Abstract
The present study takes a multilevel approach to examine developmental trajectories in risk-taking propensity. We examined the moderating role of specific executive function components, attention shifting and inhibitory control, on the link between exuberant temperament in infancy and propensity for risk-taking in childhood. Risk-taking was assessed using a task previously associated with sensation seeking and antisocial behaviors. Two hundred and ninety one infants were brought into the lab and behaviors reflecting exuberance were observed at 4, 9, 24, and 36 months of age. Executive function was assessed at 48 months of age. Risk-taking propensity was measured when children were 60 months of age. The results indicate that exuberance and attention shifting, but not inhibitory control, significantly interact to predict propensity for risk-taking. Exuberance was positively associated with risk-taking propensity among children relatively low in attention shifting but unrelated for children high in attention shifting. These findings illustrate the multifinality of developmental outcomes for temperamentally exuberant young children and point to the distinct regulatory influences of different executive functions for children of differing temperaments. Attention shifting likely affords a child the ability to consider both positive and negative consequences, and moderates the relation between early exuberance and risk-taking propensity.
Keywords: Temperament, Risk-taking, Executive function, Development, Longitudinal
Risk-taking behaviors involve potential danger or harm to the individual while also providing an opportunity to obtain reward (Leigh, 1999). Based on this definition, research has traditionally focused on antisocial behaviors, such as criminal activity, substance and tobacco use, as well as unsafe sexual practices (DiClemente, Hansen, & Ponton, 1995; Zuckerman, Ball, & Black, 1990). Despite important advances, questions still remain regarding strategies for prevention and treatment of antisocial risk-taking behaviors (Nation et al., 2003). Therefore, it is important to elucidate early behavioral and cognitive markers that predict the development of later risk-taking behaviors.
An ecologically valid behavioral measure that assesses propensity for risk-taking is the Balloon Analogue Risk Task (BART; Bornovalova et al., 2009; Lejuez et al., 2002, 2003a, 2007; Lejuez, Aklin, Bornovalova, & Moolchan, 2005; Lejuez, Aklin, Zvolensky, & Pedulla, 2003b; Wallsten, Pleskac, & Lejuez, 2005; White, Lejuez, & de Wit, 2007, 2008). The BART is a computerized task that can be used to index real-world risk-taking behavior. In this task, participants accumulate money or points by pressing a button that inflates a simulated balloon. Each balloon has an explosion point, which results in loss of all points that have been accumulated for that balloon. The BART has been associated with adolescent and adult sensation seeking, impulsivity and deficiencies in behavioral constraint (Bornovalova et al., 2009; Lejuez et al., 2002). Additionally, riskiness on the BART has been found to correlate with addictive, health, and risk behaviors, such as smoking, substance use, sexual behavior, and delinquency in young adults (Lejuez et al., 2002, 2003a) as well as in adolescents (Lejuez et al., 2003b, 2005, 2007).
Individual differences in early temperament and personality have been found to influence developmental pathways to psychiatric problems as well as pathways to normative functioning (e.g., Caspi, Moffitt, Newman, & Silva, 1996; Degnan & Fox, 2007; Kim, Cicchetti, Rogosch, & Manly, 2009; Lahat, Hong, & Fox, 2011; Pérez-Edgar & Fox, 2005a) and may play an influential role in the development of risk-taking behaviors. Indeed, Williams et al. (2010) used a multilevel approach to predict substance use from behavioral inhibition (BI), a temperament identified in infancy and characterized by heightened negative affect, vigilance towards novelty, and withdrawal from unfamiliar social situations (Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Kagan, Reznick, & Snidman, 1988; Kagan & Snidman, 1991). The findings indicated that high BI observed during infancy was associated with increased risk for substance-related problems in adolescence among boys, whereas high BI protected against substance-related problems in adolescence among girls. Additionally, high BI increased the risk for substance-related problems during adolescence among high risk-taking children. In contrast, high BI protected low risk-taking children from substance-related problems in adolescence (Williams et al., 2010). Although BI individuals display risk-taking behaviors such as substance use, it is likely that they are turning to substance use as a means for coping with social situations (Arnett, 2001), and not because of a temperamental tendency towards taking risks.
Another temperament that is likely to be associated with propensity for risk-taking behaviors is exuberance. Often contrasted with BI, temperamental exuberance has been defined by positive reactivity to novelty, approach behavior, and sociability (Putnam & Stifter, 2005). During infancy and toddlerhood, this temperament is characterized by positive affect, motor reactivity to novel stimuli, and positive social behavior (Calkins, Fox, & Marshall, 1996; Degnan et al., 2011; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Hane, Fox, Henderson, & Marshall, 2008; Park, Belsky, Putnam, & Crnic, 1997). Children with an exuberant temperament are also characterized by impulsivity, sensitivity to reward, fearlessness, and risk-taking (Fox et al., 2001; Polak-Toste & Gunnar, 2006; Rothbart & Bates, 2006).
Exuberant temperament is associated with both adaptive and maladaptive social-emotional outcomes (Eisenberg et al., 1996; Rothbart, Ahadi, & Hershey, 1994). For example, according to Depue and Collins (1999), individuals with a bias towards behavioral approach, rather than patterns of behavioral withdrawal, display positive affect and approach only when goals are not being blocked. However, when goals are blocked such individuals may display frustration and even aggression toward the blocking stimulus. Indeed, parent-rated positive affect in school-aged children has been linked to externalizing problems (Degnan et al., 2011; Eisenberg et al., 1996; Putnam & Stifter, 2005; Rothbart et al., 1994; Stifter, Putnam, & Jahromi, 2008).
There is also evidence that exuberant temperament is associated with adaptive outcomes. For example, infants who respond positively to novel sensory stimuli show less inhibition and more sociability in infancy and childhood (Fox et al., 2001; Hane et al., 2008). Additionally, preschool children characterized as confident and outgoing, characteristics associated with exuberance, displayed less behavioral control, but better social skills as adults (Caspi et al., 2003). Moreover, children with a greater likelihood of displaying a high, stable exuberant profile during early childhood were found to display greater social competence and less social reticence at 5 years of age when interacting with an unfamiliar peer (Degnan et al., 2011).
Although the underlying neurobiology involved in exuberant temperament is subject to debate, it is likely that positive reactivity and approach to novelty result from either increased activation of the behavioral activation system (BAS), decreased activation of the behavioral inhibition system (BIS), or activation in motivational systems involved in processing of anticipation and saliency of reward (Depue & Collins, 1999; Gray & McNaughton, 2000; Panksepp, 1998; Zuckerman, 1991). According to Gray (1982), increased BAS activity leads to greater approach and impulsivity. In addition, according to Depue and Collins (1999), approach behavior involves dopaminergic projections to brain regions associated with processing of reward. Indeed candidate genes that modulate dopamine, such as DRD4, DAT, and COMT have been found to play a role in impulsivity (see Kreek, Nielsen, Butelman, & LaForge, 2005) and DRD4 has also been found to be involved in risk-taking and novelty seeking (Lusher, Chandler, Ball, 2001; Schinka, Letsch, & Crawford, 2002). Therefore, increased activation in these systems may lead individuals to approach and experience greater positive affect in response to reward (Depue & Collins, 1999; Polak-Toste & Gunnar, 2006).
The multifinality of developmental pathways associated with exuberance underscores the importance of identifying possible moderators of maladaptive outcomes (Cicchetti & Rogosch, 1996). Recent research has pointed to the role of executive function (EF) in the regulation of motivated and risk-taking behavior (see Somerville & Casey, 2010 for a review). EFs are generally thought to underlie one's ability to control thoughts, behavior, impulses, create long-term goals while ignoring distracting information, and play a significant role in decision-making (Bunge & Crone, 2009; Zelazo, Carlson, & Kesek, 2008). Thus, exuberant children and adolescents with poor EF abilities may be at particular risk for negative outcomes resulting from high levels of impulsivity and risk-taking behaviors.
An additional finding that likely has considerable influence on the behaviors and outcomes of exuberant children, is that EF is often impaired in light of emotionally charged interactions. This reduced cognitive control is especially pronounced during adolescence when rates of risky behavior, such as substance use, increase (Casey, Getz, & Galvan, 2008). For example, adolescents have been found to make riskier choices compared to adults when a reward was given during decision-making (Figner, Mackinlay, Wilkening, & Weber, 2009). Furthermore, in the presence of peers, adolescents, but not adults, showed increased activation in reward-related brain regions, such as the ventral striatum and orbitofrontal cortex, and this activation predicted subsequent risk-taking in a simulated driving task. Additionally, brain areas associated with EF were less strongly recruited by adolescents than adults (Chein, Albert, O’Brien, Uckert, & Steinberg, 2011). These studies show that risky choices tend to peak during adolescence and it is likely that EF would play a similar role in younger children with high risk tendencies, such as exuberant children.
Two components of EF that may be relevant for risk taking behavior are attention shifting and inhibitory control. Attention shifting is the ability to flexibly reallocate attention within one’s internal and external environments to support goal-directed behaviors or meet task demands (Pérez-Edgar & Fox 2005b). It also involves the ability to switch between different tasks. For example, three-year-olds have been found to perseverate on a single task despite being given a new task with new rules. In contrast, by 5 years of age, most children are able to switch tasks immediately when instructed to do so (Zelazo et al., 2008). According to Lonigan and Vasey (2009), attention shifting could reduce a child’s level of fear or negative affect by facilitating the disengagement of attention from negative thoughts or threatening stimuli and focusing attention on more positive and adaptive stimuli. Similarly, attention shifting could play a role in uninhibited children's risk-taking behavior by allowing a child to focus on adaptive stimuli and keep multiple outcomes in mind (such as both reward and punishment).
Another EF ability, inhibitory control, is the capacity to inhibit a dominant response in favor of a more appropriate or subdominant response (Rothbart, Ellis, Rueda, & Posner, 2003). This EF ability could help a child modulate the expression of an inappropriate or maladaptive response, aiding in adaptive social and emotional development (Kieras, Tobin, Graziano, & Rothbart, 2005). Thus, inhibitory control could play a role in risk-taking by inhibiting maladaptive risky choices in favor of more balanced decisions. It is important to note that BI and inhibitory control represent different constructs and may interact to result in developmental outcomes. BI refers to a temperamental style characterized by high levels of fearful reactivity to novelty (e.g., Fox et al., 2005), whereas inhibitory control is a higher order cognitive process that involves the ability to inhibit and override dominant responses and behaviors in favor of more appropriate responses (Rothbart et al. 2003). Behaviorally inhibited individuals may be high or low on inhibitory control and individuals who are not behaviorally inhibited may perform well or poorly on inhibitory control tasks.
The manner in which attention shifting and inhibitory control contribute to adaptive behavior may differ as a function of a child’s style of temperamental reactivity (Eisenberg et al. 2005; White, McDermott, Degnan, Henderson, & Fox, 2011). For example, high levels of attention shifting have been found to decrease the risk for anxiety problems in children with high levels of BI, whereas high levels of inhibitory control increased this risk for anxiety symptoms (White et al., 2011).
The purpose of the current study was to examine whether attention shifting and inhibitory control play a role in risk-taking behavior on the BART among children who are prone to temperamental exuberance. While previous work has examined either one of these EF abilities independently in relation to outcomes, few have included them both in the same study. Attention shifting may play a role in participant performance on the BART, as the potential gain of accruing a greater reward must be balanced against the potential risk of losing all money accrued for that balloon (Bornovalova et al., 2009). Additionally, inhibitory control may be important for performance on the BART with regard to withholding risky pumping of balloons.
The present study provides a longitudinal investigation of the moderating role that EF plays in the relation between exuberant temperament and risk-taking propensity. We focus on the interplay between temperament and cognitive processes in order to predict propensity for risk-taking behavior, and thus present a multilevel analysis of how this behavior unfolds (Cicchetti & Toth, 2009). Applying a multilevel approach to the investigation of developmental trajectories that lead to anti-social risk behaviors is important for understanding adaptive and maladaptive developmental processes (Cicchetti & Valentino, 2007). Temperament has been thought to initially stem from a biological predisposition (Kagan, Reznick, & Snidman, 1987; Kagan & Snidman, 1991), whereas EF involves processes at the cognitive level. We expected to find a positive association between exuberance and propensity for risk taking. Furthermore, in light of the role that EF has been found to play in risk-taking behavior, we expected this association to be particularly pronounced among children relatively low in attention shifting and inhibitory control.
Methods
Participants
As part of a longitudinal study, families from a large metropolitan area in the United States were contacted by mail and were screened by phone in order to insure that infants were born full-term and had not experienced any serious illness or developmental problems. Seven hundred and seventy-nine infants, who met these criteria, were brought into the lab at 4 months of age for temperament screening, during which positive and negative affect and motor reactivity to novel stimuli were assessed (see Hane et al., 2008 for a complete description of recruitment and selection process). Of this sample, 291 infants (135 males, 156 females) representing the full range of patterns of reactivity were selected and invited to continue participation in the larger longitudinal study. Of these infants 187 (64.30%) were Caucasian, 41 (14.10%) were African American, and 63 (21.50%) were of other various ethnic backgrounds. Most mothers were at least college graduates (84.40%) or high school graduates (15.60%).
Procedures
Following the temperament screening at 4 months, infants visited the lab again at 9, 24, 36, 48, and 60 months of age. At 9-months of age behavioral and affective reactivity were assessed using emotion-eliciting paradigms adapted from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith & Rothbart, 1999). When children were 24 and 36 months, behavior and affect were observed during behavioral inhibition (Fox et al., 2001) and risk-taking (Kochanska, 1995; Pfeifer, Goldsmith, Davidson, & Rickman, 2002; Putnam & Stifter, 2005) paradigms. At 48 months, children were brought into the laboratory for various assessments including measures of EF using the Dimensional Change Card Sort (DCCS; Zelazo, 2006), Day-Night Stroop (Gerstadt, Hong, & Diamond, 1994), Grass-Snow Stroop (Carlson & Moses 2001), as well as the verbal IQ subtest of the Wechsler Preschool and Primary Scale of Intelligence (WPPSI; Wechsler, 2002). Finally, at 60 months children were brought into the laboratory for an additional assessment, which included a risk-taking propensity measure - the Youth Balloon Analogue Risk Task (BART-Y; Lejuez et al., 2007).
Measures
Longitudinal Exuberance Profiles
In order to examine associations between longitudinal patterns of exuberance and risk-taking outcomes at 60 months, the longitudinal exuberance profiles created by Degnan et al. (2011) were used. Using longitudinal exuberance profiles provides an index of the stability or level of exuberance over time. These profiles were created using a latent profile analysis (LPA; Gibson, 1959) in order to produce a measure of the probability of membership in a high exuberance profile. Measures included in the profile analysis included observed measures of positive reactivity during the temperament screening at 4 months (Hane et al., 2008), positive approach behavior at 9 months (Goldsmith & Rothbart, 1999), and positivity, approach, and sociability during the behavioral inhibition (Fox et al., 2001) and exuberance/risk-taking (Putnam & Stifter, 2005) paradigms at 24 and 36 months of age.
During the 4 month screening, infant behavior during the reactivity paradigm was subsequently coded as follows: A motor reactivity score was obtained by summing the frequencies of arm waves, arm wave bursts (several waves in rapid succession), leg kicks, leg kick bursts, back arches, and hyperextensions throughout the paradigm. A positive reactivity measure was derived from these data, which included frequencies of positive affect and motor reactivity codes. Coders achieved intra-class correlation coefficients ranging from .80 to .92 (See Hane et al., 2008).
At 9 months of age, infants completed a number of tasks from the Lab-TAB assessment (Prelocomotor Version 3.1; Goldsmith & Rothbart, 1999), including masks, unpredictable toys, puppets, and peek-a-boo. A positive approach measure, coded in epochs, was derived from these data and included the following codes: intensity of smiling, positive motor behavior, peak to joy, attention towards puppets, and approach towards stimuli. Reliabilities across 20% of the cases were achieved separately for each of the scales during each episode. Kappas ranged from .75 to .92 (M =.85) for the puppet codes, .81 to .84 (M =.83) for the peek-a-boo codes, .59 to .98 (M =.78) for the unpredictable toy codes, and .76 to .97 (M =.85) for the mask codes. Intraclass correlations (ICCs) computed for latency to joy during puppets (r =.93) and peek-a-boo (r =.84), as well as duration of attention to the puppets (r =.97), showed good inter-rater reliability (See Degnan et al., 2011).
At 24 and 36 months of age, children were assessed for exuberant affect and behavior during the standard behavioral inhibition paradigm (Fox et al., 2001; Kagan, Reznick, & Snidman, 1987), as well as an exuberance/risk-taking paradigm (Kochanska, 1995; Pfeifer et al., 2002; Putnam & Stifter, 2005). At 24 and 36 months, the behavioral inhibition paradigm included a freeplay task, a stranger approach task, a robot task, and a tunnel task. At 24 months, the exuberance/risk taking tasks included asking children to stick their hands into a black box, climb up steps to jump onto a mattress, watch a confetti popper, and approach a vacuum cleaner. At 36 months, the exuberance/risk-taking tasks included asking them to put on a blood pressure cuff, jump on a trampoline, touch a gorilla mask, climb up steps toward the wall, touch a realistic-looking snake, touch an unpredictable mechanical dragon toy, and sit close to the experimenter to read a book. Three measures were derived from data collected at 24 and 36 months of age: positivity, which included codes for smiling and positive vocalizations; approach, which included codes for speed to touch/approach the stimuli, distance from mother, speed to vocalize, activity level, number of prompts (reversed scored), degree of approach, and willingness to perform each task; and sociability, which included codes for proximity, talking, smiling, gesturing, and verbal initiations (all toward experimenter). Inter-rater reliability for these continuous measures ranged from .60 to .98. An overall subscale of Exuberance was computed at 24 and 36 months of age as the average of positivity, approach, and sociability (See Degnan et al., 2011).
Degnan et al. (2011) estimated longitudinal latent classes using the above measures of exuberance at 4, 9, 24, and 36 months. Two profiles (high exuberance and low exuberance) were found to provide the best fitting model. The “high exuberance” profile (n = 83; 28%; 50 females) displayed a higher percentage of membership in the 4-month positive group (46%), and higher levels of 9-month positive approach, 24-month exuberance, and 36-month exuberance. The “low exuberance” profile (n = 208; 72%; 105 females) displayed a lower percentage of membership in the 4-month positive group (31%), and lower levels of 9-month positive approach, 24-month exuberance, and 36-month exuberance (see Degnan et al., 2011, for more details). Individual probabilities of membership in the two profiles were computed and the present study includes the probabilities of membership in the high exuberance profile as the measure of exuberance across early childhood. The LPA was computed using all 291 participants who had data for at least one time point (i.e., 4-months of age). Reasons for missing data during these and subsequent visits included refusal by children to perform the tasks, experimenter or technical errors, difficulty scheduling laboratory visits at these ages, family relocation, and permanent attrition.
Dimensional Change Card Sort (DCCS)
At 48 months of age children were administered the DCCS (Zelazo, 2006) in order to assess attention shifting between two sets of rules. Participants were presented with two sorting boxes with a model card mounted on each box and were instructed to sort cards. In the practice phase they were trained to first sort cards according to one dimension (e.g., color) and then according to the other dimension (e.g., shape). During the testing phase children were presented with cards that vary on two dimensions (e.g., a blue star or a red truck). The sorting cards varied on two dimensions as well (e.g., a red star or a blue truck), but no sorting card matched a model card on either color or shape. In the first test phase (pre-switch) participants were asked to sort 8 cards according to one dimension (e.g., color) and in the second testing phase (post-switch) they were asked to sort the cards again according to the other dimension (e.g., shape). The order of dimension was counter-balanced, and children’s scores were computed according to the percentage of correct post-switch sorts. Of the original 291 participants, 208 had complete DCCS data. No differences in exuberance were observed between participants who had complete DCCS data and participants that did not, t(280.31) = .61, p = .54. Children who did not sort correctly on at least 80% of the pre-switch trials were excluded from the analysis (n = 27). Thus, DCCS data in the present study included 181 participants.
Day-Night Stroop
At 48 months of age children were also administered the Day-Night Stroop (Gerstadt et al. 1994). On this task children are required to inhibit a prepotent response and instead provide a subdominant response. Children were asked to say the word “Day” when presented with a picture of the moon and “Night” when presented with a picture of the sun. Children were administered up to 10 practice trials until they responded correctly to the consecutive presentation of a Day and a Night card. The task included 16 test trials, with the sun and moon pictures presented eight times each in a pseudorandom order. Of the original 291 participants, 198 had complete Day-Night Stroop data. No differences in exuberance were observed between participants who had complete Day-Night Stroop data and participants that did not, t(231.02) = −.27, p = .79. Children who did not get one of each trial type correct during practice trials or who completed less than half of the test trials were excluded from analyses (n = 24). Thus, the Day-Night Stroop data included a total of 174 participants. The variable of interest was children’s percentage of correct responses on test trials, reflecting a child’s ability to inhibit a prepotent response.
Grass-Snow Stroop
An additional inhibitory control task that was administered at 48 months was the Grass-Snow Stroop (Carlson & Moses, 2001). In this task, unlike the Day-Night Stroop, children are asked to respond by pointing rather than speaking. The experimenter first verified that the child could name the colors of grass and snow by asking “What color is grass?” and “What color is snow?” A large board was then presented that had a white card attached to the upper-left corner, a green card attached to the upper–right corner, and two foam cut-outs shaped like hands centered below the colored cards. The child was told to point to the white card when the experimenter said “grass” and to point to the green card when the experimenter said “snow.” Children received up to 10 practice trials until the child responded correctly to the consecutive presentation of both a Grass and a Snow trial. There were a total of 16 test trials, with the white and green picture cards presented 8 times each in a pseudorandom order. Of the original 291 participants, 198 had complete Grass-Snow Stroop data. No differences in exuberance were observed between participants who had complete Grass-Snow Stroop data and participants that did not, t(232.36) = −1.10, p = .27. Children who did not get one of each trial type correct during practice trials or who completed less than half of the test trials were excluded from analyses (n = 16). Thus, the Grass-Snow Stroop data included a total of 182 participants. The variable of interest was children’s percentage of correct responses on test trials, reflecting a child’s ability to inhibit a prepotent response.
Verbal IQ
The vocabulary and information subscales of the Wechsler Preschool and Primary Scale of Intelligence (WPPSI; Wechsler, 2002) were administered at 48 months of age. An age-adjusted scaled Verbal IQ score was derived from the prorated scores of the two subscales. This Verbal IQ score was included in the present analysis in order to control for separate effects that a child’s Verbal IQ may have on their performance on the EF tasks. Complete Verbal IQ data was obtained from 200 participants. No differences in exuberance were observed between participants who had complete Verbal IQ data and participants that did not, t(289) = −.09, p = .93.
Youth Balloon Analogue Risk Task (BART-Y)
At 60-months of age participants completed the Youth version of the Balloon Analogue Risk Task (Lejuez, et al., 2007). The BART-Y is a computerized risk-taking propensity measure that requires participants to inflate computer-generated balloons without popping them in order to earn points. Each pump is worth 1 point, but if a balloon explodes then all points accrued for that balloon are lost. Participants could stop pumping the balloon at any time prior to explosion and allocate the accrued points to a permanent prize meter and they were informed that they would win a prize in accordance with the points they earned. The task was divided into 2 blocks consisting of 10 balloons in each block. Dividing the task into 2 blocks allows examining differences in balloon pumping over time. The dependent measure was adjusted average number of pumps, or the average number of pumps on trials on which a balloon did not explode. This is the standard outcome measure in previous BART studies (e.g., Lejuez et al., 2002; 2007). Reliability of the task is supported by robust correlation across blocks within a single session (r = .70; Lejuez et al., 2007) and acceptable test-retest reliability across one year intervals (year 1 – year 2 r = .49; year 2 – year 3 r = .67; MacPherson, Magidson, Reynolds, Kahler, & Lejuez, 2010). The validity of the BART-Y has been assessed and the association between BART-Y and risk behaviors remained significant after controlling for demographic variables, impulsivity, and sensation-seeking (Lejuez et al., 2007). BART data in the present study was obtained from 225 participants. No differences in exuberance were observed between participants who had complete BART data and participants that did not, t(199.76) = .71, p = .47.
Results
Descriptive Statistics
Means and standard deviations of predictor (probability of membership in high exuberance profile and scores on EF measures) and outcome (adjusted average number of pumps on the BART-Y) variables are presented in Table 1.
Table 1.
Means (and standard deviations) of predictor and outcome variables
| Variable | M (SD) |
|---|---|
| Probability of high exuberance profile | .35 (.34) |
| Percent correct on post-switch trials of DCCS | 52.11 (44.13) |
| Percent correct trials on Day-Night Stroop | 68.19 (22.60) |
| Percent correct trials on Grass-Snow Stroop | 80.29 (25.32) |
| Adjusted average number of pumps on BART-Y (Block 1) | 12.98 (7.35) |
| Adjusted average number of pumps on BART-Y (Block 2) | 10.81 (6.88) |
| Adjusted average number of pumps on BART-Y (Blocks 1 & 2) | 11.77 (6.71) |
Preliminary Analyses
A series of t-tests were used to examine sex differences on all predictor and outcome variables. Significant sex differences were found for Day-Night Stroop, t(144.50) = −2.08, p < .04, and Grass-Snow Stroop, t(140.02) = −3.13, p < .002 with females (Day-Night M = 71.43, SD = 20.13; Grass-Snow M = 85.65, SD = 20.29) displaying higher scores than males (Day-Night M = 64.12, SD = 24.91; Grass-Snow M = 73.75, SD = 29.16). No other significant sex differences were found, all ps > .75. Thus, sex was included as a covariate in the regression analysis with the Stroop tasks, but was removed from all other further analyses.
Additionally, Pearson correlations were used to examine relations between predictor and outcome variables, as well as Verbal IQ (See Table 2). A significant correlation was found between adjusted average number of pumps on Block 1 and Block 2 of BART-Y, as well as between Block 1 and the total score on the BART-Y and Block 2 and the total score on the BART-Y. A paired sample t-test indicated that the average number of pumps on Block 1 was significantly higher than Block 2, t(218) = 6.37, p < .0001. Additionally, a significant correlation was found between the two Stroop tasks. Given this correlation, the Day-Night and Grass-Snow scores were averaged to create a composite measure of inhibitory control. No significant correlations were found among the DCCS, the Stroop tasks, the exuberance, or the BART adjusted average number of pumps score. Significant correlations were found between verbal IQ and the EF measures (DCCS score, Day-Night Stroop score, and Grass-Snow Stroop score). Given these effects, Verbal IQ was entered as a control variable in all the following analyses.
Table 2.
Correlation between predictor and outcome variables
| Exuberance | DCCS | Day-Night Stroop |
Grass-Snow Stroop |
Verbal IQ | BART-Y (Block 1) |
BART-Y (Block 2) |
BART-Y (Blocks 1 & 2) |
|
|---|---|---|---|---|---|---|---|---|
| Exuberance | - | .03 | .02 | .04 | .09 | .12 | .08 | .11 |
| DCCS | - | - | .11 | .10 | .18* | −.01 | −.02 | −.02 |
| Day-Night Stroop | - | - | - | .28** | .15* | −.03 | .01 | .01 |
| Grass-Snow Stroop | - | - | - | - | .30* | .07 | .02 | .05 |
| Verbal IQ | - | - | - | - | - | −.12 | −.10 | −.10 |
| BART-Y (Block 1) | - | - | - | - | - | - | .72** | .93** |
| BART-Y (Block 2) | - | - | - | - | - | - | - | .93** |
| BART-Y (Blocks 1 & 2) | - | - | - | - | - | - | - | - |
p < .05,
p < .01
Effects of Exuberance and EF on Risk-Taking Propensity
To examine the moderating role of EF in the relation between probability of high exuberance and risk-taking propensity, separate hierarchical multiple regression analyses with attention shifting and inhibitory control were computed for Block 1 and Block 2 of the BART-Y, as well as for the two blocks combined. To reduce multicollinearity and aid in interpretation, mean centered predictors were used. Next, the interaction terms were computed as the product between the mean-centered continuous measures of probability of high exuberance and attention shifting/inhibitory control. The first step of the regression analyses included Verbal IQ and the main effects of probability of high exuberance and attention shifting/inhibitory control, as well as gender for the analyses with inhibitory control. To test for significant moderation effects of attention shifting/inhibitory control on the link between probability of high exuberance and risk-taking propensity, the interaction product term between the probability of high exuberance and attention shifting/inhibitory control was entered in the second step of the regression analyses. Although the entire regression models were examined for significance, moderation hypotheses were tested by examining the second step for whether it significantly increased the variance explained by the model. Interactions were probed and plotted according to the guidelines by Aiken and West (1991). High and low levels of high exuberance probability and attention shifting/inhibitory control were computed as +/− 1 SD. Follow-up statistical tests from these probes are reported below.
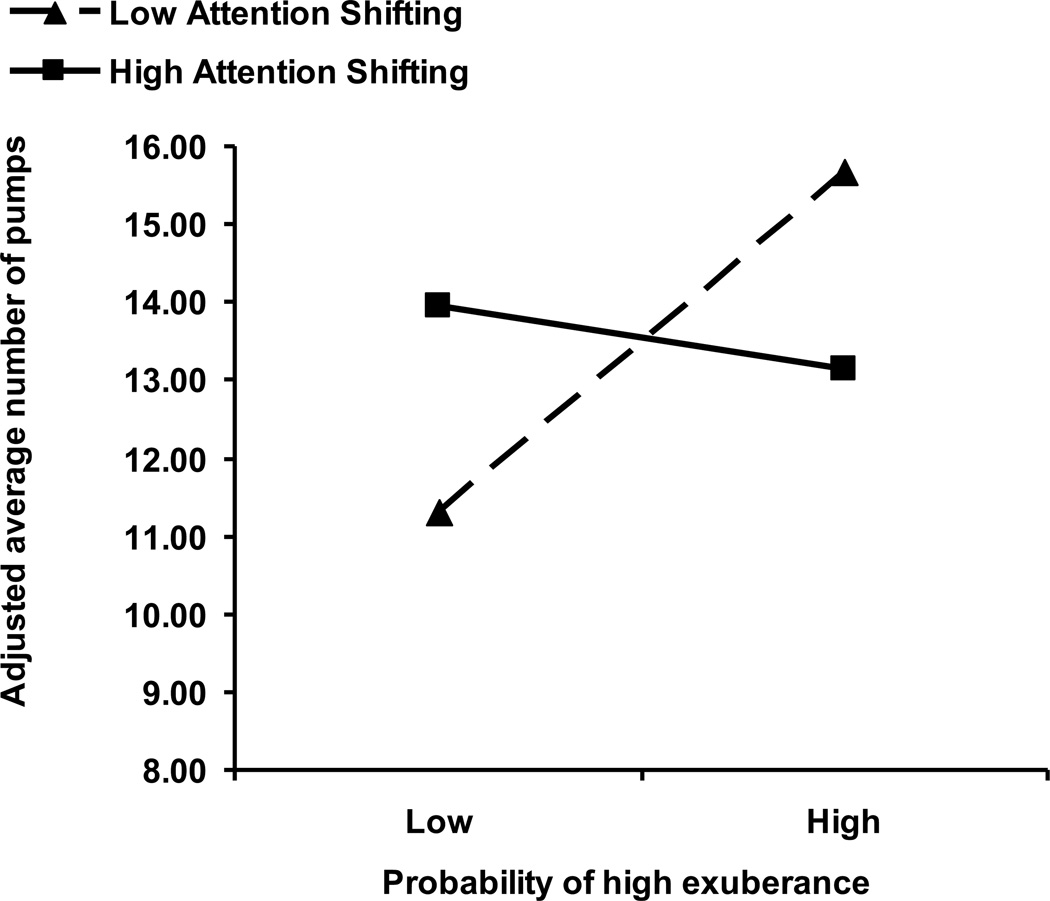
Significant findings were obtained for the regression including attention shifting predicting BART-Y Block 1 only. The addition of the interaction term between probability of high exuberance and attention shifting significantly improved the model, ΔR2 = .03, F(1,133) = 4.00, p < .05 (see Table 3 and Figure 1). The full model with the interaction term was at trend level F(4, 133) = 2.34, p = .058, explaining 7% of the variance in risk-taking propensity. Additionally, verbal IQ was found to significantly predict risk-taking propensity on the BART-Y (see Table 3). No significant results were obtained when including inhibitory control as a moderator.
Table 3.
Hierarchical multiple regression analysis predicting 5-year risk-taking propensity
| Variables by step | R2 | β (t) |
|---|---|---|
| Step 1 (df 3/134) | .04 | |
| Verbal IQ | −.17 (−1.96)* | |
| Probability of High Exuberance | .11 (1.27) | |
| Attention Shifting | .01 (.12) | |
| Step 2 (df 4/133) | .07* | |
| Verbal IQ | −.19 (−2.23)** | |
| Probability of High Exuberance | .12 (1.41) | |
| Attention Shifting | .003 (.04) | |
| Probability of High Exuberance × Attention Shifting | −.17 (−1.99)** |
p < .058,
p < .05
Figure 1.
Joint effect of probability of high exuberance and attention shifting on risk-taking propensity
Follow-up regressions revealed that probability of high exuberance was related to risk-taking propensity on BART-Y Block 1 only when children exhibited poor attention shifting. Specifically, within children who exhibited low attention shifting, probability of high exuberance predicted increased risk-taking propensity on the BART-Y, β = .29, t(133) = 2.34, p < .05. However, when children showed high attention shifting, exuberance was not related to risk-taking propensity, β = −.05, t(133) = −.46, p = .65.
Discussion
The findings from the current study indicated that attention shifting moderated the link between exuberant temperament and propensity for risk-taking behaviors during childhood. Exuberance was positively associated with propensity for risk-taking but only among children with poor attention shifting skills. Interestingly, exuberance was unrelated to risk taking propensity for children with high levels of attention shifting. At high levels of attention shifting, risk-taking propensity scores were clustered around the sample mean regardless of temperamental exuberance. Additionally, inhibitory control did not play a role in risk-taking propensity of high versus low exuberant children. These findings were obtained using a task that assessed propensity for risk-taking behaviors in childhood. Riskiness on the BART has been associated with anti-social risk behaviors, such as smoking, substance use, sexual behavior, and delinquency in adolescence and young adulthood (Lejuez et al., 2002, 2003a, 2003b, 2005, 2007).
Our study illustrates the importance of a multilevel analysis approach (Cicchetti & Toth, 2009; Cicchetti & Valentino, 2007). We predicted propensity for risk-taking, which has been associated with anti-social behaviors, by examining the complex interplay between early temperament and childhood EF. Our findings that attention shifting, but not inhibitory control, interacts with exuberance to predict risk-taking underscores the importance of using multiple methods to understand the development of adaptive and maladaptive outcomes.
Why might attention shifting skills, but not inhibitory control, moderate the relations between temperamental exuberance and risk taking? One possibility is the salience of reward or the differential weighing of reward versus punishment that is involved in the particular measure of risk-taking used in this study. It is possible that high exuberant/low attention shifting children tend to focus primarily on the reward associated with the behavior and are not able to shift their attention to consider the risk involved. Additionally, children with low exuberance who were also low on attention shifting may rigidly focus on the risk of potential loss, and are unable to shift their attention to the possibility of gaining reward. Although inhibitory control can play a protective role in avoiding risky behaviors, it is possible that for balanced performance on the BART, mere inhibitory control is not sufficient to control risk-taking, and the ability to flexibly consider different possible outcomes is necessary. These interpretations are in line with the notion that during the BART participants must weigh the potential reward versus punishment with every pump (Bornovalova et al., 2009). The results of the present study indicate that individuals exhibiting high exuberance and poor attention shifting have difficulty with this balancing of gain/loss, which results in high risk-taking behavior. In contrast, for individuals displaying low exuberance and low attention shifting, difficulty in balancing gain/loss results in low risk-taking.
Exuberance was only associated with risk-taking propensity when children were relatively low in attention shifting. These results suggest that high attention shifting is important for making balanced decisions involving risk. These findings demonstrate the multifinality of development (Cicchetti & Rogosch, 1996) and are in line with other research demonstrating that attention shifting and inhibitory control have differential influences on levels of risk or adaptation (White et al., 2011). For example, White et al. (2011) found that high levels of inhibitory control increased the risk for anxiety symptoms amongst behaviorally inhibited children, whereas high levels of attention shifting decreased the risk for anxiety problems in these children. Taken together, the findings of the current study and White et al.’s study (2011) suggest that high levels of attention shifting may serve as a protective factor in the link between temperament and negative outcomes. This conclusion may have important implications for prevention and intervention efforts in the form of training in order to improve attention shifting skills. Studies have shown that training in a specific EF task can lead to more generalized improvements in EF (e.g., Dowsett & Livesey, 2000; Kloo & Perner, 2003), and this can be relevant for developmental outcomes.
The hierarchical regression predicting risk-taking propensity from probability of high exuberance only yielded significant results for the first block of the task. Although risk-taking propensity was highly correlated across the two blocks, which supports the reliability of the BART, the adjusted average number of pumps on Block 2 was lower than on Block 1. This pattern of decrease is in line with previous work (Lejuez et al., 2007). The decrease in risk-taking propensity was an overall decrease, with few rank order differences, suggesting that the whole distribution shifted down in Block 2. These lower scores may have limited the power of the regression analysis in Block 2. One possible explanation may be that with time and after experiencing several trials with losses, all participants tend to be more conservative in their responding in order to avoid further loss and thus individual differences fade over the course of the task.
An interesting finding in the present study was the negative relation found between verbal IQ and adjusted average number of pumps in Block 1 of the BART-Y. Although verbal IQ was included in the analysis merely as a control variable, the results suggest that participants with a lower verbal IQ displayed a significantly larger number of pumps, thus showing more risk-taking behavior on the BART-Y. This finding is in line with previous research showing that lower IQ, and verbal IQ in particular, is related to higher delinquent behavior (Lynam, Moffitt, & Stouthamer-Loeber, 1993). Although the BART does not assess delinquent behavior per se, previous research with this task has shown that it is related to risk for delinquency (Lejuez et al., 2007).
The findings of the present study are consistent with research on adolescent risk-taking behavior and the role of EF (e.g., Casey et al., 2008; Cauffman, et al., 2010; Chein, et al., 2011; Figner, et al., 2009). Taken together, these studies show that reduced EF is associated with propensity for risky behaviors even during childhood. Casey et al. (2008) proposed a neurobiological model of cognitive and motivational processes to account for real-world adolescent risky behavior, including functional circuitry between striatal and prefrontal cortical regions. Furthermore, the strength of the connection between these regions was associated with the capacity to effectively engage in EF (Casey et al., 2007; Liston, et al., 2006). It is possible that attention shifting moderates temperamental exuberance through altering activation in the systems underlying positive reactivity and approach tendencies, such as the BAS and BIS systems (Depue & Collins, 1999; Gray & McNaughton, 2000; Panksepp, 1998; Zuckerman, 1991). Attention shifting may modify dopaminergic projections to neural sites involved in encoding the salience of reward stimuli, and as a result influence exuberant individuals’ balancing of motivations from reward and punishment. Future research should address this question and examine the underlying neural circuits by which attention shifting moderates the association between exuberance and propensity for risk-taking.
Several limitations of the present study should be noted. First, our sample included participants from middle-upper social economic status and it is not clear from our findings whether our results would generalize to high-risk samples. However, given that EF has been found to be impaired in children from disadvantaged backgrounds (e.g., Noble, Norman, & Farah, 2005) it is likely that temperament would interact with EF to predict risk-taking among high risk populations. Second, although riskiness on the BART has been associated with “real world” risk-taking behaviors (Lejuez et al., 2002, 2003a, 2003b, 2005, 2007), these are usually influenced by environmental variables such as peer influence (Allen, Porter, & McFarland, 2006) and parental monitoring (Borawski, Levers-Landis, Lovegreen, & Trapl, 2003). However, in the present study, the BART-Y does not account for these variables that in reality may play an important role in risk-taking. Finally, our participants completed the BART-Y at 5 years of age, and we do not know yet, whether the BART-Y at this young age would predict later “real world” risk behaviors, such as substance use and delinquency. These limitations should be addressed by future work.
In sum, the findings from the present multi-level study suggest a moderating role for certain aspects of EF in the association between exuberant temperament and propensity for risk-taking behavior in childhood. Specifically, we found that children with high exuberance across early childhood and low attention shifting in preschool displayed increased propensity for risk-taking on the BART-Y, a task previously associated with anti-social behaviors in adolescence and adulthood. Similar findings were not obtained for inhibitory control, suggesting a differential role for different EFs in young children’s risk-taking propensity. The findings of the present study suggest that exuberant temperament may serve as an early marker of later anti-social risk behaviors.
Acknowledgments
The research in this paper was supported by a grant (R37HD17899) to Nathan A. Fox.
References
- Aiken LS, West GM. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage Publishing; 1991. [Google Scholar]
- Allen JP, Porter MR, McFarland CF. Leaders and followers in adolescent close friendships: Susceptibility to peer influence as a predictor of risky behavior, friendship instability, and depression. Development and Psychopathology. 2006;18:155–172. doi: 10.1017/S0954579406060093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Adolescence and emerging adulthood: A cultural approach. Upper Saddle River, NJ: Prentice Hall; 2001. [Google Scholar]
- Borawski EA, Levers-Landis CE, Lovegreen LD, Trapl ES. Parental monitoring, negotiated unsupervised time, and parental trust: The role of perceived parenting practices in adolescent health risk behaviors. Journal of Adolescent Health. 2003;33:60–70. doi: 10.1016/s1054-139x(03)00100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Cashman-Rolls A, O'Donnell JM, Ettinger K, Richards JB, deWit H, Lejuez CW. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacology, Biochemistry, and Behavior. 2009;93:258–262. doi: 10.1016/j.pbb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Crone EA. Neural correlates of the development of cognitive control. In: Rumsey J, Ernst M, editors. Neuroimaging in Developmental Clinical Neuroscience. Cambridge University Press; 2009. pp. 22–37. [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children's theory of mind. Child Development. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, et al. Frontostriatal connectivity and its role in cognitive control in parent–child dyads with ADHD. American Journal of Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brian. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Milne B, Amell JW, Theodore RF, Moffitt TE. Children's behavioral styles at age 3 are linked to their adult personality traits at age 26. Journal of Personality. 2003;71:495–513. doi: 10.1111/1467-6494.7104001. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Archives of General Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham SJ, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46:193–207. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental outcome. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Valentino K. Toward the application of a multiple-levels-of-analysis perspective to research in development and psychopathology. In: Masten A, editor. Minnesota Symposia on Child Psychology. Vol. 34. Mahwah, NJ: Erlbaum; 2007. pp. 243–284. [Google Scholar]
- Dowsett S, Livesey DJ. The development of inhibitory control in pre-school children: Effects of “executive skills” training. Developmental Psychobiology. 2000;36:161–174. doi: 10.1002/(sici)1098-2302(200003)36:2<161::aid-dev7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Hane AA, Henderson HA, Moas OL, Reeb-Sutherland BC, Fox NA. Longitudinal stability of temperamental exuberance and social-emotional outcomes in early childhood. Developmental Psychology. 2011;47:765–780. doi: 10.1037/a0021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- DiClemente RJ, Hansen WB, Ponton LE. Handbook of adolescent health risk behavior. New York: Plenum; 1995. [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Murphy BC, Poulin R, Shepard S. The relations of regulation and emotionality to problem behavior in elementary school children. Development and Psychopathology. 1996;8:141–162. [Google Scholar]
- Eisenberg N, Sadovsky A, Spinrad TL, Fabes RA, Losoya SH, Valiente C, et al. The relations of problem behavior status to children’s negative emotionality, effortful control, and impulsivity: concurrent relations and prediction of change. Developmental Psychology. 2005;41:193–211. doi: 10.1037/0012-1649.41.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. Journal of Experimental Psychology: Learning Memory and Cognition. 2009;35:709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: performance of children 3 1/2–7 years old on a Stroop-like day-night test. Cognition. 1994;53:129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Gibson WA. Three multivariate models: factor analysis, latent structure analysis and latent profile analysis. Psychometrika. 1959;24:229–252. [Google Scholar]
- Goldsmith HH, Rothbart MK. The laboratory temperament assessment battery (Lab-TAB): Pre-locomotor version 3.1. Technical Manual. Department of Psychology, University of Oregon; 1999. [Google Scholar]
- Gray JA. The neuropsychology of anxiety. London: Oxford University Press; 1982. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An inquiry into the function of the septo-hippocampal system. 2nd ed. Oxford: Oxford University Press; 2000. [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44:1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan JJ, Reznick S, Snidman N. The physiology and psychology of behavioral Inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kagan J, Snidman N. Temperamental factors in human development. The American Psychologist. 1991;46:856–862. doi: 10.1037//0003-066x.46.8.856. [DOI] [PubMed] [Google Scholar]
- Kieras JE, Tobin RM, Graziano WG, Rothbart MK. You can’t always get what you want: effortful control and children’s responses to undesirable gifts. Psychological Science. 2005;16:391–396. doi: 10.1111/j.0956-7976.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Cicchetti D, Rogosch FA, Manly JT. Child maltreatment and trajectories of personality and behavioral functioning: Implications for the development of personality disorder. Development and Psychopathology. 2009;21:889–912. doi: 10.1017/S0954579409000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloo D, Perner J. Training transfer between card sorting and false belief understanding: Helping children apply conflicting descriptions. Child Development. 2003;74:1823–1839. doi: 10.1046/j.1467-8624.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Kochanska G. Children's temperament, mother's discipline, and security of attachment: Multiple pathways to emerging internalization. Child Development. 1995;66:597–615. [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk-taking, stress, responsivity, and vulnerability to drug abuse and addiction. Nature Neuroscience. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lahat A, Hong M, Fox NA. Behavioral inhibition: Is it a risk factor for anxiety? International Review of Psychiatry. 2011;23:248–257. doi: 10.3109/09540261.2011.590468. [DOI] [PubMed] [Google Scholar]
- Leigh BC. Peril, chance, and adventure: Concepts of risk, alcohol use and risky behavior in young adults. Addiction. 1999;94:371–383. doi: 10.1046/j.1360-0443.1999.9433717.x. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Bornovalova MA, Moolchan ET. Differences in risk-taking propensity across inner city adolescent ever and never-smokers. Nicotine & Tobacco Research. 2005;7:71–79. doi: 10.1080/14622200412331328484. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Daughters S, Zvolensky MJ, Kahler C, Gwadz M. Reliability and validity of the youth version of the Balloon Analogue Risk Task (BART-Y) in the assessment of risk-taking behavior among inner city adolescents. Journal of Clinical Child and Adolescent Psychology. 2007;36:106–111. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Strong DR, Richards JB, Kahler CW, Read JP. The balloon analogue risk task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2003a;11:26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the balloon analogue risk task (BART) as a predictor of adolescent real-world risk-taking behaviors. Journal of adolescence. 2003b;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk-taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebal Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Lynam D, Moffitt T, Stouthamer-Loeber M. Explaining the relation between IQ and delinquency: class, race, test motivation, school failure, or self-control? Journal of Abnormal Psychology. 1993;102:187–196. doi: 10.1037//0021-843x.102.2.187. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Vasey MW. Negative affectivity, effortful control, and attention to threat-relevant stimuli. Journal of Abnormal Child Psychology. 2009;37:387–399. doi: 10.1007/s10802-008-9284-y. [DOI] [PubMed] [Google Scholar]
- Lusher JM, Chandler C, Ball D. Dopamine D4 receptor gene (DRD4) is associated with Novelty Seeking (NS) and substance abuse: The saga continues. Molecular Psychiatry. 2001;6:497–499. doi: 10.1038/sj.mp.4000918. [DOI] [PubMed] [Google Scholar]
- MacPherson L, Magidson JM, Reynolds EK, Kahler CW, Lejuez CW. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcoholism: Clinical and Experimental Research. 2010;34:1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation M, Crusto C, Wandersman A, Kumpfer KL, Seybolt D, Morrissey-Kane E, et al. What works in prevention: Principles of effective prevention programs. American Psychologist. 2003;58:449–456. doi: 10.1037/0003-066x.58.6-7.449. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Park S, Belsky J, Putnam S, Crnic K. Infant emotionality, parenting, and 3-year inhibition: Exploring stability and lawful discontinuity in a male sample. Developmental Psychology. 1997;33:218–227. doi: 10.1037//0012-1649.33.2.218. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005a;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. A behavioral and electrophysiological study of children’s selective attention under neutral and affective conditions. Journal of Cognition and Development. 2005b;6:89–118. [Google Scholar]
- Pfeifer M, Goldsmith HH, Davidson RJ, Rickman M. Continuity and change in inhibited and uninhibited children. Child Development. 2002;73:1474–1485. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- Polak-Toste CP, Gunnar MR. Temperamental exuberance: correlates and consequences. In: Marshall PJ, Fox NA, editors. The Development of Social Engagement. New York: Oxford University Press; 2006. pp. 19–45. [Google Scholar]
- Putnam SP, Stifter CA. Behavioral approach-inhibition in toddlers: prediction from infancy, positive and negative affective components, and relations with behavior problems. Child Development. 2005;76:212–226. doi: 10.1111/j.1467-8624.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL. Temperament and social behavior in childhood. Merrill-Palmer Quarterly. 1994;40:21–39. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 6th ed. New York: Wiley; 2006. pp. 99–166. [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71:1113–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Letsch EA, Crawford FC. DRD4 and novelty seeking: Results of meta-analyses. American Journal of Medical Genetics. 2002;114:643–648. doi: 10.1002/ajmg.10649. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter CA, Putnam S, Jahromi L. Exuberant and inhibited toddlers: stability of temperament and risk for problem behavior. Development and Psychopathology. 2008;20:401–421. doi: 10.1017/S0954579408000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsten TS, Pleskac TJ, Lejuez CW. Modeling behavior in a clinically diagnostic sequential risk-taking task. Psychological Review. 2005;112:862–880. doi: 10.1037/0033-295X.112.4.862. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WPPSI-III administration and scoring manual. San Antonio, TX: 2002. [Google Scholar]
- White TL, Lejuez CW, de Wit H. Personality and gender differences in effects of d-amphetamine on risk taking. Experimental and Clinical Psychopharmacology. 2007;15:599–609. doi: 10.1037/1064-1297.15.6.599. [DOI] [PubMed] [Google Scholar]
- White TL, Lejuez CW, de Wit H. Test-retest characteristics of the ballon analogue risk task (BART) Experimental and Clinical Psychopharmacology. 2008;16:565–570. doi: 10.1037/a0014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology. 2011;39:735–747. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Fox NA, Lejuez CW, Reynolds EK, Henderson HA, Pérez-Edgar KE, Steinberg L, Pine DS. Early temperament, propensity for risk-taking and adolescent substance-related problems: A prospective multi-method investigation. Addictive Behaviors. 2010;35:1148–1151. doi: 10.1016/j.addbeh.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Zelazo PD. The dimensional change card sort (DCCS): a method of assessing executive function in children. Nature Protocols. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Carlson SM, Kesek A. Development of executive function in childhood. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. 2nd edition. Cambridge, MA: MIT Press; 2008. pp. 553–574. [Google Scholar]
- Zuckerman M. Psychobiology of personality. Cambridge, England: Cambridge University Press; 1991. [Google Scholar]
- Zuckerman M, Ball S, Black J. Influences of sensation seeking, gender, risk appraisal, and situational motivation on smoking. Addictive Behaviors. 1990;15:209–220. doi: 10.1016/0306-4603(90)90064-5. [DOI] [PubMed] [Google Scholar]