Abstract
Hypothesis
Cell replacement therapy in the inner ear will contribute to the functional recovery of hearing loss.
Background
Cell replacement therapy is a potentially powerful approach to replace degenerated or severely damaged spiral ganglion neurons. This study aimed at stimulating the neurite outgrowth of the implanted neurons and enhancing the potential therapeutic of inner ear cell implants.
Methods
Chronic electrical stimulation (CES) and exogenous neurotrophic growth factor (NGF) were applied to 46 guinea pigs transplanted with embryonic dorsal root ganglion (DRG) neurons four days post deafening. The animals were evaluated with the electrically-evoked auditory brain stem responses (EABRs) at experimental day 7, 11, 17, 24, 31. The animals were euthanized at day 31 and the inner ears were dissected out for immunohistochemistry investigation.
Results
Implanted DRG cells, identified by EGFP fluorescence and a neuronal marker, were found close to Rosenthal's canal in the adult inner ear for up to four weeks following transplantation. Extensive neurite projections clearly, greater than in non-treated animals, were observed to penetrate the bony modiolus and reach the spiral ganglion region in animals supplied with CES and/or NGF. There was, however, no significant difference in the thresholds of EABRs between DRG-transplanted-animals supplied with CES and/or NGF and DRG-transplanted animals without CES or NGF supplement.
Conclusions
The results suggest that CES and/or NGF can stimulate neurite outgrowth from implanted neurons, although based on EABR measurement these interventions did not induce functional connections to the central auditory pathway. Additional time or novel approaches may enhance functional responsiveness of implanted cells in the adult cochlea.
Keywords: chronic electrical stimulation, dorsal root ganglion, hair cell, hearing loss, nerve growth factor, neurite, spiral ganglion, transplantation, translational research
Introduction
The primary cause of hearing loss is damage to the sensory epithelium and neurons of the auditory system, cells which are incapable of regenerating in the adult mammalian (1,2). Extensive efforts have been made to prevent the degeneration of spiral ganglion neurons secondary to hair cell loss, including neurotrophic factors (3-6) and electrical stimulation (7-9). A cell replacement therapy (see (11,12) for reviews) aimed at replacing degenerated cochlear ganglion neurons has been suggested in a number of previous studies using embryonic nervous tissue, adult neural stem cells, undifferentiated embryonic stem cells and cells derived from inner ear (13-24). Using embryonic dorsal root ganglion (DRG) tissue it was shown that not only had the cells the ability to survive in the adult inner ear for at least ten weeks (25,26), especially when nerve growth factor (NGF) was supplemented (27), but the cells appeared also to integrate with the host auditory system. Stem cells showed relatively lower survival and appeared to integrate less well with the host tissue (28,29). Thus, at present the DRG model provides one of the best platforms for assessing to what extent different interventions, i.e. electrical stimulation, may affect implant survival and function of implanted cells.
It has been demonstrated that implanted DRG neurons can grow projections through the bony modiolus towards the spiral ganglion region (27), where they are capable to form connections with the host spiral ganglion neurons. However, apparent contact formation decreased with time, suggesting that an entirely new approach is needed to enhance neurite outgrowth from implanted neurons. It has also been reported that survival of spiral ganglion neurons following deafferentation is enhanced by electrical stimulation (7-9). In the present study, in order to stimulate, enhance and maintain the neurite outgrowth from the implanted neurons, chronic electrical stimulation (CES) and/or NGF was applied to adult guinea pigs with simultaneous implantation of embryonic mouse DRG. Electrically-evoked auditory brain stem responses (EABRs) were used to evaluate the functional integration of the implanted DRG neurons transplanted into the auditory system.
Materials and Methods
Subjects
Forty-six pigmented adult guinea pigs (270-470 g; Elm Hill Breeding Labs, Chelmsford, MA, USA) of both genders were used in this investigation. Dorsal root ganglions (DRGs) were harvested from mouse embryos (C57BL/6-TgN; Jackson Laboratory, Bar Harbor Maine, USA). All animal procedures were approved by the regional ethical committee (University of Michigan Committee on the Use and Care Of Animals). Animals were housed in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, with free access to food and water throughout the experiment. Veterinary care and animal husbandry were provided by the Unit for Laboratory Animal Medicine at the University of Michigan. Considerable efforts were made to minimize the number of animals used, and the suffering of animals involved in the study.
Experimental Design
Following initial testing using acoustic auditory brain stem responses (AABRs) and chemical deafening (see below), the animals were implanted with osmotic minipumps to apply test substances (nerve growth factos, NGF, or control substances; see below), electrodes for electrical stimulation, and, for selected groups, dorsal root ganglion (DRG) tissue. In some groups, chronic electrical stimulation (CES) was applied for about three weeks (Table 1). Functional testing was made by recording EABRs at several time-points throughout the experiment (Table 2)
Table 1. Survival of embryonic mouse dorsal root ganglion neurons in the adult auditory systems.
| Experimental group | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Interventions | HBSS | DRG+HBSS | DRG+CES+HBSS | NGF | DRG+NGF | DRG+CES+NGF |
| Number of animals per group | 7 | 7 | 8 | 8 | 8 | 8 |
| with surviving implants | NA | 4/7 | 4/8 | NA | 5/8 | 5/8 |
Table 1. Embryonic mouse DRG neurons survived four weeks following transplantation into the inner ears of adult guinea pigs. The surviving DRG implants were located in the vicinity of spiral ganglion and organ of Corti. HBSS = Hanks' balanced salt solution, DRG = dorsal root ganglion, CES = chronic electrical stimulation, NGF = nerve growth factor
Table 2. Experimental schedule and procedures.
| Experimental day | Interventions |
|---|---|
| 0 | AABR; deafening procedures |
| 4 | AABR to confirm deafening; DRG implantation in Groups B, C, E, F; Osmotic minipump cannula + electrode implantation in all groups |
| 7 | EABR; initiate CES in groups C + F |
| 11 | EABR |
| 17 | EABR + pump change |
| 24 | EABR |
| 31 | EABR; transcardial perfusion for histology |
AABR = acoustic auditory brain stem responses, EABR = electrically-evoked auditory brain stem responses, CES = chronic electrical stimulation
AABR recordings
Using the Tucker Davis system, click-evoked auditory brain stem responses (AABRs) were collected to assure normal hearing. On day 4, post-deafening, the AABR was repeated to confirm a threshold shift ≥ 60 dB SPL or the animals would be eliminated from the study. AABR to click stimuli were measured as described (9) using Tucker-Davis system. Animals were anesthetized with 10mg/kg xylazine and 40mg/kg ketamine i.m (9). Responses were recorded with subdermal recording needle electrodes placed at the vertex (active) against a reference placed at the midline of the skull approximately 2 cm anterior to bregma. A subcutaneous electrode in the thigh provided the ground. In the soundproof room, computer generated alternating polarity voltage pulses (160 μs duration, 50 pps) were delivered to a transducer positioned at the opening of the ear canal. A mean of 1024 samples of 7.7 ms electrophysiological activity following stimulation were recorded. Stimuli were provided at various intensities to determine threshold, which was defined as the lowest stimulus intensity that evoked at least a 0.2 μV replicable waveform.
Inner ear deafening in guinea pig
In order to damage the sensory epithelium the subjects were chemically deafened (30). Kanamycin (450mg/kg) was administered subcutaneously two hours prior to ethacrynic acid (50mg/kg) injection, via aseptic jugular vein cannulation while anesthetized with 10mg/kg xylazine and 40mg/kg ketamine i.m (9).
Preparation of mouse DRGs
The dissection of DRGs has been described previously (25,27,28). Briefly, donor DRG neurons were dissected from mouse embryos at embryonic day 13-14 (E13-14). The animals were from a transgenic mouse line with an EGFP cDNA under the control of a chicken β-actin promoter and cytomegalovirus enhancer (strain C57BL/6-TgN, ACTbEGFP, 1Osb from The Jackson Laboratory). Under aseptic conditions and deep anesthesia (10mg/kg xylazine and 40mg/kg ketamine i.m.), the abdomen and uterus of the pregnant mice were exposed. The embryos were excised, decapitated and transferred to tissue culture medium (DMEM, Gibco BRL Life Technologies). The spinal cord was exposed using fine tip of the 30ga needles and the DRGs were identified close to the spinal cord. Two lower lumbar DRGs were identified and dissected out on each side of the spinal cord using the fine tips of the forceps. The DRGs were then transferred to the culture medium, and kept at 4°C until transplantation.
Transplantation of DRGs into the guinea pig inner ear
In order to measure EABRs and stimulate the inner ears with CES, we surgically insert the electrodes in the inner ear of animals and secure them with screws. Guinea pigs, anesthetized as above, were given local anesthetics (1% lidocaine) and placed on a water-circulating heating pad for surgery. Using aseptic procedures, a midline skin incision was made on the dorsal surface of the head, continuing behind the left ear (postauricular) and ending at the base of the pinna. After removing the periosteum, three holes were drilled through the skull using a 1.5 mm-diameter cutting burr. Three screws were attached, using bregma as reference: 1 cm posterior at midline (active), 2 cm anterior (reference) at midline, and 1 cm lateral towards implanted ear (ground). A restraint bolt for securing the stimulator was secured by 3 small anchor screws around bregma (see diagram Mitchell et al., 1997). All screws and electrode were secured with methyl methacrylate. The bulla was opened to provide access to the round window and basal cochlear turn. A small hole was drilled at the basal cochlear turn close to the round window using a diamond burr, and two lumbar DRGs were implanted into scala tympani (Fig 1A). The cochlectomy was then sealed with a small piece of fascia. The round window was penetrated with a 30-gauge needle to ease insertion of a custom-made catheter and electrode combination device. The device was inserted so that the ball electrode ended approximately 3mm inside the cochlea, and the cannula at about 2mm. The reference electrode was placed outside the cochlea, contacting the wall of the bulla. The defect was sealed using carboxylate cement. The catheter was connected to a machined flow moderator (Prieskorn & Miller 2000) and mini-osmotic pump (Alzet Model 2002, Alza Corp., Palo Alto, CA; pump capacity 0.5 μl/h, duration 2 weeks) containing either 200 μg/ml NGF or the vehicle used to dilute the NGF, HBSS (Invitrogen) with 0.1% guinea pig serum albumin (GPSA). The pump was inserted subcutaneously on the back of the animal between the scapulae. Thirteen days following implantation, the pump was replaced under aseptic surgical conditions to assure continuous infusion.
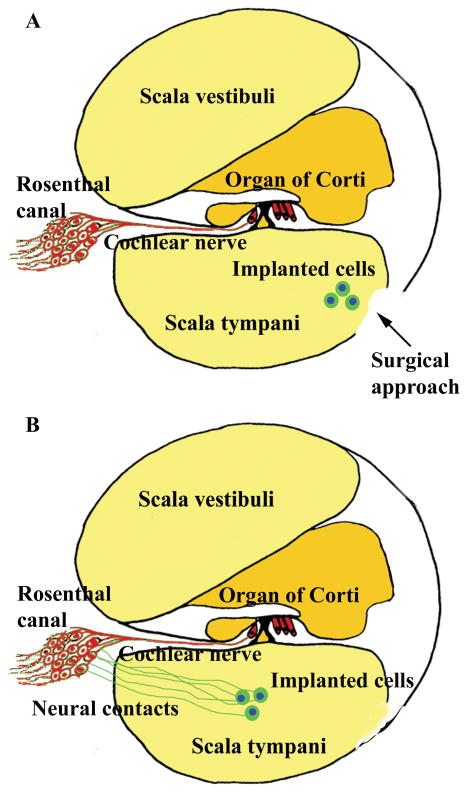
Figure 1.
Schematic figure shows the basal cochlear turn and the surgical approach for transplantation (A). The implanted cells were transplanted into scala tympani. Schematic figure shows the implanted cells survived in the inner ear and generate neurite projections towards spiral ganglion area in Rosenthal canal (B).
Immunosuppressant and antibiotics administration
In order to reduce the risk of postoperative immunological rejection and infection, the animals received daily injections of cyclosporin (0.56 mg/100 g body weight) and doxycycline (0.24 mg/100 g body weight) intraperitoneally beginning the day of surgery until the day of sacrifice.
EABR recordings
Prior to measuring the EABR, the impedance of the electrode was measured using an impedance monitor (sinusoid waveform at 1000 Hz). The animal, anesthetized as above, was placed in a sound proof booth. Stimulus current ranged from approximately 10 to 1000 μA and the neurologic response was collected from epidural recording screws connected at the following sites: active recording site - vertex, reference site - midline recording screw and ground - left screw. Using Hall's method (31) the EABR responses were summed to alternate polarity current pulses, where each pair provided charge balancing. Two thousand forty-eight responses to 50 μs computer-generated monophasic current pulses, presented at 50 PPS with an alternating polarity on each presentation were collected for analysis. Intensity of stimuli varied from P3 threshold to P1 saturation, where saturation was defined as the intensity that evokes less than a 5% increase in response amplitude from a lower stimulus intensity. The P3 thresholds (amplitude ≥ 0.2 μV) and N2-P3 amplitude input/output (I/O) function were determined.
Chronic electrical stimulation
Beginning on day 7 and continuing for 24 days (day 7-31), subjects from Groups C and F received continuous pulsatile, biphasic, charge-balanced electrical stimulation from a battery-powered, wearable custom-built stimulator (Mr. Chris Ellinger, Univ. of Michigan, Mi., USA). The stimulator plugged into the electrode connector and was secured by the restraint bolt. Stimuli were provided at a 40% duty cycle: 400μsec on, 600μsec off.
Histology
Following an overdose of pentobarbital (i.p.), the animals were transcardially perfused with 0.9% saline at 37°C followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C. The cochleas were excised, trimmed and kept in the fixative before being transferred to 0.1 M phosphate buffer, in which they were stored until further processing.
EGFP detection
The cochleas were cryo-protected with 30% sucrose overnight and then cryo-sectioned at a thickness of 12 μm (embedded in tissue freezing medium). Sections were collected every 24 μm and observed with a fluorescence microscope (Zeiss) using an appropriate filter set. Images were acquired using a Spot digital camera.
Immunohistochemistry
Neurofilament antibodies were used for immunohistochemical detection of implanted mouse neuronal tissue in sections with surviving DRG implants from the host animals. Following preincubation in goat serum (Santa Cruz Biotechnology, Inc.), the sections were incubated with neurofilament antibody (NF-L, 1:100, Santa Cruz Biotechnology, Inc.) overnight at 4°C. After rinsing, the secondary antibody goat anti-mouse IgG1-Texas red (1:200, Santa Cruz Biotechnology, Inc.) was applied to the sections at room temperature for two hours. The sections were observed as described above.
Data analysis
In addition to absolute value of the EABR thresholds, input/output (I/O) function was analyzed. The student t-test was used to compare the amplitude at the 10-1000 μA stimulation level while an ANOVA was used for the testing of EABR thresholds in the different groups.
Results
Identification and location of DRG implants
Surviving mouse DRG implants were found in scala tympani of adult guinea pig cochleas at four weeks following the implantation (Table 1 and Figs 2A, 3A, refer Fig 1A for anatomy). In addition to the GFP fluorescence, DRG neurons were labeled by antibodies against neurofilament (Figs 2B, 3B, 3E, 3H). Most DRG neurons were located in clusters and single neurons were rarely seen. The DRG implant survived in 18 out of 31 (58%) animals at four weeks following implantation (cf. Table 1), with no obvious difference in the DRG survival between the different experimental groups. All the surviving DRG implants were located in the scala tympani and close to the vicinity of Rosenthal's canal and the organ of Corti.
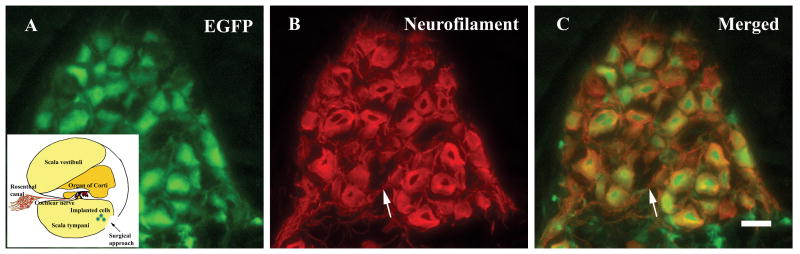
Figure 2.
Mouse DRG neurons survived four weeks following transplantation into the inner ear of adult guinea pigs supplied with chronic electrical stimulation and exogenous NGF. The insert in A shows the basal cochlear turn and the transplantation approach (arrow in insert). The EGFP fluorescence of implanted DRG neurons was readily identified (A). Surviving DRG neurons were double labeled with antibodies against neurofilament (red) (B). The merged image is shown in (C). Neurites were also observed among the surviving DRG neurons (arrow in B and C). Scale bar in C: 20 μm.
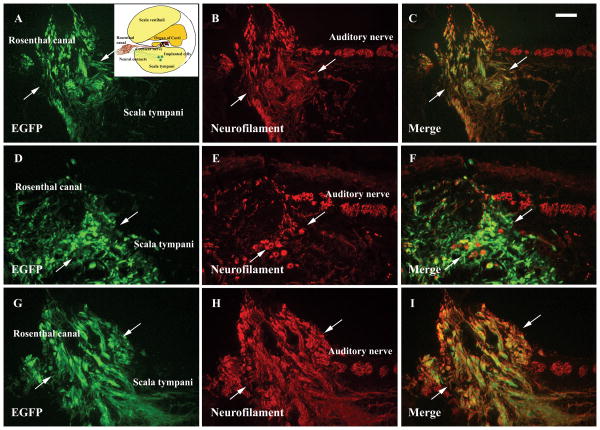
Figure 3.
Extensive neurite projections (arrows in A-I) from the implanted DRG neurons (green in A, D and G) were observed penetrating the bony modiolus and reaching Rosenthal's canal in the inner ear supplied with chronic electrical stimulation (A-C), NGF (D-F), chronic electrical stimulation together with NGF supplement (G-I). The surviving DRG neurons also expressed neuronal specific protein neurofilament which showed in red in B, E and H. The double labeling cells showed in yellow in C, F and I. Insert in A shows the schematic figure of the basal cochlear turn and the transplantation site (arrow). Scale bar in C: 100 μm.
Neurite outgrowth from implanted DRG neurons
In DRG implanted animals, exogenously applied NGF (e.g. animals in Group E and Group F) or CES (Group C) resulted in extensive neurite outgrowth from the transplanted DRG neurons (Fig. 3, refer Fig 1B for anatomy). The processes, positively labeled with neurofilament antibodies (Figs. 3B, E, H), were found to reach the osseous modiolus and penetrate through the thin bone to reach inside Rosenthal's canal (Fig. 3). There is no obvious difference in the neurite projections among the animals receiving CES (Group C) or NGF (Group E) only and the animals receiving a combination of NGF and CES (Group F). In animals without the supplement of CES or NGF (e.g., in Group B), DRG neurons were found to locate close to the modiolus but with relatively short neurites and no projections towards Rosenthal's canal.
Assessment of hearing function in animals implanted with mouse DRG
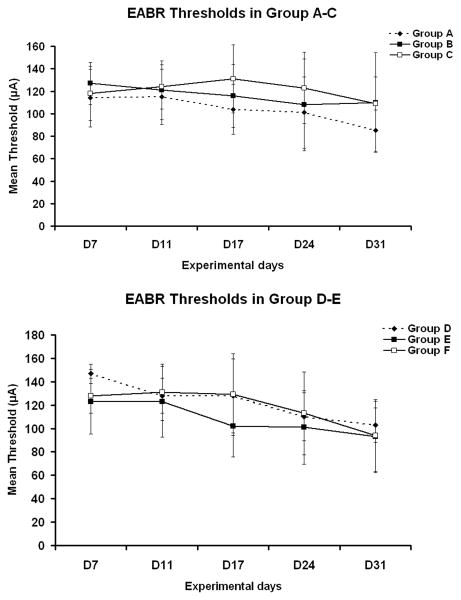
EABR thresholds were obtained at experimental days 7, 11, 17, 24 and 31 (Fig. 4). There was no significant difference in the mean EABR P3 thresholds between any of these groups (P>0.05, ANOVA). Thus, there appeared to be no obvious positive effect on EABR thresholds, neither in DRG-transplanted animals nor in animals receiving NGF and/or CES supplements. However, there were slight but not significant changes in the input/output functions. I/O functions corresponding to the six groups are shown in Figs. 5 and 6.
Figure 4.
(A) Mean P3 EABR thresholds in Experimental Groups A (HBSS group), B (DRG + HBSS), and C (DRG + HBSS + CES). There is no significant difference in the EABR threshold among these groups. (B) Mean P3 thresholds in Groups D (NGF), E (DRG + NGF), and F (DRG + NGF + CES). There is no significant difference in the EABR threshold among these groups.
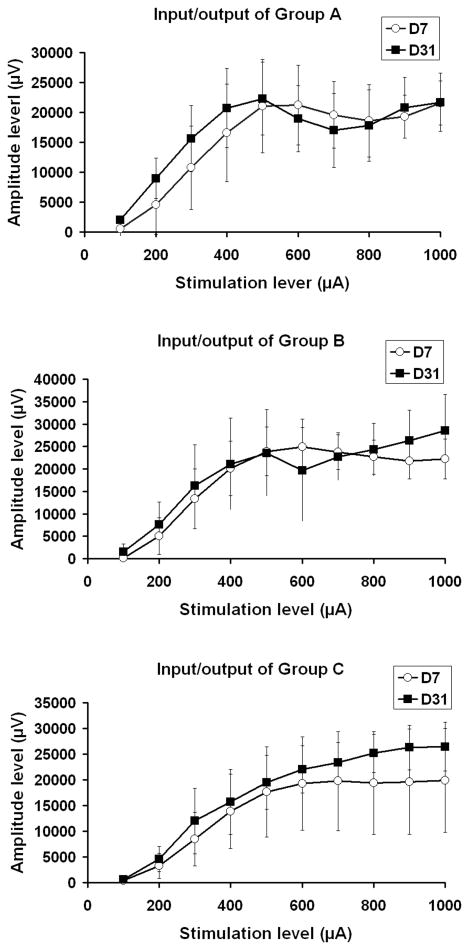
Figure 5.
Input/output functions from Groups A (HBSS), B (DRG + HBSS), and C (DRG + HBSS + CES). There was a slight difference in the amplitude at the high stimulation level between experimental days 7 and 31 in the DRG transplanted animals (e.g. Groups B, C). However, the difference was not significant using student's t-test.
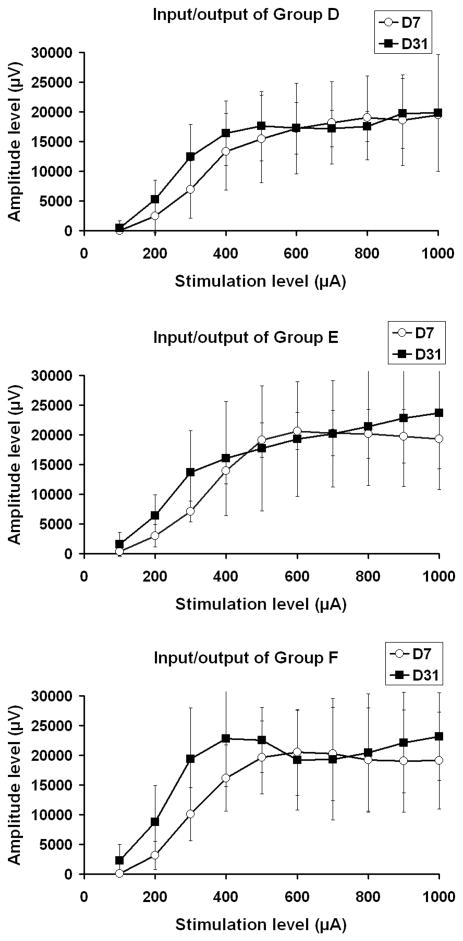
Figure 6.
Input/output functions from Groups D (NGF), E (DRG + NGF), and F (DRG + NGF + CES). There was a slight difference in the amplitude at the high stimulation level between experimental days 7 and 31 in the DRG transplanted animals (e.g. Groups- E, F). However, the difference was not significant using student's t-test.
Discussion
A cell therapy approach may offer the ability to replace auditory sensory epithelium and neurons when degenerated or severely injured (32,33). However, for this approach to be clinically feasible, implanted cells must survive, migrate to appropriate location, grow neurites, and most importantly, establish functional contacts with the appropriate host target cells. The selection of donor cells is certainly a key issue and a number of potential alternatives have been tested experimentally. Using the embryonic DRG model, we have previously shown not only the survival (25,26) and migration (25) of the implanted neurons but also distinct neurite projections between the implanted neurons and host spiral ganglion neurons (27). These observations were confirmed in the present study. We also found that no obvious difference in the DRG survival between the groups of guinea pigs transplanted with DRGs in the present study. In our previous observation (27), we found that NGF improved the survival of DRGs transplanted into the rat inner ear. We speculate that the different effect of NGF on the implant survival may be related to the difference in the species of the host animals. In the present study, neuronal tissue from mouse DRGs survived in the inner ears of adult guinea pigs for at least four-week duration. In animals supplied with chronic electrical stimulation (CES) and/or exogenous NGF, survival was accompanied by extensive neurite outgrowth where processes were found to penetrate the bony modiolus and reach the spiral ganglion region. In a previous study, neurite projections were shown in animals supplemented with exogenous NGF at three weeks following transplantation whereas there was a significant reduction at six weeks (27). In order to promote and sustain neurite outgrowth of the DRG neurons, a CES and/or NGF supplement was applied to the DRG-transplanted animals in the present study. Indeed, extensive neurite outgrowth was found in the animals supplied with CES and/or NGF. Furthermore, neurite projections were also shown to penetrate through the bony modiolus and reach Rosenthal's canal in the inner ears treated with CES and/or NGF, which suggests that these procedures possess the potential to stimulate and enhance neurite outgrowth from the implanted neurons. Compared to the previous observations of NGF treatment (27), the animals receiving both CES+NGF did not show any further neurite outgrowth, suggesting that the effect of CES and NGF to the implanted neurons is not additive. The effect of electrical stimulation (i.e., CES) is clinically very interesting in that it may suggest that combining a cellular implant with a permanent cochlear prosthesis electrode would provide a beneficial situation for the formation of new neurites and cellular contacts, and thus the basis for enhanced function.
In contrast to the effects on neurite formation, it was not possible to demonstrate any effect on EABR thresholds and I/O functions. The lack of positive findings in the functional EABR measurement is disappointing but may have several explanations. It is possible that the implanted cells failed to form fully functional contacts with the host auditory nervous system, or that the implanted cells were not electrically excitable. However, it is more likely that, even if surviving cells were both functional and structurally well integrated, they were too few to modify the electrical responsiveness of the inner ear. Probably the population of surviving cells must be much larger. It is obvious that further experiments are required to optimize the implantation procedures so that more cells survive and become integrated with the host auditory system.
Acknowledgments
The authors would like to thank H. Valentine for technical assistance and the assistance of the University of Michigan Hearing Research Core Center P30 DC-005188. This study was supported by NIH grant DC0382, the Swedish Research Council, the Foundation Tysta Skolan, the Tobias Foundation, the Petrus and Augusta Hedlund Foundation, the Swedish Foundation for International Cooperation in Research and High Education (STINT), and in part by the European Commission (FP6 Integrated Project EUROHEAR; contract grant number: LSHG-CT-20054-512063). Dr. Z. Hu was supported by fellowships from Karolinska Institutet (Sweden), Swedish Institute (Sweden) and the 2008 Adele and Harold Schaeffer/Innisfree Foundation Grant in Auditory Science from National Organization for Hearing Research (NOHR, USA).
References
- 1.Holley MC. Application of new biological approaches to stimulate sensory repair and protection. Br Med Bull. 2002;63:157–69. doi: 10.1093/bmb/63.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- 3.Ernfors P, Duan ML, ElShamy WM, et al. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–7. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 4.Miller JM, Chi DH, O'Keeffe LJ, et al. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–43. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 5.Shinohara T, Bredberg G, Ulfendahl M, et al. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–60. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staecker H, Kopke R, Malgrange B, et al. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–94. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 7.Miller JM. Physiologic measures of electrically evoked auditory system responsiveness: effects of pathology and electrical stimulation. Am J Otol. 1991;12(Suppl):28–36. discussion 43-7. [PubMed] [Google Scholar]
- 8.Miller AL, Prieskorn DM, Altschuler RA, et al. Mechanism of electrical stimulation-induced neuroprotection: effects of verapamil on protection of primary auditory afferents. Brain Res. 2003;966:218–30. doi: 10.1016/s0006-8993(02)04170-7. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell A, Miller JM, Finger PA, et al. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- 10.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–6. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Ulfendahl M. Cell replacement therapy in the inner ear. Stem Cells Dev. 2006;15:449–59. doi: 10.1089/scd.2006.15.449. [DOI] [PubMed] [Google Scholar]
- 12.Ulfendahl M, Hu Z, Olivius P, et al. A cell therapy approach to substitute neural elements in the inner ear. Physiol Behav. 2007;92:75–9. doi: 10.1016/j.physbeh.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 13.Sekiya T, Kojima K, Matsumoto M, et al. Cell transplantation to the auditory nerve and cochlear duct. Exp Neurol. 2006;198:12–24. doi: 10.1016/j.expneurol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Regala C, Duan M, Zou J, et al. Xenografted fetal dorsal root ganglion, embryonic stem cell and adult neural stem cell survival following implantation into the adult vestibulocochlear nerve. Exp Neurol. 2005;193:326–33. doi: 10.1016/j.expneurol.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Nicholl AJ, Kneebone A, Davies D, et al. Differentiation of an auditory neuronal cell line suitable for cell transplantation. Eur J Neurosci. 2005;22:343–53. doi: 10.1111/j.1460-9568.2005.04213.x. [DOI] [PubMed] [Google Scholar]
- 16.Okano T, Nakagawa T, Endo T, et al. Engraftment of embryonic stem cell-derived neurons into the cochlear modiolus. Neuroreport. 2005;16:1919–22. doi: 10.1097/01.wnr.0000187628.38010.5b. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrand MS, Dahl HH, Hardman J, et al. Survival of partially differentiated mouse embryonic stem cells in the scala media of the guinea pig cochlea. J Assoc Res Otolaryngol. 2005;6:341–54. doi: 10.1007/s10162-005-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Monedero R, Corrales CE, Cuajungco MP, et al. Reinnervation of hair cells by auditory neurons after selective removal of spiral ganglion neurons. J Neurobiol. 2006;66:319–31. doi: 10.1002/neu.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka AJ, Kondo T, Miyamoto RT, et al. In vivo and in vitro characterization of bone marrow-derived stem cells in the cochlea. Laryngoscope. 2006;116:1363–7. doi: 10.1097/01.mlg.0000225986.18790.75. [DOI] [PubMed] [Google Scholar]
- 20.Coleman B, Hardman J, Coco A, et al. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant. 2006;15:369–80. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrales CE, Pan L, Li H, et al. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J Neurobiol. 2006;66:1489–500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker MA, Corliss DA, Gray B, et al. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear Res. 2007;232:29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekiya T, Holley MC, Kojima K, et al. Transplantation of conditionally immortal auditory neuroblasts to the auditory nerve. Eur J Neurosci. 2007;25:2307–18. doi: 10.1111/j.1460-9568.2007.05478.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya K, Fujinami Y, Hoya N, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171:214–26. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z, Ulfendahl M, Olivius NP. Survival of neuronal tissue following xenograft implantation into the adult rat inner ear. Exp Neurol. 2004;185:7–14. doi: 10.1016/j.expneurol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Olivius P, Alexandrov L, Miller J, et al. Allografted fetal dorsal root ganglion neuronal survival in the guinea pig cochlea. Brain Res. 2003;979:1–6. doi: 10.1016/s0006-8993(03)02802-6. [DOI] [PubMed] [Google Scholar]
- 27.Hu Z, Ulfendahl M, Olivius NP. NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiol Dis. 2005;18:184–92. doi: 10.1016/j.nbd.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Hu Z, Ulfendahl M, Olivius NP. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 2004;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Hu Z, Wei D, Johansson CB, et al. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005;302:40–7. doi: 10.1016/j.yexcr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 30.West BA, Brummett RE, Himes DL. Interaction of kanamycin and ethacrynic acid. Severe cochlear damage in guinea pigs. Arch Otolaryngol. 1973;98:32–7. doi: 10.1001/archotol.1973.00780020036009. [DOI] [PubMed] [Google Scholar]
- 31.Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res. 1990;45:123–36. doi: 10.1016/0378-5955(90)90188-u. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Monedero R, Edge AS. Stem cells for the replacement of inner ear neurons and hair cells. Int J Dev Biol. 2007;51:655–61. doi: 10.1387/ijdb.072372rm. [DOI] [PubMed] [Google Scholar]
- 33.Coleman B, de Silva MG, Shepherd RK. Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells. 2007;25:2685–94. doi: 10.1634/stemcells.2007-0393. [DOI] [PubMed] [Google Scholar]