Abstract
Dynamic preservation strategies such as hypothermic machine perfusion are increasingly discussed to improve liver graft quality before transplantation. This review summarizes current knowledge of this perfusion technique for liver preservation. We discuss optimization of perfusion conditions and current strategies to assess graft quality during cold perfusion. Next, we provide an overview of possible pathways of protection from ischemia-reperfusion injury. Finally, we report on recent clinical applications of human hypothermic machine liver perfusion.
Keywords: Hypothermic oxygenated perfusion, Ischemia-reperfusion injury, ROS, Mitochondria
Introduction
Nearly one century ago, Alexis Carrel and Charles Lindbergh developed the first device for automatic ex vivo perfusion of small organs at body temperature with an oxygenated solution [1]. Remarkably, despite numerous technical advances since that time, no system has been able to maintain complete function of organs (livers, kidney, lungs or hearts) for more than several days outside of the body [2, 3]. In contrast, because of the simplicity and effectiveness, preservation of solid organs for transplantation has been predominantly performed by cold storage (CS) at 4 °C for the last 4 decades [4–6]. Over the past 10 years, however, the development of novel dynamic preservation techniques to improve organ quality before implantation has received increasing attention. For the liver, the main debate over machine perfusion relates to three separate approaches, differing in perfusate temperature and the degree of oxygenation: normothermic, subnormothermic and hypothermic liver perfusion. While normothermic machine perfusion (NMP) closely simulates in vivo conditions and therefore needs oxygenated diluted blood and nutritional compounds as perfusate [7–9], subnormothermic machine perfusion (SNMP) and hypothermic machine perfusion (HMP) rely on the physically dissolved oxygen in a blood-free perfusate at temperatures of 20-25 °C (subnormothermic) [10, 11] or 2-10 ° (hypothermic) [12, 13].
For the purpose of this review, we will focus on the following aspects of the current research on HMP: key questions on perfusion conditions, new insights into assessing graft function during HMP, and the underlying mechanisms of protection and associated clinical applications.
Perfusion Conditions
HMP techniques for liver preservation vary significantly in experimental setups. The most common variations relate to the degree of oxygenation, the perfusion route and flow, and the perfusate composition (Tables 1 and 2) [14].
Table 1.
Ex vivo studies on hypothermic machine liver perfusion
| Author | Year | Species | Oxygenation | Pressure | Route | Medium | Duration (h) |
|---|---|---|---|---|---|---|---|
| Continuous perfusion | |||||||
| Dutkowski [19] | 1998 | Rat | active oxygenation | 4.48 mmHg | PV | UW modified | 10, 24 |
| Dutkowski [56] | 1998 | Rat | active oxygenation | 4.48 mmHg | PV | UW modified | 10 |
| Dutkowski [55] | 1999 | Rat | active oxygenation | 4.48 mmHg | PV | UW modified | 10 |
| Southard [60] | 2000 | Rat | 100–125 torr | na | PV | UW | 24 |
| Compagnon [61] | 2001 | Rat | pO2: 90 ± 5 mmHg | PV + HA, 1–11 mmHg | HA + PV | Celsior-HES | 24-48 |
| So [62] | 2001 | Rat | active oxygenation | PV: 12 cm H2O | PV | UW | 24 |
| Minor [63] | 2002 | Rat | >500 mmHg | PV: <8 mmHg | PV | HTK | 24 |
| Lee [32] | 2002 | Rat | na | na | PV | UW-starch free | 10 |
| Dutkowski [20] | 2003 | Rat | 283 ± 4 mmHg | na | PV | UW modified | 10 |
| Lauschke [64] | 2003 | Rat | >500 mmHg | 2–4.5 Pas/ml | PV | HTK, Belzer | 24 |
| Jain [65] | 2004 | Rat | active oxygenation | PV | UW - starchfree | 1, 24 | |
| Xu [66] | 2004 | Rat | PV 1.9-2.9 mmHg | PV | 24 | ||
| Guarrera [51] | 2005 | Pig | no active | Vasosol | 12 | ||
| Bessems [67] | 2005 | Rat | na | PV 15 mmHg | PV | UW-G, Polysol | 24 |
| Bessems [68] | 2005 | Rat | na | na | PV | UW-G. Polysol | 24 |
| Xu [69] | 2005 | Rat | na | na | na | UW vs UW starch free | 24 |
| Jain [70] | 2005 | Pig | active oxygenated | PV: 10-12 mmHg, HA: 30 mmHg | HA + PV | KPS-1 | 24 |
| Bessems [71] | 2005 | Rat | 700 mmHg | PV: 20 cm H2O | PV | UW vs Polysol | 24 |
| t‘Hart [72] | 2005 | Rat | active oxygenation | PV: 4 or 8 mmHg; HA: 25 or 50 mmHg | HA + PV | UW | 24 |
| Minor [9] | 2006 | Rat | >500 mmHg | na | PV | HTK | 2, 18 |
| van der Plaats [73] | 2006 | Pig | HA 35.8, PV 19.2kPa | PV: 4 mmHg, HA: 30/20 mmHg | HA + PV | UW | 24 |
| t‘Hart [26] | 2007 | Rat | active oxygenation | HA + PV | UW | 24 | |
| Bessems [74] | 2007 | Rat | 700-800 mmHg | PV: 16 mmHg | PV | UW | 24 |
| Vekemans [75] | 2007 | Pig | no active | PV 3-5 mmHg or 7 mmHg, HA 25 mmHg | HA + PV | KPS-1 | 24 |
| Monbaliu [23] | 2007 | Pig | no active | PV: 7 mmHg, HA: 25 mmHg | HA + PV | KPS-1 | 24 |
| Manekeller [76] | 2008 | Rat | na | na | HTK, UW | 18 | |
| Jain [77] | 2008 | Rat | na | na | PV | 4 different | 5 |
| Stegemann [78] | 2009 | Rat | 500 mmHg | na | PV | HTK | 1.5 |
| Liu [79] | 2009 | Pig | 300 mmHg | PV: 3–5 mmHg, HA: 30 mmHg | HA + PV | KPS-1 | 4 |
| Stegemann [80] | 2010 | Rat | 500 mmHg PV | PV | HTK | 18 | |
| Luer [17] | 2010 | Rat | 600 vs 200 vs 50 mmHg | PV | HTK | 18 | |
| Monbaliu [81] | 2012 | Pig | PV: 7 mmHg, HA: 25 mmHg | HA + PV | KPS-1 | 2.3 | |
| Giannone [82] | 2012 | Rat | Normobar vs. hyperbar | Celsior | 24 | ||
| Dirkes [24] | 2013 | Pig | 125 mmHg | PV: 13 ± 1 mm Hg | HA + PV | UW | 24 |
| Liu [83] | 2013 | Pig | 330 ± 90 mmHg | na | HA + PV | na | 4 |
| Endischemic perfusion | |||||||
| Dutkowski [57] | 2006 | Rat | 313.4 ± 24.6 mm Hg | PV: 4.4 mmHg | PV | UW modified | 3 |
| Dutkowski [12] | 2006 | Rat | 350 mmHg | PV: 3 mmHg | PV | UW modified | 1 |
| Stegemann [78] | 2009 | Rat | 500 mmHg PV | na | PV | 1.5 | |
| Schlegel [15] | 2013 | Pig | >60 kPa | 3 mmHg | PV | UW modified | 1 |
Table 2.
In vivo studies on hypothermic machine liver perfusion
| Author | Year | Species | Oxygenation | Pressure | Route | Medium | Duration (h) |
|---|---|---|---|---|---|---|---|
| Continuous perfusion | |||||||
| Pienaar [3] | 1990 | Dog | na | 16–18 mmHg | PV | UW | 72 |
| Uchiyama [84] | 2001 | Pig | na | HA: 30-60 mmHg | HA + PV | UW-MPS | 2 |
| Iwamoto [85] | 2000 | Pig | na | PV:8 mmHg, HA 20-80 mmHg | HA + PV | na | 2 |
| Lee [13] | 2003 | Rat | na | na | PV | UW | 5 |
| Vekemans [86] | 2009 | Pig | 310 mmHg | na | na | 3 different (KPS, Aqix-Rs1) | 4 |
| Monbaliu [87] | 2011 | Pig | no active oxygenation | PV 7 mmHg, HA 30 mmHg | HA + PV | KPS-1 | 4 |
| Fondevila [88] | 2012 | Pig | HA: 90kPa | PV and HA (4 mmHg PV, 20-30 mmHg HA) | HA + PV | UW MP | 4 |
| Endischemic perfusion | |||||||
| de Rougemont [31] | 2009 | Pig | >60 kPa | 3 mmHg | PV | UW modified | 1 |
| Schlegel [21] | 2013 | Rat | 90 kPa, 750 mmHg | 3 mmHg | PV | UW modified | 1 |
| Schlegel [49•] | 2014 | Rat | 90 kPa, 750 mmHg | 3 mmHg | PV | UW modified | 1 |
| Schlegel [50] | 2014 | Rat | 90 kPa, 750 mmHg | 3 mmHg | PV | UW modified | 1 |
Is Active Oxygenation Necessary?
Oxygen consumption of liver tissue decreases with decreasing temperature, but does not completely stop under CS (4 °C) [21]. Reported perfusate oxygenation during liver HMP studies ranges between 10 and 100 kPa (Tables 1 and 2), yet the optimal level of oxygenation remains unclear. Dissolved oxygen may be sufficient at 4 °C for standard livers donated after brain death (DBD) [16]. Previous studies, however, confirm that livers exposed to warm ischemia before organ procurement, for example, donation after cardiac death (DCD) donors, have higher oxygen demands than controls [12]. It has also been shown that active oxygenation, compared to no oxygenation, is protective for DCD livers [17].
Some studies have reported oxidative stress when isolated hepatocytes are exposed to oxygen under cold conditions. This stress is due to the reaction of oxygen with increased intracellular redox-active iron ions, which triggers free radical-mediated cell injury [18]. Based on this, researchers have been reluctant to combine cold liver perfusion with oxygenation. In contrast to these reports, however, a significant number of oxygenated perfusion experiments in livers [15, 17, 19–21] and kidneys [22] have demonstrated only minimal oxidative stress during hypothermic oxygenated perfusion despite high oxygen concentrations.
Importantly, the rate of oxygen consumption during HMP decreases rapidly during the first hour and ceases after 90 min at a low baseline level because of downregulation of mitochondrial respiration despite increasing ADP and sufficient levels of substrates (oxygen and electron donors) [15, 23, 24]. Accordingly, during hypothermic oxygenated liver perfusion, mitochondria switch from a high-flux electron transfer stage at the beginning of machine perfusion to a low-flux electron transfer stage during approximately 60-90 min of perfusion. Conversely, in the complete absence of oxygen, mitochondria stay in a reduced high flux state and trigger high release of mitochondrial-derived reactive oxygen species (ROS) during the first minutes of reperfusion [25••). This in turn leads to the release of nuclear danger-associated molecular patterns (DAMPs) and subsequent downstream activation of nonparenchymal liver cells [15, 25••]. Therefore, protection of liver grafts against significant oxidative stress depends on sufficient mitochondrial oxygenation during hypothermic perfusion of livers before exposure to blood and oxygen at normothermic conditions.
Is low Perfusion Pressure Sufficient?
Another aspect of the debate regarding HMP relates to the determination of the optimal perfusion pressure necessary for cold machine liver perfusion (Tables 1 and 2). Because liver sinusoids are known to be very sensitive to endothelial shear stress, a balance is needed between the perfusion of a maximum of liver cells and minimal endothelial injury. Previous work in rat livers suggests that the reduction of portal perfusion pressure to 4 mmHg achieves both complete perfusion and no endothelial injury, while perfusion at 8 mmHg induced endothelial injury [15, 26]. Recent studies by our group in animal models and human livers confirm that a low portal perfusion pressure of 3 mmHg results in complete perfusion without evidence of sinusoidal impairment [15, 27••]. In contrast, hypothermic portal perfusion at higher pressures results in clear deterioration of endothelial cells [15]. Based on this, a significant reduction of the portal perfusion pressure to ≤3 mmHg appears to have a decisive and positive effect during cold oxygenated liver perfusion and minimizes the risk of endothelial shear stress.
Is Portal Perfusion Alone Adequate in Hypothermic Conditions?
The best perfusion route during HMP has been discussed widely. Some researchers argue that simultaneous delivery of the perfusate and oxygen through the portal vein and the hepatic artery would increase the benefit of HMP [28]. Due to the fact that portal and arterial branches join in the liver sinusoids, single portal perfusion allows for circulation to all hepatocytes in less than 1 min after the start of low-pressure cold perfusion in a pig model (Fig. 1). Considering the low oxygen demand of livers under cold temperatures, the supply of oxygen by single-portal perfusion appears sufficient, at least for hepatocytes, endothelial cells, Kupffer cells and intrahepatic interlobular biliary branches. Consistently, HMP in discarded human livers showed no difference in perfusion quality between singular perfusion of the portal vein or the hepatic artery alone versus dual perfusion [29]. Whether additional arterial hypothermic perfusion is useful for the viability of the extrahepatic bile ducts remains to be investigated. Recent studies suggest that most biliary injury is triggered through a cascade of mediators released during early reperfusion rather than by ischemia itself [21].
Fig. 1.
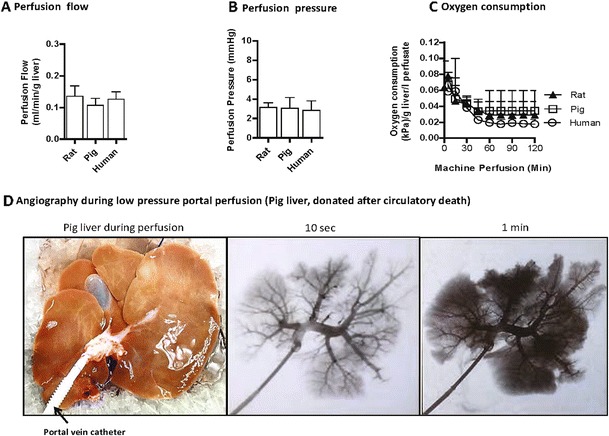
Perfusion flow at low pressure (3 mmHg) appears comparable for different species (rat, pig, human), if related to liver weight (A, B). Oxygen consumption during hypothermic machine liver perfusion decreases during the first hour of perfusion and stays at a low level during further perfusion (C). Angiography during low-pressure hypothermic perfusion of pig DCD livers demonstrates complete perfusion of all sinusoids within the first minutes by exclusively portal vein perfusion (D)
Is the Perfusion Medium Important?
The perfusates used during liver HMP in the majority of experiments are based on the original or modified UW (University of Wisconsin) solution or UW machine perfusion (MP) solution. Perfusion studies with IGL (Institut George Lopez), Celsior or HTK (Histidine-Tryptophan-Ketoglutarate) solutions have been performed (Tables 1 and 2); however, no conclusive comparison of machine liver perfusates is available. As low potassium concentrations decrease vascular resistance in hypothermia, the presence of starch increases viscosity. Therefore, solutions with low potassium and without starch appear advantageous [30], but an ideal perfusate for liver HMP has yet to be determined. Besides the fundamental implication of oxygen in the cold perfusate, additional “cleaning” effects on the sinusoidal glycocalyx are also important during liver HMP [15].
Hypothermic machine perfusion and subsequent transplantation of human livers were performed using CE-certified UW gluconate (KPS-1®, Belzer UW MP®) or with a modification of UW gluconate including α-ketoglutarate, L-arginine, N-acetylcysteine and prostaglandin E1 [16].
Is end-Ischemic Perfusion Effective?
End-ischemic perfusion is initiated after CS and transport of organs to the transplant center. Because transport of perfusion devices to the place of procurement is not needed, this concept is very practical and easy. In addition, recent research in rat, pig and human livers shows that despite a relatively long CS period of up to 8 h, end-ischemic hypothermic oxygenated perfusion protects liver grafts [12, 13, 15, 27••, 31, 32]. This effect is the result of significant uploading of liver energy stores within a short time and the rapid conversion of mitochondrial electron carriers from a reduced to oxidized stage [15]. Similar results have been reported by end-ischemic gaseous oxygen persufflation of livers and kidneys under hypothermic conditions [33, 34].
Assessment of Liver Injury During Hypothermic Machine Liver Perfusion
While several studies have analyzed organ injury during NMP [35–37], less is known about the behavior of liver grafts during HMP. Measurement of hepatocellular and mitochondrial injury during HMP provides useful information for graft assessment, but important questions remain and need to be addressed. Here we report on the current findings.
Markers of Hepatocellular Injury
Release of cytosolic hepatocyte enzymes, such as AST, ALT and LDH, is simple and can be measured in cold perfusates during liver HMP. Importantly, and as related to graft assessment, ALT release per gram of liver during HMP correlates with the release of ALT during reperfusion injury after transplantation [16]. Furthermore, effluent analysis during HMP allows the determination of several acute phase reactants [38].
The soluble fraction of cytokeratin-18 (CK18), the major filament protein in the liver, has been shown to be released into the extracellular space during liver cell death. As full-length CK19 is cleaved by caspases during apoptotic cell death, CK18 fragments are believed to present a means for monitoring epithelial apoptotic liver cell death [39•). Measurement of CK18 fragments is feasible during HMP, but future research is needed to introduce these new tests for use in regular graft assessment.
Small non-coding RNAs (21-25 nucleotides), known as microRNA (miRNA), play an important role in the regulation of gene expression. These RNAs are generated and exported from the nucleus. The most abundant liver-specific miRNA is miR-122, which is released into the circulation during hepatocyte damage [40] and is therefore detectable during HMP. Furthermore, increased miR-222 may display cholangiocyte damage and was suggested to predict cholangiopathy after liver transplantation [39•, 41, 42].
Markers of Mitochondrial Injury
Mitochondrial dysfunction causes either massive destruction of hepatocytes or sensitization of liver cells to a variety of otherwise non-lethal stress conditions. Executioner caspases and mitochondrial outer membrane permeabilization are critical for the death of hepatocytes, triggered by mitochondria [43]. The mechanisms of such mitochondria-derived injury are at least threefold:
Intracellular signaling: Several intracellular stress conditions are sensed by small members of the BCL-2 protein family (BH3-only proteins), which can activate mitochondrial outer membrane permeabilization by stimulating the pore-forming activity of BAX and BAK [43, 44].
Intramitochondrial signaling: outer membrane permeabilization can also be initiated at the inner mitochondrial membrane by an unspecific opening of the mitochondrial permeability transition pore complex (MPT-pore), resulting in the release of mitochondrial ROS and proteins, such as cytochrome C, direct inhibitor of apoptosis-binding protein with low pI (DIABLO) and apoptosis-inducing factor (AIF) [43, 45].
Extracellular signaling: death receptors of the tumor necrosis factor family (Fas) are activated by extracellular signals from the Fas ligands at the cell surface. Upon binding, these receptors form the intracellular death-inducing signaling complex (DISC) and trigger either cleavage of initiator caspase 8 or activation of pro-apoptotic proteins (Bid, Bax), resulting in the assembly of the apoptosome complex (cytochrome c, Apaf-1, caspase 9) and activation of caspase 3 [44].
Currently, there is no experience in differentiating the distinct signaling pathways of mitochondrial injury during HMP. However, future research may allow selective determination of liver epithelial injury, mitochondrial or nuclear-derived injury by measuring CK18 fragments, special miRNAs, mitochondrial membrane proteins (cytochrome c) or apoptosis-related proteins (SMAC, DIABLO) during cold machine liver perfusion.
Ischemia Reperfusion Injury of Liver Grafts
Mitochondrial Injury is a key Event
Efforts to restore blood flow in hypoxic tissue can paradoxically result in more destructive than beneficial effects, depending on the length of the ischemic period. Previous research indicates that the main damaging effects in ischemic reperfusion (I/R) injury involve reactions following restoration of blood flow to the tissue rather than the ischemia itself [25••). Under conditions of oxygen deficiency, oxygen molecules in the mitochondrial respiratory chain accept only one electron instead of four as accepted under normal conditions, therefore producing mitochondrial ROS (Fig. 2). The generation of ROS by mitochondria in turn triggers the MPT opening and subsequent nuclear damage and endoplasmic reticulum (ER) stress responses [25••]. Danger-associated molecular patterns (DAMPs) that are released by dying hepatocytes play a major role in this context [15, 46].
Fig. 2.
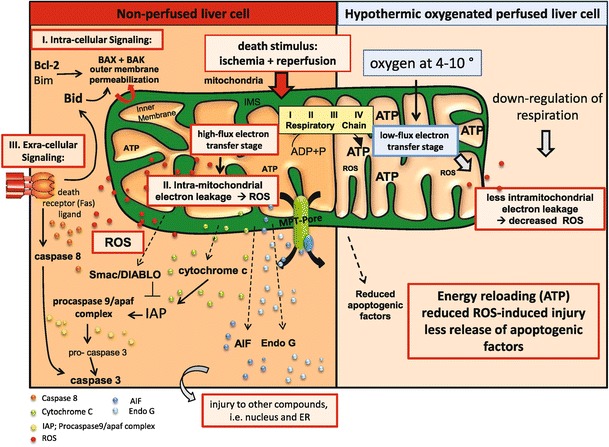
Physiological release of reactive oxygen species (ROS) occurs in mitochondria between complex II and III. During ischemia reperfusion, however, this ROS release increases significantly and can initiate opening of the mitochondrial permeability transition pore complex (MPT-pore). Subsequently, mitochondrial proteins (cytochrome C, Smac/DIABLO, endonuclease G, AIF) are released in the cytosol, which activate different cell organelles. Hypothermic oxygenated perfusion prevents the initial mitochondrial ROS release
DAMPs: Signals for Inflammation and Cell Death
DAMPs are a group of molecules that are exposed by dying cells to operate as modulators of sterile inflammation. By binding to specific pattern-recognition receptors, DAMPs exert immune-modulatory functions with a wide panel of proinflammatory factors. The high-mobility group box-1 (HMGB-1) protein is a highly conserved, abundant, non-histone nuclear protein expressed in almost all eukaryotic cells. Cellular effects of HMGB-1 are induced through signal pathway receptors, such as the receptor for advanced glycan end products (RAGE) and Toll-like receptor 4 (TLR4) [47]. Within the nucleus, HMGB-1 modulates and facilitates the transcription of many genes. Once released from cells, HMGB-1 acts as a component of the innate immune system alerting the host-to-cell stress (Fig. 3). HMGB-1 is hyperacetylated upon activation with LPS in monocytes and macrophages, while unacetylated HMGB-1 is thought to be released by necrotic cells. Further hepatotoxic effects originate from a downstream crosstalk among hepatocytes, tissue-resident immune cells (macrophages) and immune cells that are recruited to the site of injury [47].
Fig. 3.
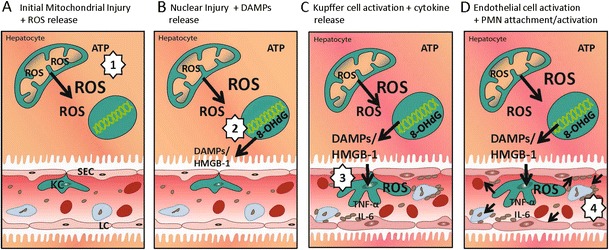
ROS release from mitochondria leads to nuclear injury and release of danger-associated molecular patterns (DAMPs) (A), which in turn stimulate Kupffer cells via Toll-like receptors once released by hepatocytes (B). Activated Kupffer cells release mediators and intravasal ROS (C), which activate downstream endothelial cells and platelets (D)
Mechanism of Protection by Hypothermic Oxygenated Perfusion
Attenuation of Oxidative Phosphorylation Pathway
An important and unexplained finding of hypothermic oxygenated liver perfusion is the discovery that it is possible to upload depleted cellular energy stores within a short perfusion time, even after significant warm and cold ischemia. While the exact mechanism of this phenomenon remains unclear, the low energy demand in hypothermic conditions and the continuous supply of oxygen favor a positive ATP balance, despite a rapid decrease in the mitochondrial respiration rate [15, 17, 48] (Fig. 2). Of note, hypothermic oxygenated liver perfusion is more effective in this respect as compared to normothermic oxygenated perfusion [49•].
Inhibition of Mitochondria-Derived ROS and Downstream Mediators
Hypothermic oxygenated machine perfusion changes the mitochondrial redox state by reversible suppression of oxidative metabolism, and subsequently decreases the initial ROS release during normothermic reperfusion [15]. Consequently, less mitochondrial injury triggers less release of mitochondrial ROS and therefore less nuclear injury via several pathways and results in less release of DAMPs into the circulation [46, 49•] (Fig. 3). In turn, Kupffer cell activation is decreased by hypothermic oxygenated perfusion with a smaller release of secondary mediators and less activation of neutrophils and platelets [25••, 49•] (Fig. 3). The effect of these reactions on the immune response (T cell activation) and biliary epithelial cells has been recently reported [21, 50].
Clinical Application of Hypothermic Liver Perfusion
The group at Columbia University in New York first perfused ten discarded human livers for 7 h through the portal vein and hepatic artery without active perfusate oxygenation, using median high flow rates of 0.7 ml/g liver /min. Liver quality was assessed by AST release in the perfusate [51]. In a subsequent clinical study, machine-perfused livers were implanted in 20 patients, with suggested improvement in early graft function, lower serum transaminase levels and shorter hospital stay compared to a historical group of cold-stored standard liver grafts donated after brain death (DBD). The study follow-up was limited to 3 months. Of importance, while the perfusate was not actively oxygenated, measurements of pO2 levels in the perfusate confirmed no change in perfusate oxygen concentrations during the entire HMP period [16], probably because of the high perfusion flow under hypothermic conditions.
Meanwhile, this group reported on 40 machine-perfused human liver grafts from DBD donors and demonstrated reduced activation of adhesions molecules as well as less activation of leukocytes in cold-perfused livers compared to cold-stored controls [52].
Two other groups [Royal Free Hospital, London [29] and KU Leuven [53] perfused discarded human livers for up to 24 h with an assessment of liver viability by liver enzyme release and morphology during cold perfusion. In one additional study, Vekemans et al. randomly allocated 27 discarded human livers to cold storage or hypothermic perfusion for 4 h (total preservation time of 15-17 h). The degree of reperfusion injury was detected during ex vivo reperfusion of all liver grafts at normal body temperature with diluted red blood cells for 2 h, only showing reduced AST and LDH release in the perfusion group as compared to cold-stored livers, but no morphological difference could be identified [54].
Over the last decade, another technique of HMP was developed from our group, delivering a hyperbaric oxygenated perfusate (>50 kPa pO2) through the portal vein at low pressure (<3 mmHg) for 1-2 h after a certain period of CS immediately prior to graft implantation. This perfusion technique has been tested in human DCD liver grafts [27••]. The application of this perfusion method, termed hypothermic oxygenated perfusion (HOPE), was the result of 15 years of experimental work in numerous animal models, which have shown strong protective effects, despite its short application through the portal vein only [12, 15, 19, 21, 31, 49•, 50, 55–57]. The rationale for applying such machine perfusion in DCD liver grafts was based on national ethical specifications that have prevented the procurement of grafts with less than 10 min warm ischemia after declaration of cardiac death (asystolic warm ischemia <10 min). In its inaugural clinical application, HOPE was applied consistently to eight extended human DCD liver grafts with prolonged asystolic warm ischemia periods up to 20 min (asystolic time) and increased donor age (median 54 years), a figure that would prevent the use of liver grafts in most centers [58, 59]. In this first human series, involving a 6-month follow-up after HOPE in DCD livers, no intrahepatic cholangiopathies were detected. Of note, and in contrast to all other perfusion strategies that need to be started at the site of procurement and require continuous pumping, this novel perfusion setting is highly simplified and low cost. Our center applied the HOPE technique in 25 human DCD livers with excellent and immediate graft function in all cases and no evidence of intrahepatic biliary injury in spite of long donor warm ischemia.
Conclusions
HMP can protect liver grafts exposed to warm and cold ischemia. This effect is probably linked closely to changes in mitochondrial electron transfer rates during cold machine perfusion and therefore depends on the abundance of oxygen during perfusion. Short-term (1-2 h) perfusion appears as effective as longer perfusion (10 h). Application of end-ischemic hypothermic oxygenated perfusion in human DCD livers was well tolerated in recent transplant series. Randomized trials are needed to further assess the full protective action of liver HMP, in particular with respect to biliary complications after liver transplantation.
Acknowledgments
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
A. Schlegel, P. Kron and P. Dutkowski declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Abbreviations
- CS
cold storage
- NMP
normothermic machine perfusion
- SNMP
subnormothermic machine perfusion
- HMP
hypothermic machine perfusion
- DCD
donation after cardiac death
- ROS
reactive oxygen species
- DAMPs
danger-associated molecular patterns
- UW
University of Wisconsin
- MP
machine perfusion
- IGL-1
Institute George Lopez-1
- HTK
histidine-tryptophan-ketoglutarate
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- LDH
lactate dehydrogenase
- BCL-2 protein
B cell lymphoma 2 protein
- BH-3
Bcl-2 homology-3
- BID
BH-3-interacting domain
- BAX
Bcl-2-associated x protein
- BAK
Bcl-2-antagonist/killer-1
- MPT Pore
mitochondria permeability transition pore
- IAP binding protein
inhibitor of apoptosis protein
- DIABLO
direct IAP-binding protein with low pI
- AIF
apoptosis-inducing factor
- CK 18
cytokeratin-18
- DISC
death-inducing signaling complex
- miRNA
micro-RNA
- ER
endoplasmic reticulum
- HMGB-1
high-mobility group box protein 1
- RAGE
receptor for advanced glycation end products
- TLR-4
roll-like receptor 4
- LPS
lipopolysaccharides
- ATP
adenosine triphosphate
- HOPE
hypothermic oxygenated perfusion
- DBD
donation after brain death
Footnotes
This article is part of the Topical Collection on Machine Preservation of the Liver
References
Papers of particular interest and published recently are highlighted below as: • Of importance •• Of major importance
- 1.Dutkowski P, de Rougemont O, Clavien PA. Alexis Carrel: genius, innovator and ideologist. Am J Transplant. 2008;8(10):1998. doi: 10.1111/j.1600-6143.2008.02364.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto N, Konishi Y, Wakashiro S, et al. Seventy-two-hour preservation of porcine liver by continuous hypothermic perfusion with UW solution in comparison with simple cold storage. J Surg Res. 1991;51(4):288. doi: 10.1016/0022-4804(91)90109-Y. [DOI] [PubMed] [Google Scholar]
- 3.Pienaar BH, Lindell SL, Van Gulik T, Southard JH, Belzer FO. Seventy-two-hour preservation of the canine liver by machine perfusion. Transplantation. 1990;49(2):258. doi: 10.1097/00007890-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 4.D'Alessandro AM, Kalayoglu M, Sollinger HW, et al. Experience with Belzer UW cold storage solution in human liver transplantation. Transplant Proc. 1990;22(2):474. [PubMed] [Google Scholar]
- 5.Isemer FE, Ludwig A, Schunck O, Bretschneider HJ, Peiper HJ. Kidney procurement with the HTK solution of Bretschneider. Transplant Proc. 1988;20(5):885. [PubMed] [Google Scholar]
- 6.Menasche P, Termignon JL, Pradier F, et al. Experimental evaluation of Celsior, a new heart preservation solution. Eur J Cardiothorac Surg. 1994;8(4):207. doi: 10.1016/1010-7940(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 7.Brockmann J, Reddy S, Coussios C, et al. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250(1):1. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 8.Fondevila C, Hessheimer AJ, Maathuis MH, et al. Superior preservation of DCD livers with continuous normothermic perfusion. Ann Surg. 2011;254(6):1000. doi: 10.1097/SLA.0b013e31822b8b2f. [DOI] [PubMed] [Google Scholar]
- 9.op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13(5):1327. doi: 10.1111/ajt.12187. [DOI] [PubMed] [Google Scholar]
- 10.Tolboom H, Izamis ML, Sharma N, et al. Subnormothermic machine perfusion at both 20 degrees C and 30 degrees C recovers ischemic rat livers for successful transplantation. J Surg Res. 2012;175(1):149. doi: 10.1016/j.jss.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruinsma BG, Yeh H, Ozer S, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant. 2014;14(6):1400. doi: 10.1111/ajt.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutkowski P, Furrer K, Tian Y, Graf R, Clavien PA. Novel short-term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non-heart beating donor. Ann Surg. 2006;244(6):968. doi: 10.1097/01.sla.0000247056.85590.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CY, Jain S, Duncan HM, et al. Survival transplantation of preserved non-heart-beating donor rat livers: preservation by hypothermic machine perfusion. Transplantation. 2003;76(10):1432. doi: 10.1097/01.TP.0000088674.23805.0F. [DOI] [PubMed] [Google Scholar]
- 14.Graham JA, Guarrera JV. “Resuscitation” of marginal liver allografts for transplantation with machine perfusion technology. J Hepatol. 2014;61(2):418. doi: 10.1016/j.jhep.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Schlegel A, Rougemont O, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58(2):278. doi: 10.1016/j.jhep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10(2):372. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 17.Luer B, Koetting M, Efferz P, Minor T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl Int. 2010;23(9):944. doi: 10.1111/j.1432-2277.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 18.Rauen U, Petrat F, Li T, De Groot H. Hypothermia injury/cold-induced apoptosis–evidence of an increase in chelatable iron causing oxidative injury in spite of low O2-/H2O2 formation. FASEB J. 2000;14(13):1953. doi: 10.1096/fj.00-0071com. [DOI] [PubMed] [Google Scholar]
- 19.Dutkowski P, Schonfeld S, Odermatt B, Heinrich T, Junginger T. Rat liver preservation by hypothermic oscillating liver perfusion compared to simple cold storage. Cryobiology. 1998;36(1):61. doi: 10.1006/cryo.1997.2066. [DOI] [PubMed] [Google Scholar]
- 20.Dutkowski P, Krug A, Krysiak M, Dunschede F, Seifert JK, Junginger T. Detection of mitochondrial electron chain carrier redox status by transhepatic light intensity during rat liver reperfusion. Cryobiology. 2003;47(2):125. doi: 10.1016/j.cryobiol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Schlegel A, Graf R, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol. 2013;59(5):984. doi: 10.1016/j.jhep.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer DP, Gallinat A, Swoboda S, et al. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation. 2014;98(9):944. doi: 10.1097/TP.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 23.Monbaliu D, Vekemans K, De Vos R, et al. Hemodynamic, biochemical, and morphological characteristics during preservation of normal porcine livers by hypothermic machine perfusion. Transplant Proc. 2007;39(8):2652. doi: 10.1016/j.transproceed.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Dirkes MC, Post IC, Heger M, van Gulik TM. A novel oxygenated machine perfusion system for preservation of the liver. Artif Organs. 2013;37(8):719. doi: 10.1111/aor.12071. [DOI] [PubMed] [Google Scholar]
- 25.••.van Golen RF, van Gulik TM, Heger M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med. 2012;52(8):1382. doi: 10.1016/j.freeradbiomed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 26.t’Hart NA, der van Plaats A, Leuvenink HG, et al. Determination of an adequate perfusion pressure for continuous dual vessel hypothermic machine perfusion of the rat liver. Transpl Int. 2007;20(4):343. doi: 10.1111/j.1432-2277.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 27.••.Dutkowski P, Schlegel A, de Oliveira M, Mullhaupt B, Neff F, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60(4):765. doi: 10.1016/j.jhep.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Op den Dries S, Sutton ME, Karimian N, et al. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS One. 2014;9(2):e88521. doi: 10.1371/journal.pone.0088521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jomaa A, Gurusamy K, Siriwardana PN, et al. Does hypothermic machine perfusion of human donor livers affect risks of sinusoidal endothelial injury and microbial infection? A feasibility study assessing flow parameters, sterility, and sinusoidal endothelial ultrastructure. Transplant Proc. 2013;45(5):1677. doi: 10.1016/j.transproceed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Schlegel A, Dutkowski P. Role of hypothermic machine perfusion in liver transplantation. Transpl Int. 2014. [DOI] [PubMed]
- 31.de Rougemont O, Breitenstein S, Leskosek B, et al. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg. 2009;250(5):674. doi: 10.1097/SLA.0b013e3181bcb1ee. [DOI] [PubMed] [Google Scholar]
- 32.Lee CY, Zhang JX, Jones JW, Jr, Southard JH, Clemens MG. Functional recovery of preserved livers following warm ischemia: improvement by machine perfusion preservation. Transplantation. 2002;74(7):944. doi: 10.1097/00007890-200210150-00008. [DOI] [PubMed] [Google Scholar]
- 33.Minor T, Koetting M, Kaiser G, Efferz P, Luer B, Paul A. Hypothermic reconditioning by gaseous oxygen improves survival after liver transplantation in the pig. Am J Transplant. 2011;11(12):2627. doi: 10.1111/j.1600-6143.2011.03731.x. [DOI] [PubMed] [Google Scholar]
- 34.Minor T, Efferz P, Luer B. Hypothermic reconditioning by gaseous oxygen persufflation after cold storage of porcine kidneys. Cryobiology. 2012;65(1):41. doi: 10.1016/j.cryobiol.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Butler AJ, Rees MA, Wight DG, et al. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation. 2002;73(8):1212. doi: 10.1097/00007890-200204270-00005. [DOI] [PubMed] [Google Scholar]
- 36.Imber CJ, St Peter SD. Lopez de Cenarruzabeitia I, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002;73(5):701. doi: 10.1097/00007890-200203150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Sutton ME. Op den Dries S, Karimian N, et al. Criteria for Viability Assessment of Discarded Human Donor Livers during Ex Vivo Normothermic Machine Perfusion. PLoS One. 2014;9(11):e110642. doi: 10.1371/journal.pone.0110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tulipan JE, Stone J, Samstein B, et al. Molecular expression of acute phase mediators is attenuated by machine preservation in human liver transplantation: preliminary analysis of effluent, serum, and liver biopsies. Surgery. 2011;150(2):352. doi: 10.1016/j.surg.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 39.•.Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol. 2014;60(5):1063. doi: 10.1016/j.jhep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Steer CJ, Subramanian S. Circulating microRNAs as biomarkers: a new frontier in diagnostics. Liver Transpl. 2012;18(3):265. doi: 10.1002/lt.23377. [DOI] [PubMed] [Google Scholar]
- 41.Verhoeven CJ, Farid WR, de Ruiter PE, et al. MicroRNA profiles in graft preservation solution are predictive of ischemic-type biliary lesions after liver transplantation. J Hepatol. 2013;59(6):1231. doi: 10.1016/j.jhep.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 42.Farid WR, Pan Q, van der Meer AJ, et al. Hepatocyte-derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transpl. 2012;18(3):290. doi: 10.1002/lt.22438. [DOI] [PubMed] [Google Scholar]
- 43.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23(16):2861. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 44.Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003;22(17):4385. doi: 10.1093/emboj/cdg423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304(3):487. doi: 10.1016/S0006-291X(03)00621-1. [DOI] [PubMed] [Google Scholar]
- 46.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14(7):1315. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Golen RF, van Gulik TM, Heger M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev. 2012;23(3):69. doi: 10.1016/j.cytogfr.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 48.van Golen RF, Reiniers MJ, van Gulik TM, Heger M. Organ cooling in liver transplantation and resection: How low should we go? Hepatology. 2014. [DOI] [PubMed]
- 49.•.Schlegel A, Kron P, Graf R, Dutkowski P, Clavien PA. Warm vs. cold perfusion technique to rescue rodent liver grafts. J Hepatol. 2014. This study provides the first comparison of hypothermic and normothermic perfusion in a model of DCD rat liver transplantation. [DOI] [PubMed]
- 50.Schlegel A, Kron P, Graf R, Clavien PA, Dutkowski P. Hypothermic Oxygenated Perfusion (HOPE) Downregulates the Immune Response in a Rat Model of Liver Transplantation. Ann Surg. 2014;260(5):931. doi: 10.1097/SLA.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 51.Guarrera JV, Estevez J, Boykin J, et al. Hypothermic machine perfusion of liver grafts for transplantation: technical development in human discard and miniature swine models. Transplant Proc. 2005;37(1):323. doi: 10.1016/j.transproceed.2004.12.094. [DOI] [PubMed] [Google Scholar]
- 52.Henry SD, Nachber E, Tulipan J, et al. Hypothermic machine preservation reduces molecular markers of ischemia/reperfusion injury in human liver transplantation. Am J Transplant. 2012;12(9):2477. doi: 10.1111/j.1600-6143.2012.04086.x. [DOI] [PubMed] [Google Scholar]
- 53.Monbaliu D, Liu Q, Libbrecht L, et al. Preserving the morphology and evaluating the quality of liver grafts by hypothermic machine perfusion: a proof-of-concept study using discarded human livers. Liver Transpl. 2012;18(12):1495. doi: 10.1002/lt.23550. [DOI] [PubMed] [Google Scholar]
- 54.Vekemans K, van Pelt J, Komuta M, et al. Attempt to rescue discarded human liver grafts by end ischemic hypothermic oxygenated machine perfusion. Transplant Proc. 2011;43(9):3455. doi: 10.1016/j.transproceed.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 55.Dutkowski P, Schonfeld S, Heinrich T, et al. Reduced oxidative stress during acellular reperfusion of the rat liver after hypothermic oscillating perfusion. Transplantation. 1999;68(1):44. doi: 10.1097/00007890-199907150-00009. [DOI] [PubMed] [Google Scholar]
- 56.Dutkowski P, Odermatt B, Heinrich T, et al. Hypothermic oscillating liver perfusion stimulates ATP synthesis prior to transplantation. J Surg Res. 1998;80(2):365. doi: 10.1006/jsre.1998.5491. [DOI] [PubMed] [Google Scholar]
- 57.Dutkowski P, Graf R, Clavien PA. Rescue of the cold preserved rat liver by hypothermic oxygenated machine perfusion. Am J Transplant. 2006;6(5 Pt 1):903. doi: 10.1111/j.1600-6143.2006.01264.x. [DOI] [PubMed] [Google Scholar]
- 58.Hong JC, Yersiz H, Kositamongkol P, et al. Liver transplantation using organ donation after cardiac death: a clinical predictive index for graft failure-free survival. Arch Surg. 2011;146(9):1017. doi: 10.1001/archsurg.2011.240. [DOI] [PubMed] [Google Scholar]
- 59.Taner CB, Bulatao IG, Perry DK, et al. Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors. Transpl Int. 2012;25(8):838. doi: 10.1111/j.1432-2277.2012.01508.x. [DOI] [PubMed] [Google Scholar]
- 60.Southard JH, Lindell S, Ametani M, Richer JP, Vos AW. Kupffer cell activation in liver preservation: cold storage vs machine perfusion. Transplant Proc. 2000;32(1):27. doi: 10.1016/S0041-1345(99)00862-3. [DOI] [PubMed] [Google Scholar]
- 61.Compagnon P, Clement B, Campion JP, Boudjema K. Effects of hypothermic machine perfusion on rat liver function depending on the route of perfusion. Transplantation. 2001;72(4):606. doi: 10.1097/00007890-200108270-00008. [DOI] [PubMed] [Google Scholar]
- 62.So PW, Fuller BJ. A comparison of the metabolic effects of continuous hypothermic perfusion or oxygenated persufflation during hypothermic storage of rat liver. Cryobiology. 2001;43(3):238. doi: 10.1006/cryo.2001.2347. [DOI] [PubMed] [Google Scholar]
- 63.Minor T, Olschewski P, Tolba RH, Akbar S, Kocalkova M, Dombrowski F. Liver preservation with HTK: salutary effect of hypothermic aerobiosis by either gaseous oxygen or machine perfusion. Clin Transplant. 2002;16(3):206. doi: 10.1034/j.1399-0012.2002.01128.x. [DOI] [PubMed] [Google Scholar]
- 64.Lauschke H, Olschewski P, Tolba R, Schulz S, Minor T. Oxygenated machine perfusion mitigates surface antigen expression and improves preservation of predamaged donor livers. Cryobiology. 2003;46(1):53. doi: 10.1016/S0011-2240(02)00164-5. [DOI] [PubMed] [Google Scholar]
- 65.Jain S, Xu H, Duncan H, et al. Ex-vivo study of flow dynamics and endothelial cell structure during extended hypothermic machine perfusion preservation of livers. Cryobiology. 2004;48(3):322. doi: 10.1016/j.cryobiol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Xu H, Lee CY, Clemens MG, Zhang JX. Pronlonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation. 2004;77(11):1676. doi: 10.1097/01.TP.0000129644.23075.71. [DOI] [PubMed] [Google Scholar]
- 67.Bessems M, Doorschodt BM, van Vliet AK, van Gulik TM. Machine perfusion preservation of the non-heart-beating donor rat livers using polysol, a new preservation solution. Transplant Proc. 2005;37(1):326. doi: 10.1016/j.transproceed.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 68.Bessems M, Doorschodt BM, Hooijschuur O, van Vliet AK, van Gulik TM. Optimization of a new preservation solution for machine perfusion of the liver: which is the preferred colloid? Transplant Proc. 2005;37(1):329. doi: 10.1016/j.transproceed.2004.12.220. [DOI] [PubMed] [Google Scholar]
- 69.Xu H, Zhang JX, Jones JW, Southard JH, Clemens MG, Lee CY. Hypothermic machine perfusion of rat livers preserves endothelial cell function. Transplant Proc. 2005;37(1):335. doi: 10.1016/j.transproceed.2004.11.075. [DOI] [PubMed] [Google Scholar]
- 70.Jain S, Lee CY, Baicu S, et al. Hepatic function in hypothermically stored porcine livers: comparison of hypothermic machine perfusion vs cold storage. Transplant Proc. 2005;37(1):340. doi: 10.1016/j.transproceed.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 71.Bessems M, Doorschodt BM, van Vliet AK, van Gulik TM. Improved rat liver preservation by hypothermic continuous machine perfusion using polysol, a new, enriched preservation solution. Liver Transpl. 2005;11(5):539. doi: 10.1002/lt.20388. [DOI] [PubMed] [Google Scholar]
- 72.t’Hart NA, van der Plaats A, Leuvenink HG, et al. Hypothermic machine perfusion of the liver and the critical balance between perfusion pressures and endothelial injury. Transplant Proc. 2005;37(1):332. doi: 10.1016/j.transproceed.2004.12.090. [DOI] [PubMed] [Google Scholar]
- 73.van der Plaats A, Maathuis MH, NA TH, et al. The Groningen hypothermic liver perfusion pump: functional evaluation of a new machine perfusion system. Ann Biomed Eng. 2006;34(12):1924. doi: 10.1007/s10439-006-9207-4. [DOI] [PubMed] [Google Scholar]
- 74.Bessems M, Doorschodt BM, Kolkert JL, et al. Preservation of steatotic livers: a comparison between cold storage and machine perfusion preservation. Liver Transpl. 2007;13(4):497. doi: 10.1002/lt.21039. [DOI] [PubMed] [Google Scholar]
- 75.Vekemans K, Liu Q, Brassil J, Komuta M, Pirenne J, Monbaliu D. Influence of flow and addition of oxygen during porcine liver hypothermic machine perfusion. Transplant Proc. 2007;39(8):2647. doi: 10.1016/j.transproceed.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Manekeller S, Schuppius A, Stegemann J, Hirner A, Minor T. Role of perfusion medium, oxygen and rheology for endoplasmic reticulum stress-induced cell death after hypothermic machine preservation of the liver. Transpl Int. 2008;21(2):169. doi: 10.1111/j.1432-2277.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 77.Jain S, Lee SH, Korneszczuk K, et al. Improved preservation of warm ischemic livers by hypothermic machine perfusion with supplemented University of Wisconsin solution. J Invest Surg. 2008;21(2):83. doi: 10.1080/08941930701883657. [DOI] [PubMed] [Google Scholar]
- 78.Stegemann J, Minor T. Energy charge restoration, mitochondrial protection and reversal of preservation induced liver injury by hypothermic oxygenation prior to reperfusion. Cryobiology. 2009;58(3):331. doi: 10.1016/j.cryobiol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Liu Q, Vekemans K, van Pelt J, et al. Discriminate liver warm ischemic injury during hypothermic machine perfusion by proton magnetic resonance spectroscopy: a study in a porcine model. Transplant Proc. 2009;41(8):3383. doi: 10.1016/j.transproceed.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 80.Stegemann J, Hirner A, Rauen U, Minor T. Use of a new modified HTK solution for machine preservation of marginal liver grafts. J Surg Res. 2010;160(1):155. doi: 10.1016/j.jss.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 81.Monbaliu DR, Debbaut C, Hillewaert WJ, et al. Flow competition between hepatic arterial and portal venous flow during hypothermic machine perfusion preservation of porcine livers. Int J Artif Organs. 2012;35(2):119. doi: 10.5301/ijao.5000038. [DOI] [PubMed] [Google Scholar]
- 82.Giannone FA, Trere D, Domenicali M, et al. An innovative hyperbaric hypothermic machine perfusion protects the liver from experimental preservation injury. ScientificWorldJournal. 2012;2012:573410. doi: 10.1100/2012/573410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Q, Vekemans K, Iania L, et al. Assessing warm ischemic injury of pig livers at hypothermic machine perfusion. J Surg Res. 2013. [DOI] [PubMed]
- 84.Uchiyama M, Matsuno N, Hama K, et al. Comparison between nonpulsatile and pulsatile machine perfusion preservation in liver transplantation from non-heart-beating donors. Transplant Proc. 2001;33(1–2):936. doi: 10.1016/S0041-1345(00)02276-4. [DOI] [PubMed] [Google Scholar]
- 85.Iwamoto H, Matsuno N, Narumi Y, et al. Beneficial effect of machine perfusion preservation on liver transplantation from non-heart-beating donors. Transplant Proc. 2000;32(7):1645. doi: 10.1016/S0041-1345(00)01437-8. [DOI] [PubMed] [Google Scholar]
- 86.Vekemans K, Liu Q, Heedfeld V, et al. Hypothermic Liver Machine Perfusion With EKPS-1 Solution vs Aqix RS-I Solution: In Vivo Feasibility Study in a Pig Transplantation Model. Transplant Proc. 2009;41(2):617. doi: 10.1016/j.transproceed.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Monbaliu D, Heedfeld V, Liu Q, et al. Hypothermic machine perfusion of the liver: is it more complex than for the kidney? Transplant Proc. 2011;43(9):3445. doi: 10.1016/j.transproceed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 88.Fondevila C, Hessheimer AJ, Maathuis MH, et al. Hypothermic oxygenated machine perfusion in porcine donation after circulatory determination of death liver transplant. Transplantation. 2012;94(1):22. doi: 10.1097/TP.0b013e31825774d7. [DOI] [PubMed] [Google Scholar]


