Abstract
While the symptomology of underactive bladder (UAB) may imply a primary dysfunction of the detrusor muscle, insights into pathophysiology indicate that both myogenic and neurogenic mechanisms need to be considered. Due to lack of proper animal models, the current understanding of the UAB pathophysiology is limited, and much of what is known about the clinical etiology of the condition has been derived from epidemiological data. We hereby review current state of the art in the understanding of the pathophysiology of and animal models used to study the UAB.
Keywords: Detrusor, Underactivity, Animal model, Surrogate
Introduction
International Continence Society defines detrusor underactivity/underactive bladder as a detrusor contraction of inadequate strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying in the absence of urethral obstruction. Prevalence of urodynamically confirmed underactive bladder (UAB) is in 9–48 % of men and 12–45 % of older women having non-neurogenic lower urinary tract symptoms [1, 2].
Bladder emptying requires complete relaxation of the internal (smooth muscle) and external (striated muscle) urethral sphincter followed by increased intravesical pressure due to detrusor smooth muscle contraction. The transition from filling phase to emptying phase of the lower urinary tract occurs voluntarily in healthy adults. Neurons from the brain and the spinal cord align together to form a neural control system to coordinate the reciprocal activity of two functional motor units involved in bladder emptying that is the detrusor and the outlet formed by the bladder neck, urethra and striated muscles of the urethral sphincter [3]. Neuron reflexes guiding the voiding are mediated by a spinobulbospinal pathway passing through a coordination center [the pontine micturition center (PMC)] located in the brain stem [3], which sets the volume at which the lower urinary tract switches from filling to emptying mode, thereby effectively determining maximum bladder capacity. The reflexes responsible for the bladder filling are organized at the spinal cord level with inputs from brainstem and cerebral centers [4].
Activation and maintenance of the micturition reflex is dependent upon normal relay of afferent information from the bladder to higher brain centers. Afferents convey the sensation of bladder fullness, which initiates activity in low-threshold mechanoceptive Aδ-fiber afferents during filling, and these afferents also convey the magnitude of detrusor contractions during the emptying phase [5]. Afferents are activated by both low (non-nociceptive) and high (nociceptive) intravesical pressures. The efferent arm of neural control in micturition involves twin autonomic branches, namely the sympathetic branch, for controlling the bladder filling and parasympathetic branch for emptying. Increased firing in mechanoceptive afferents from bladder distension during filling phase reverses the pattern of efferent outflow through firing in the sacral parasympathetic pathways and completes inhibition of sympathetic and somatic pathways. Therefore, the relay of afferent input from the bladder to the neural circuitry in the brain stem, in particular, the periaqueductal gray (PAG) and PMC is crucial to switch on the periodic transformation of the lower urinary tract from the mode of bladder filling to emptying [6–8].
Many neurotransmitters including acetylcholine, nor-epinephrine, dopamine, serotonin, excitatory and inhibitory amino acids, adenosine triphosphate, nitric oxide and neuropeptides are involved in the control of micturition [3]. Acetylcholine is the primary neurotransmitter effecting bladder emptying through its action on the muscarinic receptors on detrusor muscle [5], and the storage phase is mediated by norepinephrine released from sympathetic nerve terminals. Muscarinic receptors are classified based on molecular and pharmacological criteria into five subtypes (M1–M5), and detrusor muscle, like other forms of smooth muscle, exhibits a heterogeneous distribution of these receptor subtypes [9].
Complete voiding is initiated upon cessation of sympathetic and somatic inputs to the detrusor and sphincter, which causes sphincters to relax and the bladder neck to assume the shape of funnel, and the concomitant increased parasympathetic activity [9] causes generation of pressure for overcoming resistance generated by the collapsed urethra. Reversal in efferent outflow in both sympathetic and somatic innervation to the urethra and sphincter causes a reflex relaxation followed in a few seconds by parasympathetic-nerve-mediated detrusor contraction. Nitric oxide released from the parasympathetic nerve terminals in the urethra also cause relaxation of urethral smooth muscle during voiding. As a result, the pressure inside the bladder rises and the urethral pressure falls, which is a prerequisite for the urine expulsion. Efficient voiding is also dependent on the activity of urethral afferents responding to urine flow. Activity in urethral afferents potentiates detrusor contraction through a feed-forward mechanism. Signal from urethral afferent is fed back to the motor system at several levels of control between the end organ and cortical brain function [10].
Current understanding of UAB pathophysiology
The prevalence of UAB is higher in the aged population and in the absence of other explanations some older adults are diagnosed with idiopathic UAB [11]. Nevertheless, advanced age does not by itself lead to clinically significant UAB. However, the potential contributions made by aging to this condition become apparent when UAB is viewed in the context of its multifactorial etiology with a role for both lower urinary tract and non-genitourinary factors in its pathogenesis [2, 11]. As is common for many other multifactorial conditions and syndromes that commonly impact the health of older adults, a variety of predisposing and precipitating factors may jointly contribute [12]. Examples of risk factors that may alone or in conjunction with other considerations lead to UAB include bladder outlet obstruction; diabetes mellitus contributing to myogenic UAB; Parkinson’s disease with neurogenic UAB; multiple sclerosis; injury to the spinal cord and cauda equina (e.g., herniated disk, pelvic fractures); infectious neurological problems (e.g., AIDS, herpes zoster infection); plus pelvic surgery and radical prostatectomy, which can lead to iatrogenic UAB. Moreover, medications such as neuroleptics, calcium channel antagonists, and α-receptor agonists may also occasionally precipitate UAB in individuals who have presumably been rendered more vulnerable as a result of predisposing risk factors.
From a mechanistic point of view, the observation that advanced age is associated with an increased risk of UAB could be attributed to the existence of multiple morbidity, combined with a variety of biological changes, which likely to be relevant to UAB and which have been linked to aging and/or common late life disease conditions. Among Americans 65 years and older, more than one-half have been diagnosed with three or more chronic conditions [13]. Common chronic conditions such as diabetes mellitus are known to predispose individuals to UAB, while animal studies discussed below indicate that chronic ischemia commonly seen in the context of atherosclerosis and microvascular disease [14], a profound lack of estrogen seen in some postmenopausal women [15–18] or biological processes, which are intrinsic to aging [17, 19] could also potentially contribute to UAB by favoring the development of degenerative or biochemical changes involving key components of the detrusor muscle, its contractile machinery or its innervation. For example, there is a striking paucity of studies that would permit an examination of the relationship between menopause or estrogen status and UAB or DU. At the same time, studies using mature or aged animal models have shown that prolonged consequences of bilateral ovariectomy include relevant consequences such as axonal [18] and myocyte [18] degeneration as seen in older adults with UAB [20]; depletion of caveolae [17] with potential implications for age-associated changes in calcium signaling [19, 21] and declines in key proteins involved in smooth muscle contractility [16].
Three main hypotheses have been proposed for the mechanisms underlying UAB: neurogenic, myogenic and integrative. The neurogenic hypothesis focuses on defect in either afferent innervation or in its integrative control at spinal and supraspinal centers, which leads to poor efferent outflow. Myogenic hypothesis focuses on the periphery, in which the ability of the detrusor to contract efficiently is compromised by defect in the muscle itself.
Given the importance of an intact afferent system to voiding function, UAB may arise when the levels of afferent activity are disproportionately low for any given degree of bladder distention [22, 23]. There can be age-dependent loss of bladder volume sensitivity due to changes in neurotransmitter release from the urothelium as well as in the sensitivity and coupling of the suburothelial interstitial cell-afferent network. Afferents in the bladder and urethra can be damaged, for example, through an effect of aging or ischemia [24]. Urethral afferent dysfunction as a late consequence of diabetes [25] can also reduce or prematurely end the micturition reflex, leading to loss of voiding efficiency, as seen in diabetic cystopathy.
The myogenic hypothesis suggests that UAB results from changes within the bladder smooth muscle that lead to reduced excitability and loss of intrinsic muscle contractility in the bladder driving spontaneous contraction. Ultrastructural studies have revealed characteristic changes associated with UAB [26], which could potentially hinder efficient contraction of the detrusor muscle. Tenets of Laplace’s law dictate that rise in bladder capacity due to urine retention will by itself hinder generation of high enough intravesical pressure. Individuals with UAB may experience a greater decline in detrusor contractility than people with normal aging. When the disproportionate decline of detrusor contractility in affected individuals is not compensated by increase in release of neurotransmitters, then it can lead to UAB.
Autonomous detrusor activity detected during bladder filling [27] facilitates the generation of bladder sensation, and absence of spontaneous contractions can hinder the initiation of afferent signals, which can lead to neurogenic UAB/DU from the impaired afferent arm. It can be difficult to isolate a neurogenic or myogenic component in patients with mixed UAB. In such cases, decreased detrusor contractility will lead to reduced autonomous activity of the bladder, which then can reduce the afferent return from the bladder.
The integrative hypothesis proposes that complex interactions among smooth muscle, connective tissue, urothelium and supportive structures with peripheral nerves contribute to normal generation of localized spontaneous activity that is observed as localized contractions and stretches (micromotions) in the human bladder [28]. These findings have led to the suggestion that the detrusor muscle is functionally modular in arrangement.
Ultrastructural features
The myogenic hypothesis suggests that UAB results from changes within the bladder smooth muscle that lead to reduced excitability and loss of intrinsic muscle contractility in the bladder driving spontaneous contraction. Ultrastructural studies have revealed characteristic changes associated with UAB [20, 29], which could potentially hinder efficient contraction of the detrusor muscle. There is also evidence in ultrastructural studies that degeneration of the detrusor muscle cell and disruption in normal detrusor fascicular architecture may contribute to poorer outcomes after TURP with respect to persistent urinary retention and raised post-void residual urine.
Importance of animal models
Until recently, clinical research in the field of lower urinary tract dysfunctions was limited to cystometry or molecular and cellular research using biopsy material from willing patients. However, recent advances in diagnostic and functional imaging have allowed brain mapping to study brain responses during bladder filling and emptying of subjects [30]. Since direct human experimentation on UAB subjects is not possible for ethical reasons, hypotheses stated for UAB will require alternatives to test the derived predictions. Animal models can be used to generate novel directions of research and corroborate findings obtained in case studies or other methods.
Animals that share similar integrative physiology of the lower urinary tract and the neural control of micturition as humans provide a suitable tool to dissect the underlying mechanisms in clinical features of UAB. Animal models can be used to reproduce some or all of the facets of UAB seen clinically as a consequence of neurogenic or myogenic dysfunction to help identify suitable interventions. Animal models provide suitable platforms of “intact” biological systems for assessing results from simpler in vitro research. The clinical context of animal studies can help build a confidence in a new treatment approach before clinical testing.
Development of an animal model for UAB is hindered by the lack of a surrogate that predicts treatment outcome. The clinically meaningful endpoints are changes in urinary frequency and voided volumes. Animal models can be classified as exploratory, explanatory or predictive—depending on the type of information that they are designed to yield. Exploratory models are used to study physiological processes and pathological mechanisms in UAB in order to generate novel ideas and theories about physiological function. Explanatory models are developed and applied in an attempt to improve our understanding of the importance and relevance of findings generated by other models. Predictive or preclinical models are those used to discover and quantify the impact of novel treatments and therapeutic agents and to assess their toxicity to the living organism.
Animal models of UAB
Epidemiology has guided most of the animal research on UAB so far. UAB is mostly seen in aged patients [31]; therefore, aged animals are preferred as exploratory UAB models. All patients with neurological disorders experience some form of lower urinary tract dysfunction—no matter how mild their disease is—they inevitably develop a bladder-related problem. This has led to the development of a large range of neurological models. The inciting event of neurological defect such as cerebrovascular event or a spinal cord injury can be recapitulated to induce equivalent changes in explanatory animal models. Considering the association of UAB with diabetes, chronic effects of chemically induced diabetes on bladder function have been studied as a UAB model. Unfortunately, this approach to modeling is complicated by the fact that the bladder has a limited repertoire of responses to injury, and thus, differing etiological factors may produce a similar picture in affected individuals. Moreover, animal modeling, which focuses on addressing a single etiological factor at a time, may not suffice when seeking to define the pathophysiology of a highly complex multifactorial condition. With these considerations in mind, future animal model studies may need to explore interactions between more than one predisposing or provocative risk factors in their ability to contribute to UAB or relevant proxy measures.
Aged rodent models
Animal models of idiopathic UAB are difficult, so phenotypic models are used in which an animal expresses cystometric or voiding features resembling those seen in UAB patients. Aged mouse [22, 32] or rat models reproduce some of the UAB features, with age-dependent loss of bladder volume sensitivity manifesting as increased inter-contractile interval. Cystometry of young and old mice shows that detrusor contractility is preserved, but the response to rise in bladder volume is diminished, which is evident from an increase in intercontractile interval at 26 months. Similar findings were observed in aged rats with increased volume and pressure thresholds for voiding [33]. Age-dependent loss of bladder volume sensitivity in aged rats may be explained by the decreased response to intravesical capsaicin, which is suggestive of reduced C-fiber afferent activity in aged rat [33]. Intravesical capsaicin is expected to increase detrusor contractility because of neurokinins released from afferent nerves following activation of transient receptor potential (TRP) channels. Aged rats also exhibit a reduction in the maximal bladder pressure generated during pelvic nerve stimulation [34–36]. Staining for calcitoningene-related peptide (CGRP) and substance P in lumbosacral dorsal root ganglion DRG neurons [37] and density of pituitary adenylate cyclase-activating peptide (PACAP) innervation of the bladder base are also decreased in aged rats [38].
In addition, aged male rats exhibited urethral dysfunction and impairment of the urethrovesical coordination, which further corroborates that complete bladder emptying relies on reciprocal contraction and relaxation of smooth muscle in the bladder and urethra, respectively [39–41]. Decrease in resting urethral pressure at voiding threshold and the occurrence of a significant delay in urethral relaxation that leads to increased PVR was observed in aged male rats. The maximal responses to carbachol in the bladder body and to phenylephrine and carbachol in the urethra were also observed to be diminished in the aged male rat bladder. Significant decreases in the amplitude of neurogenic contractions were associated with fibrosis but without accompanying a decrease in nerve density in the bladder neck. Thus, these findings from aged rats implicate impairment of both afferent and efferent transmission results in incomplete voiding. There is also an upregulation of purinergic receptors in the urothelium and bladder nerve bundles compared to control rats, perhaps corresponding to the increased non-adrenergic and non-cholinergic (NANC) innervation seen in aging humans. Upregulation of β-adrenoceptors [42] is also reported in aged rat bladder [41], and the pathological relevance of altered receptor expression in UAB will be interesting to investigate. Recent findings also suggest that the cellular basis of age-related cognitive decline manifesting in cAMP signaling and KCNQ channels share some parallels with age-related decline in bladder contractility.
Peripheral versus central models
Animal models of UAB can be broadly divided into peripheral and central models based on the predominant site of the deficit. Peripheral models are those resulting from direct damage to the bladder, its peripheral innervation or blood supply, whereas central models are developed following injuries to the spinal cord, brainstem or higher centers.
Diabetic bladder dysfunction (DBD) models
DBD describes storage and voiding problems, as well as other less clinically defined phenotypes, such as decreased sensation and increased bladder capacity in both type I and type II diabetes mellitus (DM) [43–47]. Systemic administration of streptozotocin is used to induce DM in rats, which is confirmed by increases in blood glucose and urine production. Animals show time-dependent changes in cystometry with initial compensated changes similar to detrusor overactivity. The decompensated stage at 12 weeks shows features of UAB that are the result of long-term hyperglycemia-related oxidative stress and polyuria. The STZ-induced DBD is associated with increased bladder weight and residual urine, which indicates the incomplete bladder emptying. Streptozotocin-induced DBD affects Aδ-fiber afferent-dependent conscious voiding, which was evaluated in metabolic cage measurements and awake cystometry [43–47]. The impairment of C-fiber-mediated bladder nociceptive responses in the DBD bladder was revealed by reduced sensitivity of C-fiber-afferent pathways to nociceptive stimuli during 0.25 % acetic acid cystometry of rats with DBD under urethane anesthesia [46].
Genetically engineered mouse models have also been developed that display salient features of DBD relevant to UAB. Liver-specific deletion of insulin receptor substrate 1 (IRS1) and IRS2 leads to hyperglycemia by 5 weeks of life [48, 49] and development of DBD that models pathologic changes in humans with type II DM [50] Importantly, the IRS1:IRS2 double-knockout model displays bladder overactivity in young mice, but bladder underactivity in older animals. Detrusor underactivity was characterized by decreased force generation in muscle strips from older diabetic mice compared with age-matched controls in response to electrical field stimulation, carbachol and KCl-mediated depolarization [25].
Diabetes-induced urethral dysfunction
Urethral dysfunction is believed to cause changes in voiding behavior of aged male rats [39–41], which results in decreased resting urethral pressure at voiding threshold and the occurrence of a significant delay in urethral relaxation. Therefore, impaired urethral relaxation can also prolong the bladder emptying during voiding phase. Urethral dysfunction is also a consequence of diabetes, which we have studied (Fig. 1) [51, 52] as well as other labs [25, 53]. Nitric oxide-mediated relaxation of the urethra is considered to be impaired in diabetes. Other studies have reported damage to urethral afferents, which reduces or prematurely ends the micturition reflex, leading to loss of voiding efficiency, as in diabetic cystopathy. DBD is also associated with altered receptor expression [54] and changes in NANC transmission [55, 56].
Fig. 1.
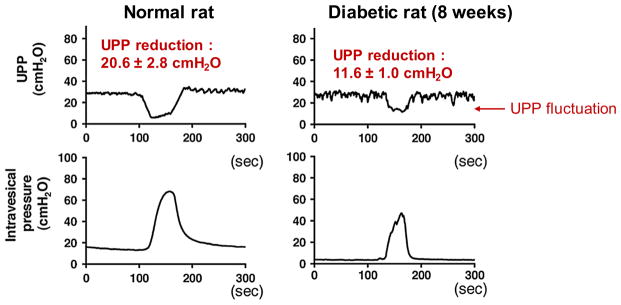
Diabetes-induced urethral dysfunction contributes to incomplete voiding. In these experiments, striated sphincter activity was suppressed by α-bungarotoxin and NO-mediated urethral relaxation was suppressed by L-NAME. Diabetic rat shows significantly reduced urethral pressure profile compared with normal rat
Ischemia and hyperlipidemia
Epidemiological studies have suggested bladder ischemia and metabolic syndrome as likely etiological factors of UAB. The underlying pathological mechanisms have not been clearly defined, but current evidence suggests that there is likely to be both a vascular and neurogenic component. Chronic bladder ischemia [57] secondary to atherosclerosis assists the progression of OAB into UAB. Cystometry of myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits show significantly shorter micturition intervals, smaller voided volume with non-voiding contractions and lower micturition pressure [58], as compared to control animals. The carbachol and electrical field stimulation-induced contractions of WHHLMI detrusor strips were also significantly decreased. This animal model would serve well to screen for drugs that seek to improve detrusor contractility.
Neurological models of UAB
Functional imaging has provided many insights into the age-related decrease in responses within the brain regions involved in interpretation of afferent inputs from the bladder. Dysfunction of the central control of the voiding reflex can lead to UAB by impacting upon key processes in perception, integration, and outflow. Considering that voluntary voiding is a learned phenomenon, age-related decline of dopamine binding has been reported in brain areas involved in cognition [59]. In fact, brain areas involved in cognition overlap with areas involved in processing afferent input from the bladder, which implicate the dopamine role in central transmission as key for UAB.
The central control of micturition is highly complex, and the voiding disturbance that develops will depend on the location and extent of the neurological injury. A number of central nervous system disorders cause voiding dysfunction in humans, including cerebrovascular events, dementia, Parkinson’s disease, multiple sclerosis and stroke. The coordination between the detrusor smooth muscle and the sphincter mechanism of the bladder occurs in the pontine region of the brainstem. On the other hand, patients with spinal cord injuries will present with a variety of lower urinary tract signs and symptoms depending on the level of injury, and whether it is partial or complete. Neurological models improve our understanding of the complex pathways controlling micturition.
Parkinson’ disease
PD is a chronic and progressive degenerative disease of the brain characterized by selective destruction of striatal dopaminergic neurons that pass from the substantia nigra pars compacta to the putamen. PD can be induced in monkeys by administering the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is selective for dopaminergic neurons in the substantia nigra. In MPTP-treated monkeys, cystometry showed that injection of a dopamine D1 receptor agonist significantly increases the bladder volume and pressure thresholds for inducing the micturition reflex, with no corresponding effect seen in normal monkeys [60, 61].
Lumbar canal stenosis (LCS) for UAB
LCS induces mechanical compression of the cauda equina by insertion of silicon rubber pieces into the L6 epidural space in female rats [62]. The mechanical compression causes degeneration of both afferent and efferent spinal nerves involved in voiding. The animal model exhibits both decreased voiding efficiency and reduced detrusor contractility.
Ventral avulsion model for UAB
Trauma to the thoracolumbar spine commonly results in injuries to the cauda equina and the lumbosacral portion of the spinal cord. A unilateral L5-S2 ventral root avulsion (VRA) injury in rats mimics a partial lesion to the cauda equina and conus medullaris [63]. Detailed cystometrogram studies found that a markedly reduced voiding efficiency was noted at 12 weeks after the VRA injury with decreased maximum amplitude to indicate reduced contractile stimuli. Concurrent external urethral sphincter (EUS) electromyography demonstrated shortened burst and prolonged silent periods associated with the elimination phase [63]. The animal model demonstrated that a 5HT1A receptor agonist, 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT), administered intravenously, elicited the supraspinal micturition reflex during cystometry.
Pelvic nerve injury for iatrogenic UAB
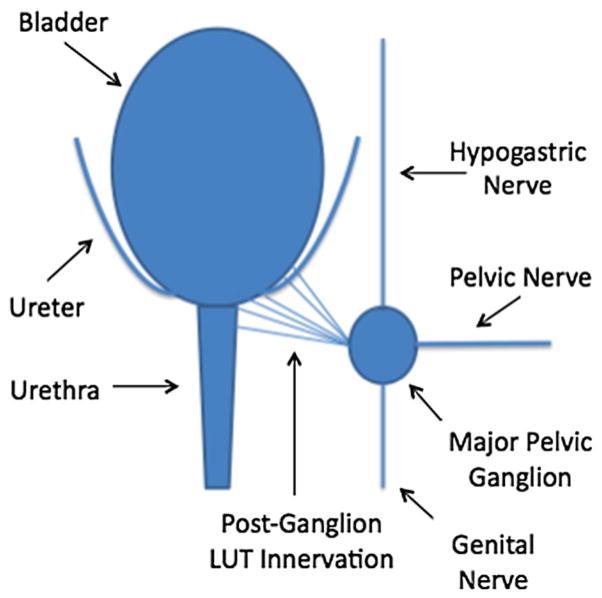
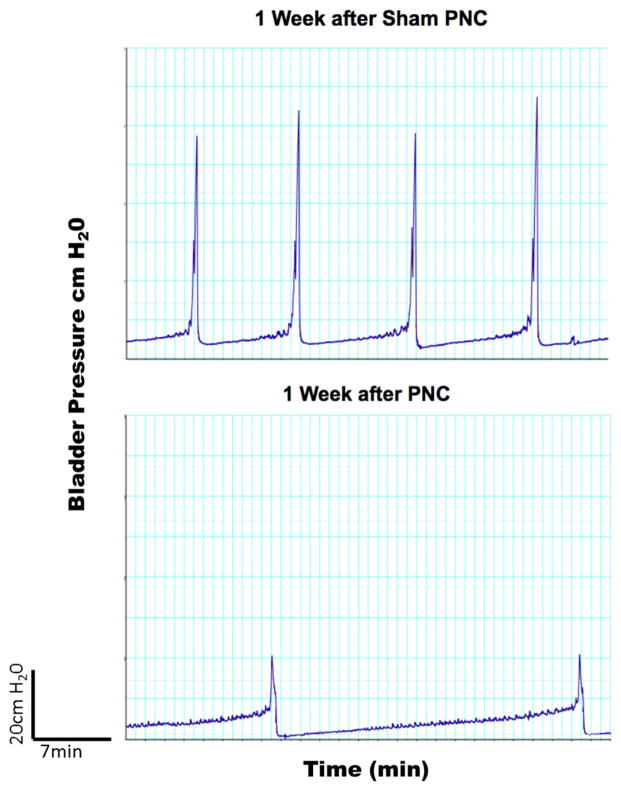
Latrogenic UAB is often associated with pelvic nerve injury induced by pelvic organ surgery. The animal model was developed for UAB by performing a bilateral pelvic nerve crush in adult female rats with a straight micro-mosquito clamp as illustrated in Fig. 2. The clamp was used to crush each pelvic nerve for a total of 30 s. The crush was performed proximal to each pelvic nerve’s entry into the major pelvic ganglion. Pelvic nerve crush injury caused significant increases in bladder capacity in cystometry performed 1 week after nerve injury as shown in Fig. 3. The model shows increased PVR and decreases in maximum voiding pressure and voiding efficiency in cystometry. Animals with pelvic nerve injury may also serve as a model of neurogenic UAB as afferent and efferent nerve dysfunction is known to be one of major causes of UAB, as discussed above.
Fig. 2.
Schematic illustration for rat model of Iatrogenic UAB-induced by brief bilateral pelvic nerve crush in adult female rats with a straight micro-mosquito clamp
Fig. 3.
Cystometric outcomes of bilateral pelvic nerve crush in female rats. Compared with sham group, the crush injury of pelvic nerves exhibited increases in PVR and decreases in maximum voiding pressure and voiding efficiency
Transgenic models
Advances in genetic engineering techniques have led to an increasing use of genetically modified organisms for research. The mouse is most commonly employed, given that its genome is completely sequenced, is easy to maintain and has a relatively short generation span. Several transgenic mouse models have demonstrated that molecular alterations in the urothelium, peripheral innervation and smooth muscle leads to significant changes in voiding function [64]. However, the full power of this technology could not be harnessed until the recent description of experimental approaches permitting the continuous comprehensive urodynamic assessment involving concurrent measurements of pressure, volume and flow in mice [22, 65].
Prostaglandin receptor knockout mouse
Prostaglandins are produced by the constitutively expressed cyclooxygenase-(COX) 1 enzyme, and an inducible isozyme, COX-2 in urothelium [66–69]. Prostaglandin E2 (PGE2) produced by this enzyme is decreased in the urine of UAB patients [70]. PGE2 mediates its effects by activating the EP family (EP1–EP4 isoforms) of G-protein-coupled receptors. PGE2 have a physiological role as it is produced by detrusor muscle in response to stretch. Mouse knockout models of the EP3 receptor showed enlarged bladder capacity [71], which could be used to screen drugs and test new hypothesis for UAB. Since EP3 knockout mice do not exhibit bladder overactivity after instillation of an EP3 receptor agonist, changes in peripheral afferent sensitivity in this model cannot be ruled out.
Purinergic receptor knockout mouse
ATP is released from human and animal urothelium in response to mechanical stretch. Prostaglandins were shown to be involved in the stretch evoked ATP release from the urothelium [72]. ATP acts as a sensory neurotransmitter by binding to purinergic receptors (P2X) on suburothelial afferent nerve endings. P2X3 receptor knockout mice appear to have reduced bladder sensation, with reduced urinary frequency and larger voided volumes [73–76].
Models of myogenic UAB
Decreased detrusor contractility in UAB can result from a lack of contractile stimulus (acetylcholine and ATP) [77] and/or a lack of tissue responsiveness due to irreversible changes in the bladder wall described as sarcopenia (loss of muscle tissue, increased collagen deposition) [26, 78]. Several factors may contribute to altered excitation–contraction coupling mechanisms including changes in the properties and density of calcium [79] and potassium channels, gap junctions and receptors in detrusor smooth muscles of aged and UAB patients.
Bladder outlet obstruction BOO
Effects similar to BOO in humans are relatively straightforward to replicate in animals. This has been achieved in a variety of animal species including the pig, rat, guinea pig and rabbit by inducing partial obstruction of the urethra using some form of ligature that either occludes/stenoses the urethra immediately or does so gradually as the animal grows [80]. Partial BOO was induced in adult female rats by ligating the proximal urethra over a 1-mm angiocatheter for 2 weeks to 6 months. Prolonged BOO caused a decrease in electric field stimulation (EFS, 2.5–40 Hz)-induced acetylcholine release and the number of nerves in the rat urinary bladder, which might contribute to detrusor underactivity (UAB) in BOO. The amount of acetylcholine was measured in the dialysate fraction obtained from a microdialysis probe inserted into the muscle strips during EFS. The reduced density of acetylcholinesterase-positive nerves in obstructed bladders without overt neurological disease can also cause insufficient efferent activation to occur.
Limitations of animal research
An ideal model would be one which replicates the symptoms, etiology and natural history of clinical UAB. Most of the animal models discussed here do not essentially mimic UAB, but bear enough similarities to make them relevant for a limited number of aspects of the human condition. It is inconceivable that any single animal model will replicate all the voiding features and consequences observed in UAB patients. Animal models need to be viewed as research tools to answer a particular experimental hypothesis rather than replicas of clinical UAB.
Studies using rodents and small laboratory animals are relatively simpler to perform and maintain than studies done using larger animals. Research done with small mammals is less expensive, more accessible and less time consuming to carry out. Irrespective of the animal species and model used, it is useful to determine the clinical relevance of the findings within the limitations of the model. Rodents can adapt to surgically induced alterations through neural plasticity and cellular adaptations. Therefore, experimental findings may represent compensatory changes or collateral effects rather than having pathophysiological relevance for UAB. It may also be that the changes seen are epiphenomena, being totally unrelated to the underlying pathological process and occurring by coincidence.
Furthermore, there are significant differences in the composition of the urinary bladder wall between small and large animal bladders, which can introduce variability in urodynamic features. The location of postganglionic parasympathetic cell bodies innervating the rat bladder is entirely in the pelvic ganglia, whereas a substantial proportion of these cell bodies are located in the bladder wall of humans and other animal species [81]. Therefore, the outcomes following partial BOO differs between species [82, 83]. Unlike rats, long-term untreated BOO does not appear to result in significant clinical decompensation of detrusor function in most men. Treatments targeted at reducing urethral resistance improved UAB symptoms [84].
The structural and functional differences discussed above are but a few examples of the interspecies variability, which are relevant to animal modeling of UAB and prevent erroneous conclusions [84]. It is important that these aspects are taken into account for identifying experimental findings that constitute general principles and are predictive of clinical efficacy from those that are unique to a model. The interspecies variability lends different strengths and weaknesses to each model and makes them suitable for studying different aspects of UAB. Regardless of the type of model used, careful validation of both the model itself and the results that it generates ensure that any findings are correctly extrapolated from animals to humans. The validation required will depend on how closely the model reproduces a disease or condition.
Conclusions
Research into the pathogenesis of detrusor underactivity and underactive bladder is hampered by the fact that it is currently an urodynamic-based diagnosis and does not appreciate the sensory afferent’s or the central nervous system’s role in UAB etiology. Despite the difficulties associated with animal modeling, there is no substitute for their use as tools to advance understanding and develop medical interventions for UAB. The various models that have been used to date have different strengths and weaknesses, and the findings should ideally be reproduced in more than one mammalian species before extrapolating data to human subjects.
Acknowledgments
Funding for this conference was made possible (in part) by 1R13AG047010 from the National Institute on Aging. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the NIH; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflict of interest Vincent Tse has conflicts of interest related to Allergan, Astellas, and American Medical Systems. Michael B Chancellor has conflicts of interest related to Allergan, Astellas, Cook, Lipella, Medtronic, Pfizer, Targacept. There are no conflicts of interest for Pradeep Tyagi, William C. de Groat, Lori A. Birder, Christopher J. Chermansky, Rosalyn M. Adam, George A. Kuchel, Philip P. Smith and Naoki Yoshimura.
Contributor Information
Pradeep Tyagi, Department of Urology, University of Pittsburgh, Pittsburgh, PA, USA.
Phillip P. Smith, Department of Surgery, University of Connecticut Health Center, Farmington, CT, USA. UConn Center on Aging, University of Connecticut Health Center, Farmington, CT, USA
George A. Kuchel, UConn Center on Aging, University of Connecticut Health Center, Farmington, CT, USA
William C. de Groat, Department of Pharmacology, University of Pittsburgh, Pittsburgh, PA, USA
Lori A. Birder, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA
Christopher J. Chermansky, Department of Urology, University of Pittsburgh, Pittsburgh, PA, USA
Rosalyn M. Adam, Department of Urology, Boston Children’s Hospital, Boston, MA, USA. Department of Surgery, Harvard Medical School, Boston, MA, USA
Vincent Tse, Department of Urology, Concord Hospital, University of Sydney, Concord, NSW, Australia.
Michael B. Chancellor, Email: chancellormb@gmail.com, Department of Urology, Beaumont Hospital, Royal Oak, MI, USA
Naoki Yoshimura, Department of Urology, University of Pittsburgh, Pittsburgh, PA, USA.
References
- 1.Jeong SJ, Kim HJ, Lee YJ, Lee JK, Lee BK, Choo YM, Oh JJ, Lee SC, Jeong CW, Yoon CY, Hong SK, Byun SS, Lee SE. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol. 2012;53(5):342–348. doi: 10.4111/kju.2012.53.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013;15(1):11–22. [PMC free article] [PubMed] [Google Scholar]
- 3.de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147(Suppl 2):S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyagi P, Thomas CA, Yoshimura N, Chancellor MB. Investigations into the presence of functional Beta1, Beta2 and Beta3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol. 2009;35(1):76–83. doi: 10.1590/s1677-55382009000100012. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi P, Chancellor MB, Li Z, De Groat WC, Yoshimura N, Fraser MO, Huang L. Urodynamic and immunohistochemical evaluation of intravesical capsaicin delivery using thermosensitive hydrogel and liposomes. J Urol. 2004;171(1):483–489. doi: 10.1097/01.ju.0000102360.11785.d7. [DOI] [PubMed] [Google Scholar]
- 6.de Groat WC. Anatomy and physiology of the lower urinary tract. Urol Clin North Am. 1993;20(3):383–401. [PubMed] [Google Scholar]
- 7.de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50(6A Suppl):36–52. doi: 10.1016/s0090-4295(97)00587-6. (discussion 53–36) [DOI] [PubMed] [Google Scholar]
- 8.de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92(2):127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 9.Tyagi S, Tyagi P, Vanle S, Yoshimura N, Chancellor MB, de Miguel F. Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J Urol. 2006;176(4 Pt 1):1673–1678. doi: 10.1016/j.juro.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 10.de Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor MB, Yoshimura N, Roppolo JR. Neural control of the urethra. Scand J Urol Nephrol Suppl. 2001;207:35–43. doi: 10.1080/003655901750174872. (discussion 106–125) [DOI] [PubMed] [Google Scholar]
- 11.Taylor JA, 3rd, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006;54(12):1920–1932. doi: 10.1111/j.1532-5415.2006.00917.x. [DOI] [PubMed] [Google Scholar]
- 12.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition–multimorbidity. J Am Med Assoc. 2012;307(23):2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida M, Masunaga K, Nagata T, Satoji Y, Shiomi M. The effects of chronic hyperlipidemia on bladder function in myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits. Neurourol Urodyn. 2010;29(7):1350–1354. doi: 10.1002/nau.20843. [DOI] [PubMed] [Google Scholar]
- 15.Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urol. 2007;69(3):436–440. doi: 10.1016/j.urology.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Ortiz RF, Wang Z, Menon C, DiSanto ME, Wein AJ, Chacko S. Estrogen modulates the expression of myosin heavy chain in detrusor smooth muscle. Am J Physiol Cell Physiol. 2001;280(3):C433–C440. doi: 10.1152/ajpcell.2001.280.3.C433. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Q, Resnick NM, Elbadawi A, Kuchel GA. Estrogen and postnatal maturation increase caveolar number and caveolin-1 protein in bladder smooth muscle cells. J Urol. 2004;171(1):467–471. doi: 10.1097/01.ju.0000099480.18735.49. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q, Ritchie J, Marouf N, Dion SB, Resnick NM, Elbadawi A, Kuchel GA. Role of ovarian hormones in the pathogenesis of impaired detrusor contractility: evidence in ovariectomized rodents. J Urol. 2001;166(3):1136–1141. [PubMed] [Google Scholar]
- 19.Lowalekar SK, Cristofaro V, Radisavljevic ZM, Yalla SV, Sullivan MP. Loss of bladder smooth muscle caveolae in the aging bladder. Neurourol Urodyn. 2012;31(4):586–592. doi: 10.1002/nau.21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haferkamp A, Dorsam J, Resnick NM, Yalla SV, Elbadawi A. Structural basis of neurogenic bladder dysfunction. III. Intrinsic detrusor innervation. J Urol. 2003;169(2):555–562. doi: 10.1097/01.ju.0000045753.02559.37. [DOI] [PubMed] [Google Scholar]
- 21.Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. II. Aging detrusor: normal versus impaired contractility. J Urol. 1993;150(5 Pt 2):1657–1667. doi: 10.1016/s0022-5347(17)35867-6. [DOI] [PubMed] [Google Scholar]
- 22.Smith PP, DeAngelis A, Kuchel GA. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol. 2012;302(5):R577–R586. doi: 10.1152/ajpregu.00508.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith PP. Aging and the underactive detrusor: a failure of activity or activation? Neurourol Urodyn. 2010;29(3):408–412. doi: 10.1002/nau.20765. [DOI] [PubMed] [Google Scholar]
- 24.Azadzoi KM, Radisavljevic ZM, Siroky MB. Effects of ischemia on tachykinin-containing nerves and neurokinin receptors in the rabbit bladder. Urology. 2008;71(5):979–983. doi: 10.1016/j.urology.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Dolber PC, Fraser MO. Differential vulnerabilities of urethral afferents in diabetes and discovery of a novel urethra-to-urethra reflex. Am J Physiol Renal Physiol. 2010;298(1):F118–F124. doi: 10.1152/ajprenal.00281.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brierly RD, Hindley RG, McLarty E, Harding DM, Thomas PJ. A prospective controlled quantitative study of ultrastructural changes in the underactive detrusor. J Urol. 2003;169(4):1374–1378. doi: 10.1097/01.ju.0000055781.07630.aa. [DOI] [PubMed] [Google Scholar]
- 27.Andersson KE. Detrusor myocyte activity and afferent signaling. Neurourol Urodyn. 2010;29(1):97–106. doi: 10.1002/nau.20784. [DOI] [PubMed] [Google Scholar]
- 28.Drake MJ, Harvey IJ, Gillespie JI, Van Duyl WA. Localized contractions in the normal human bladder and in urinary urgency. BJU Int. 2005;95(7):1002–1005. doi: 10.1111/j.1464-410X.2005.05455.x. [DOI] [PubMed] [Google Scholar]
- 29.Blatt AH, Brammah S, Tse V, Chan L. Transurethral prostate resection in patients with hypocontractile detrusor—what is the predictive value of ultrastructural detrusor changes? J Urol. 2012;188(6):2294–2299. doi: 10.1016/j.juro.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths DJ. Use of functional imaging to monitor central control of voiding in humans. Handb Exp Pharmacol. 2011;202:81–97. doi: 10.1007/978-3-642-16499-6_5. [DOI] [PubMed] [Google Scholar]
- 31.Zimmern P, Litman HJ, Nager CW, Lemack GE, Richter HE, Sirls L, Kraus SR, Sutkin G, Mueller ER. Effect of aging on storage and voiding function in women with stress-predominant urinary incontinence. J Urol. 2014 doi: 10.1016/j.juro.2014.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai HH, Boone TB, Thompson TC, Smith CP, Somogyi GT. Using caveolin-1 knockout mouse to study impaired detrusor contractility and disrupted muscarinic activity in the aging bladder. Urology. 2007;69(2):407–411. doi: 10.1016/j.urology.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Chai TC, Andersson KE, Tuttle JB, Steers WD. Altered neural control of micturition in the aged F344 rat. Urol Res. 2000;28(5):348–354. doi: 10.1007/s002400000135. [DOI] [PubMed] [Google Scholar]
- 34.Hotta H, Morrison JF, Sato A, Uchida S. The effects of aging on the rat bladder and its innervation. Jpn J Physiol. 1995;45(5):823–836. doi: 10.2170/jjphysiol.45.823. [DOI] [PubMed] [Google Scholar]
- 35.Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int. 2010;10(Suppl 1):S127–S136. doi: 10.1111/j.1447-0594.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 36.Mohammed HA, Santer RM. Distribution and changes with age of calcitonin gene-related peptide- and substance P-immunoreactive nerves of the rat urinary bladder and lumbosacral sensory neurons. Eur J Morphol. 2002;40(5):293–301. doi: 10.1076/ejom.40.5.293.28900. [DOI] [PubMed] [Google Scholar]
- 37.Mohammed H, Hannibal J, Fahrenkrug J, Santer R. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: effects of age. Urol Res. 2002;30(4):248–255. doi: 10.1007/s00240-002-0261-6. [DOI] [PubMed] [Google Scholar]
- 38.Lluel P, Deplanne V, Heudes D, Bruneval P, Palea S. Age-related changes in urethrovesical coordination in male rats: relationship with bladder instability? Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1287–R1295. doi: 10.1152/ajpregu.00499.2001. [DOI] [PubMed] [Google Scholar]
- 39.Lluel P, Palea S, Barras M, Grandadam F, Heudes D, Bruneval P, Corman B, Martin DJ. Functional and morphological modifications of the urinary bladder in aging female rats. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R964–R972. doi: 10.1152/ajpregu.2000.278.4.R964. [DOI] [PubMed] [Google Scholar]
- 40.Lluel P, Palea S, Ribiere P, Barras M, Teillet L, Corman B. Increased adrenergic contractility and decreased mRNA expression of NOS III in aging rat urinary bladders. Fundam Clin Pharmacol. 2003;17(5):633–641. doi: 10.1046/j.1472-8206.2003.00187.x. [DOI] [PubMed] [Google Scholar]
- 41.Iaina A, Serban I, Kapuler S, Gavendo S, Lindner A, Eliahou HE. Beta-adrenergic receptors in the urinary bladder of adult and developing rats. Isr J Med Sci. 1988;24(4–5):237–240. [PubMed] [Google Scholar]
- 42.Goins WF, Yoshimura N, Phelan MW, Yokoyama T, Fraser MO, Ozawa H, Bennett NJ, de Groat WC, Glorioso JC, Chancellor MB. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol. 2001;165(5):1748–1754. [PubMed] [Google Scholar]
- 43.Gray MA, Wang CC, Sacks MS, Yoshimura N, Chancellor MB, Nagatomi J. Time-dependent alterations of select genes in streptozotocin-induced diabetic rat bladder. Urology. 2008;71(6):1214–1219. doi: 10.1016/j.urology.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, de Groat WC, Yoshimura N. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004;53(10):2723–2730. doi: 10.2337/diabetes.53.10.2723. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, Kubo K, Kumon H, Groat WC, Yoshimura N. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J Urol. 2002;168(3):1259–1264. doi: 10.1097/01.ju.0000023400.17372.3e. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki K, Yoshimura N, Chancellor MB. Implications of diabetes mellitus in urology. Urol Clin North Am. 2003;30(1):1–12. doi: 10.1016/s0094-0143(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 47.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8(1):65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009;15(11):1307–1311. doi: 10.1038/nm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Cheng Z, Cristofaro V, Li J, Xiao X, Gomez P, Ge R, Gong E, Strle K, Sullivan MP, Adam RM, White MF, Olumi AF. Inhibition of TNF-alpha improves the bladder dysfunction that is associated with type 2 diabetes. Diabetes. 2012;61(8):2134–2145. doi: 10.2337/db11-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torimoto K, Fraser MO, Hirao Y, De Groat WC, Chancellor MB, Yoshimura N. Urethral dysfunction in diabetic rats. J Urol. 2004;171(5):1959–1964. doi: 10.1097/01.ju.0000121283.92963.05. [DOI] [PubMed] [Google Scholar]
- 51.Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol. 2005;173(3):1027–1032. doi: 10.1097/01.ju.0000146268.45662.36. [DOI] [PubMed] [Google Scholar]
- 52.Christ GJ, Bushman W, Fraser MO. Impact of diabetes and obesity on the prostate and urethra: implications to improved bladder dysfunction understanding and treatment. J Urol. 2009;182(6 Suppl):S38–S44. doi: 10.1016/j.juro.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Sun Y, Zhang Z, Feng X, Meng H, Li S, Zhu Y, Chen S, Wang Y, Wang J, Zhang D, Jiang X, Li N, Shi B. Cannabinoid receptors 1 and 2 are associated with bladder dysfunction in an experimental diabetic rat model. BJU Int. 2013;112(2):E143–E150. doi: 10.1111/bju.12172. [DOI] [PubMed] [Google Scholar]
- 54.Bschleipfer T, Nandigama R, Moeller S, Illig C, Weidner W, Kummer W. Bladder outlet obstruction influences mRNA expression of cholinergic receptors on sensory neurons in mice. Life Sci. 2012;91(21–22):1077–1081. doi: 10.1016/j.lfs.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Philyppov IB, Paduraru ON, Andreev YA, Grishin EV, Shuba YM. Modulation of TRPV1-dependent contractility of normal and diabetic bladder smooth muscle by analgesic toxins from sea anemone Heteractis crispa. Life Sci. 2012;91(19–20):912–920. doi: 10.1016/j.lfs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Fu LW, Longhurst JC. Role of 5-HT3 receptors in activation of abdominal sympathetic C fibre afferents during ischaemia in cats. J Physiol. 1998;509(Pt 3):729–740. doi: 10.1111/j.1469-7793.1998.729bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida M, Masunaga K, Nagata T, Satoji Y, Shiomi M. The effects of chronic hyperlipidemia on bladder function in myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits. Neurourol Urodyn. 2010;29(7):1350–1354. doi: 10.1002/nau.20843. [DOI] [PubMed] [Google Scholar]
- 58.MacDonald SW, Karlsson S, Rieckmann A, Nyberg L, Backman L. Aging-related increases in behavioral variability: relations to losses of dopamine D1 receptors. J Neurosci. 2012;32(24):8186–8191. doi: 10.1523/JNEUROSCI.5474-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi S, Muroyama A, Matsushima H, Yoshimura I, Mitsumoto Y. Oral administration of coenzyme Q(1)(0) reduces MPTP-induced loss of dopaminergic nerve terminals in the striatum in mice. Neurol Sci. 2012;33(1):195–199. doi: 10.1007/s10072-011-0627-z. [DOI] [PubMed] [Google Scholar]
- 60.Yoshimura N, Mizuta E, Kuno S, Sasa M, Yoshida O. The dopamine D1 receptor agonist SKF 38393 suppresses detrusor hyperreflexia in the monkey with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neuropharmacology. 1993;32(4):315–321. doi: 10.1016/0028-3908(93)90151-r. [DOI] [PubMed] [Google Scholar]
- 61.Sekido N, Jyoraku A, Okada H, Wakamatsu D, Matsuya H, Nishiyama H. A novel animal model of underactive bladder: analysis of lower urinary tract function in a rat lumbar canal stenosis model. Neurourol Urodyn. 2012;31(7):1190–1196. doi: 10.1002/nau.21255. [DOI] [PubMed] [Google Scholar]
- 62.Chang HH, Havton LA. Serotonergic 5-HT(1A) receptor agonist (8-OH-DPAT) ameliorates impaired micturition reflexes in a chronic ventral root avulsion model of incomplete cauda equina/conus medullaris injury. Exp Neurol. 2013;239:210–217. doi: 10.1016/j.expneurol.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R534–R547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn. 2010;29(7):1344–1349. doi: 10.1002/nau.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahnama’i MS, Biallosterski BT, de Wachter SG, Van Kerrebroeck PE, van Koeveringe GA. The distribution of the prostaglandin E receptor type 2 (EP2) in the detrusor of the guinea pig. Prostaglandins Other Lipid Mediat. 2012;99(3–4):107–115. doi: 10.1016/j.prostaglandins.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Rahnama’i MS, de Wachter SG, van Koeveringe GA, van Kerrebroeck PE, de Vente J, Gillespie JI. The relationship between prostaglandin E receptor 1 and cyclooxygenase I expression in guinea pig bladder interstitial cells: proposition of a signal propagation system. J Urol. 2011;185(1):315–322. doi: 10.1016/j.juro.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Rahnama’i MS, van Kerrebroeck PE, de Wachter SG, van Koeveringe GA. The role of prostanoids in urinary bladder physiology. Nat Rev Urol. 2012;9(5):283–290. doi: 10.1038/nrurol.2012.33. [DOI] [PubMed] [Google Scholar]
- 68.Rahnama’i MS, van Koeveringe GA, Essers PB, de Wachter SG, de Vente J, van Kerrebroeck PE, Gillespie JI. Prostaglandin receptor EP1 and EP2 site in guinea pig bladder urothelium and lamina propria. J Urol. 2010;183(3):1241–1247. doi: 10.1016/j.juro.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Kim JC, Park EY, Hong SH, Seo SI, Park YH, Hwang TK. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol. 2005;12(10):875–880. doi: 10.1111/j.1442-2042.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- 70.McCafferty GP, Misajet BA, Laping NJ, Edwards RM, Thorneloe KS. Enhanced bladder capacity and reduced prostaglandin E2-mediated bladder hyperactivity in EP3 receptor knockout mice. Am J Physiol Renal Physiol. 2008;295(2):F507–F514. doi: 10.1152/ajprenal.00054.2008. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka I, Nagase K, Tanase K, Aoki Y, Akino H, Yokoyama O. Modulation of stretch evoked adenosine triphosphate release from bladder epithelium by prostaglandin E(2) J Urol. 2011;185(1):341–346. doi: 10.1016/j.juro.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 72.Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174(3):977–982. doi: 10.1097/01.ju.0000169481.42259.54. discussion 982–973. [DOI] [PubMed] [Google Scholar]
- 73.Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/ P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567(Pt 2):621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21(15):5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407(6807):1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63(3 Suppl 1):17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Brierly RD, Hindley RG, McLarty E, Harding DM, Thomas PJ. A prospective evaluation of detrusor ultrastructural changes in bladder outlet obstruction. BJU Int. 2003;91(4):360–364. doi: 10.1046/j.1464-410x.2003.04092.x. [DOI] [PubMed] [Google Scholar]
- 78.Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Aging differentially modifies agonist-evoked mouse detrusor contraction and calcium signals. Age (Dordr) 2011;33(1):81–88. doi: 10.1007/s11357-010-9163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murakami S, Yoshida M, Masunaga K, Maeda Y, Ueda S. Change in acetylcholine release from rat bladder with partial outlet obstruction. BJU Int. 2008;101(5):633–639. doi: 10.1111/j.1464-410X.2007.07325.x. [DOI] [PubMed] [Google Scholar]
- 80.McMurray G, Casey JH, Naylor AM. Animal models in urological disease and sexual dysfunction. Br J Pharmacol. 2006;147(Suppl 2):S62–S79. doi: 10.1038/sj.bjp.0706630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gabella G. Structure of the intramural nerves of the rat bladder. J Neurocytol. 1999;28(8):615–637. doi: 10.1023/a:1007084130642. [DOI] [PubMed] [Google Scholar]
- 82.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27(3):141–155. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- 83.Chang SJ, Chiang IN, Yu HJ. The effectiveness of tamsulosin in treating women with voiding difficulty. Int J Urol. 2008;15(11):981–985. doi: 10.1111/j.1442-2042.2008.02134.x. [DOI] [PubMed] [Google Scholar]
- 84.Insel TR. From animal models to model animals. Biol Psychiatry. 2007;62(12):1337–1339. doi: 10.1016/j.biopsych.2007.10.001. [DOI] [PubMed] [Google Scholar]