Abstract
Tricyclic antidepressants (TCAs) are among the first line treatments clinically recommended against neuropathic pain. However, the mechanism by which they alleviate pain is still unclear. Pharmacological and genetic approaches evidenced a critical role of delta-opioid receptors (DORs) in the therapeutic action of chronic TCA treatment. It is however unclear whether mu-opioid receptors (MORs) are also necessary to the pain-relieving action of TCAs. The lack of highly selective MOR antagonists makes difficult to conclude based on pharmacological studies. In the present work, we thus used a genetic approach and compared mutant mice lacking MORs and their wild-type littermates. The neuropathy was induced by unilateral sciatic nerve cuffing. The threshold for mechanical response was evaluated using von Frey filaments. MOR-deficient mice displayed the same baseline for mechanical sensitivity as their wild-type littermates. After sciatic nerve cuffing, both wild-type and MOR-deficient mice displayed an ipsilateral mechanical allodynia. After about 10 days of treatment, nortriptyline suppressed this allodynia in both wild-type and MOR-deficient mice. MORs are thus not critical for nortriptyline action against neuropathic pain. An acute injection of the DOR antagonist naltrindole induced a relapse of neuropathic allodynia in both wild-type and MOR-deficient mice, thus confirming the critical role of DORs in nortriptyline action. Moreover, morphine induced an acute analgesia in control and in neuropathic wild-type mice, but was without effect in MOR-deficient mice. While MORs are crucial for morphine action, they are not critical for nortriptyline action. Our results highlight the functional difference between DORs and MORs in mechanisms of pain relief.
Keywords: Neuropathic pain, Antidepressant, Mechanical allodynia, Opioid receptors, Mice
1. Introduction
Neuropathic pain is a pain arising as a direct consequence of a lesion or disease affecting the somatosensory system (Loeser and Treede, 2008). It is generally a chronic condition, resistant to classical analgesics (Attal et al., 2006). As first line of treatment, the current pharmacotherapy includes the use of inhibitors of the noradrenaline and serotonin reuptake sites, such as tricyclic anti-depressant drugs (TCAs) (Attal et al., 2006; Dworkin et al., 2007; Moulin et al., 2007). The clinical efficacy of TCAs against this neurological disorder is well-documented (Moulin et al., 2007). These drugs are not acute analgesics but require a sustained treatment to relieve neuropathic pain, which suggests the recruitment of a secondary downstream mechanism and neuronal plasticity. This mechanism still remains to be detailed, but an implication of the endogenous opioid system has been proposed (Valverde et al., 1994; Gray et al., 1998; Marchand et al., 2003c; Mico et al., 2006; Benbouzid et al., 2008a,b).
Some studies on the acute or sub-chronic action of antidepressant drugs showed that opioid antagonists can block the antidepressant-induced analgesia (Reichenberg et al., 1985; Valverde et al., 1994; Gray et al., 1998; Su and Gebhart, 1998; Schreiber et al., 1999, 2002; Marchand et al., 2003c; Ortega-Alvaro et al., 2004; Mico et al., 2006; Benbouzid et al., 2008a,b), suggesting the involvement of the opioid system. On the contrary, other studies showed no effect of opioid antagonists on antidepressant drug action (Pick et al., 1992; Fuchs et al., 1996; Ghelardini et al., 2000; Marchand et al., 2003a,b). However, most of these studies did not address the consequences of a long-term TCA treatment, as used in human patients, or did not specifically concern neuropathic pain condition. Moreover, the lack of selectivity of some antagonists, such as naloxone, limited the possibility to identify precisely the implicated receptors. Using a murine model of neuropathic pain that is sensitive to long-term but not to acute antidepressant treatment (Benbouzid et al., 2008a,b,c; Yalcin et al., 2009a,b), we recently showed that long-term nortriptyline alleviates neuropathic allodynia by the downstream recruitment of the opioid system and more particularly by the recruitment of delta-opioid receptors (DORs) (Benbouzid et al., 2008a,b).
In the present study, we used a genetic approach to evaluate whether mu-opioid receptors (MORs) are also critical for the anti-allodynic action of long-term nortriptyline treatment. Our results demonstrate that while MORs are crucial for morphine analgesic action, they are not necessary for nortriptyline to alleviate neuropathic allodynia, which highlights the specific role played by DORs.
2. Materials and methods
2.1. Animals
The generation of mice lacking the MORs was previously described (Matthes et al., 1996), and these mice were backcrossed in a C57BL/6J background for at least 10 generations. Heterozygotes mice (MOR+/−) were bred in our animal facilities and the experiments were conducted on male MOR+/+ and MOR−/− litter-mate mice from this breeding. They were genotyped upon weaning and adult male mice weighing 25–30 g were used in the experiments. The mice were group-housed three to four per cage and maintained under a 12-h light/dark cycle (lights on at 6:00 AM) with food and water ad libitum. The animal facilities are legally registered for animal experimentation under Animal House Agreement B67-482-1. The scientists in charge of the experiments possess the French certificate authorizing experimentation on living animals, delivered by the governmental veterinary office. All procedures were performed in accordance with the guidelines for animal experimentation of the International Association for the Study of pain (IASP) and the European Communities Council Directive 86/6609/EEC.
2.2. Surgery
The neuropathy was induced by cuffing the main branch of the right sciatic nerve (Mosconi and Kruger, 1996; Pitcher et al., 1999; Benbouzid et al., 2008c). Surgeries were done under ketamine/ xylazine anesthesia (ketamine: 17 mg/mL, xylazine: 2.5 mg/mL, intraperitoneal [IP], 4 mL/kg) (Centravet, Taden, France). The common branch of the right sciatic nerve was exposed and a 2-mm section of split PE-20 polyethylene tubing (Harvard Apparatus, Les Ulis, France) was placed around it (Cuff group) (Benbouzid et al., 2008a,b,c; Yalcin et al., 2009a,b). The shaved skin was closed using suture. Sham-operated mice underwent the same surgical procedure described above without implantation of the Cuff (Sham group).
2.3. Drugs
The nortriptyline treatment began 15 days after the neuropathy was induced, and the treatment lasted at least 20 days (Benbouzid et al., 2008a,b,c; Yalcin et al., 2009a,b). During the treatment, the mice received two injections per day (morning and evening) of nortriptyline (5 mg/kg) (Sigma–Aldrich, St. Quentin Fallavier, France). The drug was dissolved in 0.9% NaCl solution that was also used for control injections. Both nortriptyline and NaCl solution were administered IP in a volume of 5 mL/kg. The injection of naltrindole hydrochloride (5 mg/kg; subcutaneously [SC]) (Sigma–Aldrich, St. Quentin Fallavier, France) was done 35 days after surgery, i.e., after 21 days of antidepressant treatment. On saline-treated animals, morphine (10 mg/kg, SC; Sigma–Aldrich, France) was acutely injected on day 40 post-surgery.
2.4. Nociceptive test
After sciatic nerve cuffing, mice display a heat thermal hyperalgesia lasting only 3 weeks while the mechanical allodynia remains stable over 2 months (Benbouzid et al., 2008a,b,c; Yalcin et al., 2009a,b). The present work thus focused on mechanical allodynia. The mechanical allodynia was determined using von Frey hairs and results were expressed in grams. Mice were placed in clear Plexiglas boxes (7 cm × 9 cm × 7 cm) on an elevated mesh screen. Calibrated von Frey microfilaments (Bioseb, France; also available from Stoelting, USA) were applied to the plantar surface of each hindpaw in a series of ascending forces up to the mechanical threshold. The filaments used in the studies were: 0.16, 0.4, 0.6, 1, 1.4, 2, 4, 6, 8, 10 and 15 grams. The highest ones were only necessary for mice under deep morphine analgesia, and morphine analgesia testing stopped at 15 g even if no response was observed. During the procedure, the filaments were tested five times per paw in ascending series until the paw withdrawal threshold was reached. This threshold was defined as the lowest of two consecutive filaments for which we observed three or more withdrawals out of the five trials (Benbouzid et al., 2008a,b,c; Yalcin et al., 2009a,b). To study the antiallodynic effect of nortriptyline treatment, we tested the mice in the morning before nortriptyline drug injection, as previously described (Benbouzid et al., 2008a,b,c; Yalcin et al., 2009a,b). The effect of acute drug injections (saline, naltrindole or morphine) was evaluated before (pre-test) and 30 min after (post-test) the considered drug injection.
2.5. Analyses
Data are expressed as mean ± SEM. Statistical analyses were performed using multifactor analysis of variance (ANOVA). The surgery procedure (Sham or Cuff) and the treatments (saline vs. drug injections) were taken as between-group factors. When needed, the time of measurement (either time course or preinjection vs. postinjection data) was taken as a within-subject factor. The Duncan test was used for post hoc comparisons. The significance level was set at p < 0.05.
3. Results
3.1. Mechanical sensitivity
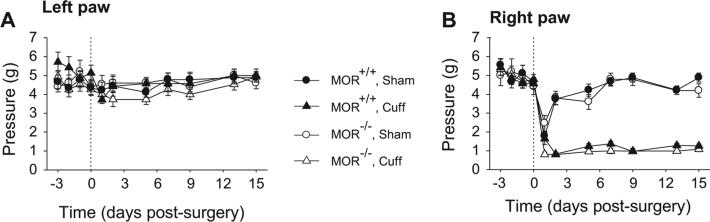
MOR-deficient mice displayed the same baseline for mechanical sensitivity as their wild-type littermates (Fig. 1A and B). The Sham surgery did not affect the paw withdrawal thresholds, while the Cuff-implanted mice displayed an ipsilateral mechanical allodynia which was present both in wild-type mice and in MOR-deficient mice (Surgery × Time interaction, MOR+/+ F6,180 = 20.2, p < 0.0001, MOR−/− F6,138 = 7.86, p < 0.0001; post hoc: Cuff < Sham at p < 0.0001 on days 2 to 15) (Fig. 1B). No difference was observed in allodynia intensity depending on the presence or absence of the MORs (Genotype effect, F6,180 = 0.9, p > 0.48).
Fig. 1.
Consequence of Cuff implantation in wild-type and in MOR-deficient mice. Adult male mice underwent surgery for unilateral Cuff implantation around the main branch of the right sciatic nerve. Sham animals underwent the same surgical procedure without Cuff implantation. The mechanical allodynia was tested using the von Frey hairs. (A) The Cuff implantation did not affect the mechanical threshold of paw contralateral to Cuff implantation (left paw). (B) However it induced an ipsilateral (right paw) allodynia in both wild-type (n = 14) and MOR-deficient (n = 15) mice. Data are expressed as mean ± SEM.
3.2. Nortriptyline effect
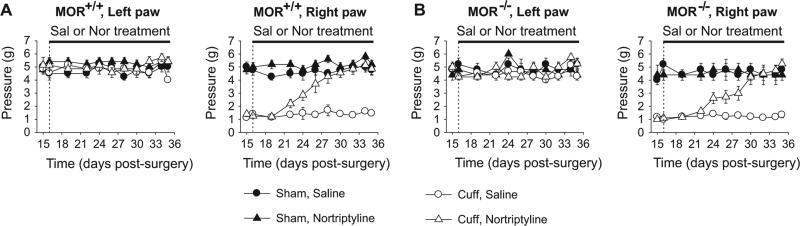
Two weeks after the Cuff insertion, we started the treatments with either nortriptyline (5 mg/kg) or the control saline solution (0.9% NaCl). The mice received two injections per day and were tested in the morning before drug injection, as previously described (Benbouzid et al., 2008a,b; Yalcin et al., 2009a). We previously reported that such treatment has no acute antalgic effect whereas it suppressed the neuropathic allodynia after 10 to 12 days of treatment (Benbouzid et al., 2008a,b; Yalcin et al., 2009a). Similarly, the nortriptyline treatment alleviated the Cuff-induced allodynia in wild-type mice after about 10 days of treatment (Surgery × Treatment × Time interaction, F9,252 = 6.14, p < 0.0001; post hoc: (CuffNor = Sham) > CuffSal at p < 0.01 on days 24 to 35) (Fig. 2A). The same antiallodynic effect was also present in MOR-deficient mice (F9,189 = 4.71, p < 0.0001; post hoc: (Cuff-Nor = Sham) > CuffSal at p < 0.05 on days 24 to 35) (Fig. 2B). In both cases, nortriptyline suppressed the Cuff-induced allodynia without affecting the mice with Sham surgery.
Fig. 2.
Nortriptyline treatment of neuropathic allodynia. Two weeks after Cuff implantation, the daily treatment with nortriptyline (5 mg/kg, i.p. twice a day) or its saline control (0.9 % NaCl) started and was maintained for 3 weeks. The hindpaw mechanical threshold was tested before the morning drug injection, using von Frey filaments. (A) In wild-type mice the treatments did not affect the mechanical threshold of the contralateral paw, but chronic nortriptyline suppressed the ipsilateral Cuff-induced allodynia. (B) Similar results were observed in MOR-deficient mice. Data are expressed in mean ± SEM. (n = 5–10 for the different Sham groups, n = 7–8 for the different Cuff groups).
3.3. Naltrindole effect
We tested the consequences of an acute injection with the DOR antagonist naltrindole (5 mg/kg) in the MOR-deficient and wild-type mice. After 3 weeks of treatment with nortriptyline or saline, the acute injection of naltrindole totally suppressed the benefit of nortriptyline treatment (MOR+/+: F1,28 = 60.45, p < 0.05; MOR−/−: F1,21 = 19.47, p < 0.05) (Fig. 3A and B). Within 30 min following the DOR antagonist injection, we observed a relapse of allodynia. This effect was present both in MOR-deficient mice and in wild-type mice. We also controlled that naltrindole per se had no effect in mice with Sham surgery, or in neuropathic mice treated with saline.
Fig. 3.
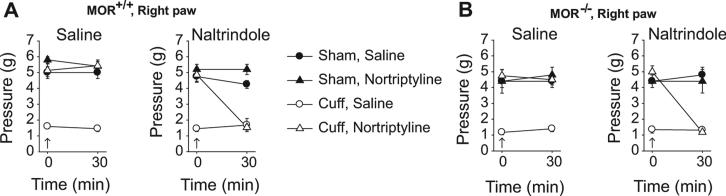
Effect of the DOR antagonist naltrindole. After 3 weeks of treatment with either nortriptyline or saline, the mice received an acute injection of saline (s.c.) and of the DOR antagonist naltrindole (5 mg/kg, s.c.). The nociceptive mechanical threshold was tested before (0 min) and 30 min after these acute injections. (A) While acute saline injection did not affect the paw withdrawal threshold, the naltrindole injection induced a relapse of the allodynia in neuropathic wild-type mice treated by nortriptyline. No effect of naltrindole was observed in Sham mice or in saline-treated neuropathic mice. (B) Similar results were obtained in MOR-deficient mice. Data are expressed in mean ± SEM. (n = 5–10 for the different Sham groups, n = 7–8 for the different Cuff groups).
3.4. Morphine effect
The acute injection of morphine (10 mg/kg) induced an acute analgesia both in Sham-operated and in Cuff-implanted wild-type mice. (Left paw: F1,22 = 142.5, p < 0.0001; right paw: F1,22 = 319.1, p < 0.0001.) (Data not shown.) The same dose of morphine had however no effect in MOR-deficient mice, whether or not neuropathic (Data not shown).
4. Discussion
In the present work, we studied the impact of MORs on a chronic nortriptyline treatment in a murine model of neuropathic pain. We demonstrated that MORs are not necessary for the anti-allodynic action of nortriptyline while they are critical for the anal-gesic action of morphine. Moreover, we showed that a DOR antagonist suppressed the antiallodynic action of nortriptyline in a MOR-independent way.
The similar baseline for mechanical sensitivity between MOR-deficient mice and their wild-type littermates is in agreement with previous reports concerning nociceptive response in these mice (Matthes et al., 1996; Fuchs et al., 1999; Kieffer and Gaveriaux-Ruff, 2002). MORs are thus not critical to set-up the thresholds for mechanical sensitivity in unchallenged situations. After induction of the neuropathy, the intensity of allodynia was also similar between MOR-deficient mice and their wild-type littermates. These results suggested that MORs do not play a critical role in the physiological control of neuropathic allodynia in our model. Conversely, a bilateral increase in MOR-deficient mice mechanical allodynia as compared to wild-type mice has been observed in a more drastic model of neuropathy, a variant of the spared nerve injury with L5 spinal nerve ligation and section (Mansikka et al., 2004). Our results are however in agreement with pharmacological data showing that systemic administration of naloxone, a broad-spectrum opioid receptor antagonist, did not influence mechanical allodynia (von Frey test) or thermal hyperalgesia (tail flick test) in other models of neuropathic pain such as the L5/L6 ligature or ischemic spinal cord injury (Hao et al., 1998; Wei et al., 1998; McGaraughty et al., 2005).
After chronic treatment with the TCA nortriptyline, the mice recovered from their neuropathic allodynia. As expected we observed this recovery in wild-type mice, but it was also present in MOR-deficient mice and the delay for therapeutic onset was unaffected by the presence of absence of MORs. These results show that MORs are not necessary for nortriptyline to exert its antiallodynic action. This contrasts with previous data on DORs. Indeed, the DOR antagonist naltrindole has been shown to acutely block the antiallodynic effect of chronic nortriptyline treatment (Benbouzid et al., 2008a,b) and this TCA is totally ineffective in DOR-deficient mice (Benbouzid et al., 2008a,b). Thus, present data suggest a striking difference between DOR and MOR implication in nortriptyline action against neuropathic pain. To strengthen this finding, we tested the effect of DOR antagonist naltrindole in the MOR-deficient and wild-type mice and showed that it blocked in both cases the anti-allodynic action of nortriptyline. It confirmed that chronic nortriptyline treatment alleviates neuropathic allodynia by recruiting an opioid tone acting on DORs. While DOR implication in this neuropathic pain treatment is critical, the implication of DORs in chronic pain processing is more subtle. In the sciatic nerve cuffing model, we previously observed no alteration of neuropathic allodynia after naltrindole administration or in DOR-deficient mice (Benbouzid et al., 2008a,b). A mild increase in mechanical allodynia and heat hyperalgesia and a more pronounced increase in cold allodynia were however observed after tight ligation of the sciatic nerve in DOR-deficient mice (Nadal et al., 2006). A delayed recovery from mechanical allodynia and heat hyperalgesia is also present in DOR-deficient mice with inflammation due to intraplantar complete Freund's adjuvant administration (Gaveriaux-Ruff et al., 2008). These results suggest a mild physiological modulation of pain response by endogenous DOR activity, but a stronger involvement of these receptors in the pharmacological control of pain. Interestingly, recent studies revealed that a DOR agonist can display an antihyperalgesic and antiallodynic effectiveness in a neuropathic pain model, which suggests that DORs could be potential therapeutic targets for neuropathic pain management (Holdridge and Cahill, 2007; Kabli and Cahill, 2007).
Even if MORs are not critical for the action of nortriptyline against neuropathic pain, they have been implicated in the action of opiates against pain. In MOR-deficient mice, morphine analgesia was thus abolished (Matthes et al., 1996; Sora et al., 1997, 1999; Fuchs et al., 1999; Schuller et al., 1999; Qiu et al., 2000) or strongly reduced (Loh et al., 1998) in models of thermal (Matthes et al., 1996; Sora et al., 1997; Loh et al., 1998; Schuller et al., 1999), mechanical (Fuchs et al., 1999), chemical (Sora et al., 1999), and inflammatory (Qiu et al., 2000) pain. In our model of neuropathic pain, the acute injection of morphine induced an acute analgesia both in Sham-operated and in Cuff-implanted wild-type mice, but it had no effect in MOR-deficient mice. These findings confirmed in a pathological condition, such as neuropathic pain, that morphine analgesia is mediated through MORs.
Our study highlights a clear-cut functional difference between DORs and MORs in mechanisms of pain relief by nortriptyline. In other situations, a differential influence of these receptors in the processing of nociceptive information can also be experimentally observed. Indeed, the expression of both receptors is segregated in the dorsal root ganglia, and their manipulation by intrathecal delivery of agonists revealed that local DORs and MORs preferentially influence mechanical and heat processing respectively (Scherrer et al., 2009). This dichotomy in mechanical and heat nociceptive controls by opioid receptors is however not present when considering the whole organism or a pathological situation. DOR agonists can for example relieve both heat hyperalgesia and mechanical allodynia in an inflammatory situation (Gaveriaux-Ruff et al., 2008; Pradhan et al., 2009), and DOR-deficient mice can display changes in both mechanical and heat responses after tight ligation of the sciatic nerve (Nadal et al., 2006). Reciprocally, MOR-deficient mice can display changes in mechanical allodynia after spared nerve injury (Mansikka et al., 2004), and a MOR agonist can affect mechanical allodynia after spare nerve ligation (Guan et al., 2008).
While MORs are mediating the acute analgesic effect of the opiate morphine in a neuropathic pain condition, they are not necessary for the effect of chronic antidepressant drug treatment. On the contrary, DORs appear to be critical for nortriptyline relief of neuropathic allodynia. These opioid receptors are however not primary targets of the antidepressant drugs. Future research will thus be needed to understand the precise anatomical and molecular links between the primary action of nortriptyline on the aminergic system and the secondary recruitment of the endogenous opioid system.
Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique (Contracts UMR7168 and UPR3212), the University of Strasbourg (Contract UMR7168) and Neurex. Publication is supported by the European Commission (ENINET Project, Contract LHSM-CT-2005-019063). Y.B. is supported by a Neurex fellowship. We thank Elisabeth Waltisperger for technical assistance and Stéphane Doridot for animal care and genotyping.
Footnotes
Competing interests
The authors have no competing interests to declare.
References
- Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13(11):1153–69. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Benbouzid M, Choucair-Jaafar N, Yalcin I, Waltisperger E, Muller A, Freund-Mercier MJ, et al. Chronic, but not acute, tricyclic antidepressant treatment alleviates neuropathic allodynia after sciatic nerve cuffing in mice. Eur J Pain. 2008a;12(8):1008–17. doi: 10.1016/j.ejpain.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Benbouzid M, Gaveriaux-Ruff C, Yalcin I, Waltisperger E, Tessier LH, Muller A, et al. Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol Psychiatr. 2008b;63(6):633–6. doi: 10.1016/j.biopsych.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, et al. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain. 2008c;12(5):591–9. doi: 10.1016/j.ejpain.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Fuchs PN, Lariviere WR, Balinsky M, Melzack R. Acute amitriptyline treatment produces non-opioid-mediated analgesia in the formalin and bee venom tests. Pathophysiology. 1996;3(4):227–31. [Google Scholar]
- Fuchs PN, Roza C, Sora I, Uhl G, Raja SN. Characterization of mechanical withdrawal responses and effects of mu-, delta- and kappa-opioid agonists in normal and mu-opioid receptor knockout mice. Brain Res. 1999;821(2):480–6. doi: 10.1016/s0006-8993(99)01060-4. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 2008;27(10):2558–67. doi: 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Bartolini A. Antinociception induced by amitriptyline and imipramine is mediated by alpha2A-adrenoceptors. Jpn J Pharmacol. 2000;82(2):130–7. doi: 10.1254/jjp.82.130. [DOI] [PubMed] [Google Scholar]
- Gray AM, Spencer PS, Sewell RD. The involvement of the opioidergic system in the antinociceptive mechanism of action of antidepressant compounds. Br J Pharmacol. 1998;124(4):669–74. doi: 10.1038/sj.bjp.0701882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, et al. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138(2):318–29. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JX, Yu W, Xu XJ. Evidence that spinal endogenous opioidergic systems control the expression of chronic pain-related behaviors in spinally injured rats. Exp Brain Res. 1998;118(2):259–68. doi: 10.1007/s002210050280. [DOI] [PubMed] [Google Scholar]
- Holdridge SV, Cahill CM. Spinal administration of a delta opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur J Pain. 2007;11(6):685–93. doi: 10.1016/j.ejpain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127(1–2):84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66(5):285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137(3):473–7. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. Mu opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54(2):321–6. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Mansikka H, Zhao C, Sheth RN, Sora I, Uhl G, Raja SN. Nerve injury induces a tonic bilateral mu-opioid receptor-mediated inhibitory effect on mechanical allodynia in mice. Anesthesiology. 2004;100(4):912–21. doi: 10.1097/00000542-200404000-00022. [DOI] [PubMed] [Google Scholar]
- Marchand F, Alloui A, Chapuy E, Hernandez A, Pelissier T, Ardid D, et al. The antihyperalgesic effect of venlafaxine in diabetic rats does not involve the opioid system. Neurosci Lett. 2003a;342(1–2):105–8. doi: 10.1016/s0304-3940(03)00270-2. [DOI] [PubMed] [Google Scholar]
- Marchand F, Alloui A, Chapuy E, Jourdan D, Pelissier T, Ardid D, et al. Evidence for a monoamine mediated, opioid-independent, antihyperalgesic effect of venlafaxine, a non-tricyclic antidepressant, in a neurogenic pain model in rats. Pain. 2003b;103(3):229–35. doi: 10.1016/S0304-3959(03)00168-4. [DOI] [PubMed] [Google Scholar]
- Marchand F, Ardid D, Chapuy E, Alloui A, Jourdan D, Eschalier A. Evidence for an involvement of supraspinal delta- and spinal mu-opioid receptors in the antihyperalgesic effect of chronically administered clomipramine in mononeuropathic rats. J Pharmacol Exp Ther. 2003c;307(1):268–74. doi: 10.1124/jpet.103.052613. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383(6603):819–23. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Honore P, Wismer CT, Mikusa J, Zhu CZ, McDonald HA, et al. Endogenous opioid mechanisms partially mediate P2X3/P2X2/3-related antinociception in rat models of inflammatory and chemogenic pain but not neuropathic pain. Br J Pharmacol. 2005;146(2):180–8. doi: 10.1038/sj.bjp.0706346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mico JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27(7):348–54. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Mosconi T, Kruger L. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morphometric analysis of axonal alterations. Pain. 1996;64(1):37–57. doi: 10.1016/0304-3959(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain – consensus statement and guidelines from the Canadian Pain Society. Pain Res Manage. 2007;12(1):13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal X, Banos JE, Kieffer BL, Maldonado R. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur J Neurosci. 2006;23(3):830–4. doi: 10.1111/j.1460-9568.2006.04569.x. [DOI] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Acebes I, Saracibar G, Echevarria E, Casis L, Mico JA. Effect of the antidepressant nefazodone on the density of cells expressing mu-opioid receptors in discrete brain areas processing sensory and affective dimensions of pain. Psychopharmacology (Berl. 2004;176(3–4):305–11. doi: 10.1007/s00213-004-1894-7. [DOI] [PubMed] [Google Scholar]
- Pick CG, Paul D, Eison MS, Pasternak GW. Potentiation of opioid analgesia by the antidepressant nefazodone. Eur J Pharmacol. 1992;211(3):375–81. doi: 10.1016/0014-2999(92)90395-k. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Ritchie J, Henry JL. Nerve constriction in the rat: model of neuropathic, surgical and central pain. Pain. 1999;83(1):37–46. doi: 10.1016/s0304-3959(99)00085-8. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS ONE. 2009;4(5):e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Sora I, Ren K, Uhl G, Dubner R. Enhanced delta-opioid receptor-mediated antinociception in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2000;387(2):163–9. doi: 10.1016/s0014-2999(99)00813-4. [DOI] [PubMed] [Google Scholar]
- Reichenberg K, Gaillard-Plaza G, Montastruc JL. Influence of naloxone on the antinociceptive effects of some antidepressant drugs. Arch Int Pharmacodyn Ther. 1985;275(1):78–85. [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137(6):1148–59. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Backer MM, Pick CG. The antinociceptive effect of venlafaxine in mice is mediated through opioid and adrenergic mechanisms. Neurosci Lett. 1999;273(2):85–8. doi: 10.1016/s0304-3940(99)00627-8. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects – a possible opioid involvement in severe depression? J Mol Neurosci. 2002;18(1– 2):143–9. doi: 10.1385/JMN:18:1-2:143. [DOI] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, et al. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2(2):151–6. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, et al. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94(4):1544–9. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Li XF, Funada M, Kinsey S, Uhl GR. Visceral chemical nociception in mice lacking mu-opioid receptors: effects of morphine, SNC80 and U-50, 488. Eur J Pharmacol. 1999;366(2–3):R3–5. doi: 10.1016/s0014-2999(98)00933-9. [DOI] [PubMed] [Google Scholar]
- Su X, Gebhart GF. Effects of tricyclic antidepressants on mechanosensitive pelvic nerve afferent fibers innervating the rat colon. Pain. 1998;76(1–2):105–14. doi: 10.1016/s0304-3959(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Valverde O, Mico JA, Maldonado R, Mellado M, Gibert-Rahola J. Participation of opioid and monoaminergic mechanisms on the antinociceptive effect induced by tricyclic antidepressants in two behavioural pain tests in mice. Prog Neuropsychopharmacol Biol Psychiatr. 1994;18(6):1073–92. doi: 10.1016/0278-5846(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Wei H, Panula P, Pertovaara A. A differential modulation of allodynia, hyperalgesia and nociception by neuropeptide FF in the periaqueductal gray of neuropathic rats: interactions with morphine and naloxone. Neuroscience. 1998;86(1):311–9. doi: 10.1016/s0306-4522(98)00027-x. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Choucair-Jaafar N, Benbouzid M, Tessier LH, Muller A, Hein L, et al. Beta(2)-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann Neurol. 2009a;65(2):218–25. doi: 10.1002/ana.21542. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Tessier LH, Petit-Demouliere N, Doridot S, Hein L, Freund-Mercier MJ, et al. Beta2-adrenoceptors are essential for desipramine, venlafaxine or reboxetine action in neuropathic pain. Neurobiol Dis. 2009b;33(3):386–94. doi: 10.1016/j.nbd.2008.11.003. [DOI] [PubMed] [Google Scholar]