Abstract
Objective
Although marital separation and divorce are associated with many negative health outcomes, few studies examine the psychophysiological mechanisms that may give rise to these outcomes. This study examined changes in resting blood pressure (BP) as a function of sleep complaints in recently divorced adults.
Method
Recently separated adults (n = 138; 38 men) completed a self-report measure of sleep complaints and a resting blood pressure (BP) assessment in the laboratory at three occasions across 7.5 months.
Results
Multilevel analyses revealed that although sleep complaints were not associated with concurrent BP, sleep complaints predicted significant increases in both systolic and diastolic BP at the subsequent laboratory visit. In addition, time since the separation from an ex-partner moderated the association between sleep complaints at baseline and resting systolic blood pressure (SBP) 3 months later. People who reported high sleep complaints 10 weeks or more after their separation demonstrated greater increases in SBP.
Conclusions
In recently separated adults, greater sleep complaints may index increased risk for future increases in BP. This work helps pinpoint one potential mechanistic pathway linking marital separation with an important, health-relevant biological outcome.
Keywords: blood pressure, divorce, longitudinal health outcomes, marital separation, sleep
Marital separation and divorce are associated with many negative social, psychological, and physical health outcomes (Amato, 2010), including increased risk for all-cause mortality (Sbarra, Law, & Portley, 2011; Shor, Roelfs, Bugyi, & Schwartz, 2012). Recently divorced adults reporting greater separation-related emotional distress, for example, evidence greater resting blood pressure (BP) in the laboratory (Sbarra, Law, Lee, & Mason, 2009). In addition, the transition out of marriage is associated with increased risk for developing diagnosable hypertension over time (Wang, 2005). Although psychological stress and negative affect may account for some of the variance linking marital separation to distal health and health-relevant biomarkers, other mechanistic pathways are relevant as well, including changes in self-care and health behaviors (Sbarra, Hasselmo, & Nojopranoto, 2012; Sbarra et al., 2011). Here we focus on sleep, a quintessential health behavior, and investigate whether and how self-reported sleep complaints associate with resting BP across 7.5 months in a sample of recently separated adults.
Dyadic Perspectives on Relationships and Sleep
A growing theoretical literature outlines bidirectional associations among sleep and relationship processes and their collective effect on health (Troxel, 2010; Troxel, Robles, Hall, & Buysse, 2007). For example, in their conceptual biopsychosocial framework, Troxel and colleagues (2007) postulate that intimate partners protect against social isolation and psychopathology, serve as strong environmental cues for engaging in health-promoting behaviors, and buffer against the negative effects of chronic stress (Uchino, 2006).
Although sleep is often viewed as a private, individual behavior, Troxel (2010) describes sleep as “a fundamental attachment behavior, in that it is a behavioral state that requires a relative cessation of awareness and down-regulation of vigilance … processes that are optimized when one feels a sense of physical and emotional safety and security” (p. 3). Consistent with this perspective, Diamond, Hicks, and Otter-Henderson (2008) found that married adults reported subjectively worse sleep on nights spent away from their partners. Relationships offer important environmental cues, or social Zeitgebers (Leonhard & Randler, 2009), that signal sleep. Thus, social disruptions, be they short-term work travel or permanent marital separation, likely alter the environmental availability of these cues.
The limited literature on sleep and relationships suggests that changes in sleep following marital separation likely reflect the affective response following relationship dissolution (Brown et al., 1996; Troxel et al., 2010). When relationships end, many people experience a combination of psychological distress and physiological dysregulation characterized by heightened autonomic nervous system activity (Diamond, 2001). It is this state of psychological distress, negative affect, and physiological hyperactivation that is most conducive to creating sleep disturbance. From this perspective, social disruptions (e.g., divorce) constitute a double-threat to salubrious health behaviors: The loss of a partner can decrease the likelihood of social control of health behaviors and instantiate biological dysregulation that renders it difficult to maintain positive health behaviors (Sbarra & Hazan, 2008; Umberson, 1992).
These ideas are consistent with Amato’s (2000) divorce-stress-adjustment model, which posits that the end of marriage is a stressful transition that can evolve from a short-term crisis into a chronic stress, depending on the individual and interpersonal resources available to cope with the relationship transition (Lorenz, Wickrama, Conger, & Elder, 2006). Sleep is one such resource that, when depleted, can impact a person’s coping ability and overall health. Sleep is both integral to emotion regulation (Walker, 2009) and sensitive to disturbances in mood (Baglioni, Spiegelhalder, Lombardo, & Riemann, 2010). Emerging evidence suggests that being separated/divorced is associated with substantially elevated risk for severe insomnia and sleep maintenance problems (Minowa, Okawa, & Uchiyama, 2000). What remains unknown, however, is whether sleep complaints following the end of marriage uniquely predict declining physiological function or simply correlate with (or follow from) separation-related psychological distress. If poor sleep is to be considered a unique vulnerability for poor health-related outcomes per Amato’s (2000) model, it must demonstrate unique predictive utility, over-and-above separation-related psychological distress.
Sleep and Cardiovascular Health
Troxel and colleagues’ (2007) framework suggests that the protective effects of relationships on health may be explained, in part, by high-quality sleep. Amato’s (2000) divorce-stress-adjustment model would hold that sleep disruptions constitute an individual vulnerability to prolonged stress following relationship dissolution. Sleep is salubrious, and both acute and chronic sleep restriction are associated with significant alterations in systems impacting cardiovascular functioning. Alterations include greater sympathetic control in the autonomic nervous system (Meerlo, Sgoifo, & Suchecki, 2008), increases in circulating inflammatory markers of cardiovascular risk (Meier-Ewert et al., 2004), and increases in resting BP (Mullington, Haack, Toth, Serrador, & Meier-Ewert, 2009).
Indeed, the cardiovascular system is sensitive to fluctuation in sleep, and as little as one night of sleep restriction (4 hours in bed) can result in a 4–7 mmHg increase in systolic blood pressure (SBP) the next morning (Tochikubo, Ikeda, Miyajima, & Ishii, 1996). Ten days of sleep restriction can result in SBP increases of 22 mmHg (Meier-Ewert et al., 2004). Epidemiological studies report similar associations; Sleep duration under 7 hours per night has been correlated with increased prevalence (Gottlieb et al., 2006) and incidence of hypertension (SBP > 140 mmHg, diastolic blood pressure [DBP] > 90 mmHg; Cappuccio et al., 2007). Interestingly, short sleep duration and low sleep efficiency have stronger associations with heightened SBP and DBP longitudinally over 5 years than cross-sectionally (Knutson et al., 2009), suggesting that chronic sleep restriction likely exerts a compounding negative effect on cardiovascular functioning over time.
Present Study
Here, we examined associations between self-reported sleep complaints and changes in resting blood pressure over 7.5 months in recently separated adults. Despite an expanding body of literature dedicated to understanding the intricacies of how close relationships and sleep ultimately impact health (Troxel, 2010; Troxel et al., 2010; Troxel et al., 2007), no studies have investigated long-term health outcomes associated with sleep complaints following marital separation. Consistent with data showing that poor sleep may portend future cardiovascular morbidities (Knutson et al., 2009), we hypothesized that sleep complaints would correlate positively with blood pressure concurrently. We also predicted a lagged temporal effect such that greater sleep complaints at an earlier occasion would be associated with increases in resting blood pressure at the subsequent visit. Furthermore, we predicted that these associations would be independent of participants’ separation-related emotional distress. This latter test would help to determine whether sleep complaints constitute a risk or correlate of separation-related stress, and thus contribute to refining Amato’s (2000) divorce-stress-adjustment model, while also deepening our understanding of the ways in which marital separation may impact health-relevant functioning.
Method
Participants
Participants were 138 (n = 38 men) community-dwelling adults with a mean age of 40.65 years (SD = 9.75 years; range = 19–63 years), who had physically separated from their former partner an average of 16 weeks before the initial laboratory visit (SD = 8 weeks; range = 2–46 weeks). Participants were recruited through various local divorce recovery support groups, newspaper advertisements, and family and conciliation courts. Twenty percent (n = 27) of participants were legally divorced, 40% (n = 56) were physically separated with no legal actions filed, 19% (n = 26) had filed divorce papers, 13% (n = 18) had divorce proceedings underway, and the remaining 8% (n = 11) did not report on their separation status. Forty-one percent (n = 56) reported that they initiated the separation, 54% (n = 74) reported that their partner initiated separation, and 6% (n = 8) chose not to report on who initiated the separation. Seventy-five percent (n = 104) of the sample described themselves as White (non-Hispanic), 13% (n = 18) as Hispanic, 1% (n = 2) as African American, 1% (n = 2) as Asian, 1% (n = 1) as Native American, 4% (n = 6) as other, and 4% (n = 5) chose not to provide race data. Fifty-two percent (n = 70) of the sample reported earning <$30,000 in gross annual income. The average body mass index (BMI) for the sample was 25.60 (SD = 5.22), which is between the upper limits of a healthy BMI and the lower limits of an overweight BMI.
Procedure
The University of Arizona Institutional Review Board approved the study protocol. All participants signed an informed consent form prior to study participation. Eligible participants must have physically separated from their partner within the past 5 months and must have cohabitated with their former partner for at least 2 years. Eligible participants were ages 18 to 65 years, had never been diagnosed with a psychiatric disorder, reported good health, were not using blood pressure medications, and were not pregnant (women only). Of 297 screened participants, 178 were eligible. Common reasons for exclusion included having been separated for longer than 5 months (n = 68) and not having cohabitated for at least 2 years (n = 24).
Of 178 eligible participants, 138 completed an initial laboratory visit (Visit 1 [V1]). After V1, 34 participants were not invited to be part of the longitudinal sample. The remaining participants were followed 3 months later at V2, and then were randomized to participate in a final visit (V3), which occurred at either 6 or 9 months after the initial visit.1
Participants completed demographic and self-report measures before V1 and were asked to refrain from using caffeine or tobacco at least four hours before all study visits. At V1, researchers consented the participant and then participants completed a stream-of-consciousness (SOC) recording about their separation experience; although the SOC is not part of this study (Mason, Sbarra, & Mehl, 2010), we mention this aspect of the procedure to be clear that participants had spent time talking about their separation before the BP assessment. We took a number of steps to ensure our resting BP assessments were completed after a period of distraction following the SOC. The SOC was followed by several minutes of quiet rest, and set-up of the physiological measurement equipment spanned roughly another 15 minutes. After equipment set-up, participants were instructed to relax while they viewed a mildly positive nature video for four minutes, which constituted the measure of resting blood pressure at study entry. We used the neutral nature video to divert attention from any divorce-related psychological distress (which would likely have impacted blood pressure readings) to a more neutral topic. This method is consistent with the relatively standard procedure of mildly distracting participants during baseline blood pressure readings, which shows superior baseline blood pressure stability and generalizability (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992). We repeated this procedure at V2 (3 months later) and at V3 (the final study visit), which occurred an average of 7.5 months after V1.
Measures
Pittsburgh Sleep Quality Index (PSQI)
The Pittsburg Sleep Quality Index (PSQI) is a 19-item scale that assesses sleep quality over a 1-month time period. It is a widely used self-report measure of sleep quality. The PSQI includes seven subscales, three of which are subjective sleep quality, sleep duration, and sleep disturbance. Subjective sleep quality is assessed by ranking sleep quality (very good, fairly good, fairly bad, very bad) during the past month, sleep duration is assessed by the question (during the past month, how many hours of sleep do you actually get at night?), and sleep disturbance questions ask about frequency of certain sleep disruptions during the past month, such as (how often have you had trouble sleeping because you cannot get to sleep within 30 minutes, cough or snore loudly, or have bad dreams.) Our primary index of sleep complaints was the Global PSQI total, which assesses overall sleep quality. Scores range from 0 to 21, with greater scores indicating greater sleep complaints. Scores of five or greater represent the cutoff for clinically impaired sleep (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The PSQI has high test–retest reliability and validity (internal consistency at baseline in the current study: α = .73) for determining sleep duration in patients with primary insomnia, when comparing to subjective patient-report daily sleep logs (Backhaus, Junghanns, Broocks, Riemann, & Hohagen, 2002). Participants completed the PSQI at all visits.
Resting blood pressure (BP)
We assessed BP at all study visits with a noninvasive tonometry device placed over the radial artery on the wrist while participants viewed a neutral 4-min nature video. This device provides frequent, real-time updates of systolic (SBP) and diastolic (DBP) blood pressure (Vasotrac AMP 205, Medwave Inc., Arden Hills, MN). The Vasotrac device measures SBP at the peak pressure in the arteries at the beginning of the cardiac cycle and DBP at the lowest pressure of the resting phase of the cycle. The Vasotrac uses frequent compression and decompression of the radial artery at the wrist to detect the zero-load state around which the pressure signals are measured. The device uses this information to detect and then display arterial pressure and waveform every 12 to 15 beats. The Vasotrac was calibrated against radial catheter measures of BP and demonstrated excellent convergent validity, mean R2 for SBP and DBP = .95 (Belani et al., 1999). Research assistants placed the tonometry device over the radial artery of the participants’ nondominant arm and participants placed their arm on a table in front of them for the duration of the laboratory visit. We scored BP data using Mindware Technology’s BP 2.6 postprocessing software. We computed minute-by-minute means for SBP and DBP and averaged these values over the course of the 4-minute period to create a single index of resting BP for each study visit.
Covariates
We assessed basic demographic data including sex, age, self-reported race, general health (Do you have more health problems than the average person?), BMI, and gross annual income to include as covariates in analyses, given their previously documented associations with blood pressure (Sbarra et al., 2009). We also assessed information about who initiated the separation (Who was responsible for the end of the relationship?) rated on a 4-point scale from (You were totally responsible to your partner was totally responsible), length of time since the separation at each study visit (measured in weeks), length of marriage before separation (measured in months), and the legal status of the separation (in the present analyses, we compared participants who were legally divorced to all other participants). These relationship-specific covariates are previously documented indicators of divorce adjustment (Sbarra et al., 2009) and may also predict physical and health outcomes following marital separation (Hewitt & Turrell, 2011).
To assess whether having sleep complaints is a unique predictor of BP over-and-above separation-related psychological distress, we accounted for participants’ scores on the revised Impact of Events Scale (IES-R) at each study visit. The IES-R is an internally consistent (α = .93 in a current study), 22-item questionnaire that taps emotional reactions and psychological distress related to stressful life events. It has demonstrated validity for measuring individual differences in adults’ psychological responses to divorce (Sbarra et al., 2009). The combined hyperarousal and intrusion subscale, which assesses the emotional intrusiveness and physiological hyperarousal related to the participants’ marital separation, served as our index of overall psychological adjustment to the separation experience (Weiss & Marmar, 2004). Items include the following: Any reminders brought back feelings of it, I feel irritable and angry, and I was jumpy and easily startled.
Data Analysis
To account for the nonindependence inherent to data collected from the same participants over time, we first examined the extent to which variance in our outcomes was attributable to between-and within-participant factors by computing intraclass correlations (ICCs; Singer & Willett, 2003). Examining the ICCs computed from unconditional means models predicting our two outcomes of interest, SBP and DBP, revealed that the data violated the nonindependence assumption of multiple regression, and OLS regression models would therefore lead to inaccurate statistical results (Singer, 1998). Specifically, the ICCs for SBP (ICC = .41) and DBP (ICC = .47) indicate that 40.75% and 47.28% of the variances in SBP and DBP, respectively, are due to between-participants factors. Thus, more than 50% of the variance in each SBP and DBP are attributable to within-participant factors, and mixed regression is an appropriate statistical procedure with which to test our hypotheses. After examining data for any potential impacts of attrition, we conducted mixed regression analyses (Preacher, Wichman, MacCallum, & Briggs, 2008; Singer & Willett, 2003) using SPSS MIXED (SPSS Version 20.0) and maximum likelihood estimation (MLE). MLE procedures allowed us to use all available data in the model from all study occasions. We grand-mean centered all person-level variables (level 2), and to facilitate interpretation, did not center the time-varying variables (level 1). We first examined unconditional means and unconditional growth models to define the functional forms for SBP and DBP before entering our predictor of interest, sleep complaints, into each model. Because of the large within-occasion variability in time since the separation (TSS), we included TSS (in weeks) as a level-1 covariate in all of the augmented models. Following Simmons, Nelson and Simonsohn’s (2011) recommendations, we then entered covariates into our final models. Finally, we reversed predictor and outcome variables to assess model directionality.
Results
Of the 138 participants who completed an initial visit (V1), 104 participants were invited back for the next laboratory visit. Ninety-one participants completed V2, and 79 completed the final visit (V3). Participants who completed at least two visits (n = 91) did not differ from participants who completed only one study visit with respect to V1 SBP, DBP, sleep complaints, psychological distress, or covariates (V1 SBP, t(112) = 0.04, p = .97; V1 DBP, t(112) = −0.09, p = .93; V1 PSQI, t(136) = 0.95, p = .35; V1 IES-R, t(131) = 1.22, p = .22; Biological sex, U = 1743.00, z = −0.97, p = .33; BMI, t(130) = −.29, p = .77; Age, t(131) = −1.37, p = .17; Health, t(131) = 0.44, p = .66).
Correlations among the PSQI, SBP, and DBP across all visits appear in Table 1. We have also included a full correlation table with all study variables as an online supplement to this paper. SBP values were positively correlated across visits, from V1 to V2 (r = .64), V1 to V3 (r = .68), and V2 to V3 (r = .86); DBP values were less correlated (r = .29, .48, and .50, respectively). Sleep complaints were also positively correlated with both SBP (r = .43) and DBP (r = .37) from V1 to V2, with participants who reported greater sleep complaints at V1 evidencing higher SBP and DBP values at V2. The mean global score for sleep complaints on the PSQI at V1 was 7.70 (SD = 4.01) and fell to 5.25 (SD = 3.54) by V3. Both scores fall above the cutoff score of 5 for clinically significant sleep complaints, and on average, participants in this sample reported significantly greater sleep complaints relative to healthy adult populations (see Table 1; Buysse et al., 1989). Figure 1 displays the concurrent associations between blood pressure and time since participants’ separation from their ex-partner as a function of their self-reported sleep complaints. In the figure, the clinical cutoff score for the PSQI (= 5) distinguishes high from low sleep complaints.
Table 1.
Correlations Among Predictor and Outcome Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1.PSQI V1 | — | ||||||||
| 2.PSQI V2 | .65** | — | |||||||
| 3.PSQI V3 | .68** | .86** | — | ||||||
| 4.SBP V1 | .10 | .10 | .01 | — | |||||
| 5.SBP V2 | .43** | .23* | .21 | .30* | — | ||||
| 6.SBP V3 | .23 | .20 | .15 | .42** | .46** | — | |||
| 7.DBP V1 | .09 | .10 | .03 | .94** | .20 | .39** | — | ||
| 8.DBP V2 | .37** | .16 | .21 | .34** | .95** | .51** | .29* | — | |
| 9.DBP V3 | .21 | .20 | .12 | .54** | .42** | .94** | .48** | .50** | — |
| M | 7.70 | 5.47 | 5.26 | 138.52 | 137.65 | 137.32 | 80.10 | 79.28 | 79.82 |
| SD | 4.10 | 3.64 | 3.54 | 17.82 | 18.57 | 19.36 | 12.97 | 12.09 | 12.05 |
Note. V1 PSQI = Visit 1 Pittsburg Sleep Quality Index; V2 PSQI = Visit 2; V3 PSQI = Visit 3; V1 SBP = Visit 1 systolic blood pressure; V2 SBP = Visit 2; V3 SBP = Visit 3; V1 DBP = Visit 1 diastolic blood pressure; V2 DBP = Visit 2; V3 DBP = Visit 3.
p < .05.
p < .01.
Figure 1.
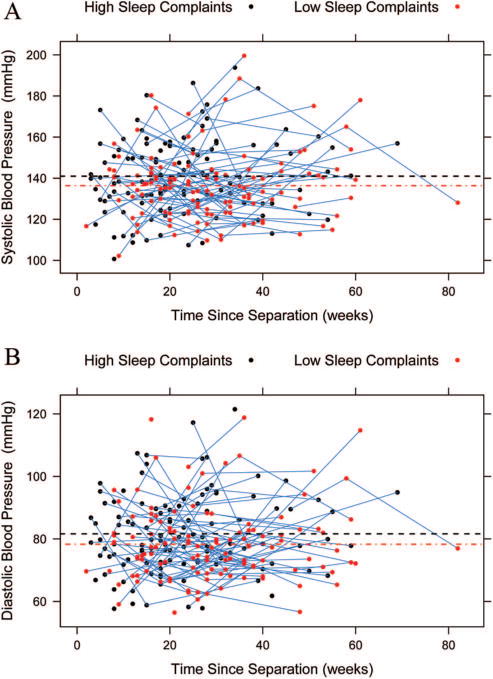
Raw associations between blood pressure and time since participants’ separation from their ex-partner (in weeks) as a function of self-reported sleep complaints. High and low sleep complaints were determined using the clinical cutoff for the PSQI (Buysse et al., 1989). Panel A illustrates the association for systolic blood pressure (SBP) and Panel B for diastolic blood pressure (DBP).
Unconditional Means Models
A series of unconditional means (random intercept) revealed substantial variability around the grand means for PSQI (b = 6.68, p < .001), SBP (b = 138.07, p < .001), and DBP (b = 79.76, p < .001).
Unconditional Growth Models
We next examined unconditional growth models predicting our primary outcomes, SBP and DBP, as a function of time since the separation (in weeks). Neither SBP (b = .04, p = .58) nor DBP (b = 0.02, p = .73) evidenced significant variability in rates of individuals’ rates of change over time. We therefore proceeded using random intercept models (Singer & Willett, 2003).
Conditional Random Intercept Models
We next examined a series of random intercept models predicting SBP and DBP from the PSQI and the covariates included in Table 1. For SBP and DBP, we conducted two series of models. In the first, we examined concurrent associations between sleep and both SBP and DBP, and in the second, we examined the lagged associations, in which we predicted SBP and DBP from the PSQI assessed at the previous study visit.
SBP
Sleep complaints were not significantly associated with concurrent SBP (bconcur = 0.54, SE(b) = 0.31, p = .084; Table 2, Model 1). Sleep complaints assessed one occasion earlier, however, significantly predicted subsequent SBP (blag = 1.43, SE(b) = 0.48, p = .004), and this association held after accounting for concurrent sleep complaints (Table 2, Model 2), concurrent psychological distress and the other covariates (blag = 1.41, SE(b) = 0.59, p = .019; Table 2, Model 3), and lagged psychological distress. Across the entire study period, sleep complaints at an earlier visit predicted significant increases in SBP at the subsequent follow-up visit (Model 3). These analyses show that each standard deviation increase in PSQI sleep complaints correlates with a 6.09 mmHg increase in SBP at the next visit.2
Table 2.
Unstandardized Regression Coefficients From Models Predicting Systolic/Diastolic Blood From Pittsburgh Sleep Quality Index
| Outcome: SBP
|
Outcome: DBP
|
|||||
|---|---|---|---|---|---|---|
| Model | 1 | 2 | 3 | 4 | 5 | 6 |
| Intercept | 134.53** | 129.04** | 127.12** | 77.30** | 75.05** | 75.48** |
| TSS | 0.23ϒ | 0.13 | ||||
| V3 assign | −2.07 | −1.32 | ||||
| Sex | 2.00 | 3.27** | ||||
| Age | −0.08 | −0.22 | ||||
| BMI | 0.44 | 0.15 | ||||
| Health | −0.99 | 0.05 | ||||
| Income | 2.32 | −0.87 | ||||
| Rel length | 0.06* | .06** | ||||
| Resp end | 2.85 | 1.63 | ||||
| Rel sit | 1.93 | 0.44 | ||||
| Race | −0.15 | 3.47ϒ | ||||
| IES-R | 3.34 | 0.44 | ||||
| PSQI | 0.54*** | −0.14 | −0.46 | 0.27 | 0.03 | −0.57 |
| PSQI-L | 1.43** | 1.41* | .71* | .89* | ||
Note. TSS = time since marital separation (in weeks) at Visit 1; V3 assign = whether participant was assigned to a 6-month or 9-month final visit; Sex = (male = 1, female = 1); BMI = body mass index at Visit 1; Health = I have more health problems than others at Visit 1; Income = gross yearly income (1 = <$30,000, 2 = $30,000 – $50,000, 3 = $50,000 –$100,000, 4 = > $100,000) at Visit 1; Rel length = length of relationship before separation (in months) at Visit 1; Resp end = Who was responsible for ending the relationship? at Visit 1; Rel sit = legal status of relationship (−1 not legally divorced, 1 = legally divorced) at Visit 1; Race (−1 = non-white, 1 = white); IES-R = Index of Events Scale–Revised at Visit 1; PSQI = Pittsburgh Sleep Quality Index across all 3 visits; PSQI-L = Pittsburgh Sleep Quality index lagged by one visit. Model 1 = Concurrent sleep predicting SBP; Model 2 = Lagged sleep predicting SBP, accounting for concurrent sleep; Model 3 = Lagged sleep predicting SBP, accounting for concurrent sleep, covariates, and concurrent psychological distress; Model 4 = Concurrent sleep predicting DBP; Model 5 = Lagged sleep predicting DBP, accounting for concurrent sleep; Model 6 = Lagged sleep predicting DBP, accounting for concurrent sleep, covariates, and concurrent psychological stress.
p < .05.
p < .01.
p < .09.
Although we observed a significant lagged effect from sleep complaints at a prior occasion to SBP 3 months later, we did not observe a strong concurrent association between PSQI scores and SBP. Because the effects of sleep complaints appear to accrue over time, one possible explanation is that the concurrent associations are dependent on time since separation (TSS). We found no evidence for a V1-PSQI × TSS interaction predicting V1 SBP scores (b = −.02, SE(b) = 0.07, p = .79). Further exploration of the prospective effect did, however, reveal a significant V1-PSQI × TSS interaction predicting SBP at V2 after accounting for SBP at V1 and each of the other main effects (b = .20, SE(b) = 0.09, p = .05). Results of this analysis appear in Figure 2 for people ±1 SD above/below the mean on the time since separation variable. Using the Johnson-Neyman technique to derive the region of significance for the conditional effect (Hayes & Matthes, 2009), we determined that the conditional effect of high sleep complaints on SBP 3 months later was significant down to 10 weeks after the physical separation from one’s ex-partner. Thus, participants reporting substantial sleep complaints 10 weeks or longer after their separation evidenced increases in SBP from V1 to V2; there were no significant increases in SBP across sleep disturbance levels for people less than 10 weeks after their separation.3 Having identified this effect, we then examined the PSQI × TSS interaction in the full multilevel model where each of these variables was a level-1, time-varying predictor of SBP. We found no evidence for this interaction effect in the full multilevel model, suggesting that the moderating effect of TSS on future increases in SBP occurs in the first few months after a marital separation (i.e., the interaction effect did not persist into our later follow-up visits).
Figure 2.
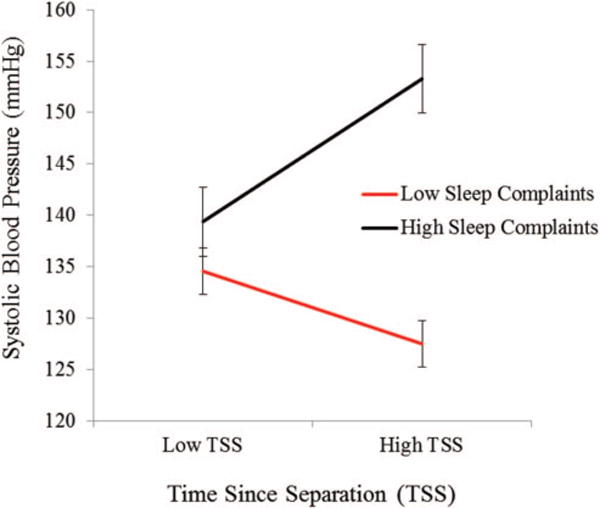
Systolic blood pressure (SBP) at V2 as a function time since separation (TSS) in weeks and PSQI sleep complaints at V1. The figure illustrates the combined effects of TSS and sleep complaints on SBP 3 months later for people who are ±1 SD above/below the mean on both the continuous predictor variables.
DBP
Sleep complaints did not significantly predict concurrent DBP (bconcur = 0.40, SE(b) = 0.23, p = .078; Table 2, Model 4). Sleep complaints assessed one occasion earlier, however, significantly predicted subsequent DBP (blag = 0.71, SE(b) = 0.32, p = .028), and this association held after accounting for concurrent sleep complaints (Table 2, Model 5) as well as concurrent DBP, concurrent psychological distress, and covariates (blag = 0.89, SE(b) = 0.36, p = .016; Model 6). Similar to the above SBP analyses, Model 6 indicates that across the 9-month study period, sleep complaints 3 months earlier predicted significant increases in DBP. This finding also held after accounting for lagged psychological distress. This model demonstrated directionality: We reversed the predictor (sleep complaints) and the outcome (DBP), and DBP did not predict sleep complaints in Model 4 (bconcur = .02, ns), Model 5 (blag = .03, ns), or Model 6 (blag =.0103, ns).4
PSQI Subscales
As a follow-up to our primary analyses, we investigated whether three subscales of the PSQI (subjective sleep quality, sleep duration, and sleep disturbance) independently predicted SBP or DBP. Neither subjective sleep quality nor sleep duration significantly predicted subsequent SBP (blag = 3.14, SE(b) = 2.51, p = .21; blag = 1.73, SE(b) = 2.07, p = .41, respectively) or DBP (blag = 1.05, SE(b) = 1.58, p = .51; blag = .73, SE(b) = 1.28, p = .57, respectively) after accounting for concurrent sleep quality or sleep duration, concurrent psychological distress, and covariates. Sleep disturbance assessed one occasion earlier trended toward significantly predicting subsequent SBP (blag = 6.80, SE(b) = 3.61, p = .06) after accounting for concurrent sleep disturbance, concurrent psychological distress, and covariates; this trend did not hold for DBP (blag = 2.58, SE(b) = 2.26, p = .26). The sleep disturbance subscale indexes frequency of problems initiating or maintaining sleep, and these findings suggest that sleep disturbance may exert a stronger effect on SBP than subjective sleep quality or duration.
Discussion
Although most people are resilient in the face of divorce and some people may even experience health benefits by ending an unhappy marriage (Sbarra et al., 2012), epidemiological data indicate that marital dissolution is a clear risk for poor health. To examine whether alterations in health behaviors may shed light on this association, we investigated changes in resting BP over 7.5 months as a function of sleep complaints in a sample of recently separated adults. We found little evidence for concurrent associations between sleep complaints and BP; however, data supported our hypothesis that sleep complaints would prospectively predict increases in resting SBP and DBP over the study period. Finally, in a focused analysis of changes in SBP across 3 months, we observed that the effect of sleep complaints on prospective increases in SBP within this window depended on the length of time that had passed since the initial physical separation from one’s ex-partner. In particular, participants who entered the study with high sleep complaints and whose separation occurred 10 weeks or more before enrollment evidenced increases in SBP, whereas the other participants did not.
The prospective finding remained significant after accounting for concurrent sleep complaints and a range of important covariates, including BMI, age, participants’ sex, self-reported health, initiator status, relationship length, time since the separation, and divorce-related psychological adjustment. Decrements in sleep quality are highly comorbid with psychological distress and depression, and by accounting for divorce-related psychological distress, our findings suggest that sleep exerts an effect on BP that is independent of underlying distress or depressive symptoms. After accounting for covariates, each standard deviation increase in sleep complaints on the PSQI corresponded to a 6.09-point increase in subsequent SBP and a 3.96 point increase in subsequent DBP; these estimates represent the average lagged effects from the PSQI to future BP scores across the entire period. In addition, we found evidence for directional specificity: Neither SBP nor DBP significantly predicted future sleep complaints. Upon investigating three PSQI subscales, we found that self-reported sleep disturbance (number of times a person experienced difficulty initiating or maintaining sleep) predicted lagged SBP more strongly than either self-reported sleep quality or sleep duration. This suggests that difficulty falling asleep and nighttime awakenings likely put one at greater risk following marital separation. Although sleep complaints decreased over time, the average participant in this study continued to report clinically meaningful sleep problems at V3, which occurred nearly 8 months after entry into the study and approximately a year after participants reported having physically separated from their ex-partner. For this sample of separated and divorcing adults, sleep complaints remained an ongoing part of their lives well after their marital separation.
Per Amato’s (2000) divorce-stress-adjustment model, analyses presented here demonstrate that sleep complaints constitute a unique prospective risk for increases in resting BP, independent of separation-related psychological distress. The findings are also consistent with Troxel et al.’s (2007) dyadic framework for understanding the associations among sleep, relationships, and health, which posits that high quality relationships may exert their positive health effects through sleep quality, and that sleep is a critical attachment behavior. When relationships dissolve, it follows that health-related markers may covary with the ability to regain and maintain high quality sleep, and analyses we report here support this logic. The average participant in this sample entered the study with a PSQI total score approximating that typically observed among people experiencing major depression, and fell nearly five standard deviations above the average score for sleep complaints reported by healthy adults (Buysse et al., 1989).
Several pathways may explain how sleep influences resting BP. One such pathway, autonomic functioning, plays an important role in regulating blood pressure homeostasis. The autonomic nervous system undergoes marked changes during the transition from wake to non-REM sleep, with a shift favoring greater parasympathetic activity and decreased sympathetic activity (Burgess, Trinder, Kim, & Luke, 1997). Similarly, resting blood pressure levels follow a diurnal rhythm that is naturally highest during the day and decreases by 10–20% during sleep (Kario, Schwartz, & Pickering, 2000). Thus, with fewer hours of sleep, the body spends more time under sympathetic burden and experiences fewer opportunities for blood pressure to naturally drop, often resulting in increased average 24-hr resting blood pressure (Gangwisch et al., 2006).
Sleep restriction (and subsequent sympathetic activation) may also act on the cardiovascular system by elevating circulating proinflammatory cytokines and promoting systemic cardiovascular inflammation (Gonzalez & Selwyn, 2003; Shearer et al., 2001). Metabolism and appetite regulation are also intricately linked to sympathetic outflow. Sleep restriction precedes elevated sympathetic activation concomitant with the downregulation in leptin, an appetite-suppressing hormone, and upregulation in ghrelin, an appetite-stimulating hormone (Spiegel et al., 2004). Alterations to the secretion of these metabolic hormones can disrupt appetite and hunger regulation, which may promote increases in weight status and BMI. Taken together, these processes may work synergistically to increase blood pressure following extended periods of sleep complaints.
Blood pressure reliably marks preclinical disease states and is a unique prospective predictor of adverse cardiovascular events, including the development of cardiovascular heart disease (Franklin et al., 2001), stroke (Gu, Burt, Paulose-Ram, Yoon, & Gillum, 2008), and kidney failure (Sarnak et al., 2005). Data presented here showed that romantically separated adults who reported more sleep complaints evidenced clinically relevant increases in BP. We observed that participants who reported 1 standard deviation above the mean on sleep complaints at baseline evidenced SBP over 140 mmHg 3 months later, which falls within the hypertensive range (140/90 mmHg; (Wang & Wang, 2004).
One question of interest from the current study centers on the lack of a concurrent PSQI-BP association. Why did we observe a significant prospective effect for the PSQI on BP increases, but not a significant concurrent association? To the extent that sleep complaints set in motion a series of processes that drive future increases in BP (e.g., sympathetic activation, metabolic alterations, weight fluctuations), these processes may take time to unfold. To explore this idea, we examined the possibility that the concurrent PSQI-BP association would depend on time since separation (TSS); we did not observe this effect. This analysis is limited, however, as it cannot account for sleep complaints prior to study entry. We did, however, find evidence that time since the separation moderated the impact of sleep complaints on SBP from V1 to V2, but this effect did not hold in the full multilevel model. Taken together, results suggest that sleep complaints may exert a prospective effect on resting BP absent of a concurrent effect and that this association may depend on how long participants report sleep complaints following their marital separation. We have no reason to believe that the period between V1 and V2 in our study represents a critical period in which the TSS should moderate the PSQI-BP associations. It is more likely that the full multilevel model was limited in its power to detect the same interaction across all assessments due to participant attrition over time, and the issue awaits further research.
The precise nature of the underlying (potentially) causal lag between sleep complaints and resting blood pressure remains unknown. This gap in knowledge is of considerable importance when seeking to design future, prospective mediational research (Cole & Maxwell, 2003). Our study shows this association can be examined within a 3- to 5-month window, but we have yet to discover the temporal resolution that will best allow researchers to observe potential mediating links among sleep complaints and BP. Future research should therefore seek to replicate these findings by a) including potential mediators that may explain the sleep complaint-BP association, and b) varying the potential causal lags to include varying temporal resolutions, such as 1-week, 1-month, 3-month, and 6-month windows of assessment. In addition, in the current study, we measured BMI at only one occasion, and future research examining whether the lagged-sleep/BP association operated through changes in BMI would be worthwhile.
Limitations
Results presented here should be interpreted in light of several limitations. Although data suggest that adults who report sleep complaints following marital separation may be at risk for increases in resting BP, this study did not include a comparison group. There is therefore no way to uncover whether the effects of interest are specific to marital separation. Second, although our sample size was large for a prospective psychophysiological study, it was relatively homogenous and contained relatively few men. Although the PSQI and the IES-R are validated measures of sleep quality and psychological distress, they are self-report measures and are vulnerable to subject bias. Furthermore, the PSQI is unable to identify reasons for sleep disruption, such as sleep disordered breathing, which has significant effects on cardiovascular functioning above and beyond sleep disruption (Nieto et al., 2000). Future study designs would benefit from objective sleep assessment, such as using actigraphy and screening for sleep-disordered breathing (which we did not screen for). Researchers who prospectively examine associations among sleep, psychological distress, and blood pressure longitudinally should strive to provide information about whether individuals who exhibit sleep complaints and high blood pressure following separation also exhibit these characteristics before their separation. Finally, a variety of unmeasured variables (e.g., changes in diet, exercise, and/or alcohol consumption) may explain variability in blood pressure, and researchers should incorporate these variables in future research.
Conclusion
This study examined associations among self-reported sleep complaints and changes in BP over nine months in recently separated adults. Sleep complaints predicted future increases in resting BP (but were not associated with concurrent BP). In an exploratory analysis, we found that the association between sleep complaints and changes in SBP depended on the time since separation from one’s ex-partner. People who reported ongoing, high levels of sleep complaints 10 weeks or longer after their separation evidenced the greatest increases in resting SBP. If replicated, these findings suggest that clinicians should attend to sleep complaints that persist beyond 3 months postseparation, as this may signal the onset of a more chronic course of insomnia and related health concerns. Promoting a consistent sleep/wake schedule with adequate sleep time, addressing nighttime rumination and emotional hyperactivation, and increasing sleep efficiency may help this vulnerable population protect against future negative health outcomes following their marital separation.
Supplementary Material
Acknowledgments
We thank Richard Bootzin for valuable contributions to the paper. The first and third authors’ work on this paper was supported by a grant from the National Institute of Child Health and Human Development (HD069498), and the overall project was funded by grants from the National Institute of Mental Health (MH074637) and the National Institute on Aging (AG028454 and AG036895).
Footnotes
We used a planned missingness design (McArdle, 1994) to ease participant burden (and thus decrease study attrition) across multiple laboratory visits. For these analyses, we grouped participants in the two follow-up sessions together. Thus, V3 visit took place an average of 7.5 months after V1. For all prospective analyses, we included a dummy-coded variable indicating to which follow-up period participants were assigned. There were no group differences on any of the V1 measures for participants assigned to the different follow-up conditions.
This model demonstrated directionality: We reversed the predictor (sleep complaints) and the outcome (SBP), and SBP did not predict sleep complaints in Model 1 (bconcur = .02, ns), Model 2 (blag = .02, ns), or Model 3 (blag = .02, ns).
The V1-PSQI × TSS interaction predicting DBP at V2 after accounting for DBP at V1 and each of the other main effects was not significant, b = −.10, SE(b) = 0.07, p = .10.
We examined the Sex × Lagged-PSQI interaction in Models 3 and 6 after accounting for each main effect. The interaction effect was significant in each model, with stronger associations between lagged sleep complaints and blood pressure increases observed for men. However, when we examined this interaction effect with only the main effects of sex, PSQI, and lagged-PSQI in the model, the interaction effect was no longer significant. These results suggest a classic suppression effect within the regression analysis—the interaction effect is only significant when other covariates are included in the model.
Contributor Information
Kendra N. Krietsch, Department of Clinical and Health Psychology, University of Florida
Ashley E. Mason, Osher Center for Integrative Medicine, University of California San Francisco
David A. Sbarra, Department of Psychology, University of Arizona
References
- Amato PR. The consequences of divorce for adults and children. Journal of Marriage and the Family. 2000;62:1269–1287. doi: 10.1111/j.1741-3737.2000.01269.x. [DOI] [Google Scholar]
- Amato PR. Research on divorce: Continuing trends and new developments. Journal of Marriage and Family. 2010;72:650–666. doi: 10.1111/j.1741-3737.2010.00723.x. [DOI] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research. 2002;53:737–740. doi: 10.1016/S0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: A focus on insomnia. Sleep Medicine Reviews. 2010;14:227–238. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Belani K, Ozaki M, Hynson J, Hartmann T, Reyford H, Martino JM, Miller R. A new noninvasive method to measure blood pressure: Results of a multicenter trial. Anesthesiology. 1999;91:686. doi: 10.1097/00000542-199909000-00021. [DOI] [PubMed] [Google Scholar]
- Brown LF, Reynolds CF, Monk TH, Prigerson HG, Dew MA, Houck PR, Kupfer DJ. Social rhythm stability following late-life spousal bereavement: Associations with depression and sleep impairment. Psychiatry Research. 1996;62:161–169. doi: 10.1016/0165-1781(96)02914-9. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. American Journal of Physiology. 1997;273:1761–1768. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: The Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA, Maxwell SE. Testing mediational models with longitudinal data: Questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology. 2003;112:558–577. doi: 10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- Diamond LM. Contributions of psychophysiology to research on adult attachment: Review and recommendations. Personality and Social Psychology Review. 2001;5:276–295. doi: 10.1207/S15327957PSPR0504_1. [DOI] [Google Scholar]
- Diamond LM, Hicks AM, Otter-Henderson KD. Every time you go away: Changes in affect, behavior, and physiology associated with travel-related separations from romantic partners. Journal of Personality and Social Psychology. 2008;95:385–403. doi: 10.1037/0022-3514.95.2.385. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.CIR.103.9.1245. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Malaspina D. Short sleep duration as a risk factor for hypertension: Analyses of the First National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. American Journal of Medicine. 2003;115:99S–106S. doi: 10.1016/j.amjmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: The Sleep Heart Health Study. Sleep: Journal of Sleep and Sleep Disorders Research. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among US adults: The third National Health and Nutrition Examination Survey mortality follow-up study. Annals of epidemiology. 2008;18:302–309. doi: 10.1016/j.annepidem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hewitt B, Turrell G. Short-term functional health and well-being after marital separation: Does initiator status make a difference? American journal of epidemiology. 2011;173:1308–1318. doi: 10.1093/aje/kwr007. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology. 1992;29:742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alpha-adrenergic blocker, doxazosin: Results from the HALT study. Hypertension. 2000;35:787–794. doi: 10.1161/01.HYP.35.3.787. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Rathouz PJ, Yan LJL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: The CARDIA Sleep Study. Archives of Internal Medicine. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard C, Randler C. In sync with the family: Children and partners influence the sleep-wake circadian rhythm and social habits of women. Chronobiology International. 2009;26:510–525. doi: 10.1080/07420520902821101. [DOI] [PubMed] [Google Scholar]
- Lorenz FO, Wickrama K, Conger RD, Elder GH. The short-term and decade-long effects of divorce on women’s midlife health. Journal of Health and Social Behavior. 2006;47:111–125. doi: 10.1177/002214650604700202. [DOI] [PubMed] [Google Scholar]
- Mason AE, Sbarra DA, Mehl MR. Thin-slicing divorce: Thirty seconds of information predict changes in psychological adjustment over 90 days. Psychological Science. 2010;21:1420–1422. doi: 10.1177/0956797610381507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ. Structural factor analysis experiments with incomplete data. Multivariate Behavioral Research. 1994;29:409–454. doi: 10.1207/s15327906mbr2904_5. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Medicine Reviews. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Minowa M, Okawa M, Uchiyama M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemo-graphic factors in the general Japanese adult population. Journal of Epidemiology/Japan Epidemiological Association. 2000;10:79–86. doi: 10.2188/jea.10.79. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Progress in Cardiovascular Diseases. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA Journal of the American Medical Association. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Preacher K, Wichman A, MacCallum R, Briggs N. Latent growth curve modeling: Quantitative applications in the social sciences. Thousand Oaks, CA: Sage; 2008. [Google Scholar]
- Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS. The effect of a lower target blood pressure on the progression of kidney disease: Long-term follow-up of the modification of diet in renal disease study. Annals of internal medicine. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Hasselmo K, Nojopranoto W. Divorce and death: A case study for health psychology. Social and Personality Psychology Compass. 2012;6:905–919. doi: 10.1111/spc3.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: An integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Personality and Social Psychology Review. 2008;12:141–167. doi: 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Law RW, Lee LA, Mason AE. Marital dissolution and blood pressure reactivity: Evidence for the specificity of emotional intrusion-hyperarousal and task-rated emotional difficulty. Psychosomatic Medicine. 2009;71:532–540. doi: 10.1097/PSY.0b013e3181a23eee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra DA, Law RW, Portley RM. Divorce and death: A meta-analysis and research agenda for clinical, social, and health psychology. Perspectives on Psychological Science. 2011;6:454–474. doi: 10.1177/1745691611414724. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. Journal of Allergy and Clinical Immunology. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Shor E, Roelfs DJ, Bugyi P, Schwartz JE. Meta-analysis of marital dissolution and mortality: Reevaluating the intersection of gender and age. Social Science & Medicine. 2012;75:46–59. doi: 10.1016/j.socscimed.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science. 2011;22:1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. doi: 10.2307/1165280. [DOI] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford, UK: Oxford University Press; 2003. [DOI] [Google Scholar]
- Spiegel K, Leproult R, L’Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology & Metabolism: Clinical and Experimental. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–1324. doi: 10.1161/01.HYP.27.6.1318. [DOI] [PubMed] [Google Scholar]
- Troxel WM. It’s more than sex: Exploring the dyadic nature of sleep and implications for health. Psychosomatic Medicine. 2010;72:578–586. doi: 10.1097/PSY.0b013e3181de7ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Buysse DJ, Matthews KA, Kravitz HM, Bromberger JT, Sowers M, Hall MH. Marital/cohabitation status and history in relation to sleep in midlife women. Sleep: Journal of Sleep and Sleep Disorders Research. 2010;33:973–981. doi: 10.1093/sleep/33.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: Examining the covariation between relationship quality and sleep. Sleep Medicine Reviews. 2007;11:389–404. doi: 10.1016/j.smrv.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Umberson D. Gender, marital status and the social control of health behavior. Social Science & Medicine. 1992;34:907–917. doi: 10.1016/0277-9536(92)90259-S. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Wang H. Effects of marital status and transition on hypertension in Chinese women: A longitudinal study; Paper presented at the Annual Meeting of the Population Association of America; Philadelphia, PA. 2005. [Google Scholar]
- Wang H, Amato PR. Predictors of divorce adjustment: Stressors, resources, and definitions. Journal of Marriage and the Family. 2000;62:655–668. doi: 10.1111/j.1741-3737.2000.00655.x. [DOI] [Google Scholar]
- Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: New challenges of the old problem. Archives of Internal Medicine. 2004;164:2126–2134. doi: 10.1001/archinte.164.19.2126. [DOI] [PubMed] [Google Scholar]
- Weiss DS, Marmar CR. Assessing psychological trauma and PTSD. Vol. 2. New York, NY: Springer; 2004. The impact of event scale-revised; pp. 168–189. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


