Abstract
LiOOt-Bu is an effective oxidant for converting the penultimate organometallic intermediate generated in a titanium alkoxide-mediated [2+2+2] reaction cascade to an allylic alcohol. Oxidation of the presumed allylic titanium species is highly regioselective, providing direct access to substituted hydroindanes containing a primary allylic alcohol. In addition to demonstrating the feasibility of this oxidation process, we document the ability to convert the primary allylic alcohol products to angularly substituted cis-fused hydroindanes.
Keywords: Metallacycle-mediated cross-coupling, Cascade reaction, Oxidation, Hydroindanes, [2+2+2]
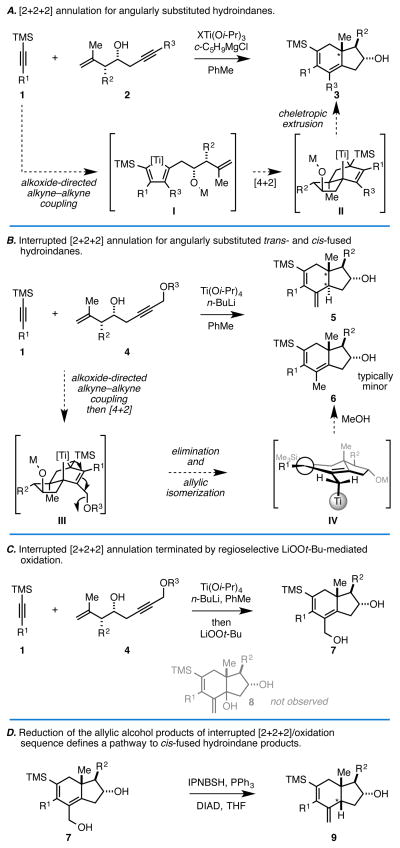
Metallacycle-mediated cross-coupling, processes that allow for the union of unsaturated substrates through the formation of metallacyclopentane intermediates by formal [2+2+1], is growing as a particularly powerful mode of chemical reactivity for stereoselective synthesis.1 While early discoveries were reported over sixty years ago, challenges associated with the control of reactivity and selectivity have lingered, negatively impacting the potential utility of this type of reactivity in complex molecule synthesis. Over the last decade a great variety of metallaycle-mediated coupling reactions for the union of alkynes with aldehdyes/imines have emerged,2 with recent advances demonstrating that coupling between alkenes, alkynes and allenes can now be accomplished with regio- and stereocontrol.1,3 In studies associated with our desire to develop this latter area of coupling technology, we recently discovered a metallacycle-mediated annulation reaction between TMS-alkynes (1) and 1,6-enynes (2) that produces angularly substituted hydroindanes (3) with outstanding levels of diastereoselectivity (> 20:1; Figure 1A).4 Experiments aimed at understanding the mechanism for this process led to the proposal that this [2+2+2] annulation process proceeded by initial alkoxide-directed alkyne–alkyne coupling (→ I),5 followed by intramolecular [4+2] cycloaddition (→ II), and finally cheletropic extrusion of the metal center. When the enyne coupling partner possesses a distal propargylic ether (4), we found that the annulation sequence is interrupted by an elimination that traps the otherwise fleeting organometallic intermediate III (Figure 1B). The result of this elimination is the production of an allylic titanium species IV that is converted to trans-fused hydroindane products by protonation (trans-selectivity is highest when R1 is branched).6 While successful, this process often delivers a mixture of hydroindane products that are contaminated with an undesired alkene isomer that likely results from protonation at the primary carbon of the allylic metal system (→ 6; – this isomer is often difficult to remove from the trans-fused hydroindane product). Here, we demonstrate that the penultimate organometallic intermediate generated in this alkoxide-directed metallacycle-mediated [2+2+2] annulation/elimination process (i.e. IV) can be regioselectively oxidized with LiOOt-Bu to deliver a hydroindane product containing a primary allylic alcohol (7) (Figure 1C). Outside of describing the first oxidation of such an intermediate in a metallacycle-mediated cascade reaction, subsequent reduction by way of an intermediate allylic diazene stereoselectively delivers cis-fused hydroindane products (9) (Figure 1D).
Figure 1.
Metallacycle-mediated convergent assembly of densely functionalized hydroindanes: Early discoveries (A and B), the development of an interrupted annulation-oxidation pathway (C), and the realization of a sequence to generate cis-fused and angularly substituted hydroindanes (D).
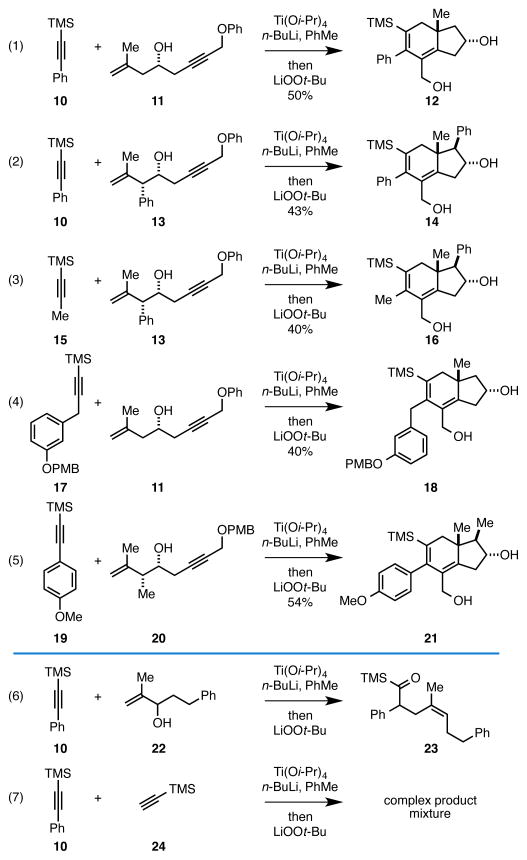
Our studies began by investigating the coupling reaction of TMS-alkyne 10 with the phenoxy-substituted enyne 11. Here, we employed the now standard reaction conditions for [2+2+2] annulation, where exposure of 10 to the combination of Ti(Oi-Pr)4 and n-BuLi (2 equiv) 7 is followed by introduction of the Li-alkoxide of enyne 11 (Scheme 1, eq 1). After warming from −78 °C to rt, a solution of LiOOt-Bu8 is added. To our delight, the substituted hydroindane 12 that contains a primary allylic alcohol was produced as a single regio- and stereoisomer in 50% yield. While we anticipated the high regioselectivity associated with differential functionalization of the TMS-alkyne, and high diastereoselection of the process, we were delighted to observe the similarly high selectivity in regioselective oxidation. In this case, we did not identify any products derived from oxidation at the ring fusion (i.e. 8, Figure 1C). While the yield of the process was 50%, the efficiency of the oxidation is high – termination of the annulation sequence with methanol delivers hydroindane products in 63% yield [52% of a 5:1 mixture of isomers (trans:cis) and 11% of the endocyclic diene; i.e. 6, Figure 1B].6
Scheme 1.
Tandem [2+2+2], elimination, and oxidation en route to angularly substituted hydroindanes: The oxidation procedure is not successful with alkyne–allylic alcohol or alkyne–alkyne coupling reactions.
As illustrated in equations 2–5 of Scheme 1, the oxidative termination of this [2+2+2] annulation reaction is effective for a variety of substrates, defining a concise route to a range of angularly substituted hydroindane products. As depicted in eqs 6 and 7 of Scheme 1, this procedure for oxidation has not been successful in the context of related titanium alkoxide-mediated coupling reactions. For example, with the identical conditions being employed for generation of the initial Ti-alkyne complex, coupling of TMS-alkyne 10 with allylic alcohol 22, followed by addition of LiOOt-Bu did not deliver the expected acylsilane 23 (eq 6) -- only the product of simple reductive cross-coupling was observed.9 Oxidative termination of an alkyne–alkyne coupling process was similarly ineffective (eq 7).
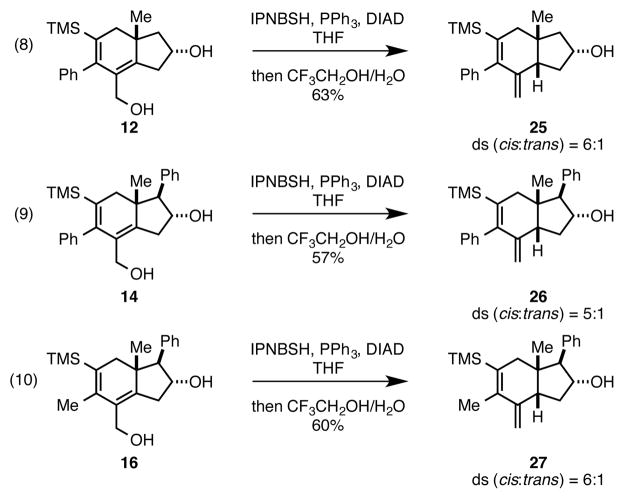
With this interrupted [2+2+2] annulation/oxidation in hand, we quickly turned our attention to exploring the reactivity of the primary allylic alcohol-containing products in reductive transposition chemistry. As illustrated in Scheme 2, we treated the annualtion product 12 with N-isopropylidene-N′-2-nitrobenzenesulfonyl hydrazine (IPNBSH), PPh3, and DIAD in THF.10 To our delight, this process delivered the cis-fused hydroindane 25 in 63% (ds = 6:1). This is in contrast to our earlier studies that demonstrated trans-selectivity (ds = 1:5) if this annulation reaction is quenched with methanol.6 Selectivity for formation of the cis-fused hydroindane product was also observed in reductive transposition of the structurally related allylic alcohols 14 and 16. Here, the cis-fused hydroindane products 26 and 27 were produced in 57 and 60% yields (ds = 5:1 and 6:1, respectively).
Scheme 2.
Stereoselective reductive transposition of the allylic alcohol-containing products delivers cis-fused hydroindanes.
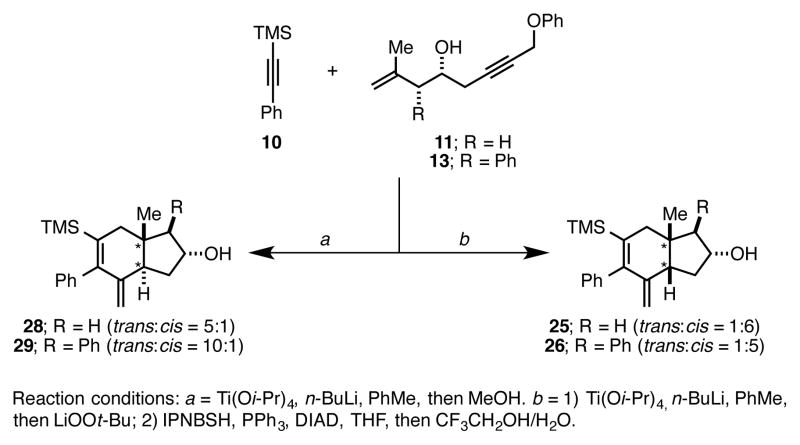
In conclusion, we have demonstrated that LiOOt-Bu is an effective terminal oxidant in an “interrupted” titanium alkoxide-mediated [2+2+2] annulation. Unlike termination of the annulation process by protonation, this oxidation proceeds with exquisite levels of regioselectivity and affords primary allylic alcohol-containing hydroindane products. These stereodefined allylic alcohols are good substrates for IPNBSH-mediated reductive transposition, paving a stereoselective convergent pathway to densely functionalized and angularly substituted cis-fused hydroindanes. For substrates that are known to deliver trans-fused hydroindanes from Ti-mediated [2+2+2] and quenching with MeOH (i.e. 11 → 28, and 13 → 29; Figure 2),6 the present LiOOt-Bu oxidative termination of the [2+2+2] and subsequent IPNBSH-mediated reduction defines a complementary approach for production of the isomeric cis-fused products (i.e. 11 → 25, and 13 → 26).
Figure 2.
Stereodivergent strategies for the synthesis of angularly substituted hydroindanes.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support of this work by the National Institutes of Health –NIGMS (GM80266).
Footnotes
This manuscript is submitted in memory and celebration of Professor Harry H. Wasserman. The corresponding author of this work is grateful to have experienced Harry’s kindness, patience and love of organic chemistry.
Experimental procedures, NMR spectra, and tabulated spectroscopic data for new compounds (PDF) are included as supporting information for this manuscript.
References and notes
- 1.For a recent review of early transition metal-mediated coupling processes in this broader class of reactivity, see: Micalizio GC. In: Comprehensive Organic Synthesis II. Knochel P, Molander GA, editors. Vol. 5. Elsevier; Amsterdam: 2014. pp. 1660–1737.
- 2.(a) Malik HA, Baxter RD, Montgomery J. In: Catalysis Without Precious Metals. 1. Bullock RM, editor. Wiley-VCH; Weinheim: 2010. pp. 181–210. [Google Scholar]; (b) Montgomery J, Sormunen GJ. Top Curr Chem. 2007;279:1–23. [Google Scholar]; (c) Ng SS, Ho CY, Schleicher KD, Jamison TF. Pure Appl Chem. 2008;80:929–939. doi: 10.1351/pac200880050929. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Moslin RM, Miller-Moslin K, Jamison TF. Chem Commun. 2007:4441–4449. doi: 10.1039/b707737h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Patman RL, Bower JF, Kim IS, Krische MJ. Aldrichimica Acta. 2008;41:95–104. [PMC free article] [PubMed] [Google Scholar]; (f) Skucas E, Ngai MY, Komanduri V, Krische MJ. Acc Chem Res. 2007;40:1394–1401. doi: 10.1021/ar7001123. [DOI] [PubMed] [Google Scholar]
- 3.(a) Reichard HA, Micalizio GC. Chem Sci. 2011;2:573–589. doi: 10.1039/C0SC00394H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Reichard HA, McLaughlin M, Chen MZ, Micalizio GC. Eur J Org Chem. 2010:391–409. doi: 10.1002/ejoc.200901094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greszler SN, Reichard HA, Micalizio GC. J Am Chem Soc. 2012;134:2766–2774. doi: 10.1021/ja2105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan J, Micalizio GC. J Am Chem Soc. 2006;128:2764–2765. doi: 10.1021/ja057352w. [DOI] [PubMed] [Google Scholar]
- 6.Jeso V, Aquino C, Cheng X, Mizoguchi H, Nakashige M, Micalizio GC. J Am Chem Soc. 2014;136:8209–8212. doi: 10.1021/ja504374j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Obora Y, Moriya H, Tokunaga M, Tsuji Y. Chem Commun. 2003:2820–2821. doi: 10.1039/b310565b. [DOI] [PubMed] [Google Scholar]; (b) Rassadin VA, Six Y. Tetrahedron. 2014;70:787–794. [Google Scholar]
- 8.Minko Y, Marek I. Org Biomol Chem. 2014;12:1535–1546. doi: 10.1039/c3ob42349b. [DOI] [PubMed] [Google Scholar]
- 9.Kolundzic F, Micalizio GC. J Am Chem Soc. 2007;129:15112–15113. doi: 10.1021/ja075678u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Movassaghi M, Ahmad OK. J Org Chem. 2007;72:1838–1841. doi: 10.1021/jo062325p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Movassaghi M, Piizzi G, Siegel DS, Piersanti G. Angew Chem Int Ed. 2006;45:5859–5863. doi: 10.1002/anie.200602011. [DOI] [PubMed] [Google Scholar]; (c) Myers AG, Zheng B, Movassaghi M. J Org Chem. 1997;62:7507. doi: 10.1021/jo9710137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.