remained in their home cage (time = 0), or were exposed to the novel environment for 15, 30 or 60 min, after which a blood sample was taken from a lateral tail vein. Values represent the group mean ± SEM. *P < 0.05 compared to SED group.
Experiment 1B
Data from Experiment 1B suggest that the differences in the HPA axis response in SED and RUN rats in response to 30-min exposure to a novel environment are not the result of a difference in the time course of the response. The dynamics of corticosterone release after novel environment exposure were similar for SED and RUN rats, peaking at 15–30 min (Fig. 1c). Univariate anova with exercise and time as factors revealed a significant effect of exercise (F1,40 = 22.374; P < 0.001), time (F3,40 = 16.091; P < 0.001) and a time by exercise interaction (F3,40 = 3.247; P = 0.032). Post-hoc analysis revealed a significant difference compared to baseline at both the 15- and 30-min time points but, by 60 min, levels of corticosterone were not significantly different from baseline (P = 0.470). There was no significant difference in baseline levels of corticosterone between SED (1.30 ± 0.41 µg/dl) and RUN (0.64 ± 0.30 µg/dl) rats (P = 0.216). Levels of corticosterone were significantly higher in SED rats compared to RUN rats at the 15-min (P = 0.011) and 30-min (P = 0.016) time points, but were not significantly different from each other by the 60-min time point (P = 0.113).
Experiment 2: Effect of 6 weeks wheel running on HPA axis responses to a saline injection
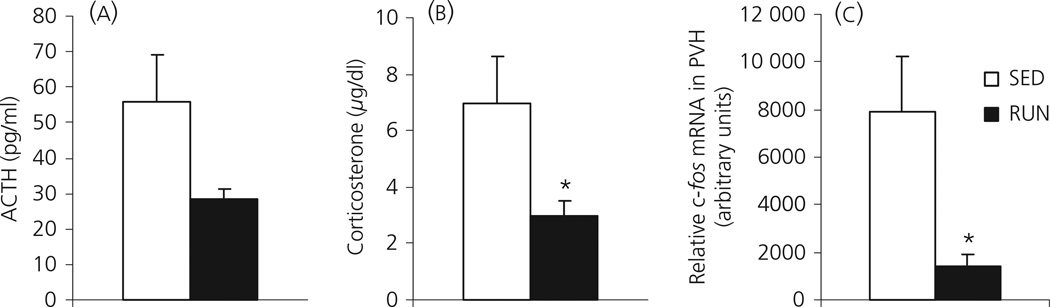
SED rats tended to have higher ACTH levels 30 min after the saline injection (55.7 ± 13.2 pg/ml) compared to RUN rats (28.6 ±2.7 pg/ml), although this did not reach statistical significance (F1, 10 = 4.567; P = 0.058; one-way anova on log-transformed data (because of unequal variance; Fig. 2a). SED rats had significantly higher levels of corticosterone (6.97 ± 1.67 µg/dl) 30 min after the i.p. saline injection compared to RUN rats (2.97 ± 0.52 µg/dl; F1, 10 = 5.237; P = 0.045; Fig. 2b). In addition, levels of c-fos mRNA in the PVH were significantly higher in SED rats (7,876 ± 2364 arbitrary units), 30 min after the saline injection compared to RUN rats (1,378 ± 563 arbitrary units; F1, 10 = 5.955; P = 0.037; Fig. 2c).
Fig. 2.
Graphs to show (a) plasma adrenocorticotrophic hormone (ACTH); (b) plasma corticosterone and (c) relative levels of c-fos mRNA in the paraventricular nucleus of the hypothalamus (PVH), 30 min after an i.p. 0.9% saline injection (Experiment 2), in rats that had continuous access to a running wheel in their home cage for 6 weeks (RUN) or rats housed under sedentary conditions for 6 weeks, in similar cages but without a running wheel (SED). Values represent the group mean ± SEM. *P < 0.05 compared to SED group.
Experiment 3: Effect of 6 weeks of wheel running on the HPA axis responses to 85 dBA noise
Exposure to 85 dBA noise resulted in a significant increase in ACTH (F3,42 = 3.688; P = 0.019; Fig. 3a) and corticosterone (F3,42 = 7.832; P< 0.001; Fig. 3b) over time, with maximum levels observed between 15 and 30 min after the start of noise. There was a significant effect of exercise on both ACTH (F1, 42 = 7.500; P = 0.009) and corticosterone (F1, 42 = 4.949; P = 0.032), with RUN rats having lower plasma levels of both hormones in response to 85 dBA noise exposure compared to SED animals. There were no significant differences between SED and RUN rats for baseline plasma levels of either hormone (F1, 10 = 2.774; P = 0.127 for ACTH; F1, 10 = 0.001; P = 0.976 for corticosterone). The pattern of the ACTH and corticosterone response was similar between SED and RUN groups, with no exercise by time interaction (F3,42 = 0.703; P = 0.556 for ACTH; F3,42 = 1.830; P = 0.156 for corticosterone).
Fig. 3.
Graphs to show the effect of exposure to 85 dBA noise (Experiment 3) on plasma levels of (a) adrenocorticotrophic hormone (ACTH) and (b) corticosterone, in rats that had continuous access to a running wheel in their home cage for 6 weeks (RUN) or rats housed under sedentary conditions for 6 weeks, in similar cages but without a running wheel (SED). Separate groups of rats were exposed to background noise conditions (approximately 60 dBA; 0-min group) or 85 dBA white noise for 15 or 30 min. The 60-min group was exposed to 85 dBA noise for 30 min, followed by 30 min under background noise conditions. The solid black bar represents the noise exposure. Values represent the group mean ± SEM. *P < 0.05 compared to SED group (main effect of exercise)
Experiment 4: Effect of 1, 3 or 6 weeks of wheel running on the HPA axis responses to 85 dBA noise
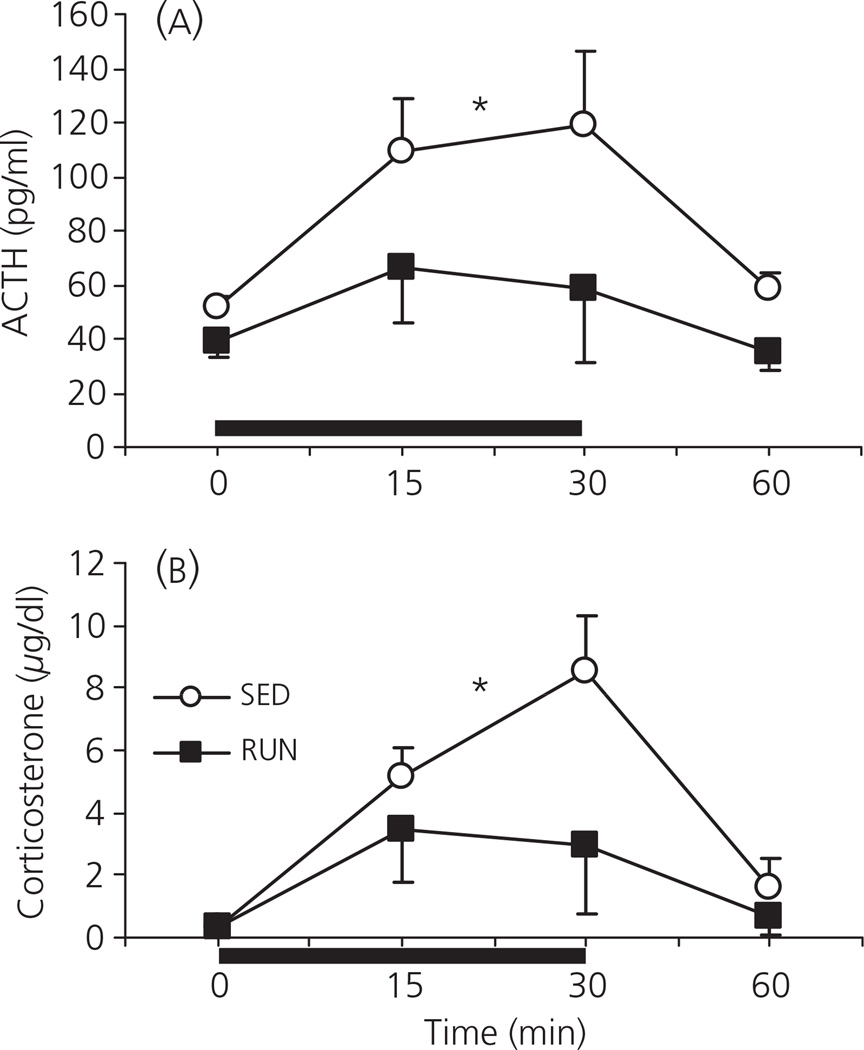
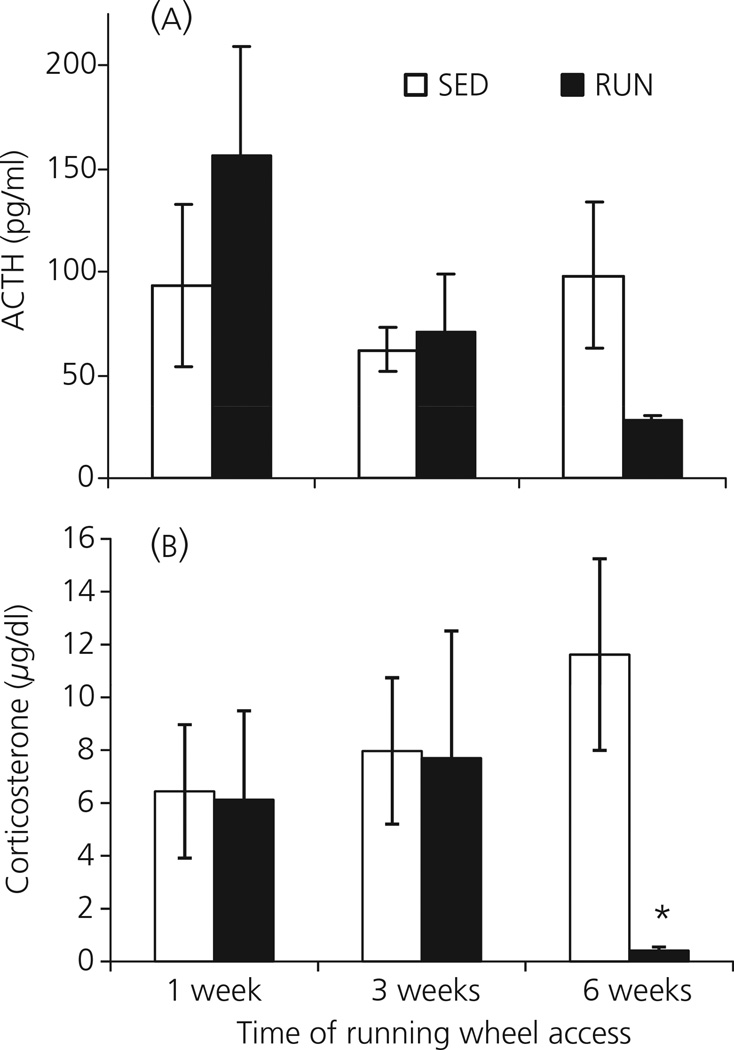
The effect of either 1, 3 or 6 weeks of wheel running on the ACTH response to 30 min of 85 dBA noise is shown in Fig. 4(A). There was no effect of time on the ACTH response to 85 dBA noise in SED rats (F2,21 = 0.045; P = 0.956). However, there was a significant effect of time on the ACTH response to 85 dBA noise in RUN rats (F2,21 = 5.997; P = 0.009), with the response at 6 weeks significantly lower than at either 1 or 3 weeks. Although there was a trend for a lower ACTH response in RUN (27.8 ± 2.9 pg/ml) compared to SED (98.4 ± 35.6 pg/ml) rats after 6 weeks access to the running wheel, the difference was not significant in this experiment (F1, 14 = 4.60; P = 0.059).
Fig. 4.
Graphs to show the effect of exposure to 30 min of 85 dBA noise (Experiment 4) on plasma levels of (a) adrenocorticotrophic hormone (ACTH) and (b) corticosterone, in separate groups of rats that had continuous access to a running wheel in their home cage for either 1, 3 or 6 weeks (RUN) or rats housed under sedentary conditions for the same time periods, in similar cages but without a running wheel (SED). Values represent the group mean ± SEM. *P < 0.001 compared to the 6-week SED group.
There was a significant effect of exercise on the corticosterone response to 85 dBA noise (F1, 42 = 5.838; P = 0.020; Fig. 4b). Oneway anovas for each time point revealed that corticosterone levels at 1 week (SED: 6.44 ± 2.53 µg/dl; RUN: 6.12 ± 3.37 µg/dl) and 3 weeks (SED: 7.97 ± 2.77 µg/dl; RUN: 7.70 ± 4.81 µg/dl) were not significantly different. By contrast, 6 weeks of access to the running wheel resulted in a significantly smaller corticosterone response to 30 min of 85 dBA noise (0.42 ± 0.13 µg/dl) compared to the SED group (11.62 ± 3.63 µg/dl; F1, 14 = 21.02; P < 0.001).
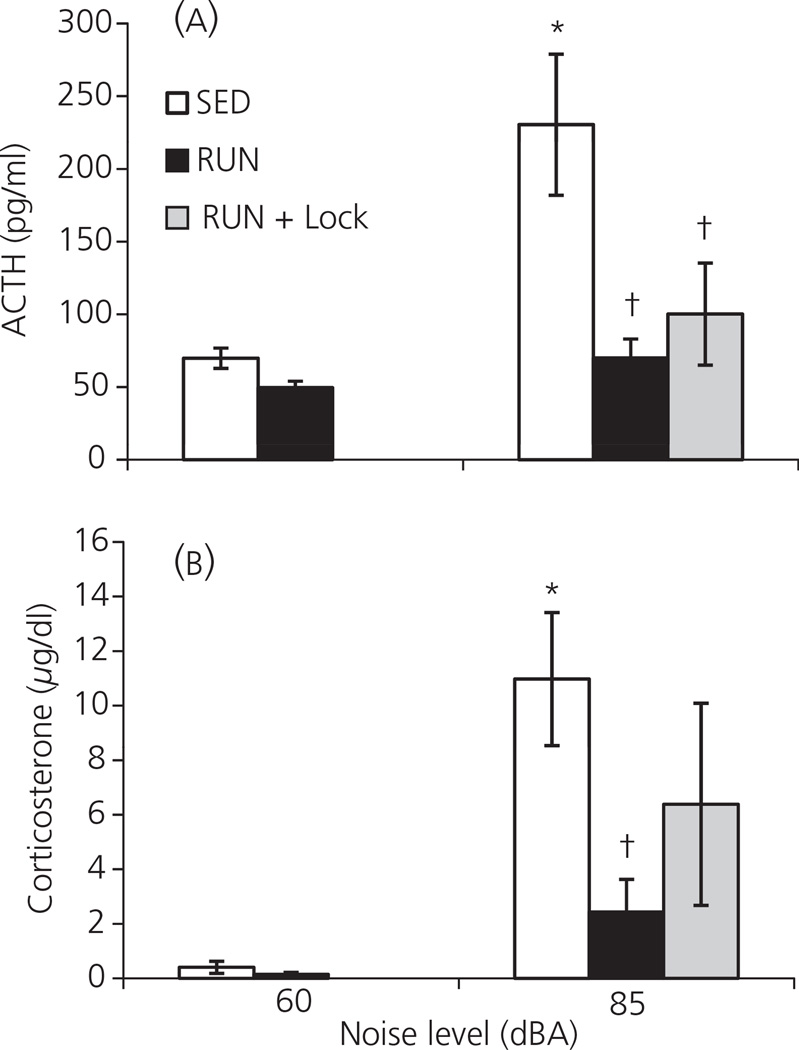
Experiment 5: Effect of 6 weeks of wheel running, and locking the wheel during noise exposure, on the HPA axis response to 85 dBA noise
In this experiment, one-way anova revealed there was a significant effect of group on plasma ACTH levels (F4,37 = 6.656; P < 0.001; Fig. 5a). As seen previously (Experiments 1B, 3), post-hoc analysis revealed there was no baseline difference in ACTH between SED (69.8 ± 7.0 pg/ml) and RUN (49.6 ± 4.5 pg/ml) rats (P = 0.631). Exposure to 30 min of 85 dBA noise resulted in a significant increase in ACTH in SED rats (230.4 ± 48.5 pg/ml; P < 0.001). By contrast, the RUN group with access to the wheel during the time of noise exposure (RUN: 70.0 ± 13.0 pg/ml) and the RUN group with a locked wheel at the time of noise exposure (RUN + Lock: 100.1 ± 35.1) did not respond to the noise with a significant elevation in ACTH (P = 0.629 and 0.234, respectively). In addition, the RUN and RUN + Lock groups had significantly lower plasma levels of ACTH compared to the SED group in response to 85 dBA noise (P < 0.001 and P = 0.003, respectively).
Fig. 5.
Graphs to show the effect of exposure to 30 min of 85 dBA noise (Experiment 5) on plasma levels of (a) adrenocorticotrophic hormone (ACTH) and (b) corticosterone, in rats that had continuous access to a running wheel in their home cage for 6 weeks (RUN) or rats housed under sedentary conditions for the same time period, in similar cages but without a running wheel (SED). Half the RUN rats had access to running wheel during noise exposure (RUN) while the other half had the running wheel locked during noise exposure (RUN + Lock). Values represent the group mean ± SEM *P < 0.01 compared to the SED 60 dBA group. †P < 0.01 compared to the SED 85 dBA group.
The data were broadly similar for corticosterone (Fig. 5b), with one-way anova revealing a significant effect of group on plasma corticosterone levels (F4,37 =5.112; P = 0.002). Post-hoc analysis revealed there was no significant difference in baseline plasma levels of corticosterone between SED (0.4 ± 0.2 µg/dl) and RUN (0.2 ± 0.1 µg/dl) rats (P = 0.933). SED rats responded to 30 min of 85 dBA noise with a significant increase in corticosterone (11.0 ± 2.4 µg/dl; P = 0.001). By contrast, RUN rats that had access to the wheel during noise presentation did not respond with increased corticosterone in response to noise (2.4 ± 1.2 µg/dl; P = 0. 459), and this level was significantly lower compared to the SED 85 dBA group (P = 0.006). Levels of corticosterone in the RUN + Lock + 85 dBA group were intermediate (6.4 ± 3.7 µg/dl): they were not significantly different from either the SED (P = 0.123) or RUN (P = 0.205) groups that were presented with noise. It should be noted that there was a large standard deviation in the RUN + Lock group, driven by two rats that had an unusually large corticosterone response to the noise (24.0 and 22.6 µg/dl). The other six animals in the RUN + Lock + 85 dBA group had a mean corticosterone level of 0.7 ± 0.4 µg/dl.
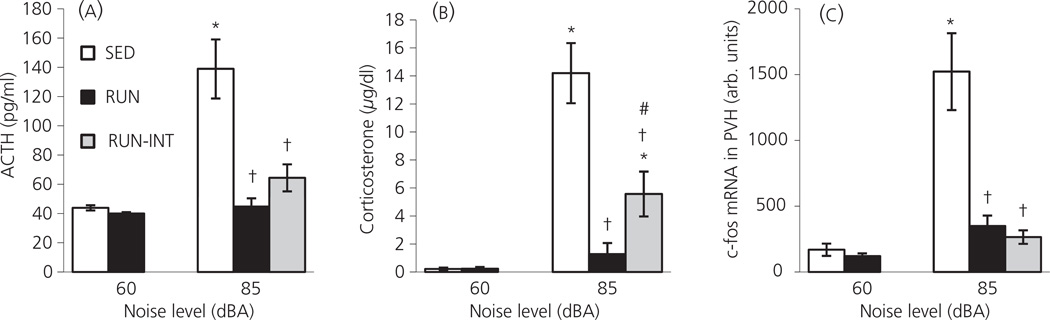
Experiment 6: Effect of 6 weeks of continuous versus intermittent access to the running wheel on the HPA axis responses to 85 dBA noise
In this experiment there was a significant effect of noise (F1, 36 = 31.65; P < 0.001), exercise (F1, 36 = 20.51; P < 0.001) and a noise by exercise interaction (F1,36 = 17.17; P < 0.001) for plasma ACTH levels (Fig. 6a). As was seen previously (Fig. 5b), post-hoc analysis revealed there was no baseline difference in plasma ACTH levels between SED (43.9 ± 1.8 pg/ml) and RUN (40.0 ± 0.9 pg/ml) rats (P = 0.798). SED rats responded to the 30 min of 85 dBA noise presentation with a significant increase in ACTH (138.9 ± 20.2 pg/ml; P < 0.001). By contrast, noise presentation did not result in a significant increase in ACTH above baseline levels for either the RUN (44.7 ± 5.7 pg/ml; P = 0.759) or intermittent RUN (64.4 ± 9.2 pg/ml; P = 0.103) groups. Both the RUN and intermittent RUN groups had significantly lower ACTH levels in response to 85 dBA noise compared to the SED group (P < 0.001 for both), and there was no difference between the RUN and intermittent RUN groups exposed to noise (P = 0.181).
Fig. 6.
Graphs to show the effect of exposure to 30 min of 85 dBA noise (Experiment 6) on (a) plasma adrenocorticotrophic hormone (ACTH); (b) plasma corticosterone and (c) relative levels of c-fos mRNA in the paraventricular nucleus of the hypothalamus (PVH), in rats that had continuous access to a running wheel in their home cage for 6 weeks (RUN), intermittent access (24 h out of 72 h) to a running wheel in their home cage for 6 weeks (RUN-INT) or were housed under sedentary conditions for the same time period, in similar cages but without a running wheel (SED). Values represent the group mean ± SEM *P < 0.01 compared to the SED and RUN 60 dBA groups. †P < 0.001 compared to the SED 85 dBA group. #P < 0.05 compared to the RUN 85 dBA group.
Similar data were obtained for plasma corticosterone (Fig. 6b). There was a significant effect of noise (F1, 36 = 47.54; P < 0.001), exercise (F1,36 =18.63; P < 0.001) and a noise by exercise interaction (F1, 36 = 18.76; P < 0.001) for plasma corticosterone levels. Post-hoc analysis showed that baseline levels of plasma corticosterone were not significantly different between SED (0.21 ± 0.10 µg/dl) and RUN (0.23 ± 0.13 µg/dl) rats (P = 0.992). SED rats responded to the 85 dBA noise presentation with a significant increase in corticosterone (14.20 ± 2.15 µg/dl; P < 0.001). By contrast, RUN rats did not respond with a significant increase in corticosterone (1.28 ± 0.79 µg/dl; P = 0.556). Although the intermittent RUN group did respond to 85 dBA noise with a significant increase in corticosterone (5.57 ± 1.60 µg/dl; P = 0.005 compared to both 60 dBA groups), and had significantly higher plasma corticosterone levels than the RUN + 85 dBA group (P = 0.021), both the RUN and intermittent RUN groups had a significantly lower corticosterone response to 85 dBA noise than the SED group (P < 0.001 for both groups).
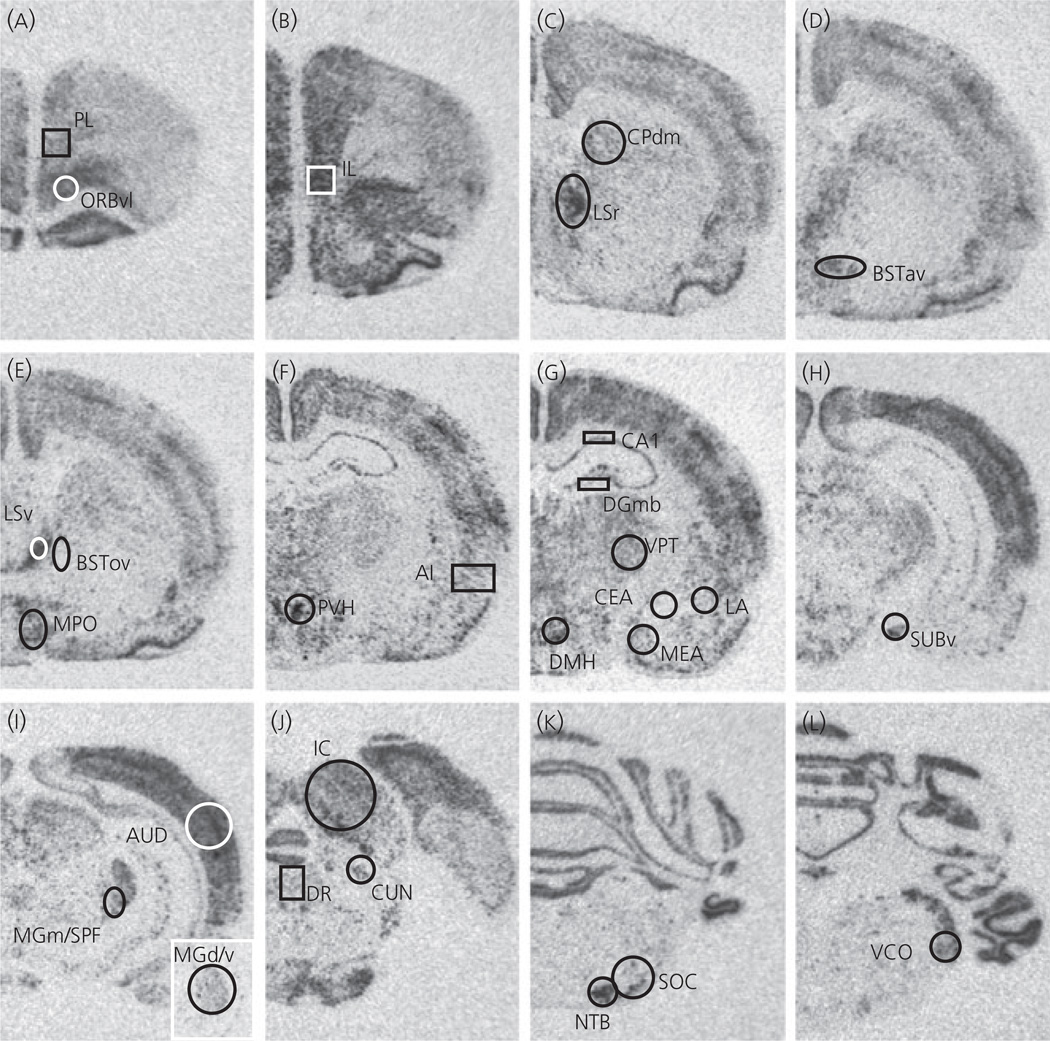
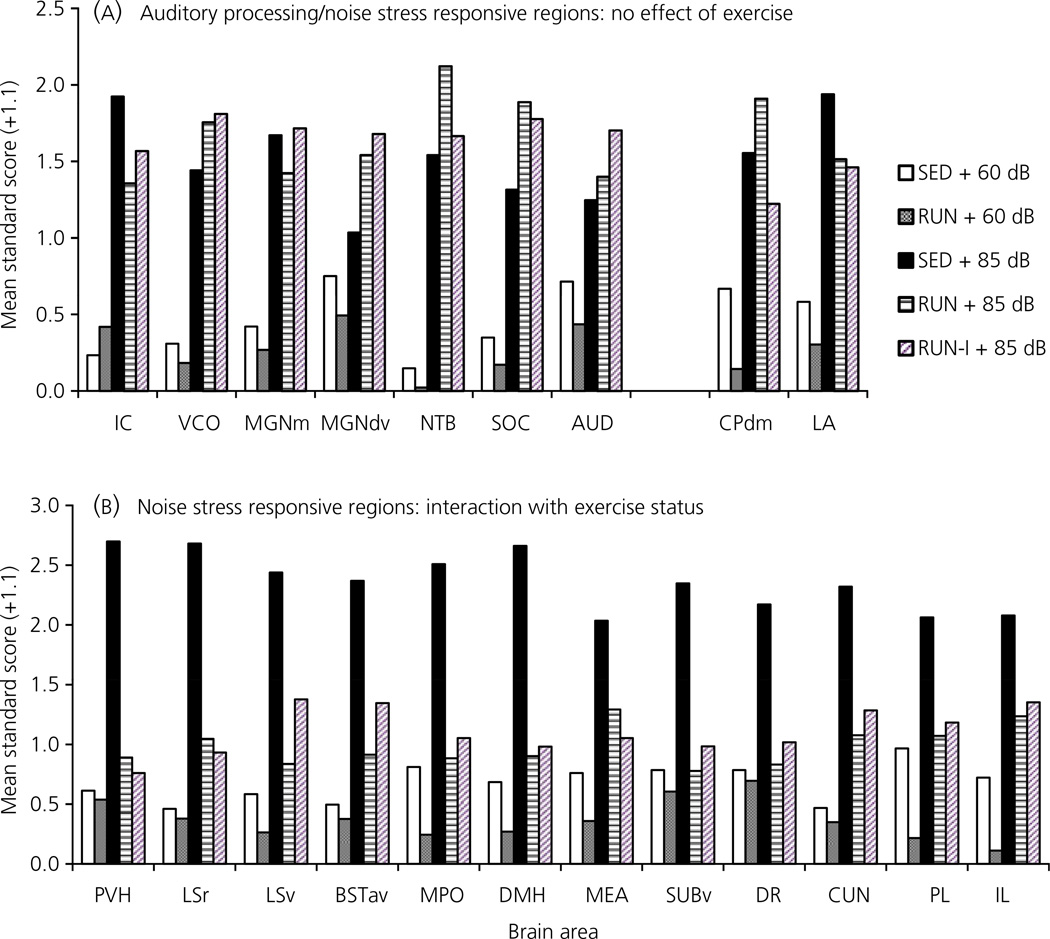
An analysis of c-fos mRNA revealed different patterns of expression depending on the brain region (Table 4). Photomicrographs of c-fos mRNA in a SED rat after 85 dBA noise, together with the templates used for analysis of each brain region, are indicated in Fig. 7. The following auditory regions were analysed: inferior colliculus (IC), ventral cochlear nucleus (VCO), dorsal/ventral parts of the medial geniculate complex (MGd/v), medial part of the medial geniculate complex together with the subparafascicular nucleus of the thalamus, parvocellular part (MGm/SPF), nucleus of the trapezoid body (NTB), superior olivary complex (SOC) and primary auditory cortex (AUD). There were no differences in baseline (60 dBA) levels of c-fos mRNA between SED and RUN rats for any auditory region analysed. With the exception of the MGd/v and AUD all auditory regions expressed significantly higher levels of c-fos mRNA in response to 85 dBA noise, regardless of exercise condition (P < 0.01). For the MGd/v and AUD regions, c-fos mRNA expression did not differ significantly between 60 dBA and 85 dBA conditions for SED rats (P = 0.494; 0.243 respectively), whereas both the RUN (P = 0.019; 0.031 respectively) and Intermittent RUN (P = 0.018; 0.012 respectively) groups had significantly increased levels of c-fos mRNA in response to 85 dBA noise compared to the 60 dBA control group. However, it should be noted that there were no significant differences in c-fos mRNA levels in response to 85 dBA noise between the SED, RUN or Intermittent RUN groups for any auditory region analysed. The pattern of c-fos mRNA expression in auditory regions is shown in Fig. 8(A). A second pattern of c-fos mRNA expression (Fig. 8B) emerged in other brain regions, many of which have previously been shown to be responsive (in terms of c-fos mRNA) to levels of noise associated with HPA axis activation [23, 28]. Broadly, the paraventricular nucleus of the hypothalamus (PVH; also shown in Fig. 6C), anteroventral area of the anterior bed nucleus of the stria terminalis (BSTav), rostral lateral septum (LSr), ventral lateral septum (LSv; previously referred to as the septohypothalamic nucleus [23, 28]), the medial preoptic area (MPO), the dorsomedial nucleus of the hypothalamus (DMH), the medial nucleus of the amygdala (MEA), the ventral subiculum (SUBv), the dorsal raphe nucleus (DR), the cuneiform nucleus (CUN), the prelimbic cortex (PL) and the infralimbic cortex (IL), showed an increased c-fos mRNA response to 85 dBA noise in the SED group. In these brain regions, compared to the SED group, the RUN and Intermittent RUN groups expressed significantly less c-fos mRNA in response to 85 dBA noise, despite having similar c-fos mRNA levels in all auditory regions analysed. The dorsomedial caudoputamen (CPdm) and lateral nucleus of the amygdala (LA) also expressed increased c-fos mRNA in response to 85 dBA noise in the SED group but, in contrast to the other ‘stress responsive’ regions listed above, expressed similar levels of c-fos mRNA in response to 85 dBA noise in the RUN and Intermittent RUN groups (Fig. 8A). The other brain regions analysed did not show an increase in c-fos mRNA expression in response to noise, with one way anovas for group showing no significant differences. The following brain regions did not show significant changes in c-fos mRNA: ventrolateral orbital frontal cortex (ORBvl; P = 0.059), the oval nucleus of the bed nucleus of the stria terminalis (BSTov; P = 0.842), the central nucleus of the amygdala (CEA; P = 0.099), agranular insular cortex (AI; P = 0.147), the CA1 region of the hippocampus (CA1; P = 0.403) and the medial blade of the anterior dentate gyrus (DGmb; P = 0.192). The ventral posterior nucleus of the thalamus (VPT) showed a significant effect of group on the levels of c-fos expression (P = 0.01), with levels in the 60 dBA RUN group being significantly lower than in all the other groups, although there were no significant differences between the SED, RUN or Intermittent RUN groups levels in the c-fos mRNA response to 85 dBA noise. A trend for this pattern was also observed in other brain regions, namely the CEA, the CA1 and DGmb, although the differences were not significant. There were no significant differences between the RUN and Intermittent RUN groups for c-fos mRNA expression for any brain region studied. It should be noted that statistical analysis of c-fos mRNA levels was performed on integrated density values, calculated as described in the Materials and methods section. However, for illustrative purposes, mean standard scores (with a standard deviation of 1) are used in Fig. 8, to show multiple brain regions on the same graph more clearly.
Table 4.
Relative Expression of c-fos mRNA in Different Rat Brain Regions After a 30-min Exposure to Background (60 dBA) or 85 dBA White Noise (Experiment 6).
| Brain region | SED + 60 dBA | SED + 85 dBA | RUN + 60 dBA | RUN + 85 dBA | RUN-I + 85 dBA |
|---|---|---|---|---|---|
| Auditory regions | |||||
| IC* | 4,419 ± 552 | 13,525 ± 2,019† | 5,419 ± 1546 | 10,472 ± 1,667‡ | 11,612 ± 1,281‡ |
| VCO* | 335 ± 82 | 1,763 ± 314† | 178 ± 63 | 2,159 ± 278‡ | 2,229 ± 590‡ |
| NTB* | 199 ± 63 | 1,882 ± 334† | 47 ± 18† | 2,584 ± 219‡§ | 2,033 ± 310‡ |
| SOC* | 105 ± 29 | 493 ± 137† | 33 ± 9† | 773 ± 82‡ | 678 ± 165‡ |
| MGm/SPF* | 60 ± 3 | 143 ± 21† | 50 ± 13 | 127 ± 18‡ | 146 ± 30‡ |
| MGd/v | 2,168 ± 391 | 2,633 ± 437 | 1,748 ± 392 | 3,462 ± 683 | 3,688 ± 713 |
| AUD | 7,240 ± 930 | 8,854 ± 790 | 6,392 ± 947 | 9,318 ± 1,071 | 10,238 ± 1,216 |
| ‘Stress responsive’ regions | |||||
| PVH* | 169 ± 47 | 1,523 ± 292† | 121 ± 21 | 350 ± 79§ | 265 ± 51§ |
| LSr* | 220 ± 68 | 4,145 ± 449† | 76 ± 36 | 1,256 ± 552‡§ | 1,054 ± 346‡§ |
| LSv* | 258 ± 74 | 1,599 ± 208† | 27 ± 7† | 441 ± 208‡§ | 831 ± 235‡§ |
| BSTav* | 126 ± 28 | 824 ± 129† | 81 ± 32 | 281 ± 71‡§ | 442 ± 148‡§ |
| MPO* | 616 ± 116 | 1,959 ± 296† | 168 ± 25† | 674 ± 148‡§ | 808 ± 239‡§ |
| DMH* | 209 ± 31 | 907 ± 133† | 63 ± 10† | 285 ± 80‡§ | 314 ± 45‡§ |
| MEA* | 52 ± 9 | 127 ± 25† | 29 ± 6 | 83 ± 26‡§ | 69 ± 14‡§ |
| SUBv* | 87 ± 28 | 583 ± 182† | 30 ± 7† | 85 ± 31§ | 150 ± 77‡§ |
| DR* | 55 ± 11 | 202 ± 64† | 45 ± 22 | 60 ± 18§ | 79 ± 20§ |
| CUN* | 52 ± 6 | 175 ± 28† | 44 ± 12 | 93 ± 18‡§ | 106 ± 15‡§ |
| PL* | 957 ± 182 | 2,020 ± 395† | 229 ± 73† | 1,058 ± 309‡§ | 1,167 ± 364‡§ |
| IL* | 1,093 ± 141 | 2,523 ± 325† | 449 ± 130† | 1,634 ± 342‡§ | 1,757 ± 415‡ |
| CPdm* | 880 ± 139 | 1,665 ± 256† | 417 ± 152† | 1,980 ± 372‡ | 1,373 ± 278‡ |
| LA* | 144 ± 26 | 371 ± 39† | 97 ± 23 | 300 ± 88‡ | 291 ± 39‡ |
| Other brain regions | |||||
| BSTov | 148 ± 48 | 123 ± 43 | 87 ± 26 | 123 ± 40 | 143 ± 41 |
| CEA | 46 ± 11 | 40 ± 6 | 15 ± 3 | 49 ± 15 | 51 ± 9 |
| ORBvl | 1,418 ± 81 | 1,631 ± 100 | 1,186 ± 71 | 1,442 ± 151 | 1,151 ± 164 |
| AI | 225 ± 29 | 237 ± 42 | 139 ± 31 | 161 ±33 | 144 ± 35 |
| VPT* | 616 ± 158 | 619 ±112 | 132 ± 27† | 566 ± 146‡ | 754 ± 113‡ |
| CA1 | 174 ± 42 | 165 ± 52 | 80 ± 30 | 204 ± 48 | 155 ± 40 |
| DGmb | 245 ± 61 | 247 ± 55 | 110 ± 28 | 186 ± 40 | 260 ± 42 |
Prior to this exposure, SED rats were housed without a running wheel, RUN rats were housed with continual access to a running wheel and Intermittent RUN (RUN-I) rats were housed with intermittent access to a running wheel (24 h out of each 72-h period) for 6 weeks. Data are expressed in arbitrary units ± SEM and n = 8 per group. For abbreviations, see Table 5. For areas analysed, see Fig. 7. Note that, as a result of the difference in size of the template used, and the fact that not all areas were analysed from the same in situ hybridisation study, different brain areas cannot be compared directly.
P < 0.01 after one-way anova for Group. Post-hoc analysis (Fishers least significant difference): P < 0.05 for SED + 85 dBA or RUN + 60 dBA versus SED + 60 dBA group;
P < 0.05 for RUN + 85 dBA or RUN-I + 85 dBA versus RUN + 60 dBA group;
P < 0.05 for RUN + 85 dBA or RUN-I + 85 dBA versus SED + 85 dBA group.
Note that there were no significant differences between the RUN-I + 85 dBA and RUN + 85 dBA groups.
Fig. 7.
Photomicrographs showing c-fos mRNA expression after 30 min exposure to 85 dBA noise (Experiment 6), in a rat housed under sedentary conditions for 6 weeks. Templates used for semi-quantitative analysis are shown for (a) prelimbic cortex (PL) and ventrolateral orbitofrontal cortex (ORBvl); (b) infralimbic cortex (IL); (c) dorsomedial caudate putamen (CPdm) and rostral lateral septum (LSr); (d) anteroventral area of the anterior bed nucleus of the stria terminalis (BSTav); (e) ventral lateral septum (LSv), oval nucleus of the bed nucleus of the stria terminalis (BSTov) and medial preoptic area (MPO); (f) paraventricular nucleus of the hypothalamus (PVH) and agranular insular cortex (AI); (g) CA1 region of the hippocampus (CA1), medial blade of the anterior dentate gyrus (DGmb), ventral posterior thalamus (VPT), central nucleus of the amygdala (CEA), lateral nucleus of the amygdala (LA), medial nucleus of the amygdala (MEA) and dorsomedial nucleus of the hypothalamus (DMH); (h) ventral subiculum (SUBv); (i) auditory cortex (AUD) and medial division of the medial geniculate complex with subparafascicular nucleus of the thalamus, parvocellular part (MGm/SPF). The insert is taken at a more rostral region of the medial geniculate complex to show the dorsal/ventral region analysed (MGd/v); (j) inferior colliculus (IC), dorsal raphe nucleus (DR) and cuneiform nucleus (CUN); (k) nucleus of the trapezoid body (NTB) and superior olivary complex (SOC); (l) ventral cochlear nucleus (VCO).
Fig. 8.
Graphs to show the effect of exposure to 30 min of 85 dBA noise (Experiment 6) on c-fos mRNA expression in (a) auditory brain regions, or regions that responded to noise with increased c-fos mRNA expression, but did not show an interaction with exercise status and (b) brain regions previously associated with stress, that responded to noise with increased c-fos mRNA expression AND showed an interaction with exercise status, in rats that had continuous access to a running wheel in their home cage for 6 weeks (RUN), intermittent access (24 h out of 72 h) to a running wheel in their home cage for 6 weeks (RUN-I) or were housed under sedentary conditions for the same time period, in similar cages but without a running wheel (SED). To show patterns of expression on the same graph, levels of c-fos mRNA are expressed as mean standard score + 1.1. The mean standard score was calculated as: (group mean – ‘population’ mean)/‘population’ SD, with the ‘population’ referring to all animals in the experiment. All standard deviations for mean standard scores = 1 (and are not shown). For the regions analysed and abbreviations, see Fig. 7 and Table 5, respectively.
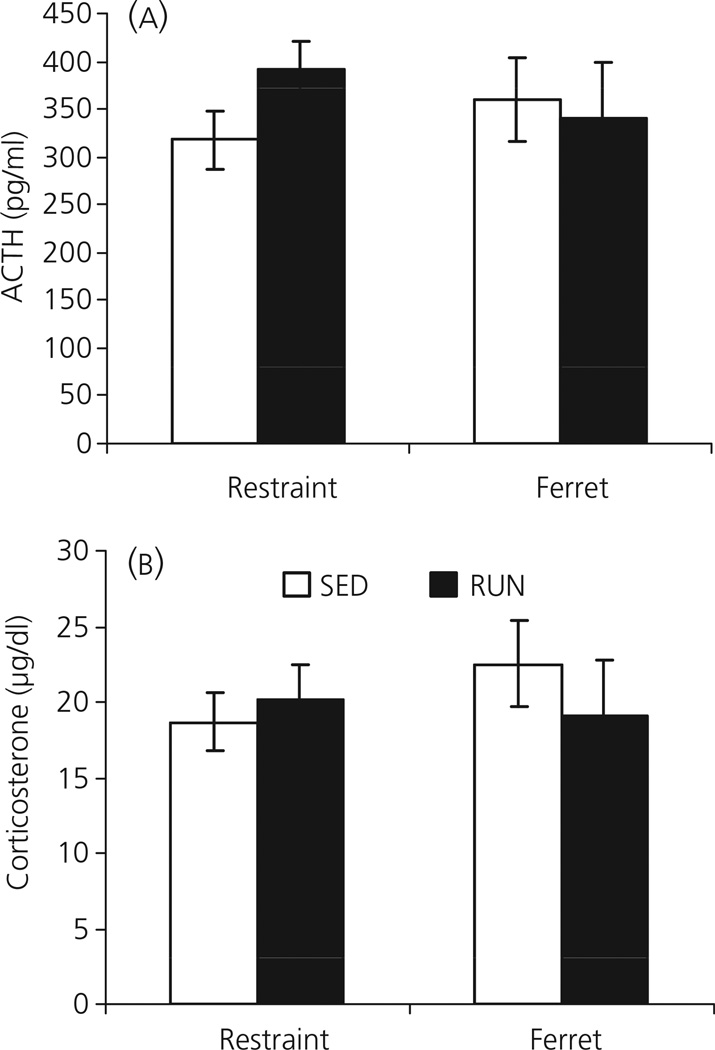
Experiment 7: Effect of 6 weeks of wheel running on the HPA axis response to restraint or ferret (predator) odoor
Consistent with previous experiments, exposure to either restraint or ferret (predator) odour resulted in high plasma levels of both ACTH (Fig. 9a) and corticosterone (Fig. 9b) in SED rats. However, similar levels of plasma ACTH and corticosterone were observed in RUN rats, with no significant differences between SED and RUN rats in the levels of either hormone in response to either ferret odour (ACTH: F1,18 = 0.065; P = 0.802; corticosterone: F1,18 = 0.588; P = 0.453) or restraint stress (ACTH: F1,18 = 2.897; P = 0.106; corticosterone: F1,18 = 0.220; P = 0.645).
Fig. 9.
Graphs to show the effect of exposure to 30 min of restraint or ferret odour (Experiment 7) on (a) plasma adrenocorticotrophic hormone (ACTH) and (b) plasma corticosterone, in rats that had continuous access to a running wheel in their home cage for 6 weeks (RUN) or were housed under sedentary conditions for the same time period, in similar cages but without a running wheel (SED). Values represent the group mean ± SEM. There were no significant differences between SED and RUN groups.
Discussion
The data presented indicate that 6 weeks of voluntary wheel running, in rats, is sufficient to reduce the HPA axis response to low-intensity stressors. That access to the wheel during stressor exposure was not necessary for HPA axis response attenuation, and that 1 or 3 weeks of running wheel access was insufficient to produce this effect, suggests that relatively stable and slow-developing plastic changes are responsible for the HPA axis response attenuation. Daily exercise was not necessary for this effect; intermittent access to the running wheel over 6 weeks resulted in a similar HPA axis response reduction. The data further suggest that regulation of the HPA axis after 6 weeks of voluntary exercise may occur at a central level, without alteration in primary sensory processing. If these data extend to humans, one way in which regular exercise may counteract the effects of stress is to constrain the HPA axis response to low-intensity stressors that occur frequently over the course of a day.
Previous studies have provided mixed data regarding the effect of exercise on HPA axis responses to acute stress. Treadmill training has been shown to increase the ACTH response to footshock [7] or immobilisation [6]. It should be noted that treadmill training is not voluntary, often involves the use of aversive stimuli, and is generally carried out during the light phase when rodents are least active. For these reasons, treadmill training in rodents is considered to be a psychological stressor [29, 30], and sensitisation of the HPA axis to a heterotypic stressor has been well documented [31]. By contrast, it is exceptionally rare for a rodent not to run when provided with an activity wheel [32]. Although this type of exercise also stimulates the HPA axis initially, adaptation occurs relatively rapidly [15]. Because rodents voluntarily choose to run on the wheel, and because wheel running has been shown to be rewarding [33], it is assumed that this type of exercise is not a chronic psychological stressor. Thymic involution and adrenal hypertrophy has been associated with elevated plasma corticosterone levels, which can occur after exercise. Although there was evidence of thymic involution and adrenal hypertrophy in some experiments, differences in organ weights were not observed consistently. There was no correlation between gland weights and the distance run over the course of each experiment (data not shown). Similarly, differences in gland weights were not necessary for observed differences in HPA axis responsiveness.
Previous studies using voluntary wheel running in mice or rats have shown either no change, or an exacerbation of ACTH and/or corticosterone responses, to inescapable tail shock [10], footshock [8, 9], forced swim [11, 12], restraint [12–14], (but see also [15]), or relatively high-intensity noise [16]. In the present study, we observed similar ACTH and corticosterone responses to 30 min of restraint or ferret (predator) odour exposure in rats living under sedentary conditions or with access to a running wheel for 6 weeks, consistent with the majority of the literature. However, additional reports have described a decreased ACTH response to cage switch stress in rats after 4 weeks of treadmill or swim training [17], and decreased corticosterone responses to novel environment exposure after 4 weeks of wheel running in mice and rats [11–13]. It has been suggested that the decreased corticosterone response to novelty occurs because novelty is a psychological stressor, whereas shock, swim and restraint stress all have a physical component. However, voluntary exercise did not decrease the HPA axis response to loud (98 dBA) noise [16] or predator odour exposure (present study), which are also considered to be psychological stressors. Because novel environment exposure results in a submaximal HPA axis response, we hypothesised that the effect of voluntary exercise depends on the intensity, rather than the type, of stressor. The present data support this hypothesis, with a decrease in HPA axis response being observed after exposure to a novel environment, moderate intensity (85 dBA) noise, or an i.p. saline injection, in rats that had access to a running wheel for 6 weeks.
The data presented indicate that access to the wheel during stressor exposure is not necessary for the decrease in HPA axis response to either novelty or 85 dBA noise exposure after 6 weeks of wheel access, suggesting the presence of plastic changes in the HPA axis or circuits that regulate this system. However, it should be noted that two of the eight animals in the locked wheel group exposed to 85 dBA noise had corticosterone levels much higher than observed even in the sedentary group. Although a definitive conclusion cannot be drawn, this suggests that, for a subset of rats, the ability to run during stressor exposure may provide an additional coping mechanism, and having the wheel present but locked may be an additional stressor.
The fact that 6 weeks, but not 1 or 3 weeks of wheel running were required to attenuate the HPA axis response suggests relatively slow-developing plastic changes are responsible for the effect. This time course is similar to the observations of Greenwood et al. [18], who found that 6 weeks, but not 3 weeks, of wheel running prevented the exaggerated fear responses and shuttle box escape deficits resulting from prior inescapable tail shock. By contrast, not all effects of exercise on HPA axis responses appear to require such extended periods of exercise. Our group has found that the facilitated HPA axis habituation to loud noise stress observed after 6 weeks of wheel running [16] is also observed after 1 week of wheel access (S. K. Sasse, C. V. Masini, T.J. Nyhuis, M. Fleshner, H. E. W. Day & S. Campeau, unpublished observations).
These studies also provide evidence that daily exercise is not required for limiting the HPA axis response to a low-intensity stressor, with intermittent access to the running wheel being sufficient to reduce the ACTH and corticosterone response to 85 dBA noise exposure. In these experiments, all rats had access to the running wheel for 24 h prior to testing. It is not known at present whether the data would be different if the wheel had been locked for the Intermittent RUN group prior to testing. The fact that more than 3 weeks of wheel running is necessary for the HPA axis response reduction to 85 dBA noise exposure suggests that wheel running may induce long-term changes that could conceivably persist for some time after wheel access is removed. Although this has not been tested, the present data indicate that, under these specific experimental conditions, daily exercise is not required to reduce the HPA axis response to an 85 dBA noise exposure. It should be noted that, for the intermittent RUN group, not only was the time of exercise reduced because access to the wheel was restricted, but the intensity of the exercise was apparently reduced also, with smaller distances run during each period of wheel access. In addition, there was no correlation between the distance run (total distance over the 6-week period) and ACTH or corticosterone release in any of the experiments (data not shown). Together, these data suggest even small amounts of exercise over a period of time may be beneficial in conferring resistance to stress. This is consistent with data from humans indicating that indicate several weeks of exercise are generally required to have health benefits, although the activity need not be intense [34, 35].
Baseline levels of both ACTH and corticosterone appeared similar between SED and RUN groups. This is consistent with most previous studies [10, 11, 13–15, 36], although a decreased level of ACTH at the nadir of the light cycle has been observed [12]. The time course of ACTH and corticosterone release was similar in SED and RUN rats, which suggests that 6 weeks of wheel running constrains the initial HPA axis response, rather than alters hormone release as a result of altered glucocorticoid negative feedback for example.
Previously published data have suggested that constraint of HPA axis response to novelty occurs mainly at the adrenal level [11]. Importantly, the present data suggest that HPA axis attenuation may occur, at least in part, at a central level. A number of brain regions previously associated with the HPA axis response to noise [23, 28], and other psychological stressors [24, 37–39], exhibited lower levels of c-fos mRNA expression in response to 85 dBA noise in rats with running wheel access for 6 weeks (either continuous or intermittent) compared to sedentary rats. This effect was not observed in all brain regions, however, and, in particular, regions associated with primary auditory processing did not express lower levels of c-fos mRNA expression in response to 85 dBA noise in RUN compared to SED animals. Although c-fos mRNA expression does habituate after repeated homotypic (same) stress presentation [40, 41], it is unlikely that the selective reduction in c-fos mRNA observed in response to noise presentation reflects habituation to the stress of exercise. To our knowledge, there is no evidence for generalised habituation across different stressors. Indeed, after habituation to one stressor, both HPA axis and c-fos mRNA responses to a heterotypic (different) stressor are as great, or even sensitised [31, 42]. Although just one measure of neuronal activity, these data suggest that regulation of the HPA axis response after long-term voluntary activity may occur at a central level, and upstream of primary sensory pathways.
Although voluntary wheel running constrains the acute HPA axis response to low-intensity but not high-intensity stressors, this nonetheless is likely to have a significant physiological impact, particularly if similar data are found in humans, who generally experience low-intensity stressors multiple times on a daily basis. If cortisol levels rise after each stressor experience, this could potentially be detrimental to health [43], and the ability to limit cortisol release, by a long-term programme of exercise, could be one way in which exercise could provide stress resilience and health benefits. It will also be important to determine whether prior exercise can diminish other responses to acute low-intensity stressors, such as increased heart rate, which would be consistent with a general reduction in stress responsiveness after exercise.
The way in which exercise reduces HPA axis responses is far from clear, but possibly involves a central mechanism. Given that the c-fos mRNA response to noise was similar in primary auditory processing regions in SED and RUN rats, but was lower in stress related regions in RUN rats, it is possible that prior exercise results in differential gating of sensory information. The fact that voluntary wheel running is rewarding in rats [44] may be an alternative way in which prior exercise may ‘buffer’ the effect of a stressor exposure. Indeed, intake of a sucrose solution or palatable foods has also been shown to diminish the HPA axis response to stressor exposure in rats [45, 46]. It is becoming clear that metabolic factors can influence stress responsiveness and, in these experiments, RUN rats generally had significantly lower body weight gain than SED rats. An exception was observed in Experiment 2, where the difference in body weight was not significant between SED and RUN groups. Interestingly, in this experiment, the difference in plasma ACTH after a saline injection was not different between SED and RUN rats also, although this may also reflect the relatively low number of animals per group. It should also be noted that, in previous studies, decreased HPA axis responses have been associated with increased adiposity [45], so it is not clear at present how the lower adiposity associated with RUN rats could contribute to the reduced HPA axis responsiveness to low-intensity stressors. It would also be of interest to determine whether enriching the home environment in alternative ways would result in a similar reduction in HPA axis responsiveness. Preliminary data from our laboratory suggest that the effect of exercise does not interact with social housing conditions however, with single- and pair-housed rats demonstrating similar ACTH and corticosterone responses at baseline and in response to a 30-min novel environment exposure. That is, single-housed rats did not show an increased HPA axis response compared to pair-housed rats for either SED or RUN conditions. Similarly, compared to single-housed rats, pair-housing did not reduce HPA axis responses for either SED or RUN conditions (M. Fountain, T. J. Nyhuis, J. A. Babb, S. K. Sasse, S. Campeau & H. E. Day, unpublished observations). Nonetheless, it is possible that enriching the home environment in other ways could result in HPA axis response reduction.
In summary, the data obtained in the present study provide evidence that long-term voluntary wheel running can constrain the HPA axis response to low-intensity stressors, whereas responses to more intense stressor exposures remain intact. This effect is dependent on the duration of prior exercise, but does not require daily exercise, or exercise during stressor exposure. Voluntary wheel running resulted in lower c-fos mRNA expression in response to low-intensity noise stress in a number of stress-related brain areas, but not in auditory nuclei, suggesting the effect may be centrally mediated, upstream of primary sensory inputs. The nature of the plastic changes responsible for this effect remains to be determined.
Table 5.
List of Abbreviations.
| AI | Agranular insular cortex |
| AUD | Primary auditory cortex |
| BSTav | Anteroventral area of the anterior bed nucleus of the stria terminalis |
| BSTov | Oval nucleus of the bed nucleus of the stria terminalis |
| CA1 | CA1 region of the hippocampus |
| CEA | Central nucleus of the amygdala |
| CPdm | Dorsomedial caudoputamen |
| CUN | Cuneiform nucleus |
| DGmb | Medial blade of the dentate gyrus |
| DMH | Dorsomedial nucleus of the hypothalamus |
| DR | Dorsal raphe nucleus |
| HPA | Hypothalamic pituitary adrenal |
| IC | Inferior colliculus |
| IL | Infralimbic cortex |
| LA | Lateral nucleus of the amygdala |
| LSr | Rostral lateral septum |
| LSv | Ventral lateral septum |
| MEA | Medial nucleus of the amygdala |
| MGd/v | Dorsal/ventral parts of the medial geniculate complex |
| MGm/SPF | Medial part of the medial geniculate complex/subparafascicular nucleus of the thalamus, parvocellular part |
| MPO | Medial preoptic area |
| NTB | Nucleus of the trapezoid body |
| ORBvl | Ventrolateral orbital frontal cortex |
| PL | Prelimbic cortex |
| PVH | Paraventricular nucleus of the hypothalamus |
| RUN | Rats housed with constant access to a running wheel in the home cage |
| RUN-I | Rats housed with intermittent (24 out of 72 h) access to a running wheel in the home cage |
| SED | Rats housed under sedentary conditions, without a running wheel |
| SOC | Superior olivary complex |
| SUBv | Ventral subiculum |
| VCO | Ventral cochlear nucleus |
| VPT | Ventral posterior nucleus of the thalamus |
Acknowledgements
These studies were supported by a NARSAD Young Investigator Award (H.E.W.D.), 5R01MH067988 (H.E.W.D.) and 5R03NS054358 (S.C.).
References
- 1.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 3.Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc Sport Sci Rev. 2005;33:120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- 5.Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R484–R489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- 6.White-Welkley JE, Bunnell BN, Mougey EH, Meyerhoff JL, Dishman RK. Treadmill exercise training and estradiol differentially modulate hypothalamic-pituitary-adrenal cortical responses to acute running and immobilization. Physiol Behav. 1995;57:533–540. doi: 10.1016/0031-9384(94)00348-9. [DOI] [PubMed] [Google Scholar]
- 7.White-Welkley JE, Warren GL, Bunnell BN, Mougey EH, Meyerhoff JL, Dishman RK. Treadmill exercise training and estradiol increase plasma ACTH and prolactin after novel footshock. J Appl Physiol. 1996;80:931–939. doi: 10.1152/jappl.1996.80.3.931. [DOI] [PubMed] [Google Scholar]
- 8.Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res Bull. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- 9.Dishman RK, Warren JM, Youngstedt SD, Yoo H, Bunnell BN, Mougey EH, Meyerhoff JL, Jaso-Friedmann L, Evans DL. Activity-wheel running attenuates suppression of natural killer cell activity after footshock. J Appl Physiol. 1995;78:1547–1554. doi: 10.1152/jappl.1995.78.4.1547. [DOI] [PubMed] [Google Scholar]
- 10.Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, Smith TP, Fleshner M. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am J Physiol Regul Integr Comp Physiol. 2003;284:R520–R530. doi: 10.1152/ajpregu.00513.2002. [DOI] [PubMed] [Google Scholar]
- 11.Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- 12.Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- 13.Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. J Neuroendocrinol. 2006;18:915–925. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 14.Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- 16.Sasse SK, Greenwood BN, Masini CV, Nyhuis TJ, Fleshner M, Day HE, Campeau S. Chronic voluntary wheel running facilitates corticosterone response habituation to repeated audiogenic stress exposure in male rats. Stress. 2008;11:425–437. doi: 10.1080/10253890801887453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe T, Morimoto A, Sakata Y, Tan N, Morimoto K, Murakami N. Running training attenuates the ACTH responses in rats to swimming and cage-switch stress. J Appl Physiol. 1992;73:2452–2456. doi: 10.1152/jappl.1992.73.6.2452. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 19.Day HE, Nyhuis TJ, Sasse SK, Masini CV, Campeau S. Intermittent Voluntary Wheel Running is Sufficient to Reduce HPA Axis Responses to Low Intensity Noise Stress. Vol. Program No. 782.11. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. Online, 2008. [Google Scholar]
- 20.Day HE, Wolf EM, Herlihy L, Campeau S. The Effect of Voluntary Exercise on the Acute HPA Axis Response to Mild Stress in Rats. Vol. Program No. 563.20. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Online., 2006. [Google Scholar]
- 21.Selye H. The Stress of Life. New York, NY: McGraw-Hill Book Company Inc; 1956. [Google Scholar]
- 22.Day HE, Nebel S, Sasse S, Campeau S. Inhibition of the central extended amygdala by loud noise and restraint stress. Eur J Neurosci. 2005;21:441–454. doi: 10.1111/j.1460-9568.2005.03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burow A, Day HE, Campeau S. A detailed characterization of loud noise stress: intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Res. 2005;1062:63–73. doi: 10.1016/j.brainres.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masini CV, Sauer S, White J, Day HE, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol Behav. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day HE, Akil H. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology. 1996;63:207–218. doi: 10.1159/000126959. [DOI] [PubMed] [Google Scholar]
- 27.Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 28.Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- 29.Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, Fleshner M, Spencer RL, Moore RL. Short-term treadmill running in the rat: what kind of stressor is it? J Appl Physiol. 2007;103:1979–1985. doi: 10.1152/japplphysiol.00706.2007. [DOI] [PubMed] [Google Scholar]
- 30.Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1321–R1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- 31.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary- adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 32.Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- 33.Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- 34.Kokkinos P. Physical activity and cardiovascular disease prevention: current recommendations. Angiology. 2008;59:26S–29S. doi: 10.1177/0003319708318582. [DOI] [PubMed] [Google Scholar]
- 35.Stathopoulou G, Powers MB, Berry AC, Smits JA, Otto MW. Exercise interventions for mental health: a quantitative and qualitative review. Clin Psychol Sci Prac. 2006;13:179–193. [Google Scholar]
- 36.Fleshner M. Exercise and neuroendocrine regulation of antibody production: protective effect of physical activity on stress-induced suppression of the specific antibody response. Int J Sports Med. 2000;21(Suppl 1):S14–S19. doi: 10.1055/s-2000-1454. [DOI] [PubMed] [Google Scholar]
- 37.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 38.Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- 39.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 40.Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Weinberg MS, Bhatt AP, Girotti M, Masini CV, Day HE, Campeau S, Spencer RL. Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinology. 2009;150:749–761. doi: 10.1210/en.2008-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]