Abstract
Background
Decontamination, cleaning, and reuse of filtering facepiece respirators (FFRs) has been proposed to mitigate an acute FFR shortage during a public health emergency. Our study evaluates the ability of commercially available wipe products to clean FFRs contaminated with either infectious or noninfectious aerosols.
Methods
Three models of surgical N95 FFRs were contaminated with aerosols of mucin or viable Staphylococcus aureus then cleaned with hypochlorite, benzalkonium chloride, or nonantimicrobial wipes. After cleaning, FFRs were separated into components (nose pad, fabrics, and perforated strip), and contaminants were extracted and quantified. Filtration performance was assessed for cleaned FFRs.
Results
Mucin removal was <1 log for all wipe products on all components. Inert wipes achieved ~1-log attenuation in viable S aureus on fabrics from all FFR models—removal was less effective from nose pads and perforated edges. Both antimicrobial wipes achieved 3–5-log attenuation on most components, with smaller reductions on nose pads and greater reductions on perforated strips. Particle penetration following cleaning yielded mean values <5%. The highest penetrations were observed in FFRs cleaned with benzalkonium chloride wipes.
Conclusions
FFRs can be disinfected using antimicrobial wipe products, but not effectively cleaned with the wipes evaluated in this study. This study provides informative data for the development of better FFRs and applicable cleaning products.
Keywords: Aerosol, Bioaerosol, Decontamination, Influenza, Pandemic, Saliva
A filtering facepiece respirator (FFR) is standard personal protective equipment to protect health care workers from respiratory threats such as pandemic influenza and tuberculosis.1,2 An FFR in use will likely be contaminated through aerosol exposure, rendering it a fomite. During normal operations, an FFR should not significantly contribute to disease transmission because it is disposed of after each patient exposure. However, continual wear during a public health emergency increases the likelihood of an FFR acting as a fomite. Secondary bacterial infections are a major factor in mortality rates of influenza pandemics; thus, protecting individuals from viruses and bacteria (eg, during influenza pandemics) is important. Bacteria are typically more robust than viruses, so research focusing on bacteria should suggest ways to lower the chance that an FFR will act as a fomite.
For a pandemic lasting 42 days, the Centers for Disease Control and Prevention (CDC) estimate that US health care workers will require more than 90 million FFRs, implying a supply shortage.3 Such shortages could also occur during and following a bioweapon attack. Smallpox (Variola major) and pneumonic plague (Yersinia pestis) are highly contagious agents considered offensive bioweapons. FFR shortages resulting from a biowarfare attack should be confined to a local area and shorter in duration than during an influenza pandemic. An emergency measure proposed to alleviate acute FFR shortages on any scale is decontamination, cleaning, and reuse.3 Experimental data assessing feasibility of this option is needed to guide regulatory and legal decisions. Heimbuch et al4 and Lore et al5 demonstrated 3 energetic decontamination methods—microwave-generated steam, low-temperature moist heat, and ultraviolet germicidal irradiation—that inactivate H1N1 and H5N1 influenza viruses without significantly affecting FFR fit or function.6,7 Other chemical and energetic methods have also shown promise for decontamination of FFRs,8–10 but we found no studies that addressed decontamination of bacterial agents on FFRs.
The US Food and Drug Administration (FDA) requires cleaning and sterilization of reprocessed medical devices and demonstration of their functional performance,11 but no reported data describe efficacy and compatibility of cleaning methods with FFRs. Sterilization and functional performance are relatively easy to assess; cleaning is harder to measure and no criteria are defined for “cleaned.” The Medical Device User Fee and Modernization Act (MDUFMA) regards the common definition of a clean device—no visual contamination present—insufficient and requires that an objective, measurable endpoint be specified.11 MDUFMA specifies no cleaning requirements for contaminants (eg, protein, microbe, and chemical), but requires that the reprocessor establish cleaning endpoints and the rationale for their selection. MDUFMA’s only reference to a quantifiable value—sterilization following cleaning must achieve a sterility assurance level of 10−6—may not apply to FFRs (non-sterile devices), leaving the criteria for both cleaning and disinfection to be defined.
FDA labels National Institute for Occupational Safety and Health (NIOSH)-approved surgical N95 respirators as single-use items, and no data have been reported from efforts to clean them. FFRs are porous, and therefore typically harder to clean than solid surfaces. Damage caused by cleaning is also a significant concern. Traditional methods to clean elastomeric respirators include washing with soap and treatment with disinfectants and disinfecting wipes.12,13 Literature provided by respirator manufacturers clearly states that cleaning procedures should not be used on the filtering element and doing so disqualifies them as the FFR is the filtering element. New FFR cleaning methods are needed that are simple to perform, effectively remove the soil load, do not degrade the level of protection, require short regeneration times, and do not impart toxic residues. Long regeneration times eliminate methods that extensively wet the FFR. Soap washes and alcoholic solutions are also eliminated because they degrade FFR performance.9 We chose to evaluate 3 wipe-based products as a readily available, inexpensive, and presumably nonaggressive cleaning technique with short FFR regeneration times.
This study was an off-label use of both the FFRs and the wipes, and the results are only an exploration of the concept of reuse. Neither endorsement nor censure of any products tested nor of the concept of cleaning and reusing FFRs is implied. We examined physical removal of deposited contaminants; measurements of disinfection were included because 2 wipe products include antimicrobial agents. Because bacteria typically tolerate environmental challenges better than viruses, we expect behavior of the bacteria tested to represent or underestimate sensitivity of a virus under similar conditions. This remains to be verified by additional testing.
MATERIALS AND METHODS
Contamination
Two challenge aerosols were applied to FFRs in separate tests, per American Society for Testing and Materials method 2721-10.14 Staphylococcus aureus (ATCC 6538) was inoculated onto a trypticase soy agar plate and incubated overnight at 37°C. A swab of cells from the plate inoculated 50 mL trypticase soy broth in a 250-mL flask. The flask was incubated for ~18 hours at 37°C at 220 rpm. After incubation, the stock was removed from the incubator and diluted 1:2,000 in an artificial saliva buffer.14
Cleaning studies
Three NIOSH-approved N95 respirators cleared as medical devices by FDA were selected for this study (Table 1). All 3 models are commonly used in US hospitals. Wipe products selected for this study were 504/07065 Respirator Cleaning Wipes (3M Company, St Paul, MN),15 which contain benzalkonium chloride (BAC); Hype-Wipes (Current Technologies, Inc, Crawfordsville, IN),16 which contain 0.9% hypochlorite (OCL); and Pampers wipes (Proctor & Gamble, Cincinnati, OH),17 which contain no active antimicrobial ingredients (ie, inert). BAC and other quaternary ammonium disinfectants commonly appear in wipe products; the examples chosen are labeled for use on respirators. OCL was shown to decontaminate FFRs without significantly degrading performance, but created odor and oxidation problems.8,9 The OCL wipe was included to measure the ability of a limited application (wiping vs immersion) to remove contaminants and minimize incompatibilities with FFRs. Alcohol- and soap-based wipe products were avoided because they are known to decrease FFR performance.9
Table 1.
Filtering facepiece respirator (FFR) components evaluated
| Code | Manufacturer | Model | Shape | Components tested | |
|---|---|---|---|---|---|
| FFR A | 3M* | 1860S | Cup | Internal | Fabric Nose pad |
| External | Fabric | ||||
| FFR B | 3M* | 1870 | Flat-fold | Internal | Fabric Nose pad |
| External | Fabric | ||||
| FFR C | Kimberly-Clark† | PFR | Duck bill | Internal | Fabric Perforated edge strip |
| External | Fabric Perforated edge strip |
||||
The 3M Company, St Paul, MN.
Kimberly-Clark Corporation, Irving, TX.
Each FFR is comprised of different materials for which cleaning efficiencies vary (Table 1). S aureus was applied to both interior and exterior FFR surfaces (in separate experiments) to provide sufficient sensitivity for reliable analysis. Mucin was applied as a heavy loading (~1 mg/cm2) only to exterior surfaces. FFR A was used as received. Only the flat front panel of FFR B and only 1 of the side panels (not containing the metal nose clip) of FFR C were used. No straps or metal nose clips were evaluated. For each independent test, 5 FFRs were loaded—3 cleaned as described below and 2 used to quantify the challenge. Two independent tests were performed for each condition, hence n = 6 for each FFR-wipe combination. After loading, FFRs were incubated at ~22°C for 30 minutes to clear aerosols from the test chamber. Each of the 3 test FFRs was wiped 3 times in turn with 4 faces of a fresh wipe product folded over twice. Total cleaning time per FFR was ~30 seconds; to ensure relatively constant wiping pressure and cleaning technique throughout the study, 1 technician cleaned all FFRs.
After cleaning (or set time for uncleaned samples), FFRs were incubated 15 minutes at room temperature before quantification of contaminants. A 38-mm round—hole punch (McMaster-Carr, Robbinsville, NJ), was used to cut 4 coupons from the external (to the wearer) surfaces of FFRs A and B, and 3 from the (internal) surfaces that would be exposed to the wearer’s respiratory secretions; the nose cushion was removed and evaluated as a fourth sample. Three 38-mm coupons each were cut from internal and external fabrics of FFR C; a fourth sample was the perforated edge strip of the FFR. For mucin testing, each coupon was placed in a 50-mL centrifuge tube containing 10 mL sterile water and extracted for 10 minutes using a vortex mixer. A QuantiPro protein assay kit (Sigma, St Louis, MO) determined mucin recovery. For S aureus testing, the same extraction procedure was executed in 10 mL extraction buffer (1 M glycine, 0.1% Tween 80 in 1X phosphate-buffered saline). The extract was plated on trypticase soy agar using a Whitley Automatic Spiral Plater (Microbiology International, Waltham, MA). Plates were incubated at 37°C for ~18 hours. After incubation, colony-forming units (CFUs) on the plates were enumerated using a Protocol Colony Counter (Microbiology International, Waltham, MA).
Filter performance after 3 cleaning cycles was evaluated for intact triplicate samples of each FFR model. For all thrice-cleaned FFR samples, a model 8130 automated filter tester (TSI Inc, Shoreview, MN) measured initial percent filter penetration by a polydisperse, solid aerosol of sodium chloride—count median diameter 0.075 ± 0.020 μm, geometric standard deviation <1.86 and mass median aerodynamic diameter ~300 nm—that meets particle size distribution criteria in 42 CFR 84 Subpart K, Section 84.18118 for NIOSH certification. All tests were conducted with a continuous airflow of 85 ± 4 L/minute. Particle penetration through N95 FFRs was determined using a transparent plastic box (20 cm × 20 cm × 10 cm) placed between the filter chucks (sample holding flange mechanism on the automated filter tester). At the center of the box’s removable top and bottom transparent plastic plates (20 cm × 20 cm) was a circular hole (25 cm2). The N95 FFR was placed on the bottom plate with the concave side facing the hole and sealed in place with melted beeswax.
Data analysis
Data were analyzed using conventional statistical tools in Prism 5 software (Graph Pad, La Jolla, CA). The S aureus and mucin cleaning efficiencies of similar components were compared using an unpaired, 2-tailed t-test at the 95% confidence interval. Filtration performances of wipes were compared using a 1-way analysis of variance or ANOVA for each FFR model.
RESULTS
The mean loading concentration of mucin on FFR samples was ~1 mg/cm2. No mucin was detected in replicates using the OCL wipes, which we attribute to interference of hypochlorite with the protein assay, either directly or by reacting with the mucin. The removal efficiency (RE) of mucin by BAC and inert wipes ranged from 21.47%–76.41% (Table 2). Poorest REs were found using the BAC wipes on FFR C—respective REs for the external fabric and perforated strip were 21.47% and 25.41%. The inert wipe removed mucin more effectively than the BAC wipe, up to 76.41%, but removed only 38.87% from FFR C’s edge strip.
Table 2.
Cleaning of filtering facemask respirators (FFRs) contaminated with mucin
| Wipe product | FFR* and component | Mean reduction | |
|---|---|---|---|
| BAC | FFR A exterior | 53.64% ± 8.62% | |
| 3M 504/07065 Respirator Cleaning Wipe† | FFR B exterior | 43.88% ± 6.30% | |
| FFR C exterior | Edge strip | 25.41% ± 7.06% | |
| Fabric | 21.47% ± 7.87% | ||
| Inert | FFR A exterior | 76.41% ± 6.92% | |
| Pampers wipe‡ | FFR B exterior | 66.96% ± 2.68% | |
| FFR C exterior | Edge strip | 38.87% ± 10.0% | |
| Fabric | 61.94% ± 8.93% | ||
BAC, benzalkonium chloride; Inert, no active antimicrobial ingredients.
FFR A, 3M 1860S (3M Company, St Paul, MN), FFR B, 3M 1870 (3M Company, St Paul, MN), and FFR C, KC PFR (Kimberly-Clark Corporation, Irving, TX).
3M Company, St Paul, MN.
Proctor & Gamble, Cincinnati, OH.
Reduction in viable S aureus varied among wipe—FFR component pairs (Table 3). The mean loading concentration of S aureus on FFR samples was 6.72 × 105 CFU/cm2. The inert wipes captured 81.56%–96.53% of S aureus from the base fabrics of all FFR models tested. REs were low for the exterior surface of perforated edge strips from FFR C (59.37%), and FFR B’s nose pad (69.28%). OCL wipes reduced viability below the detection limit (>5-log attenuation) for 7 of 10 samples among the 3 FFR models. Two remaining samples (interior fabrics of FFRs B and C) lost >4 logs in viability, the last sample (nose pad of FFR B) showing the smallest decrease (98.98%) of the sample set. BAC wipes produced 2 samples below the detection limit (interior surface of perforated edge strip from FFR C, interior fabric of FFR B); 5 other samples showed 3–5 log reductions in viability. Attenuation on FFR B’s nose pad again was the least (68.92%) of the sample set.
Table 3.
Cleaning/disinfection of filtering facepiece respirators (FFR) contaminated with Staphylococcus aureus
| Wipe product | FFR* | Component | Mean reduction | |
|---|---|---|---|---|
| Inert | FFR A | Exterior | 95.80% ± 0.70% | |
| Pampers wipe† | Interior | Nose pad | 90.95% ± 1.51% | |
| Fabric | 90.01% ± 1.24% | |||
| FFR B | Exterior | 94.70% ± 1.72% | ||
| Interior | Nose pad | 69.28% ± 11.10% | ||
| Fabric | 92.34% ± 4.13% | |||
| FFR C | Exterior | Edge strip | 59.37% ± 8.61% | |
| Fabric | 81.56% ± 4.91% | |||
| Interior | Edge Strip | 85.24% ± 4.81% | ||
| Fabric | 96.53% ± 1.40% | |||
| BAC | FFR A | Exterior | 99.72% ± 0.32% | |
| 3M 504/07065 Respirator Cleaning Wipe‡ | Interior | Nose pad | 98.60% ± 0.78% | |
| FFR fabric | 95.37% ± 4.25% | |||
| FFR B | Exterior | 99.96% ± 0.04% | ||
| Interior | Nose pad | 68.92% ± 13.10% | ||
| Fabric | >99.999% | |||
| FFR C | Exterior | Edge strip | 99.994% ± 0.002% | |
| Fabric | 99.998% ± 0.005% | |||
| Interior | Edge strip | >99.999% | ||
| Fabric | 99.845% ± 0.060% | |||
| OCL | FFR A | Exterior | >99.999% | |
| Hype-Wipe§ | Interior | Nose pad | >99.999% | |
| Fabric | >99.999% | |||
| FFR B | Exterior | >99.999% | ||
| Interior | Nose pad | 98.98% ± 0.17% | ||
| Fabric | 99.997% ± 0.002% | |||
| FFR C | Exterior | Edge strip | >99.999% | |
| Fabric | >99.999% | |||
| Interior | Edge strip | >99.999% | ||
| Fabric | 99.998% ± 0.001% | |||
Inert, no active antimicrobial ingredients; BAC, benzalkonium chloride; OCL, 0.9% hypochlorite.
FFR A, 3M 1860S (3M Company, St Paul, MN), FFR B, 3M 1870 (3M Company, St Paul, MN), and FFR C, KC PFR (Kimberly-Clark Corporation, Irving, TX).
Proctor & Gamble, Cincinnati, OH.
3M Company, St Paul, MN.
Hype Wipe, Current Technologies, Inc, Crawfordsville, IN.
Mean particle penetration of each thrice-cleaned FFR model (Fig 1) was <5%, NIOSH’s N95 certification criterion. For all 3 FFR models tested, BAC wipes caused more penetration than the other wipes; for FFRs A and B, this difference was significant (P < .05). Of the models tested, FFR C showed the greatest penetration—1 replicate exceeded the 5% threshold (5.6%) after cleaning with a BAC wipe—and the differences were not significant.
Fig 1.
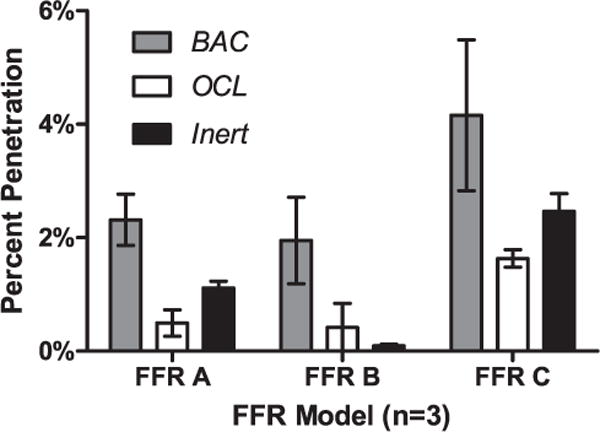
Particle penetration of filtering facepiece respirators (FFRs) following cleaning 3 times with wipe products. FFR A 1860S (3M Company, St Paul, MN), FFR B 3M 1870 (3M Company, St Paul, MN), and FFR C KC PFR (Kimberly-Clark Corporation, Irving, TX). BAC, benzalkonium chloride (3M 504/07065 Respirator Cleaning Wipe, 3M Company, St Paul, MN); OCL, 0.9% hypochlorite (Hype-Wipe, Current Technology Inc, Crawfordsville, IN); Inert, no active antimicrobial ingredients (Pampers Wipe, Proctor & Gamble, Cincinnati, OH).
DISCUSSION
FFR decontamination and reuse is a controversial strategy proposed to mitigate an acute FFR shortage during a medical crisis such as pandemic influenza. For single-use FFRs, this study explores options for cleaning, a step required by the FDA for reusable medical devices, a category that includes FFRs reused by medical personnel. We report the efficacy of commercially available, low-cost methods that might be used to clean FFRs during a critical supply shortage, and the effect of these methods on FFR performance. The study is exploratory and intended to prompt future investigation. Because both the FFRs and the wipe products are being examined outside their intended contexts of application, our results and conclusions are purely informational and are not to be taken as product evaluations or recommendations about reuse.
The inert wipes removed contaminants only physically, providing a baseline value of removal efficiency of contaminants from FFRs using a simple wiping technique—1-log removal of mucin. The heavy loading (~1 mg/cm2) used to enhance sensitivity of analysis might have raised this value slightly. Protein from human breath condensate accumulates at ~0.34 μg/minute breathing time.19 If one assumes a constant 8-hour wear time, the upper limit of protein contamination inside the FFR is 163 μg. Except in a direct contamination event (eg, sneezing and coughing), exterior FFR loading will be much less, but no precedent exists. In the context of cleaning, decontamination, and reuse of FFRs, removal of nonviable protein must be secondary to disinfection and should be balanced against decontamination and removal of infectious agents. This discussion should not be extended to applications reusing other devices because of factors not discussed herein; for example, allergens and endotoxins may cause adverse health reactions.
Inert wipes removed S aureus slightly more efficiently than mucin—1 log of S aureus from all FFR fabrics except the exterior of FFR C (81.56%)—but the results were statistically significant (all Ps < .0018). Nose pads and perforated strip of FFR C were cleaned less effectively, as expected due to their material properties and roughness. One-log removal left ~1.5 × 103 CFU/cm2 on the FFR. In operational use, the interior (wearer’s side) of FFRs will likely experience loading concentrations used in this study, so the observed endpoint is a realistic estimate. In most real-world scenarios, external concentration will be lower; exterior loading of FFRs worn for 20 minutes in hospital rooms after discharge was 3–30 CFU/cm2 (Heimbuch et al, unpublished data, 2013). Loading concentrations will increase with wear time, and FFRs worn under different operational conditions may experience different loading concentrations. The reuse scenario assumes that device users will reuse only their own devices and thus be exposed to only their own flora, and this study presupposed a decontamination step following the cleaning step. Based on FFR usage in a hospital setting (Heimbuch et al, unpublished data, 2013) residues on cleaned external surfaces would be <30 CFU/cm2. Because FFRs are not sterile devices and bacteria tend not to reaerosolize from fibers, this endpoint might be acceptable; however, user risk imposed by this level of contamination must be evaluated.
As a direct remedy, OCL and BAC wipes contain an antimicrobial agent that augments physical removal (cleaning) with a disinfection (kill) mechanism to reduce viable counts of S aureus. Hypochlorite in OCL wipes produced below detection limit values (>99.99% attenuation) for 7 of 10 surfaces (Table 3). OCL wipes effectively disinfected the perforated edge strip of FFR C and the nose pad of FFR A, on which inert wipes were only marginally effective. Hypochlorite solution was likely absorbed by FFR C’s edge strips and FFR A’s nose pad, providing greater exposure. The polyurethane nose pad20 of FFR B showed the least decontamination by OCL (98.98%); if physical cleaning caused ~60% of net removal, OCL contributed only 39% to reduction of S aureus counts on the nose pad. Amide groups of polyurethanes compete for hypochlorite, decreasing availability for surface decontamination and creating a chloramide that may act as a weaker disinfectant.
BAC wipes decontaminated less effectively than OCL wipes, giving below detection limit results for only 4 of 10 samples—edge strips and exterior fabric of FFR C, and the exterior fabric of FFR B. Deposition of BAC on the rough surfaces likely aided disinfection of the edge strip. FFR A’s nose pad, cleaned below the detection limit by OCL wipes, lost only 98.6% of viability when cleaned by the BAC wipe, suggesting that its primary mechanism to reduce viable S aureus on this surface is physical removal and that the urethane-derived chloramide is active, possibly as the actual disinfectant. Overall, BAC wipes disinfected FFR A less effectively than the other FFR models, which we presume indicates incompatibility of BAC with the device’s material properties. Material properties of the internal and external fabrics of FFRs B and C differ, as did their cleaning efficiencies. Physical removal of contaminants by inert wipes was fairly constant on these surfaces, supporting the idea of material incompatibility with BAC. Less mucin was removed by BAC wipes than by inert wipes, but both followed a similar trend. The BAC wipes’ cleaning efficiency of the external fabric from FFR C was notably poor (21.47%); roughly half the cleaning achieved on similar samples from the other 2 FFR models. FFR C’s external surface was also cleaned at lowest efficiency by the inert wipe—but by only ~15%. It appears that material properties of the external surface of FFR C are less receptive than the other 2 models to cleaning methods of this study. All 3 are surgical FFRs with fluid-resistant exterior surfaces, but properties of the fluid-resistant coatings may not be identical and can, in principle, be designed to influence cleanability.
Physical degradation of FFRs following cleaning appear to be negligible. No degradation or blemishing was observed of filtration media, nose pads, or nose clips. Measurements of particle penetration through FFRs following cleaning support a conclusion that physical damage caused by cleaning and abrasion was not problematic. The BAC wipe caused 1 FFR C to exceed 5% penetration and caused statistically greater penetration through FFRs A and B than did the other 2 wipes. The increase in penetration is attributed to the antimicrobial/cleaning solution, which includes BAC and Tween. Tween is also present in the inert wipe; both products increased penetration through FFR C. BAC is a quaternary ammonium compound, and likely to interact with the charged surface of the electret medium and contribute to the decay in filtration performance observed for all 3 FFR models. Tween, a nonionic detergent, could also affect performance of electret media—some detergents have been shown to degrade the performance of FFRs.9 The similarity of particle penetration values for the 3 masks tested after cleaning with BAC and inert wipes (Fig 1) suggests dependence of the effect (presumably the availability) of the detergent on characteristics of the facing material.
CONCLUSIONS
Our study—a preliminary evaluation of FFR cleanability using available technologies—focused on mucin and included S aureus, but we consider the data generally applicable to all microbial agents. An airborne respiratory pathogen (eg, influenza virus) would be coated in mucin and thus expected to behave similarly to the mucin protein. Feasibility of the concept of reuse has been reinforced here, but more studies are needed before such a practice can be approved or recommended.
Several experimental factors limit the overall applicability of the data. However, the data we present broaden the body of work on decontamination and reuse of FFRs and invite some measure of optimism. FFRs tested withstood significant physical handling and abrasion, and physical removal of both S aureus and mucin was demonstrated. The significance of 1-log reduction in contamination and availability of residual contaminants below FFR surfaces is unclear. FFRs are not sterile devices, so levels of cleaning achieved should be put in context with loading concentrations observed during field use of FFRs.20 The FFRs were successfully disinfected by wipes that contain antimicrobial agents, against atypically highly concentrated challenges needed to permit measurement of 5-log reductions. A growing body of positive results encourages optimism that such a strategy can be practical for extending wear periods.
Both BAC and OCL displayed liabilities that limit their prospects for this application. BAC caused partial disinfection, but also degradation of filtration performance, which will exceed 5% penetration after only 2 or 3 cleaning cycles. Immersion of FFRs in 10% household bleach affected FFR performance only minimally, but blemished the FFRs, oxidized metal parts, and imparted an odor.8,9 Selective topical application of more-dilute hypochlorite in a wipe greatly ameliorated oxidative damage. Wearers did not evaluate odor, but OCL wipes might serve as a 1-step remedy. A different detergent might perform more satisfactorily in practice, as might a more-repellent FFR surface.
These results will augment the ongoing process of developing a next generation of respiratory protection products.21 Multiuse FFRs are not currently marketed, but there is no regulatory impediment to developing such a device.22 A reusable FFR and its cleaning process would require NIOSH certification and FDA clearance. Cleaning and disinfection will be required according to MDUFMA, and the data in this study provide insight into design considerations for such a device. Materials used in nose pads of both 3M FFRs are incompatible with hypochlorite. This trend might not extend to other disinfectants and is another matter for further investigation. Surface roughness clearly lowered cleaning efficiency, as shown by ineffective cleaning of FFR C’s edge strip by inert wipes. However, this texture promoted concentration of antimicrobial agents, which locally enhanced disinfection. Successful development of a reusable FFR will require judicious selection of material properties and a design that allows for concurrent development of an effective cleaning and disinfection strategy. Selection of the antimicrobial agent must also be compatible with electret media and other respirator surfaces. We focused on the FFR material and nose pads; elastic straps are a subject for future studies.
The CDC, NIOSH, FDA, and Department of Defense have not recommended FFR decontamination and reuse because the practice is inconsistent with established regulations. NIOSH respirator certification regulations include no provisions for decontamination,18 so reusing FFRs in this manner will void their NIOSH approval.
Acknowledgments
The findings and conclusions of this article are those of the authors and do not necessarily reflect the views of NIOSH or FDA. Mention of any company name or product does not constitute endorsement by NIOSH and the mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
This work was funded by the Food and Drug Administration Centers for Devices and Radiologic Health through an interagency agreement with the Air Force Research Laboratory.
Footnotes
Conflicts of interest: None to report.
References
- 1.Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. doi: 10.1016/j.ajic.2007.10.007. Available from: http://www.cdc.gov/hicpac/2007IP/2007isolationPrecautions.html. Accessed November 1, 2013. [DOI] [PMC free article] [PubMed]
- 2.Occupational Safety and Health Administration. Pandemic influenza preparedness and response guidance for healthcare workers and healthcare employers. Available from: https://www.osha.gov/Publications/3328-05-2007-English.html. Accessed November 1, 2013.
- 3.Bailar JC, Burke DS, Brosseau LM, Cohen HJ, Gallagher EJ, et al. Reusability of facemasks during an influenza pandemic. Washington [DC]: Institute of Medicine, National Academies Press; 2006. [Google Scholar]
- 4.Heimbuch BK, Wallace WH, Kinney K, Lumley AE, Wu C-Y, Woo MH, et al. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2010;38:3–8. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Lore MB, Heimbuch BK, Brown TL, Wander JD, Hinrichs SH. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 6.Bergman MS, Viscusi DJ, Heimbuch BK, Wander JD, Sambol AR, Shaffer RE. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J Engineered Fiber Fabric. 2010;5:33–41. [Google Scholar]
- 7.Viscusi AJ, Bergman MS, Novak DA, Faulkner KA, Palmiero AJ, Powell J, et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, smell, comfort, and donning ease. J Occup Environ Hyg. 2011;8:426–36. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 8.Salter W, Kinney K, Wallace W, Lumley L, Heimbuch BK, Wander JD. Analysis of residual chemical on filtering facepiece respirators after decontamination. J Occup Environ Hyg. 2010;7:437–45. doi: 10.1080/15459624.2010.484794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viscusi DJ, King WP, Shaffer RE. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Resp Prot. 2007;24:93–107. [Google Scholar]
- 10.Viscusi DJ, Bergman MS, Eimer BC, Shaffer RE. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–27. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medical Device User Fee and Modernization Act of 2002. Pub Law 107–250. Available from: http://olpa.od.nih.gov/legislation/107/publiclaws/meddvcfeemdrn.asp. Accessed November 1, 2013.
- 12.National Institute for Occupational Safety and Health, Respirator and Cleaning Maintenance Guidance. Available from: http://www.cdc.gov/niosh/npptl/cleaning.html, Accessed May 25, 2010.
- 13.Appendix B-2 to § 1910.134. Respirator cleaning procedures (mandatory) Available from: http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=9782&p_table=STANDARDS. Accessed May 25, 2010.
- 14.ASTM E2721–10: Standard test method for evaluating of the effectiveness of decontamination procedures for surfaces when challenged with droplets containing human pathogenic viruses. West Conshohocken [PA]: American Society for Testing and Materials International; 2010. [Google Scholar]
- 15.504 Respirator Cleaning Wipe description. Available from: http://solutions.3m.com/wps/portal/3M/en_US/3M-PPE-Safety-Solutions/Personal-Protective-Equipment/Products/Product-Catalog/~/3M-Respirator-Cleaning-Wipe-504-07065-AAD-Respiratory-Protection-System-Component-500-EA-Case?N=4294930766+5011378&&Nr=AND(hrcy_id%3AGSS0T2RDG4gs_MN4QBZ4GJN_N2RL3FHWVK_GPD0K8BC31gv&rt=d). Accessed May 25, 2010.
- 16.Materials safety data sheet. Hype-Wipe (Disinfecting Towel with Bleach) Available from: http://www.daigger.com/store/hype-wipe-disinfecting-bleach-towelettes8482a/14255?section=0. Accessed May 25, 2010.
- 17.Material Safety Data Sheet # PGMSDS BC-07. Pampers Unscented Natural Aloe Wipes. Available from: http://www.setonresourcecenter.com/msdshazcom/htdocs//MSDS/Retail/P/Pampers%20Natural%20Aloe%20Unscented%20Wipes.pdf. Accessed November 3, 2013.
- 18.Approval of Respiratory Protective Devices. Title 42, CFR, Part 84. Available from: http://www.gpo.gov/fdsys/pkg/CFR-2004-title42-vol1/xml/CFR-2004-title42-vol1-part84.xml. Accessed November 3, 2013.
- 19.Bloemen K, Lissens G, Desager K, Schoeters G. Determinants of variability of protein content, volume and pH of exhaled breath condensate. Respir Med. 2007;101:1331–7. doi: 10.1016/j.rmed.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 20.3M Health Care Particulate Respirator and Surgical Mask 1870, 1870+ Available from: http://solutions.3m.com/wps/portal/3M/en_US/3M-PPE-Safety-Solutions/Personal-Protective-Equipment/Products/Product-Catalog/~/3M-Health-Care-Particulate-Respirator-and-Surgical-Mask-1870-120-case?N=4294930053+5011378&&Nr=AND(hrcy_id%3AQG9NNN488Ggs_BP0KPGHZ89_N2RL3FHWVK_GPD0K8BC31gv)&rt=d. Accessed November 3, 2013.
- 21.Radonovich L. Better respiratory protection equipment using advanced technologies for healthcare employees. 2011 Available from: http://www.publichealth.va.gov/docs/cohic/project-breathe-report-2009.pdf. Accessed December 14, 2011.
- 22.Heimbuch BK, Harnish D. Discussions on short-term and long-term solutions to mitigate a shortage of filtering facepiece respirators caused by pandemic influenza, final report from interagency meeting, food and drug administration-centers for devices and radiologic health. Available from: http://www.ara.com/Capabilities/docs/publications/FFR-Shortages06202011.pdf. Accessed November 27, 2013.


