Abstract
Prostate cancer (PCa) is one of the most common neoplasms that metastasize to bone. The aim of this study was to determine if osteoclasts play a role in the seeding of disseminated tumor cells to the bone marrow by mobilizing hematopoietic stem cells (HSCs) out of their marrow niche. Human PC-3Luc cells were introduced into male SCID mice by intracardiac (i.c.) injection after mice were treated with the antiresorptive agent Zoledronic Acid (bisphosphonate (BP)) and/or AMD3100, which mobilizes HSCs out of the marrow. Short term homing of PC-3 was assessed at 24 hours by QPCR for human Alu and luciferase and HSC number was determined by FACS. Mice also received pre and/or post treatments of BP by intraperiteneal (i.p.) injections, in addition to PC-3luc by intratibial (i.t.) injections. TRAP assays were used to determine the osteoclast (OC) number in both studies. AMD3100 enhanced the release of HSCs from the bone marrow, while BP increased the retention of HSCs. PCa entry into bone was facilitated in AMD3100, BP, and AMD3100+BP treatments. Before PCa injection, the number of TRAP+ OC was increased in mice treated with AMD3100, while treatment with BP resulted in relatively lower TRAP+ OCs. TRAP+ OCs were not detected in the AMD3100 + BP treatment. After PCa injection, however, the number of TRAP+ OCs was dramatically increased, but did not differ significantly amongst the treatment groups. The pre and post BP treatments in the Nude mice decreased the size of PCa lesions in the tibia compared to the control. The results indicate that OC activation is not necessary for PCa metastasis to bone at the earliest stages. These findings are critical in proving that OCs' contribution to metastasis occur during the growth phase of the tumor rather than at the initiation phase.
Keywords: Prostate Cancer, Disseminated Tumor Cells, Osteoblasts, Osteoclasts, Cancer Stem Cells
Introduction
Breast and prostate cancer (PCa) represent solid tumors that frequently metastasize to the bone marrow, causing alterations of bone integrity and function, and resulting in a major source of morbidity and mortality. The process of metastasis has been characterized as a multistep cascade, in which tumor cells must first undergo an epithelial to mesenchymal transition to a more motile phenotype, enter vascular or lymphatic structures, and ultimately reverse the transition at the site of metastasis. Previous studies have demonstrated that SDF-1 (stromal cell-derived factor-1 or CXCL12) and its receptor, CXCR4 represent a major mechanism in PCa’s metastasis to bone[1, 2]. We have recently shown that the binding of CXCL12 to CXCR4 activates the expression of receptors which facilitate the localization of disseminated tumor cells (DTCs) to the bone marrow microenvironment [3, 4].
More recently, we demonstrated that circulating PCa cells target the ‘niche,’ in marrow that houses hematopoietic stem cells (HSC) [5]. We used a micrometastasis model of PCa metastasis to demonstrate that tumor cells directly compete with HSCs for occupancy of the endosteal HSC niche in during bone marrow transplantation [5]. In fact, based upon observations of their proximity to osteoblasts, it appears very likely that cancer stem cells (CSCs) expressing the CD133+/CD44+ phenotype preferentially compete for occupancy of the osteoblastic HSC niche [5]. Once in the niche, metastatic cells, like HSCs, may be mobilized back into the peripheral blood using agents that mobilize HSCs [5]. Critically, HSCs co-localize with DTCs to the endosteal bone surfaces, and when the number of HSC niches was altered, DTC numbers in marrow responded accordingly [5, 6]. Importantly, increasing numbers of DTCs in marrow resulted in HSCs mobilizing out of the marrow and into progenitor cell pools and the peripheral blood. Together, these findings demonstrate that PCa and HSCs compete for the endosteal HSC niche and use the same mechanisms to access and egress the niche, and provide direct evidence that this HSC niche plays a central role in bone metastases.
The mechanism by which HSCs are mobilized into the peripheral blood in response to cytokines has been an area of active investigation. Recent work has demonstrated that secretion of MMP-9, cathepsin G, and cathepsin K by osteoclasts results in the digestion of CXCL12, resulting in the release of HSCs into the blood1. Likewise, osteoclastic activity appears to be essential to PCa metastasis. In part, as PCa cells may express the receptor activator of nuclear factor kB ligand, or RANKL, which stimulates osteoclasts and bone resorption osteoclastic activity may be induced facilitating metastasis and growth [1, 2]. Since osteoclasts activate HSC mobilization, the creation of empty niches may make an increase in PCa metastasis more likely[7].
Paralleling the role that they play in HSC biology, osteoclasts are also involved in metastastic growth of solid tumors in bone, including breast and prostate cancer. In fact, the activation of osteoclastic activity by tumor cells releases growth factors and cytokines sequestered in bone matrix, which contributes to the growth of the tumors, a process termed “the vicious cycle.” In this model, tumor cell–host interactions result in boney lesions which are predominately osteoclastic (breast cancer) or osteoblastic (PCa) boney lesions.
Based upon the active role that osteoclasts play in regulating HSC retention in the niche and in tumor growth, we speculated that osteoclasts may play a central role in the initial seeding of tumor cells into the HSC niche. Accordingly, we used our ability to track tumor cells with QPCR in the context of osteoclast inhibition with bisphosphonates to question whether osteoclasts participate in the earliest seeding of PCa into the HSC niche - an activity independent of their role in stimulating tumor growth via the “vicious cycle”. The clinical implication is that if osteoclasts play a role in the initial seeding of the HSC niche by solid tumors, then the use of agents which modify osteoclastic activities (e.g. bisphosphonates, cathepsin V inhibitors and decoy receptor for osteoblastic RANKL) should be initiated far earlier for individuals with high grade tumor types.
Methods
Cell culture
PC-3 (CRL-1435) prostate cancer cell line was obtained from the American Type Culture Collection (Rockville, MD). The metastatic subline LNCaP C4-2B was originally isolated from a lymph node of a patient with disseminated bony and lymph node involvement. PC-3Luc cells were constructed by stably transducing PC-3 cells with a GFP-luciferase lentivirus, as previously described. Cells were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin-streptomycin (Invitrogen), and maintained at 37°C, 5% CO2, and 100% humidity.
In vivo metastasis assays
All experimental procedures were approved by the University of Michigan Committee for the Use and Care of Animals (UCUCA). In the first set of animal investigations, male 5–6 week old severe combined immune deficient mice (SCID) were injected with 1×106 PCa cells by intracardiac injection (i.c.). under isoflurane anesthesia (Figure 1A). In order to mobilize HSCs out of the niche, mice were administered AMD3100 (AMD) (Sigma-Aldrich, St. Louis, MO) or vehicle (0.9% saline) by intraperitoneal injection at 5 mg/kg (100 µl) per day for 5 days preceeding the tumor inoculation. To limit osteoclastic activity, which could participate in PCa seeding to the niche, some of the animals were administered Zoledronic Acid (ZA, a bisphosphonate) (Novartis Pharma AG, Basel, Switzerland)[8–10] by intravenous injection (3 µg/kg/day) for 3 and 7 days preceding the i.c. injection of tumor cells. Additional groups included no treatment controls and animals treated with both AMD3100 and Zoledronic Acid.
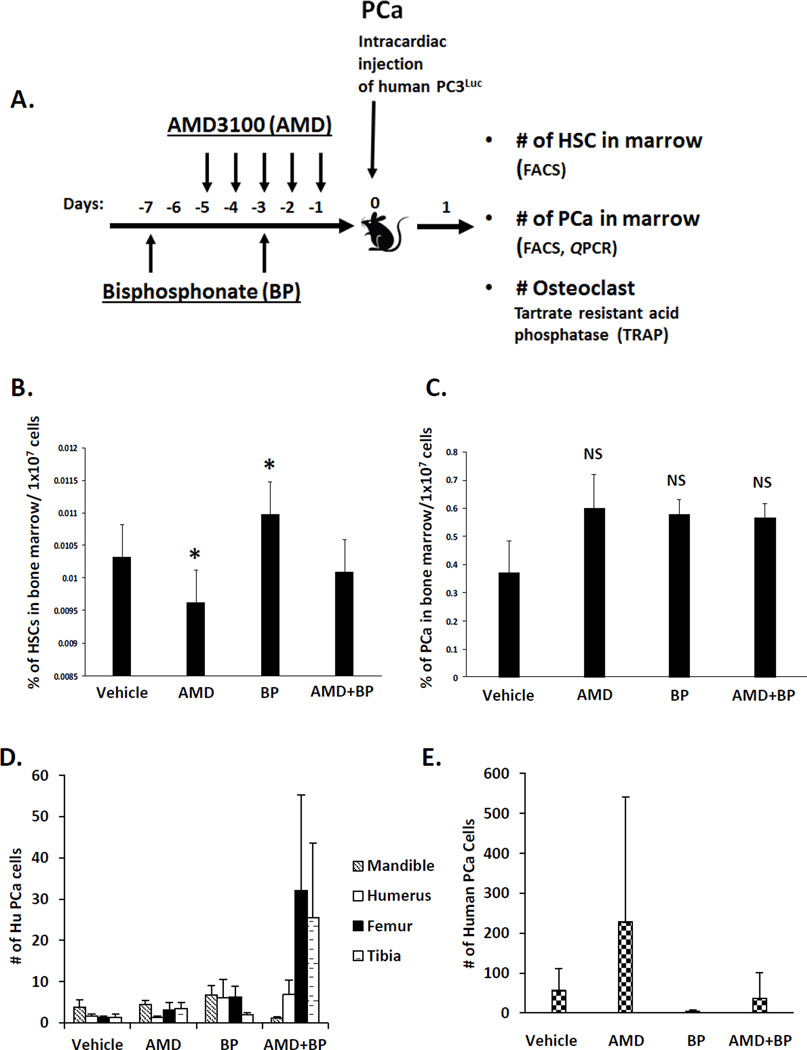
Figure 1. Osteoclasts are not critical for early colonization of DTCs into the bone marrow.
(A) Experimental outline illustrating the methods and injection strategy for delivery of vehicle, AMD3100 (AMD), and Zoledronic acid (ZA) treatments along with inoculation of human PCa into mice. (B) HSC numbers in bone marrow were analyzed by FACS. (C) The short term homing capabilities of DTCs in marrow were evaluated after 24 hours by quantifying HLA-expressing PCa cells in marrow. Data are presented as the mean ± standard error (n=5). (D, E). Short term homing of prostate cancer in vivo to bone by real time PCR (QPCR). The short term homing capabilities were evaluated after 24 hours by assessing QPCR for Alu and data were normalized to total mouse β-actin. Data are presented as the mean ± standard error (n=5) where significance was determined by using a Kruskal-Wallis test and Dunn’s multiple comparisons with the level of significance set at *P<0.05.
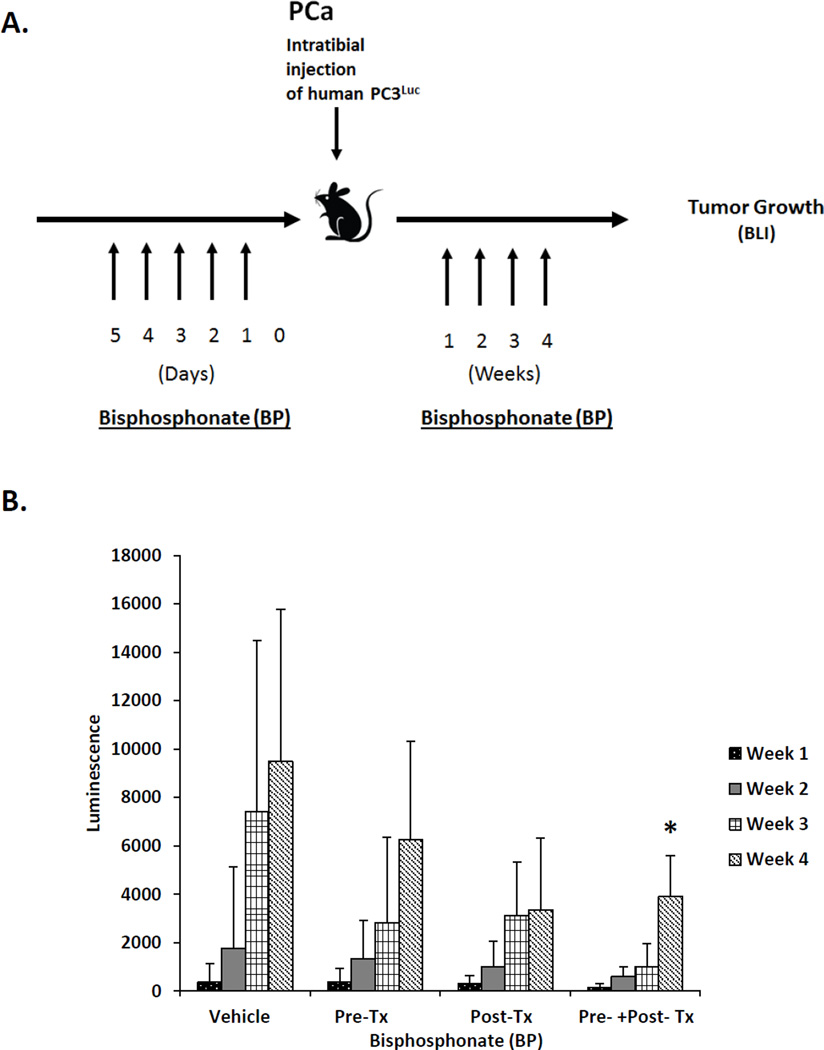
A second set of studies was designed to evaluate the role of osteoclasts after PCa cells had arrived in the bone marrow. For this study, PC-3Luc cells were inoculated into male nude mice by intratibial (i.t.) injection. Recipient mice were randomized into treatment groups which included pretreatment with vehicle or Zoledronic acid (3 µg/kg/day) for 5 days preceding i.t. injection of tumor cells, or twice per week for 4 weeks following i.t. injection; or treatment with Zoledronic acid or vehicle preceding and following tumor inoculation. All mice were sacrificed at 4 weeks following i.t. tumor inoculation. BioLuminescent Imaging was performed once a week for the four weeks following the i.t. injection at the University of Michigan Small Animal Imaging Resource facility using a CCD IVIS system with a 50-mm lens (Xenogen Corp.) and the results were analyzed using LivingImage software (Xenogen). Here the mice were injected with luciferin (40 mg/mL) i.p. and ventral images were acquired 15 min postinjection under 1.75% isofluorane/air anesthesia. Total tumor burden of each animal was calculated using regions of interest (ROI) that encompassed the entire animal. For tibia-specific measurements, ROI values were recorded for each individual tibia. The size of tibia-specific lesions was quantified by luminescent signal evident at each week, and the luminescence value of the lesion at week 4 were used in results.
Real time PCR (QPCR)
Tumor cell numbers were assessed using several primer/probe sets including those targeting human Alu TaqMan probes (F–5′-CAT GGT GAA ACC CCG TCT CTA-3′, R5′-GCC TCA GCC TCC CGA GTA G-3′, TaqMan probe–5′-FAM-ATT AGC CGG GCG TGG TGG CG-TAMRA-3′, Applied Biosystems). The data were normalized to mouse tissue β-Actin (Mm00607939_s1). QPCR was performed using standard techniques. RT products were analyzed by QPCR in TaqMan® Gene Expression Assays, using 15.0 µl of TaqMan® Universal PCR Master Mix (Applied Biosystems), 1.5 µl of TaqMan® Gene Expression Assay (forward and reverse primers at 18 µM and Taqman probe at 5 µM), 1 µl of the RT product, and 12.5 µl of RNAse/DNAse-free water in a total volume of 30 µl. Reactions without template and/or enzyme were used as negative controls. The 2nd step PCR reaction (95°C for 30 seconds, 60°C) ran for 40 cycles after an initial single cycle of 95°C for 15 minutes. The PCR product was detected using an ABI PRISM 7700 instrument (Applied Biosystems). RNA quantity (CR) was normalized to the housekeeping gene β-Actin control by using the formula CR=2(40-Ct of sample)-(40-Ct of control). The threshold cycle (Ct) is the cycle at which a significant increase in fluorescence occurs.
Fluorescence-activated cell sorting (FACS)
The flow cytometric analyses and fluorescence-activated cell sorting (FACS) were performed on a FACS Aria dual-laser flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and data were analyzed with DIVA software (Becton Dickinson). BD cytometer setup and tracking beads kit (BD Biosciences, Franklin Lakes, NJ, Cat no. 642412) are used for the daily instrument standardization and validation procedure. Sorting calibration was performed before each sort by drop-delay using Accudrop beads (BD Biosciences, Cat no. 345249), populations for sorting were gated by forward and side scatter to eliminate the presence of doublets. Sorting of these gated cells was done using a 100-µm nozzle at 20 psi in purity mode. Cells were triturated and filtered through a nylon screen (40 µm; BD Falcon, Bedford, MA) to obtain single-cell suspensions.
For determining HSCs the cells were incubated first with an antibody cocktail of anti-CD150PE (Clone TC15-12F12.2, BioLengend, San Diego, CA), CD48FITC (Clone BCM-1) and CD41FITC (Clone MWReg30), cKitBIO (Clone 2B8) and SCA-1APC (Clone E13-161.7) for twenty minutes on ice, then rinsed and stained with anti-Biotin MicroBeads (Miltenyl Biotec, Auburn, CA) and streptavidin-APC-Cy7 conjugated secondary antibody for another twenty minutes. Cells were enriched for cKit+ using an AutoMACS machine (Miltenyl Biotec, Auburn, CA). The enriched cells were resuspended in 2 mg/ml 7-AAD (eBioscience, San Diego, CA) to discriminate live from dead cells. Only live (7-AAD) cells were included in analyses and sorts. Hematopoietic stem cells were sorted on a FACS Vantage dual laser flow-cytometer (Becton Dickinson, San Jose, CA) by gating on cells that are Sca-1+cKit+CD150+CD41−CD48−.
For DTCs, the bone marrow was flushed from the femurs of euthanized mice and depleted of hematopoietic-lineage cells using a Lineage Cell Depletion Kit (Miltenyi Biotec, 130-092-211) was used. DTCs were identified in lineage depleted murine marrow using a fluorescein isothiocyanate (FITC)-human leukocyte antigens-A, B, and C loci (HLA-ABC) antibody (Biolegend, San Diego, CA, Cat no. 311404).
TRAP staining
Mouse femurs were fixed in 10% neutral buffered formalin (Sigma HT50) for 24 h at room temperature. Following fixation, the samples were decalcified in 10% EDTA, pH 7.5, for 20 days and embedded in paraffin. Deparaffinized 5µm sections were rehydrated and stained for TRAP activity ((Kamiya Biolmedical Comp, Seattle WA, KT-008) according to the manufacturer’s instructions[2].TRAP positive, multinucleated cells were quantified in random high power fields under light microscopy at 20X.
Statistical analyses
All experiments were performed at least three times with similar results and representative assays shown. Numerical data is expressed as mean ± standard error. Statistical analysis was performed by ANOVA or Student’s t test using the GraphPad Instat statistical program (GraphPad Software, San Diego, CA) with significance at P < 0.05. For the QPCR assays, a Kruskal-Wallis test and Dunn’s multiple comparisons tests were utilized with the level of significance set at P < 0.05.
Results
The purpose of these studies was to determine the role that osteoclasts play in the early dissemination of tumor cells to the bone marrow niche. Previously, we demonstrated that DTCs target the HSC niche in marrow as a site for homing, so for these studies we used AMD3100 to mobilized HSCs and progenitor populations out of the niche to provide additional sites for early metastasis of DTCs (Figure 1A). Similarly, the bisphosphonate Zoledronic acid was employed to limit osteoclastic activity during the initial seeding of DTCs into the marrow. As shown in Figure 1B, HSC numbers identified by FACS in marrow decreased in response to AMD3100. Surprisingly, bisphosphonate treatment resulted in enhanced levels of HSCs present in the bone marrow, presumably by inhibiting the normal osteoclastic role in stem cell mobilization. The combined treatment of both AMD3100 and Zoledronic acid did not result in a change of HSC numbers in marrow relative to vehicle.
Mobilization of HSCs out of the niche with AMD3100 increased the number of DTCs identified by FACS staining in the bone marrow niche, albeit not to a significant degree in this short term homing study (Figure 1C). Bisphosphonate treatment also tended to increase the overall number of DTCs present in the marrow, suggesting that osteoclasts may not be critical for the initial colonization of the marrow by DTCs. Consistent with the other groups, the combined treatment of animals with AMD3100 and Zoledronic acid facilitated more DTCs in the marrow relative to vehicle alone (Figures 1C); however, these changes were not found to result in significant differences between the treatment and the non-treatment groups, or between treatment groups (Figure 1C).
After sacrificing the animals, the long bones and selected tissues were isolated and tumor cells quantified using QPCR for human Alu against a standard curve of human PCa cells mixed with murine marrow, and normalized against total mouse β-actin. As shown in Figure 1D for the mandible, humerus, femur and tibia, there were no significant differences in the number of DTCs present across the treatment groups. In the peripheral blood, while there were no significant differences in the number of PCa identified across the treatment groups, there was a trend for more PCa cells in the blood following AMD3100 treatments, and less following Zoledronic acid administration (Figure 1 E).
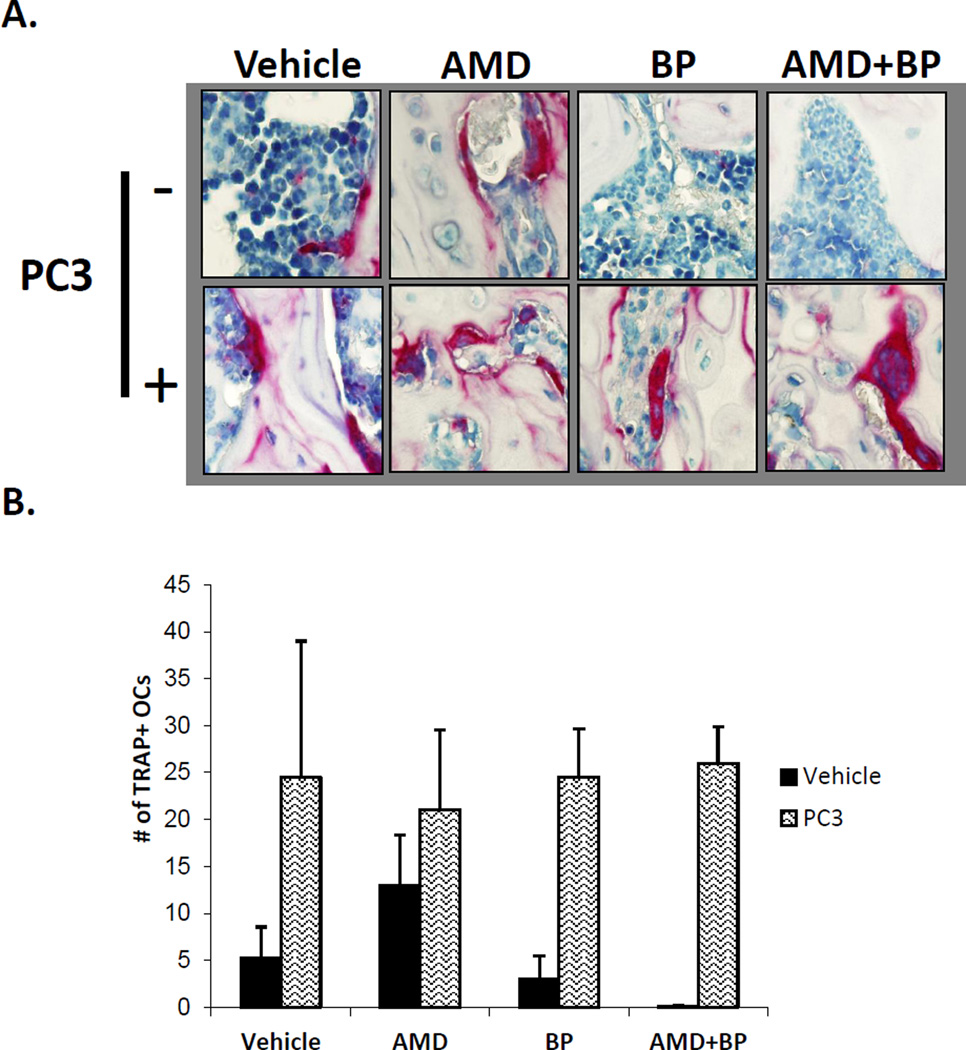
To validate that the treatment with Zoledronic acid resulted in osteoclast inhibition, immunohistochemistry for TRAP was performed in long bone samples. Compared to untreated controls, AMD3100 treatment alone resulted in the enhanced expression of TRAP positive multinucleated cells in the long bones of the animals, whereas pretreatment with Zoledronic Acid reduced the basal levels of TRAP staining in the long bones, however these differences were not statistically significant. Importantly, when PCa was not present, AMD3100 treatment in conjunction with the bisphosphonate reduced the expression of TRAP staining compared to AMD3100 treatment alone. As demonstrated in Figure 2A and quantified in Figure 2B, a significant increase in TRAP staining was evident in the vehicle treated animals even as early as 24h after injection of PCa. This dramatic increase in TRAP suggests that the presence of PCa cells is rapidly able to activate osteoclastic activity, and this activity remained elevated despite treatment with AMD3100, Zoledronic acid, alone or in combination.
Figure 2. Quantification of osteoclasts during short term PCa homing.
After fixation and longitudinal sectioning across an entire femur, TRAP staining was performed. Osteoclasts were defined as TRAP staining positive cells with ≥ 3 nucli across. (A). Co-administration of Bisphosphonate and AMD3100 reduces the osteoclast numbers in the absence of prostate cancer but did not exert the same effect in the presence of prostate cancer. The femurs were decalcified and stained for TRAP activity, as shown in Figure 2A. (B). Quantification of Figure 2A. Data are presented as the mean ± standard error (n=5).
To validate that our model was sufficient to determine whether osteoclasts play a role in early tumor dissemination, we further explored the role of osteoclasts in a model where tumor cells are placed directly into the marrow (Figure 3A). Following i.t. injection, tumor growth in the vehicle treated animals followed the expected pattern of tumor growth and by 4 weeks the BLI signal intensity prompted us to terminate the study (Figure 3B). Pretreatment of animals with Zoledronic acid prior to inoculation with PCa reduced overall tumor growth in the i.t. model, however these differences did not rise to the level of statistical significance. Similarly, treatment of the animals with Zoledronic acid after tumor inoculation reduced overall tumor growth during the four-week study, but these differences did not prove to be significant relative to the vehicle treated animals. Osteoclast inhibition prior to and after inoculation of tumor cells directly into the tibia did however significantly blunt tumor growth compared to vehicle treated controls in this model.
Figure 3. Osteoclastic activity participates in supporting tumor growth after dissemination.
(A) Experimental outline illustrating the methods and injection strategy for injection of Zoledronic acid (ZA) (bisphosphonate) treatments. (B). Quantification of tumor growth over time by bioluminescent imaging following AMD and ZA treatments. The data demonstrate that tumor growth was best inhibited by the combined treatment of Zolodronic acid delivered pre and post tumor implantation. The mice were imaged every 4 weeks, with Bio-luminescent Imaging, after intra-tibial injection of PC3-Luc cells. Data is presented as the mean ± standard error (n=3).
Discussion
The use of osteoclast inhibitors is a mainstay of cancer therapy for those individuals with skeletal metastases. Conventional wisdom dictates that inhibition of osteoclast activity in a metastatic environment may help to reduce growth of tumors in bone. Previously, we established an experimental model, which demonstrated that DTCs of PCa are able to compete for occupancy of the HSC niche[5]. Osteoclasts are thought to play a role in HSC mobilization, and are also known to support tumor growth in bone through activation of what has been termed a “vicious cycle”, in which bone resorption releases factors which stimulate tumor growth, which in turn stimulates osteoclastic activity. We addressed whether an additional function of osteoclastic activity is involved in regulating the initial seeding of DTCs into the HSC niche.
The results from this study demonstrate that osteoclastic inhibition with bisphosphonate did not influence the number of DTCs identified in the bone marrow of animals with in 24h of inoculation. Yet when tumors were preestablished in the bones of the animals, osteoclastic inhibition played a role in limiting tumor growth. Within the limits of our studies, we observed that osteoclasts are not necessary for the initial establishment of DTCs from PCa in the marrow (Figure 4). While osteoclasts are apparently not involved in these initial processes, it is likely that multiple cells and molecules regulate the establishment of initial footholds for DTCs in marrow. Many of these are likely to include factors which regulate the adhesion and migration out of the circulation and into the bone marrow parenchyma, including endothelial cells, vascular endothelial adhesion molecules (e.g. VCAM-1), and HSC-regulating factors, including stromal-derived factor-1 (SDF-1 or CXCL12), as well as physical factors including the number and occupancy of HSC niches. Yet, osteoclasts are clearly important for tumor growth within the skeleton, as has been shown clinically with potent osteoclast inhibitors (bisphosphonates and denosumab), which decrease the risk of skeletal related events (SRE) and thus improve quality of life and survival (Figure 4).
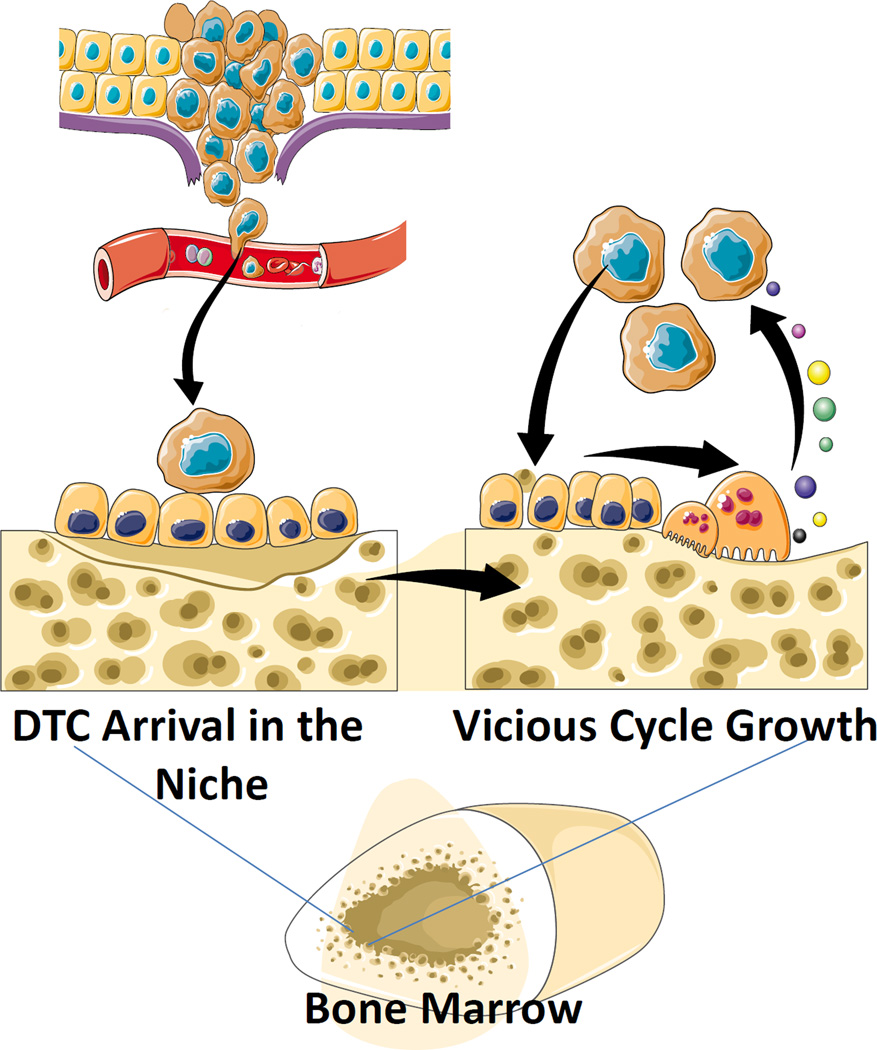
Figure 4. Model For Osteoclastic Support for DTC Dissemination and Metastatic Growth.
Osteoclasts play a significant role in regulating HSC number in bone marrow by regulating HSC egress during mobilizing events. Conversely, occupancy of the HSC niche by DTCs during the initial seeding of DTCs is not regulated significantly by osteoclastic activity. Yet once in the niche, osteoclastic activity participates in the support of tumor growth in marrow as described in the Vicious Cycle Theory [13]
One important caveat, which pertains to our findings and is of particular note, is that osteoclastic TRAP activity was increased in the marrow of the experimental animals as early as 24h after introduction of PCa into the circulation. This also occurred in animals that were treated with bisphosphonate prior to tumor inoculation. These observations suggest that preexisting osteoclast precursors or mature osteoclasts in our animals were not completely inhibited by our treatments and thus we can not completely rule out that osteoclasts may play in regulating early metastatic seeding. However, our findings that AMD3100 and bisphosphonates do regulate HSC mobilization as predicted, suggests that perhaps the activities of osteoclasts may have many different functions, some of which are not exclusively related to bone turnover. For example, osteoclasts may produce factors which regulate HSC homing independent of tissue resorption. However this notion requires further proof and would prove difficult to dissociate from the known role that HSCs play in bone formation[11].
From a clinical perspective, our findings suggest that osteoclast inhibition therapy for patients with primary disease, aimed at preventing the establishment of DTCs in bone, is not likely to prove beneficial. There are several reasons for this conclusion. First and foremost, by the time of diagnosis of PCa, tumor cells have already disseminated, in the majority of cases[12]. The reason for this is unclear other than to surmise that shedding of tumor cells from a primary tumor must occur relatively early in tumor progression. Thus, if conventional wisdom prevails, in order to have an effect on the prevention of the establishment of DTCs in marrow, it would be necessary to place all men on prophylactic osteoclastic inhibition therapy; for which enforcement would be impossible and benefits might well be minimal. Moreover, most men who are diagnosed with PCa are cured with local/regional or systemic therapeutics, so addition of DTC prophylaxis for most men would not provide any additional therapeutic gain. However for those who do progress to develop skeletal related events (SREs), osteoclastic inhibition used to target dissemination events would probably not be effective since, as our study shows, the establishment of DTCs in marrow appears to be osteoclast-independent.
There are however constraints to our studies. First is the inherent limitation of examining the biology of human PCa in a murine model, which requires the use of immune deficient mice. Moreover, the intracardiac (i.c.) and intratibial (i.t.) injection models bypass the establishment of true circulating tumor cells (CTCs) from a primary tumor in the marrow, and therefore also bypass the mechanisms that may regulate osteoclastic activity prior to the arrival of CTCs/DTCs. This is a real possibility given the rapid induction of TRAP staining in the marrows of animals injected with PCa 24h prior to sacrifice. Second, the bisphosphonate used in this animal system did not produce absolute elimination of osteoclastic activity. Given the limited numbers of DTCs found in these studies in the marrow of the untreated animals, incomplete blockade is likely to produce bias in our data interpretation.
In summary, our data suggests that osteoclasts are not critical in the earliest stages of metastasis to the bone marrow. Therefore the data suggests that targeting osteoclastic activity prior to DTC seeding is not likely to prove beneficial in a clinical setting. However, once established in bone, osteoclastic activities do play a significant role in driving tumor growth once tumor cells become established in bone. Moreover, our data suggests that osteoclastic induction may be an early response of osteoclastic precursors early in skeletal metastasis, and therefore the induction of these precursors could be indicative of subsequent growth and therefore may serve as an early predictor of the clinical course of disease. Clearly further investigation is warranted.
Acknowledgments
J. L. Zalucha was funded by a student fellowship by the American Association for Dental Research (AADR), and the University of Michigan School of Dentistry Student Research and Pathways Programs. This work is directly supported by the National Cancer Institute (CA093900 to K.J. Pienta and R.S. Taichman, CA163124 to Y. Shiozawa, K.J. Pienta, and R.S. Taichman, U54CA143803 to K.J. Pienta, CA143055 to K.J. Pienta), the Department of Defense (W81XWH-14-1-0403 to Y. Shiozawa, and W81XWH-11-1-0636 to K.J. Pienta, K.J. Pienta, and R.S. Taichman), and the Prostate Cancer Foundation (Y. Shiozawa, K.J. Pienta, and R.S. Taichman). K.J. Pienta receives support as an American Cancer Society Clinical Research Professor.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Resources
- 1.Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annual review of immunology. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- 2.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nature medicine. 2006;12(6):657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. The Journal of biological chemistry. 2008;283(7):4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 4.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. The Prostate. 2007;67(1):61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 5.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. The Journal of clinical investigation. 2011;121(4):1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 7.Shiozawa Y. Solid Tumors target the HSC niche to establish metastatic footholds in the marrow. 2008 [Google Scholar]
- 8.Wang HL, Weber D, McCauley LK. Effect of long-term oral bisphosphonates on implant wound healing: literature review and a case report. Journal of periodontology. 2007;78(3):584–594. doi: 10.1902/jop.2007.060239. [DOI] [PubMed] [Google Scholar]
- 9.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146(4):1727–1736. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 10.Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42(4):806–818. doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem cells. 2008;26(8):2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(2):677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Molecular cancer therapeutics. 2007;6(10):2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]