Abstract
Phage that display a surface peptide with the NGR sequence motif home selectively to tumor vasculature in vivo. A drug coupled to an NGR peptide has more potent antitumor effects than the free drug [W. Arap et al., Science (Washington DC), 279: 377–380, 1998]. We show here that the receptor for the NGR peptides in tumor vasculature is aminopeptidase N (APN; also called CD13). NGR phage specifically bound to immunocaptured APN and to cells engineered to express APN on their surface. Antibodies against APN inhibited in vivo tumor homing by the NGR phage. Immunohistochemical staining showed that APN expression is up-regulated in endothelial cells within mouse and human tumors. In another tissue that undergoes angiogenesis, corpus luteum, blood vessels also expressed APN, but APN was not detected in blood vessels of various other normal tissues stained under the same conditions. APN antagonists specifically inhibited angiogenesis in chorioallantoic membranes and in the retina and suppressed tumor growth. Thus, APN is involved in angiogenesis and can serve as a target for delivering drugs into tumors and for inhibiting angiogenesis.
INTRODUCTION
Angiogenesis, the formation of new blood vessels, is a rate-limiting step in solid tumor growth (1–3). Angiogenic blood vessels express markers that are either present at very low levels or are entirely absent in normal blood vessels. Such markers include the αvβ3 and αvβ5 integrins (4, 5), certain receptors for vascular growth factors (6, 7), matrix metalloproteases (8, 9), and other types of molecules, such as a high-molecular-weight proteoglycan (10–12). The growth factor receptors and integrins are not only markers for angiogenesis, they also play an important functional role in this process (13, 14).
We have previously probed tumor vasculature by screening random peptide libraries displayed on phage for the ability of the phage to home to tumors in vivo. Selection of phage from the libraries in tumor-bearing mice yielded three peptide motifs capable of homing to tumor vasculature: an RGD (arginine-glycine-aspartic acid) motif embedded in a double-cyclic peptide (termed RGD-4C), an NGR (asparagine-glycine-arginine) motif, and a GSL (glycine-serine-leucine) motif. Coupling an anticancer drug or a proapoptotic peptide to the RGD-4C or CNGRC peptides yielded compounds with increased efficacies against tumors and lowered toxicity to normal tissues in mice (15, 16). Of the ~25 known integrins, many recognize the RGD motif as a central feature of their binding site in their extracellular matrix ligands (17). The binding specificity of an RGD peptide for the individual integrins depends on the sequence surrounding the RGD motif and on the conformation of the peptide (17). The RGD-4C peptide binds selectively to the αvβ3 and αvβ5 integrins (18), which are specifically expressed in angiogenesis (3, 4) and serve as receptors for RGD-4C (15, 19). A cyclic NGR peptide, CNGRC, homes into tumors more effectively than linear peptides containing the NGR motif (15).
The NGR motif resembles RGD, and NGR peptides can bind to integrins, but the affinity of NGR peptides to integrins is lower than the affinity of RGD-integrin binding (18, 20). Despite the integrin binding by the NGR peptides, the RGD-4C peptide does not effectively compete with the tumor homing of a phage displaying the NGR motif (15), indicating that the receptor for NGR is different from the receptors for the RGD-4C peptide, the αv integrins.
Here we show that peptides containing the NGR motif bind to APN5 (also known as CD13; Ref. 21). APN is a membrane-spanning, Mr 140,000 cell surface protein that is expressed in various epithelial cells and in macrophages (21–23). APN is thought to play a role in chemokine processing and tumor invasion (24–26). We also show that the only vascular structures with detectable APN are tumor blood vessels and other types of vessels undergoing angiogenesis. In addition, APN antagonists are antiangiogenic in vivo. These findings indicate that APN plays a functional role in angiogenesis.
MATERIALS AND METHODS
Antibodies, Enzyme Inhibitors, and Cell Lines
The antihuman APN antibody WM15 was from PharMingen. Antimouse APN antibodies R3-63 and 2M-7 have been described (27). The anti-APN antibody RC38 was a gift from Dr. E. Oosterwijk (University Hospital of Nijmegen, the Netherlands; Ref. 28). The APN inhibitors, bestatin and actinonin, and the serine protease inhibitor, leupeptin, were from Sigma Chemical Co; bFGF and tumor necrosis factor-α were from R&D Systems. MDA-MB-435 human breast carcinoma cells and Molt-4 human T cell leukemia cells were from American Type Culture Collection. KS1767 human Kaposi’s sarcoma cells were obtained from Dr. J. A. Levy (University of California, San Francisco, CA).
Transfection of APN cDNA and Cell Surface Expression of APN
MDA-MB-435 cells were transfected by the calcium phosphate method with 20 μg of full-length APN or CD20 cDNA in the retroviral vector pZIP(SV)X-1 (a gift from Dr. Richard Mulligan, Children’s Hospital, Boston, MA) or the vector alone, as described (29). Molt-4 cells were electroporated with the APN cDNA inserted in the RcRSV mammalian expression vector (Invitrogen). Cells expressing high levels of APN on the cell surface were selected by culturing in G418-containing medium, followed by three sequential rounds of fluorescence-activated cell sorter sorting with the WM15 anti-APN antibody.
Isolation of APN and Assay of Enzymatic Activity
APN was extracted with 50 mM octylglucoside from KS1767 Kaposi’s sarcoma xenografts grown in nude mice (15). APN activity was measured with Ala-pNA and Leu-pNA substrates (29) in the presence or absence of the metalloenzyme inhibitor o-phenatroline (Sigma).
Phage Binding Assays
Phage that expressed peptides fused to the p111 surface protein (30) were used in binding assays as described (18, 31). In these studies, the NGR phage were CNGRCVSGCAGRC and CVLNGRMEC, referred to as NGR-phage, (15). To test phage binding to APN, APN was immunocaptured to microtiter wells coated with 10 μg/ml of purified anti-APN antibody WM15 or with BSA. APN was bound to the antibody from an octylglucoside extract of KS1767 Kaposi’s sarcoma tumors grown in nude mice. These KS1767 cells express APN on their surface (result not shown). The extract was incubated in antibody-coated and control wells for 1 h, the wells were washed, and 2 X 109 TUs of phage was added. After a 2-h incubation at room temperature, the wells were washed, and bound phage were quantitated by plating with bacteria (31).
Cytotoxicity Assay in Vitro
MDA-MB-435 cells and their APN-transfectants were exposed to dox or a dox-CNGRC peptide conjugate (15) in 96-well microtiter plates at 10 μg/well of the drug. After 20 min of drug exposure, unbound drug was removed through extensive washing with DMEM, and fresh medium was added. Surviving cells were quantitated at 24 h as described (31).
In Vivo Tumor Studies
MDA-MB-435 mammary fat pad tumors were grown to a diameter of 0.5–1 cm3 (15). The homing of i.v. injected phage to tumors was assessed by coinjecting 250 μg of affinity-purified R3-63 antibody or normal rat IgG with the phage. As an internal control, phage with no insert and an ampicillin marker were used (32). In tumor growth studies, mice with size-matched MDA-MB-435 tumors were treated i.p. with APN inhibitors in 200 μl of DMEM or DMEM alone, and tumor volume was monitored. Tissues were processed for immunohistochemistry as described (15). The animal procedures were carried out under Avertin (0.017 ml/g) anesthesia. All animal experimentation was reviewed and approved by the Animal Research Committee of The Burnham Institute.
Angiogenesis Assays
In the retinal neovascularization model (33), 1-week-old mice were exposed to 75% oxygen in air for 5 days. After 5 more days in room air, the proliferative neovascular response in the retina was quantified in H&E-stained sections by counting the nuclei of neovessels extending from the retina into the vitreous in 6-μm cross-sections. CAM assays were performed as described (34).
Immunohistology
Mouse tissues were processed as described (15). Human tissue samples were fixed in 4% paraformaldehyde, and sections were stained with various antibodies by using immunoperoxidase detection with the Vectastain ABC Elite kit (Vector Laboratories) and the Metal-enhanced DAB substrate (Pierce). Alternatively, fluorescently labeled antimouse IgG (Sigma) was used for detecting the primary antibody.
RESULTS
Binding of NGR Phage to APN
We have previously identified the RGD-binding peptide motif, WN/D DGWL (31). This motif resembles a site in certain integrin β subunits that participates in binding RGD-containing integrin ligands. This motif is also present in the extracellular domain of APN and some other aminopeptidases. Given the similarity of the RGD and NGR motifs, we hypothesized that the receptor for the NGR phage in tumor vasculature might be an aminopeptidase.
We tested the binding of NGR phage to APN immunocaptured onto microtiter wells. Two different NGR phage bound specifically to APN-containing wells, whereas the tumor-homing RGD-4C phage and another RGD phage showed no binding (Fig. 1A). The antibody used to capture APN did not bind the NGR phage without bound APN (not shown). The specificity of the binding was further examined by inhibiting the APN binding of one of the NGR phage with soluble peptides. Soluble CNGRC peptide blocked the binding of NGR phage to APN. Two other cyclic peptides had no effect (Fig. 1B).
Fig. 1.
Specific binding of NGR phage to isolated APN, to APN-transfected cells, and to APN in tumors. A, binding of phage to immunocaptured APN. Phage displaying the RGD or the NGR peptide in different sequence contexts were added at 2 X 109 TUs/well to microtiter wells that had been coated with anti-APN and treated with APN-containing cell extract or with BSA. Data represent the means of bound phage from triplicate wells; bars, SE. B, Inhibition of the binding of NGR phage to APN by soluble NGR peptide. Phage (2 X 109 TUs) were added to microtiter wells, together with 200 μg/well of the indicated peptides, and bound phage were quantitated (means from triplicate wells; bars, SE). C, Binding of NGR phage to APN-transfected cells. APN-transfected and control cells were incubated with CNGRC phage (2 X 109 TUs) and the indicated amounts of the CNGRC or CARAC (control) peptide, and bound phage were quantitated (means from triplicate wells; bars, SE). D, Inhibition of tumor homing of NGR phage by anti-APN. Mice bearing size-matched MDA-MB-435 tumors were coninjected with 109 TUs/mouse of the indicated phage, together with anti-APN IgG or normal rat IgG. The number of phage TUs recovered from the tumors is shown (means; n = 3; bars, SE).
Molt-4 T-cell leukemia and MDA-MB-435 breast carcinoma cells transfected with APN cDNA (29), but not cells transfected with empty vector or with CD20 cDNA, bound the NGR phage (Fig. 1C and data not shown). The binding of NGR phage to cells expressing APN was blocked by the CNGRC peptide in a dose-dependent manner but was not blocked by a control peptide of a similar general structure (CARAC).
Homing of NGR-Phage
The in vivo homing of the CNGRC phage to tumors was blocked by coinjection of a rat antimouse APN antibody (R3-63; Fig. 1D). This antibody is capable of inhibiting the enzymatic activity of APN (27). Tumor homing of RGD-4C phage was not affected by R3-63, and normal rat IgG had no effect on the homing of either phage.
Cytotoxicity of NGR Peptide Conjugate
A conjugate of the CNGRC peptide and the cytotoxic drug dox was selectively toxic to APN-expressing cells. Under conditions where the exposure of APN-transfected and control MDA-MB-435 cells to the conjugate was limited to 20 min by washing away the conjugate, the conjugate killed a significant fraction of the APN-expressing cells. APN-negative cells were essentially unaffected (Fig. 2). Free dox and dox coupled to the CARAC control peptide showed no significant toxicity upon this short incubation. The doxorubicin- RGD-4C conjugate was toxic to cells, regardless of their APN expression. This outcome is in agreement with the expression of the a.v[33 integrin by the MDA-MB-435 cells (15). The selectivity of the CNGRC conjugate was lost when the incubation with the drug was prolonged; all dox compounds were highly toxic to both types of cells. These results provide further evidence for the binding of NGR to APN at cell surfaces.
Fig. 2.
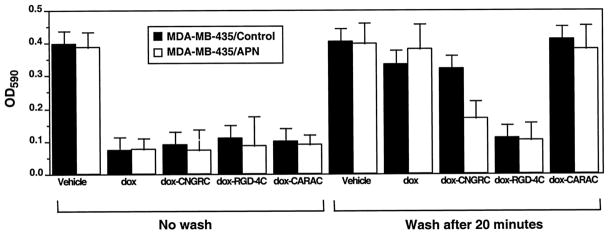
An NGR peptide-dox conjugate is selectively toxic to APN-positive cells. APN-transfected and parental MDA-MB-435 cells were exposed either to dox or dox-CNGRC, dox-RGD-4C, or dox-CARAC conjugates. The treatment with the drug was either for the duration of the 24-h incubation (No wash), or the drug was removed by washing cells after 20 min. Incubation continued for another 24 h. Surviving cells were quantitated at the end of the 24-h incubation period. Bars, SE.
APN Expression in Angiogenesis
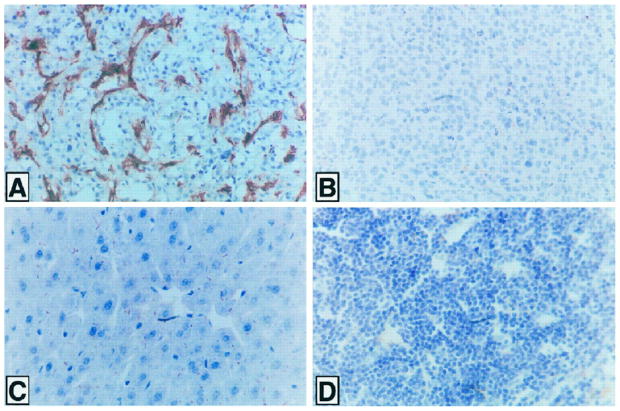
We next studied the expression of APN in endothelial cells to determine whether its expression would agree with it being the homing receptor in tumors for the NGR phage. Immunohistochemical staining showed strong mouse APN expression in the vasculature of tumors formed by the MDA-MB-435 breast carcinoma cells in nude mice (Fig. 3A). An antibody specific for human APN did not stain these tumors (Fig. 3B), which agrees with the lack of APN expression by these cells in vitro, as shown. The blood vessels in all normal mouse tissues examined were negative for APN (Fig. 3, C and D show liver and spleen, respectively).
Fig. 3.
Immunoperoxidase staining for APN in tumor and normal tissues in mice. A, tumor grown from MDA-MB-435 human breast carcinoma cells in a nude mouse. Antimouse APN shows positive staining in the tumor blood vessels. B, tumor cells and blood vessels in this xenograft are negative with antihuman APN, which does not react with mouse APN. Mouse liver (C) and spleen (D) show no significant staining with the antimouse APN antibody.
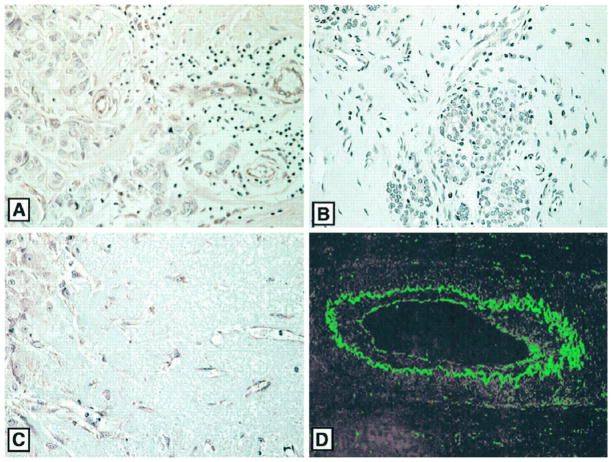
APN immunostaining was also found in the blood vessels, including capillaries, of various tumors from patients. Fig. 4A shows vascular APN staining in a human breast carcinoma. The vasculature in human malignant gliomas and lymph node metastases from multiple tumor types was also positive for APN (data not shown). The blood vessels in various normal human tissues were essentially negative for APN. Faint staining was sometimes seen in the endothelial cells of arteries but not in capillaries; Fig. 4B shows such staining for normal breast tissue. Blood vessels in corpus luteum expressed APN (Fig. 4C). As the blood vessels in this tissue undergo angiogenesis (35), this finding suggests that the expression of APN in blood vessels is related to angiogenesis.
Fig. 4.
APN expression in human angiogenesis. Immunoperoxidase staining shows strong APN expression in endothelial cells and the subendothelium of blood vessels in a human breast carcinoma (A) and in corpus luteum undergoing angiogenesis (C). The blood vessels in normal breast tissue are essentially negative (B). D is a confocal immunofluorescence image showing anti-APN staining of a medium-sized vessel in a human carcinoma. APN staining is present both at the endothelial surface and in a subendothelial layer. A–C, X300; D, X500.
Confocal immunofluorescence microscopy showed that endothelial cells and subendothelial layers of the vessels (presumably pericytes and possibly smooth muscle cells) expressed APN in tumors (Fig. 4D). The subendothelial APN staining colocalized with staining for APA, a marker of pericytes and smooth muscle cells (28) in blood vessels (not shown).
In the described tissue localization studies, two different antibodies, one against human APN (WM15) and the other against mouse APN (R3-63), were used. They both showed similar localization of APN in human and mouse tissues. Overlay of tissue sections with the CNGRC phage (detected by an anti-M13 antibody) revealed a similar staining pattern as anti-APN. Moreover, preincubating human tumor tissue sections with NGR phage blocked staining with the WM15 anti-APN antibody, whereas staining with the RC38 anti-APA was not affected. Conversely, preincubation of tumor tissue sections with the WM15 anti-APN antibody blocked the binding of the NGR phage to tissue sections (results not shown).
Functional Role of APN in Angiogenesis
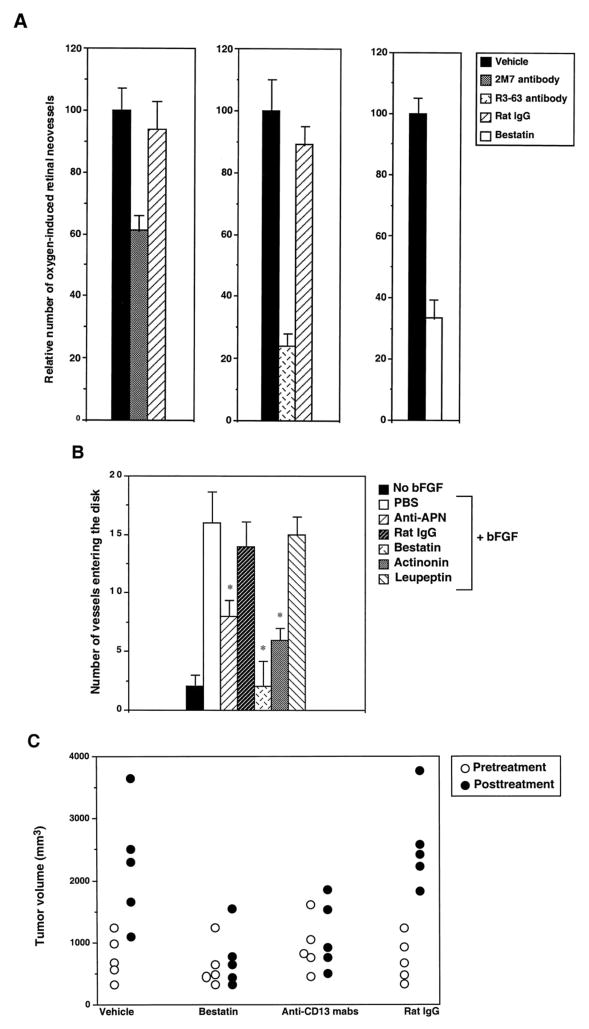
To test the functional role of the APN enzymatic activity in angiogenesis, we studied the effects of APN inhibitors in angiogenesis models. Two antibodies capable of inhibiting the enzymatic activity of APN, antimouse APN R3-63 and 2M-7, both inhibited hypoxia-induced retinal neovascularization (33) upon systemic treatment of mice (Fig. 5A). A chemical inhibitor, bestatin, had a similar effect.
Fig. 5.
Inhibition of angiogenesis by APN inhibitors. A, mice developing hypoxia-induced retinal neovasculature were treated i.v. with PBS (Vehicle), antimouse APN antibodies or normal rat IgG (250 μg/mouse), or bestatin (200 μg/mouse). The number of retinal neovessels in mice treated with PBS was set at 100%. Data represent means; n = 3; bars, SE. The reduction in blood vessel number was statistically significant for the anti-APN antibodies and bestatin (P < 0.01). B, CAMs were treated with PBS, bFGF, or bFGF together with anti-APN R3-63, normal rat IgG, bestatin, actinonin, or leupeptin. There were significantly fewer vessels in the CAMs treated with the APN inhibitors, whereas the serine protease inhibitor, leupeptin, had no effect. Columns, means; n = 8; bars, SE. *, t test, P < 0.05 relative to controls. C, mice bearing size-matched MDA-MB-435-derived breast carcinoma xenografts were divided into four groups of five animals and treated with DMEM, bestatin (250 μg/mouse), a mixture of R3-63 and 2M-7 anti- bodies, or normal rat IgG (125 μg/mouse) given i.p. once a week for 3 weeks. Tumor volumes at the start of the experiment (Pretreatment) and 3 weeks later (Posttreatment) are shown. Similar results were observed in two independent experiments. The tumors in the groups treated with bestatin and anti-APN had a significantly smaller volume posttreatment than the control groups (t test, P < 0.05).
Immunostaining of the CAM showed that the R3-63 antibody recognizes CAM (chicken) vasculature, making it possible to test its effect on bFGF-induced angiogenesis in the CAM (34). R3-63 significantly suppressed vessel growth in the CAM assay, as did bestatin and another chemical inhibitor, actinonin (Fig. 5B). Leupeptin, which does not inhibit APN activity, had no effect.
Systemic treatment of mice with two rat antimouse APN antibodies inhibited the growth of breast carcinoma xenografts derived from MDA-MB-435 cells, whereas normal rat IgG had no effect (Fig. 5C). Because these cells do not express APN in vitro or in vivo (Fig. 3B), the anti-APN effects are attributable to APN expressed in tumor blood vessels. Treatment with bestatin also inhibited tumor growth (Fig. 5C).
DISCUSSION
We show here that tumor-homing NGR peptides bind to APN. We also show that APN is a new marker for angiogenic vasculature, and that it is functionally important in angiogenesis. Our binding assays and in vivo homing experiments show that NGR peptides bind selectively to APN. Phage displaying these peptides interacted with immunocaptured APN and APN-transfected cells in culture. This binding is specific; in each case, the binding was inhibited by the cognate soluble peptide. Furthermore, anti-APN antibody inhibited in vivo homing of NGR phage to tumors, strongly suggesting that APN is the receptor for NGR peptides in tumors.
The expression pattern of APN agrees with its proposed role as the receptor for the NGR peptides in tumor vasculature; APN is specifically expressed in endothelial and subendothelial cells in angiogenesis. Various types of tumors in two species, analyzed with two monoclonal anti-APN antibodies and with an NGR phage overlay, consistently revealed APN expression in tumor vasculature. The vascular APN expression was independent of whether the tumor cells expressed APN. We also found strong APN expression in the blood vessels of corpus luteum and have shown in other work that retinal neovasculature expresses APN.6 In each case, tumors, corpus luteum, and retinal neovascularization, the vasculature is undergoing angiogenesis (1–3, 33, 35). Thus, APN expression correlates with angiogenesis. The expression of APN in angiogenesis may depend on growth factors and cytokines, because tumor necrosis factor-α and bFGF up-regulate APN in cultured endothelial cells (36).7
We did not find APN expression in the vasculature of any normal tissues, including the blood vessels in the brain. However, others have found APN in the pericytes associated with the blood-brain barrier (37). Although we are uncertain as to the cause, this discrepancy may relate to expression levels, which appear to be far higher in angiogenesis than in resting blood vessels. Importantly, both studies found the endothelial cells in the brain to lack APN.
Despite the presence of APN in various epithelial cells (22), NGR phage injected i.v. home specifically to tumors and other sites of angiogenesis, presumably because phage are not able to traverse blood vessels to the epithelia. Low-molecular-weight drugs have been targeted previously to tumors as NGR peptide conjugates without apparent epithelial toxicity (15, 16). It may be that tumor vessels take up much of the conjugate before it diffuses into tissues. In addition, the homing peptide moiety may be proteolytically destroyed after the conjugate leaves the circulation, preventing specific uptake of the drug by APN-positive cells.
Our data show that APN is not only a previously unrecognized marker of angiogenic endothelial cells but is also functionally important in angiogenesis. We find that APN inhibitors, such as inhibitory antibodies, bestatin and actinonin, suppressed angiogenesis. In accord with earlier studies (25, 26) the APN inhibitors also suppressed tumor growth. Our results suggest that inhibition of angiogenesis is likely to be a factor in the antitumor activity of these compounds.
The role of APN in angiogenesis may be to facilitate endothelial cell invasion of tissue, which is an essential component of angiogenesis (38, 39). Experiments with tumor cells have shown that APN expression can increase invasiveness (26). Because compounds that are enzymatic inhibitors of APN inhibit angiogenesis, this putative invasion-promoting effect would appear to be related to the enzymatic activity of APN. Another possibility is that APN could modulate the activity of a growth factor or cytokine. Such activities are common among aminopeptidases, including APN (24, 36, 40, 41).
NGR-containing ligands show promise for targeting phage, drugs, and peptides to tumor vasculature (15, 16). The identification of APN as the vascular receptor responsible for the homing of NGR peptides to tumors will open new possibilities for refining such targeting.
Acknowledgments
Supported by Grants DAMD-17-98-1-8041 (to R. P.) and DAMD-17-99-1-8164 (to W. A.) from the Department of Defense and Grant CA74238 (to E. R.) and Cancer Center Support Grant CA30199 from the National Cancer Institute.
We thank Dr. Eva Engvall for comments on the manuscript; Drs. Frank J. Coufal, Edward Monosov, and Egbert Oosterwijk for reagents and microscopy; and Carlotta Cavazos and Bradley H. Restel for technical assistance.
The abbreviations used are
- APN
aminopeptidase N
- APA
aminopeptidase A
- bFGF
basic fibroblast growth factor
- TU
transforming unit
- dox
doxorubicin
- CAM
chorioallantoic membrane.
Footnotes
W. Arap, M. Hagedorn, R. Pasqualini, and E. Ruoslahti, unpublished results.
R. Pasqualini, unpublished results.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αv integrins. Science (Washington DC) 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 5.Hammes HP, Brownlee M, Jonczyk A, Sutter A, Preissner KT. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med. 1996;2:529–533. doi: 10.1038/nm0596-529. [DOI] [PubMed] [Google Scholar]
- 6.Korpelainen EI, Alitalo K. Signaling angiogenesis and lymphangiogenesis. Curr Opin Cell Biol. 1998;10:159–164. doi: 10.1016/s0955-0674(98)80137-3. [DOI] [PubMed] [Google Scholar]
- 7.Lappi DA. Tumor targeting through fibroblast growth factor receptors. Semin Cancer Biol. 1995;6:279–288. doi: 10.1006/scbi.1995.0036. [DOI] [PubMed] [Google Scholar]
- 8.Hass TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- 9.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S, Ruiter DJ. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990;136:1393–1405. [PMC free article] [PubMed] [Google Scholar]
- 11.Burg MA, Pasqualini R, Arap W, Ruoslahti E, Stallcup WB. NG2 proteoglycan-binding peptides target tumor neovasculature. Cancer Res. 1999;59:2869–2874. [PubMed] [Google Scholar]
- 12.Arap W, Pasqualini R, Ruoslahti E. Chemotherapy targeted to tumor vasculature. Curr Opin Oncol. 1998;10:560–565. doi: 10.1097/00001622-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 14.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science (Washington DC) 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 15.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature. Science (Washington DC) 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 16.Ellerby E, Arap W, Kain R, Andrusiak R, Ellerby L, Del Rio G, Krajewski S, Lombardo CR, Ruoslahti E, Bredesen DE, Pasqualini R. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 17.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 18.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Bio/Technology. 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 19.Pasqualini R, Koivunen E, Ruoslahti E. αv integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 20.Healy JM, Murayama O, Maeda T, Yoshino K, Sekiguchi K, Kikuchi M. Peptide ligands for integrin αvβ3 selected from random phage display libraries. Biochemistry. 1995;34:3948–3955. doi: 10.1021/bi00012a012. [DOI] [PubMed] [Google Scholar]
- 21.Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1993;82:1052–1070. [PubMed] [Google Scholar]
- 22.Look AT, Ashmun RA, Shapiro LH, Peiper SC. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Investig. 1989;83:1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen J, Kokholm K, Noren O, Sjostrom H. Structure and expression of aminopeptidase N. Adv Exp Med Biol. 1997;421:47–57. doi: 10.1007/978-1-4757-9613-1_7. [DOI] [PubMed] [Google Scholar]
- 24.Lendeckel U, Arndt M, Wex T, Ansorge S. Role of alanyl aminopeptidase in growth and function of human T cells. Int J Mol Med. 1999;4:17–27. [PubMed] [Google Scholar]
- 25.Saiki I, Fujii H, Yoneda J, Abe F, Nakajima M, Tsuruo T, Azuma I. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer. 1993;54:137–143. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii H, Nakajima M, Saiki I, Yoneda J, Azuma I, Tsuruo T. Human melanoma invasion and metastasis enhancement by high expression of aminopeptidase N/CD13. Clin Exp Metastasis. 1995;13:337–344. doi: 10.1007/BF00121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stryhn A, Norén O, Sjöstrom H, Werdelin O. A mouse aminopeptidase N is a marker for antigen-presenting cells and appears to be co-expressed with major histocompatibility complex class II molecules. Eur J Immunol. 1993;23:2358–2364. doi: 10.1002/eji.1830230946. [DOI] [PubMed] [Google Scholar]
- 28.Schlingemann RO, Oosterwijk E, Wesseling P, Rietveld FJ, Ruiter DJ. Aminopeptidase A is a constituent of activated pericytes in angiogenesis. J Pathol. 1996;179:436–442. doi: 10.1002/(SICI)1096-9896(199608)179:4<436::AID-PATH611>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Ashmun RA, Look AT. Metalloprotease activity of CD13/aminopeptidase N on the surface of human myeloid cells. Blood. 1990;75:462–469. [PubMed] [Google Scholar]
- 30.Smith GP, Scott JK. Libraries of peptides and proteins displayed in filamentous phage. Methods Enzymol. 1993;217:228–257. doi: 10.1016/0076-6879(93)17065-d. [DOI] [PubMed] [Google Scholar]
- 31.Pasqualini R, Koivunen E, Ruoslahti E. A peptide isolated from phage display libraries is a structural and functional mimic of an RGD-binding site on integrins. J Cell Biol. 1995;130:1189–1196. doi: 10.1083/jcb.130.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Investig. 1998;102:430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Investig Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 34.Jain RK, Schlenger K, Hockel M, Yuan F. Quantitative angiogenesis assays: progress and problems. Nat Med. 1997;3:1203–1208. doi: 10.1038/nm1197-1203. [DOI] [PubMed] [Google Scholar]
- 35.Klauber N, Rohan RM, Flynn E, D’Amato RJ. Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med. 1997;3:443–446. doi: 10.1038/nm0497-443. [DOI] [PubMed] [Google Scholar]
- 36.van Hal PT, Hopstaken-Broos JP, Prins A, Favaloro EJ, Huijbens RJ, Hilvering C, Figdor CG, Hoogsteden HC. Potential indirect anti-inflammatory effects of IL-4. Stimulation of human monocytes, macrophages, and endothelial cells by IL-4 increases aminopeptidase-N activity (CD13; EC 3.4.11.2) J Immunol. 1994;153:2718–2728. [PubMed] [Google Scholar]
- 37.Kunz J, Krause D, Kremer M, Dermietzel R. The 140-kDa protein of blood-brain barrier-associated pericytes is identical to aminopeptidase N. J Neurochem. 1994;62:2375–2386. doi: 10.1046/j.1471-4159.1994.62062375.x. [DOI] [PubMed] [Google Scholar]
- 38.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 39.Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22:251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 40.Taylor A. Aminopeptidases: structure and function. FASEB J. 1993;7:290–298. doi: 10.1096/fasebj.7.2.8440407. [DOI] [PubMed] [Google Scholar]
- 41.Ward PE, Benter IF, Dick L, Wilk S. Metabolism of vasoactive peptides by plasma and purified renal aminopeptidase N. J Biol Chem. 1990;264:5480–5487. doi: 10.1016/0006-2952(90)90348-o. [DOI] [PubMed] [Google Scholar]