Abstract
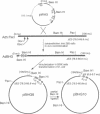
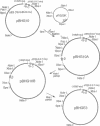
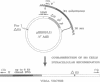
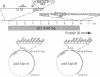
Human adenoviruses (Ads) are attracting considerable attention because of their potential utility for gene transfer and gene therapy, for development of live viral vectored vaccines, and for protein expression in mammalian cells. Engineering Ad vectors for these applications requires a variety of reagents in the form of Ads and bacterial plasmids containing viral DNA sequences and requires different strategies for construction of vectors for different purposes. To simplify Ad vector construction and develop a procedure with maximum flexibility, efficiency, and cloning capacity, we have developed a vector system based on use of Ad5 DNA sequences cloned in bacterial plasmids. Expanded deletions in early region 1 (3180 bp) and early region 3 (2690 or 3132 bp) can be combined in a single vector that should have a capacity for inserts of up to 8.3 kb, enough to accommodate the majority of cDNAs encoding proteins with regulatory elements. Genes can be inserted into either early region 1 or 3 or both and mutations or deletions can be readily introduced elsewhere in the viral genome. To illustrate the flexibility of the system, we have introduced a wild-type early region 3 into the vectors, and to illustrate the high capacity for inserts, we have isolated a vector with two genes totaling 7.8 kb.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss L. E., Vales L. D. Promoter of the adenovirus polypeptide IX gene: similarity to E1B and inactivation by substitution of the simian virus 40 TATA element. J Virol. 1991 Feb;65(2):598–605. doi: 10.1128/jvi.65.2.598-605.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D. S., Graham F. L. Insertional mutagenesis using a synthetic lac operator. Gene. 1989 Oct 30;82(2):201–208. doi: 10.1016/0378-1119(89)90045-0. [DOI] [PubMed] [Google Scholar]
- Bautista D. S., Hitt M., McGrory J., Graham F. L. Isolation and characterization of insertion mutants in E1A of adenovirus type 5. Virology. 1991 Jun;182(2):578–596. doi: 10.1016/0042-6822(91)90599-7. [DOI] [PubMed] [Google Scholar]
- Berkner K. L. Expression of heterologous sequences in adenoviral vectors. Curr Top Microbiol Immunol. 1992;158:39–66. doi: 10.1007/978-3-642-75608-5_3. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett A. J., Prevec L., Graham F. L. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993 Oct;67(10):5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Daniell E. Genome structure of incomplete particles of adenovirus. J Virol. 1976 Aug;19(2):685–708. doi: 10.1128/jvi.19.2.685-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Choudhury G., Haj-Ahmad Y., Brinkley P., Rudy J., Graham F. L. Human adenovirus cloning vectors based on infectious bacterial plasmids. Gene. 1986;50(1-3):161–171. doi: 10.1016/0378-1119(86)90321-5. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury G., Haj-Ahmad Y., Graham F. L. Protein IX, a minor component of the human adenovirus capsid, is essential for the packaging of full length genomes. EMBO J. 1987 Jun;6(6):1733–1739. doi: 10.1002/j.1460-2075.1987.tb02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L. Covalently closed circles of human adenovirus DNA are infectious. EMBO J. 1984 Dec 1;3(12):2917–2922. doi: 10.1002/j.1460-2075.1984.tb02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gräble M., Hearing P. Adenovirus type 5 packaging domain is composed of a repeated element that is functionally redundant. J Virol. 1990 May;64(5):2047–2056. doi: 10.1128/jvi.64.5.2047-2056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräble M., Hearing P. cis and trans requirements for the selective packaging of adenovirus type 5 DNA. J Virol. 1992 Feb;66(2):723–731. doi: 10.1128/jvi.66.2.723-731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Ahmad Y., Graham F. L. Development of a helper-independent human adenovirus vector and its use in the transfer of the herpes simplex virus thymidine kinase gene. J Virol. 1986 Jan;57(1):267–274. doi: 10.1128/jvi.57.1.267-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell. 1980 Jul;20(3):787–795. doi: 10.1016/0092-8674(80)90325-6. [DOI] [PubMed] [Google Scholar]
- Hearing P., Samulski R. J., Wishart W. L., Shenk T. Identification of a repeated sequence element required for efficient encapsidation of the adenovirus type 5 chromosome. J Virol. 1987 Aug;61(8):2555–2558. doi: 10.1128/jvi.61.8.2555-2558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A enhancer contains two functionally distinct domains: one is specific for E1A and the other modulates all early units in cis. Cell. 1986 Apr 25;45(2):229–236. doi: 10.1016/0092-8674(86)90387-9. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Ghosh-Choudhury G., Smiley J. R., Fallis L., Graham F. L. Abundant expression of herpes simplex virus glycoprotein gB using an adenovirus vector. Virology. 1988 May;164(1):1–14. doi: 10.1016/0042-6822(88)90613-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Leach D. R., Stahl F. W. Viability of lambda phages carrying a perfect palindrome in the absence of recombination nucleases. 1983 Sep 29-Oct 5Nature. 305(5933):448–451. doi: 10.1038/305448a0. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. In vivo consequences of plasmid topology. Nature. 1981 Jul 23;292(5821):380–382. doi: 10.1038/292380a0. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- McKinnon R. D., Bacchetti S., Graham F. L. Tn5 mutagenesis of the transforming genes of human adenovirus type 5. Gene. 1982 Jul-Aug;19(1):33–42. doi: 10.1016/0378-1119(82)90186-x. [DOI] [PubMed] [Google Scholar]
- Mittal S. K., McDermott M. R., Johnson D. C., Prevec L., Graham F. L. Monitoring foreign gene expression by a human adenovirus-based vector using the firefly luciferase gene as a reporter. Virus Res. 1993 Apr;28(1):67–90. doi: 10.1016/0168-1702(93)90090-a. [DOI] [PubMed] [Google Scholar]
- Morsy M. A., Alford E. L., Bett A., Graham F. L., Caskey C. T. Efficient adenoviral-mediated ornithine transcarbamylase expression in deficient mouse and human hepatocytes. J Clin Invest. 1993 Sep;92(3):1580–1586. doi: 10.1172/JCI116739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz P. L., Young C. S. Polarity in adenovirus recombination. Virology. 1984 Jun;135(2):503–514. doi: 10.1016/0042-6822(84)90204-6. [DOI] [PubMed] [Google Scholar]
- Ruben M., Bacchetti S., Graham F. Covalently closed circles of adenovirus 5 DNA. Nature. 1983 Jan 13;301(5896):172–174. doi: 10.1038/301172a0. [DOI] [PubMed] [Google Scholar]
- Tibbetts C. Viral DNA sequences from incomplete particles of human adenovirus type 7. Cell. 1977 Sep;12(1):243–249. doi: 10.1016/0092-8674(77)90202-1. [DOI] [PubMed] [Google Scholar]