Abstract
The treatment and management of advanced urothelial carcinoma of the bladder is a considerable therapeutic challenge. Prospective, randomized clinical trial data demonstrate a survival advantage for those patients that receive neoadjuvant chemotherapy prior to radical cystectomy. Despite the overall survival benefits, results from both institutional and administrative datasets suggest that historical use of a neoadjuvant chemotherapy paradigm is remarkably low. This review will evaluate the recent trends in pre-operative chemotherapy utilization that suggest small, but progressively increased use–currently on the order of 20% of radical cystectomy patients. Additionally, this analysis will explore the various processes and structural barriers that preclude its receipt such as patient age and comorbidity, as well as physician preference, delay to potentially curable surgery, geographic region, distance to treatment facility, and socioeconomic status.
Keywords: bladder cancer, neoadjuvant therapy, chemotherapy, utilization, review
Introduction
A decade has passed since Grossman and colleagues released the results of the seminal SWOG 8710 clinical trial.[1] This prospective analysis noted small but significant survival benefits for patients who received neoadjuvant chemotherapy (NAC) prior to radical cystectomy (RC). Importantly, that analysis cited a 14% improvement in 5-year overall survival, a 30-month mean survival advantage, and a 23% increase in pT0 rates compared to those patients that received radical cystectomy alone. These findings have been supported by multiple other series, using a variety of agents, which have noted similarly modest but significant benefits of a NAC paradigm for urothelial carcinoma of the bladder (UC). [2–7]
Supporters of NAC cite the critically important benefit of tumor downstaging, earlier treatment of clinically undetected micrometastatic disease, improved patient performance status prior to radical cystectomy, and enhanced dose delivery prior to surgery. [8–10] However, critics of NAC note: chemotherapy prior to potentially curative surgery risks disease progression, most saliently for chemotherapy non-responders; the benefits of NAC do not outweigh the risks (mortality rates of 1–3%); the poor correlation of clinical and pathologic staging for bladder cancer precludes an accurate pre-operative assessment of appropriate NAC candidates; and cure rates for pT2 disease are quite high after radical cystectomy alone. [10–14]
Historical Patterns of Neoadjuvant Chemotherapy Use
Given these conflicting sentiments, there is disagreement about the optimal timing, dosing, agents, and utility of chemotherapy for clinically localized UC. Perhaps not surprisingly, utilization of perioperative chemotherapy, with NAC in particular, has been low. Several series have investigated the use of chemotherapeutic regimens prior to the release of Grossman, et. al’s [1] data. Using SEER-Medicare linked administrative data, Porter and colleagues [15] evaluated perioperative chemotherapy use from 1992–2002. These results demonstrate dramatically low implementation of NAC, with rates of 1.2% to 11% during the study timeframe, for Stage 2 to Stage 4 UC, respectively. These authors noted considerable variability in use of chemotherapies based on SEER region as well as temporal variation in the type of chemotherapy used, with increasing use of gemcitabine and carboplatin at the end of the study period. The data on individual chemotherapies, while likely representing realistic temporal trends, should be interpreted with some caution given validation studies within the same dataset suggesting high sensitivity and specificity for any chemotherapy claim, but low reliability of billing for a specific agent. [16,17]
The low utilization of chemotherapy for UC has been confirmed by other authors using administrative datasets, such as the National Cancer Database (NCDB) maintained by the American College of Surgeons and the American Cancer Society. David et. al [18] evaluated perioperative chemotherapy use for 7,161 Stage III UC patients treated with RC. Data were evaluated from 1998 to 2003 within the NCDB. Perioperative chemotherapy in this series was defined somewhat restrictively as within 4 months of RC. These authors noted a relatively meager utilization rate of 11.6% for any chemotherapy and 1.2% for NAC specifically. Within the same dataset, though using expanded eligibility criteria, Fedeli and colleagues [19] evaluated patterns of care for 40,388 patients diagnosed with Stage II through Stage IV muscle-invasive UC. They noted temporal trends of increased NAC, ranging from 6% in 2003 to 13% in 2007. These researchers also noted considerable regional variation in utilization rates of chemotherapy as well as high rates of partial cystectomy (7%–10%) and use of primary chemotherapy (15.7%–19.9%) without attempt at curative treatment via RC or radiation.
Taken together, the aforementioned data suggest relatively low historical utilization of perioperative chemotherapy– specifically NAC– prior to the release of the SWOG 8710 data. While these results are somewhat disturbing given the level 1 evidence supporting the use of NAC, several authors have noted in recent publications and abstracts, continued small but progressive increases in NAC utilization.
Recent Utilization Trends
One of the concerning patterns of care raised in the previously discussed administrative series is that NAC use tends to be concentrated in high-volume, academic medical centers. In order to clarify the utilization of NAC in a tertiary referral center, Raj and colleagues [20] at University of Texas, Southwestern Medical Center evaluated 238 patients at their institution that underwent RC between years 2003 and 2008. The authors determined that 145 of those patients were eligible for NAC or diagnosed as clinical Stage ≥ 2. They noted modestly increased utilization in their institutional series, with 22% of eligible patients receiving some form of NAC, while 17% received specifically cisplatin-based chemotherapy. Cited factors associated with the withholding of NAC were patient factors such as age, comorbidity, or preference, in addition to physician concerns regarding the toxicity of chemotherapy and the presence of apparent clinically localized disease. This series confirmed the significant downstaging associated with a NAC regimen, noting a pT0 rate of 28%, compared to 8% for those that did not receive pre-operative chemotherapy. In this institutional series, NAC was not associated with improved disease-specific or overall survival.
Using the national Veterans Affairs Clinical Cancer Registry, Sandu et. al [21] have presented data investigating the use of NAC within the Veterans Health Administration (VA). These researchers evaluated all patients in the VA diagnosed with clinical Stage ≥ 2 from 1997 to 2007, which resulted in a cohort of 3,336 patients, 36.3% of whom were treated with RC. Mean NAC use within the surgical cohort was 6.3%. However, temporal trends of NAC utilization increased from 3% in 2003 to 14% in 2007. Within this dataset, odds of NAC receipt in 2007 was nearly 2.4 fold higher compared to NAC utilization in 2003. Independent predictors of NAC use in this series were more recent diagnosis and older age.
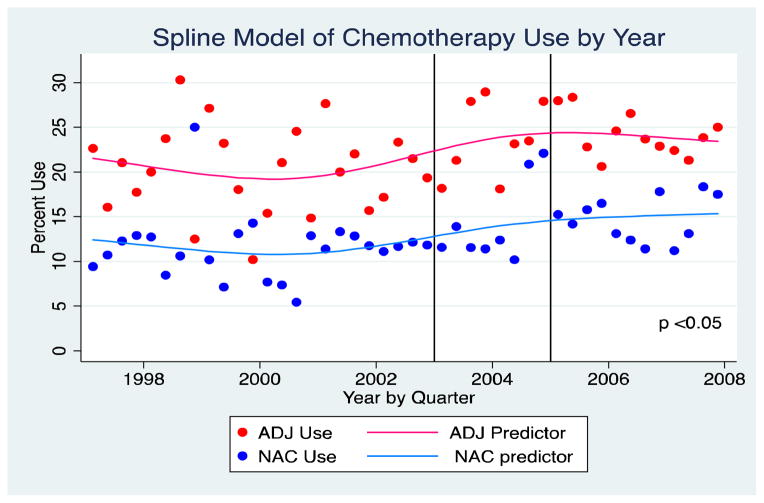
Expanding on previous SEER-Medicare data, Keegan, et al. [22] have presented an update of this cohort focused specifically on the temporal trends of perioperative chemotherapy utilization after SWOG 8710. This dataset was comprised of 143,243 patients with incident diagnosis of UC of the bladder between years 1997 and 2007, with follow-up data through 2009. After exclusions, 4,183 of these patients underwent RC. Medicare claims were assessed for chemotherapy utilization by quarter within the 6-month period prior to and after RC. 65.7% of patients within this cohort underwent RC and had no claim for any chemotherapeutic agent. NAC use increased over the time course of this evaluation, from 11.1% in 1997 to 15.2% in 2007. (Figure 1) However, adjuvant chemotherapy utilization remained nearly 2-fold higher than NAC use across gender, race, comorbidity, and all age groups with the exception of the most elderly- those greater than 85 years of age. Married subjects were more likely to receive chemotherapy, suggesting the importance that social support networks may play in the decision to undergo chemotherapy. Lower comorbidity, younger age, and North Central SEER regions were independent predictors of increased utilization of NAC. The odds of receipt of NAC was 42% higher (OR 1.42; 95%CI 1.17–72) after the release of the SWOG 8710 data. However, these data suggest there remains considerable regional variation in the use of NAC and there appears to be disproportionately higher incidence of no nodes or Nx pathology in the NAC group, which raises concerns regarding the adequacy of surgical resection within a NAC cohort.
Figure 1.
Temporal Trends in Perioperative Chemotherapy for Bladder Cancer-From Keegan, et. al [22]
Most recently, Zaid and colleagues [23] evaluated the NCDB for variation in the utilization of NAC from 2006 to 2010. These researchers identified 5,692 patients that were diagnosed with clinical T2 bladder cancer and subsequently underwent RC. Their data demonstrated overall NAC utilization of 16.9% during the study period. There were persistent temporal trends of increased use of NAC, with proportional use of 10.2% in 2006 to 20.9% in 2010. Consistent with previous reports, these data also revealed significant downstaging among those patients who received pre-operative chemotherapy compared to those that underwent immediate RC, 31.2% vs 7.6%, respectively. In multivariate analysis, younger age, higher clinical stage, lower comorbidity, higher income, Northeast location, and treatment in an academic facility were independent predictors of NAC. Congruent with other administrative series, Zaid et al’s data also highlight worrisome patterns of care given the decreased receipt of NAC for those patients of lower socioeconomic status, the uninsured, those treated in community hospitals, and the elderly. These findings further underscore the need to minimize the barriers to appropriate care for classically underserved groups.
Discussion
Advanced bladder cancer represents a considerable treatment and management challenge. Given that overall survival for those patients with pT3 disease has improved little in the last 30 years [24] and that recurrence rates at 5 years may exceed 50% [25], any protocol with proven benefits such as NAC deserves careful consideration. While the gains in overall survival noted by Grossman et al. and others are relatively small, their findings represent a significant clinical advance for the field of urologic oncology, to the extent that some authors have called for the use of NAC as a quality of care measure for bladder cancer. [26,27]
Historical data from administrative series suggest underutilized perioperative chemotherapy in general and NAC in particular. Critics of NAC cite high cure rates for pT2 tumors with surgery alone, that the marginal benefits of NAC do not exceed the risks, the clinical staging of bladder is a poor predictor of eventual pathologic stage, and that any delay of surgery risks disease progression, particularly for those that do not have a response to chemotherapy. [10,11,13] Despite these reservations, utilization of NAC does appear to be increasing subsequent to the release of the seminal SWOG 8710 findings, albeit very slowly. Utilization rates in recent large, population based series suggest proportional use on the order of 20%, with perhaps comparatively higher usage in academic centers [20,28] (Table 1)
Table 1.
Chemotherapy Use Across Multiple Series
| Study | Study Dates | Use at Start of Study | Use at End of Study |
|---|---|---|---|
| Porter, et. al [15] | 1992–2002 | — | 7% * |
| Fedeli, et. al [19] | 1998–2003 | 6% | 13% |
| David, et. al [18] | 2003–2007 | — | 1.2% * |
| Keegan, et. al [22] | 1997–2008 | 11% | 15% |
| Sandhu, et. al [21] | 2003–2007 | 3% | 14% |
| Raj, et. al [20] | 2003–2008 | — | 22% ~ |
| Zaid, et. al [23] | 2006–2010 | 7.6% | 20.9% |
Mean use for pT3
Mean use in series
Despite the apparent underutilization of NAC, it is important to point out that not all candidates for RC are suitable for NAC. It is in fact difficult to determine the true number of appropriate patients who should receive NAC. In a survey conducted of all active members of the Society for Urologic Oncology regarding their practice patterns and opinions regarding NAC, Cowan and colleagues [29] noted that urologic oncologists cite age, comorbidity, delay in surgery, and modest marginal benefit as the principle determinants to eschew NAC. Intriguingly, only 65% of responding urologic oncologists discussed NAC with 90% of their patients for whom RC was an option. Moreover, many medical oncologists will not offer cisplatin-based chemotherapy to patients with a glomerular filtration rate (GFR) less than 60 mL/minute. While there is debate regarding the best formula to clinically measure GFR, [30,31] it appears that upwards of 40% of patients may be disqualified from NAC based on insufficient renal function alone [32,33], not to mention the significant proportion of patients that undergo cystectomy for non-muscle invasive disease, for which NAC is thought to be of little benefit.
One of several benefits of administrative datasets, such as SEER-Medicare and the NCDB, [34–36] is that they collect large volumes of population-based data in a longitudinal fashion. Additionally, these databases provide an excellent representation of patterns of care across broad regions, demographic categories, and institutional types. However, given that the majority of data regarding the use of perioperative chemotherapy for UC is gleaned from these population-based datasets, there are limitations to consider and conclusions should be made in a measured fashion. Inherent limitations to administrative data include systematic biases and unmeasured confounders created by omitted variable bias, attribution bias, missing data, the lack of granular comorbidity data, and imprecise or absent documentation of specific chemotherapy regimes. These factors may influence the ability to draw precise conclusions regarding true patterns of care for NAC. Nevertheless, the weight of the data from the NCDB, SEER-Medicare, VA registries, and institutional series suggest that the proportional use of NAC is increasing, apparently in response to level 1 data, although it remains underutilized.
Conclusion
Historical patterns of care regarding the use of NAC suggest dramatic underutilization. However, data from a variety of sources indicate that use of NAC is increasing–on the order of 20% of those undergoing RC– in apparent response to level 1 data demonstrating the overall survival benefits of a NAC paradigm. Nonetheless, these data also highlight that there remain persistent structural and process barriers to the use of NAC. Ultimately, the future promise is to appropriately stratify those individuals with the highest likelihood to respond to chemotherapy as well as precisely and prospectively target these patients in order to reduce disease recurrence and improve overall survival. [37]
Acknowledgments
This work was supported in part by the National Institutes of Health, K-12 Paul Calabresi Career Development Award for Clinical Oncology, CA-90625 to KAK
References
Papers of particular interest are highlighted as:
* Of importance
** Of major Importance
- 1**.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. Seminal SWOG 8710 clinical trial demonstrating overall survival and significant downstaging attributed to NAC regimen compared to RC alone. [DOI] [PubMed] [Google Scholar]
- 2.Advanced Bladder Cancer Overview Collaboration. Neoadjuvant chemotherapy for invasive bladder cancer. Cochrane Database Syst Rev. 2005:CD005246. doi: 10.1002/14651858.CD005246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Advanced Bladder Cancer Collaboration. Neoadjuvant chemotherapy for invasive bladder cancer. Cochrane Database Syst Rev. 2009:1–32. [Google Scholar]
- 4.Dash A, Pettus JA, Herr HW, Bochner BH, Dalbagni G, Donat SM, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113:2471–7. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DC, Mackler NJ, Dunn RL, Hussain M, Wood D, Lee CT, et al. Phase II trial of paclitaxel, carboplatin and gemcitabine in patients with locally advanced carcinoma of the bladder. J Urol. 2008;180:2384–8. doi: 10.1016/j.juro.2008.08.075. discussion 2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairey AS, Daneshmand S, Quinn D, Dorff T, Dorin R, Lieskovsky G, et al. Neoadjuvant chemotherapy with gemcitabine/cisplatin vs. methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive urothelial carcinoma of the bladder: A retrospective analysis from the University of Southern California. Urol Oncol. 2012 doi: 10.1016/j.urolonc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, et al. A Systematic Review of Neoadjuvant and Adjuvant Chemotherapy for Muscle-invasive Bladder Cancer. European Urology. 2012;62:523–33. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. The Lancet. 1999;354:533–40. [PubMed] [Google Scholar]
- 9.Schultz PK, Herr HW, Zhang ZF, Bajorin DF, Seidman A, Sarkis A, et al. Neoadjuvant chemotherapy for invasive bladder cancer: prognostic factors for survival of patients treated with M-VAC with 5-year follow-up. J Clin Oncol. 1994;12:1394–401. doi: 10.1200/JCO.1994.12.7.1394. [DOI] [PubMed] [Google Scholar]
- 10.de Vere White RW, Katz MH, Steinberg GD. The case for neoadjuvant chemotherapy and cystectomy for muscle invasive bladder cancer. J Urol. 2009;181:1994–7. doi: 10.1016/j.juro.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann J, Retz M, Stöckle M. Chemotherapy in the post-MVAC era: the case for adjuvant chemotherapy. World J Urol. 2002;20:144–50. doi: 10.1007/s00345-002-0252-9. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann J, Retz M, Wiemers C, Beck J, Thüroff J, Weining C, et al. Adjuvant cisplatin plus methotrexate versus methotrexate, vinblastine, epirubicin, and cisplatin in locally advanced bladder cancer: results of a randomized, multicenter, phase III trial (AUO-AB 05/95) J Clin Oncol. 2005;23:4963–74. doi: 10.1200/JCO.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 13.Raghavan D, Quinn D, Skinner DG, Stein JP. Surgery and adjunctive chemotherapy for invasive bladder cancer. Surg Oncol. 2002;11:55–63. doi: 10.1016/s0960-7404(02)00007-5. [DOI] [PubMed] [Google Scholar]
- 14.Raghavan D, Burgess E, Gaston KE, Haake MR, Riggs SB. Neoadjuvant and adjuvant chemotherapy approaches for invasive bladder cancer. Seminars in Oncology. 2012;39:588–97. doi: 10.1053/j.seminoncol.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Porter MP, Kerrigan MC, Donato BMK, Ramsey SD. Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urologic Oncology: Seminars and Original Investigations. 2011;29:252–8. doi: 10.1016/j.urolonc.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Lund JL, Stürmer T, Harlan LC, Sanoff HK, Sandler RS, Brookhart MA, et al. Identifying Specific Chemotherapeutic Agents in Medicare Data: A Validation Study. Med Care. 2011:1. doi: 10.1097/MLR.0b013e31823ab60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 18.David KA, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Low Incidence of Perioperative Chemotherapy for Stage III Bladder Cancer 1998 to 2003: A Report From the National Cancer Data Base. J Urol. 2007;178:451–4. doi: 10.1016/j.juro.2007.03.101. [DOI] [PubMed] [Google Scholar]
- 19.Fedeli U, Fedewa SA, Ward EM. Treatment of Muscle Invasive Bladder Cancer: Evidence From the National Cancer Database, 2003 to 2007. J Urol. 2011;185:72–8. doi: 10.1016/j.juro.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 20*.Raj GV, Karavadia S, Schlomer B, Arriaga Y, Lotan Y, Sagalowsky A, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2010;117:276–82. doi: 10.1002/cncr.25429. Patterns of care for perioperative chemotherapy use in high-volume tertiary care facility. Patient age, comorbidity, and apparent clinically localized disease significant predictors for withholding NAC. [DOI] [PubMed] [Google Scholar]
- 21*.Sandhu GS, Luo S, Zeringue A, Carson KR, Nepple KR, Strope SA, et al. Utilization and predictors of neoadjuvant chemotherapy for muscle-invasive bladder cancer in the Veterans Health Administration. J Clin Oncol. 2012;30(suppl):Abst 4594. Recent data demonstrating increased use of NAC in VA system consistent with evidence based practice. [Google Scholar]
- 22*.Keegan KA, Morgan TM, Resnick MJ, Anderson CB, Ni S, Davis R, et al. Changing Utilization of Neoadjuvant and Adjuvant Chemotherapies for Muscle-Invasive Urothelial Carcinoma After Publication of Landmark Manuscripts. J Urol. 2012;187:e216–7. Abst 527. Recent data to address the specific impact of level 1 data on utilization of perioperative chemotherapy. 42% higher odds of NAC utilization after 2003, the year of SWOG 8710 data. [Google Scholar]
- 23*.Zaid HB, Patel SG, Stimson CJ, Resnick MJ, Cookson MS, Barocas DA, Chang SS. Trends in the Utilization of Neoadjuvant Chemotherapy in Muscle-invasive Bladder Cancer: Results From the National Cancer Database. Urology. 2013 doi: 10.1016/j.urology.2013.07.072. in press. Large scale, administrative data demonstrating increased utilization of NAC from 10.2% to 20.9%, and significant tumor downstaging associated with NAC protocol. [DOI] [PubMed] [Google Scholar]
- 24.Herr HW, Dotan Z, Donat SM, Bajorin DF. Defining optimal therapy for muscle invasive bladder cancer. J Uro. 2007;177:437–43. doi: 10.1016/j.juro.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery JS, Miller DC, Weizer AZ. Quality indicators in the management of bladder cancer. J Natl Compr Canc Netw. 2013;11:492–500. doi: 10.6004/jnccn.2013.0061. [DOI] [PubMed] [Google Scholar]
- 27.Cooperberg MR, Porter MP, Konety BR. Candidate quality of care indicators for localized bladder cancer. Urologic Oncology: Seminars and Original Investigations. 2009;27:435–42. doi: 10.1016/j.urolonc.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Feifer A, Taylor JM, Shouery M, et al. Multi-institutional quality-of-care initiative for nonmetastatic, muscle-invasive, transitional cell carcinoma of the bladder: Phase I. J Clin Oncol. 2011;29(Suppl 7):Abstr 240. [Google Scholar]
- 29.Cowan N, Chen Y, La Rochelle J, Amling C, Koppie T. Neoadjuvant Chemotherapy Use in Bladder Cancer: A Survey of Current Practice and Opinions. J Urol. 2013;189:e587–8. Abst 1435. doi: 10.1155/2014/746298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao C-K, Moshier E, Seng SM, Godbold J, Grossman S, Winston J, et al. Impact of the CKD-EPI equation for estimating renal function on eligibility for cisplatin-based chemotherapy in patients with urothelial cancer. Clinical Genitourinary Cancer. 2012;10:15–20. doi: 10.1016/j.clgc.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for Cisplatin-based chemotherapy in bladder cancer. Journal of Clinical Oncology. 2006;24:3095–100. doi: 10.1200/JCO.2005.04.3091. [DOI] [PubMed] [Google Scholar]
- 32.Canter D, Viterbo R, Kutikov A, Wong Y-N, Plimack E, Zhu F, et al. Baseline renal function status limits patient eligibility to receive perioperative chemotherapy for invasive bladder cancer and is minimally affected by radical cystectomy. Urology. 2011;77:160–5. doi: 10.1016/j.urology.2010.03.091. [DOI] [PubMed] [Google Scholar]
- 33.Dash A, Galsky MD, Vickers AJ, Serio AM, Koppie TM, Dalbagni G, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–13. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 34.Warren JL, Klabunde CN, Schrag D, Bach PB, riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 35.Newman LA, Lee CT, Parekh LP, Stewart AK, Thomas CR, Beltran RA, et al. Use of the National Cancer Data Base to develop clinical trials accrual targets that are appropriate for minority ethnicity patients: a report from the American College of Surgeons Oncology Group (ACOSOG) Special Population Committee. Cancer. 2006;106:188–95. doi: 10.1002/cncr.21592. [DOI] [PubMed] [Google Scholar]
- 36.Menck HR, Bland KI, Eyre HJ, Cunningham MP, Fremgen A, Murphy GP, et al. CA: a Cancer Journal for Clinicians. 1998;48:134–45. doi: 10.3322/canjclin.48.3.134. [DOI] [PubMed] [Google Scholar]
- 37.Lotan Y, Bagrodia A, Passoni N, Rachakonda V, Kapur P, Arriaga Y, et al. Prospective evaluation of a molecular marker panel for prediction of recurrence and cancer-specific survival after radical cystectomy. European Urology. 2013;64:465–71. doi: 10.1016/j.eururo.2013.03.043. [DOI] [PubMed] [Google Scholar]