Abstract
Research regarding musculoskeletal injury risk has focused primarily on anatomical, neuromuscular, hormonal, and environmental risk factors; however, subsequent injury risk screening and intervention programs have been largely limited to neuromuscular factors and have faced challenges in both implementation and efficacy. Recent studies indicate that poor neurocognitive performance, either at baseline or in the aftermath of a concussion, is associated with elevated risk of musculoskeletal injury. Despite the relatively limited current understanding regarding the nature of the relationship between different aspects of neurocognitive performance and musculoskeletal injury risk, this is a promising area of research that may yield significant advances in musculoskeletal injury risk stratification, rehabilitation, and prevention.
Introduction
Participation in athletics has grown significantly over the past few decades. These increases have been observed throughout the lifespan (42,48), across gender (48), and across different categories of able-bodiedness (12). These changes in the rate of participation have given rise to a concomitant increase in the number of musculoskeletal injuries (2,16,29). Due to the significant physical, psychological, and economic costs that can be associated with these injuries (21,40), it is important to determine the full scope of risk factors associated with musculoskeletal injury to develop injury risk prevention and rehabilitation strategies.
Perhaps the best example is that of anterior cruciate ligament (ACL) injury, for which investigations have identified numerous possible risk factors among several different categories. These include anatomical (e g., femoral intercondyle notch width) (43), neuromuscular (e g., altered biomechanics during high-risk athletic tasks) (14), hormonal (e g., cyclical changes in joint laxity) (8), and environmental (e g., playing surface type and condition, level of competition) (4) risk factors. The identification of these risk factors has subsequently led to the development of several injury risk stratification and prevention protocols (6,15).
Yet despite the promise of these protocols, injury rates remain high. Such injury prevention programs have primarily focused on identifying and correcting high-risk neuromuscular patterns. This is partly due to the demonstrated protective effects of neuromuscular-based interventions but is also due to the inherent limitations with other identified risk factors (such as hormonal and anatomical) for use in clinically feasible interventions. Unfortunately, the implementation of evidence-based neuromuscular interventions also may be limited by the time required and the level of protocol adherence needed for efficacy (33,34). These barriers to implementation may be ameliorated by the use of clinically feasible neuromuscular-based screening protocols to identify athletes at high risk for ACL injury (7); however, such tools have not yet solidly demonstrated efficacy in prospective studies (32).
In light of this current state, there is a need to reconsider the areas of focus for musculoskeletal injury risk identification and prevention. One understudied area that possesses strong potential to affect musculoskeletal injury risk is neurocognitive performance. Neurocognitive performance may influence musculoskeletal injury risk through a variety of mechanisms and is potentially modifiable. In this review, we discuss the emerging evidence demonstrating the importance of different aspects of neurocognitive performance on musculoskeletal injury risk and factors associated with elevated risk of injury.
A Definition of Neurocognitive Performance in the Context of Sports Performance
Prior to examining its role in injury risk, the term “neurocognitive performance” must first be defined in the context of sports performance. In its most general form, this would be inclusive of aspects such as language, intelligence, and social functioning, which may not be germane to injury risk. We will thus use a more limited working definition of the term “neurocognitive performance” as containing the following dimensions: visual attention, self-monitoring, agility/fine motor performance, processing speed/reaction time, and dual-tasking (Table).
Table.
Dimensions of neurocognitive performance in the sport performance context.
| Dimension | Working Definition |
|---|---|
| Visual attention | The ability to concentrate on visual input to the exclusion of other less essential stimuli |
| Self-monitoring | The ability to focus on proprioceptive/kinesthetic feedback |
| Agility/fine motor skill | The ability to make minor adjustments in motor activity |
| Processing speed/reaction time | The ability to engage in stimulus-response behavior within an intended time frame |
| Dual tasking | The ability to engage in two activities at the same time to maximize goal attainment |
These neurocognitive dimensions are likely highly intertwined with neuromuscular control, motor learning, and other aspects critical for the performance and safety of the athlete. Athletics demands initiating and maintaining appropriate performance of dynamic activities in a complicated and rapidly changing environment. The success of each action is contingent on voluntary and involuntary motor commands modulated by sensory processing, attention, and motor planning. Appropriate function in these neurocognitive dimensions would allow the athlete to successfully and safely accomplish motor tasks. Dimensions such as attention and processing speed help the athlete survey the playing environment for potential obstacles that might threaten their immediate goals. Quick reaction time and dual tasking allow the athlete to adjust to rapidly altering playing environments while simultaneously maximizing task performance. These responsive motor acts can then be completed as intended in a stable, coordinated fashion when aided by adept self-monitoring and fine motor skills.
Conversely, deficiencies in these neurocognitive dimensions may not allow the athlete to correctly interpret or react to an evolving playing environment. This may result in greater likelihood of engaging in motor acts that are at high risk for injury. Consider the scenario of an American Football wide receiver jumping for a catch during a pass play. The receiver is required to 1) maintain visual attention on the incoming ball, 2) monitor and adjust his body positioning (possibly in response to perturbations by a defender), 3) process and react to the evolving positioning of defenders to advance the ball, and 4) accomplish these tasks in quick overlapping time frames. Deficits in neurocognition may reduce the chance of a successful catch, place the athlete in high injury risk position during landing, and/or leave the athlete in a position vulnerable to receiving hits from defenders. This Figure provides an illustration of how neurocognitive performance may impact musculoskeletal injury risk.
Figure.
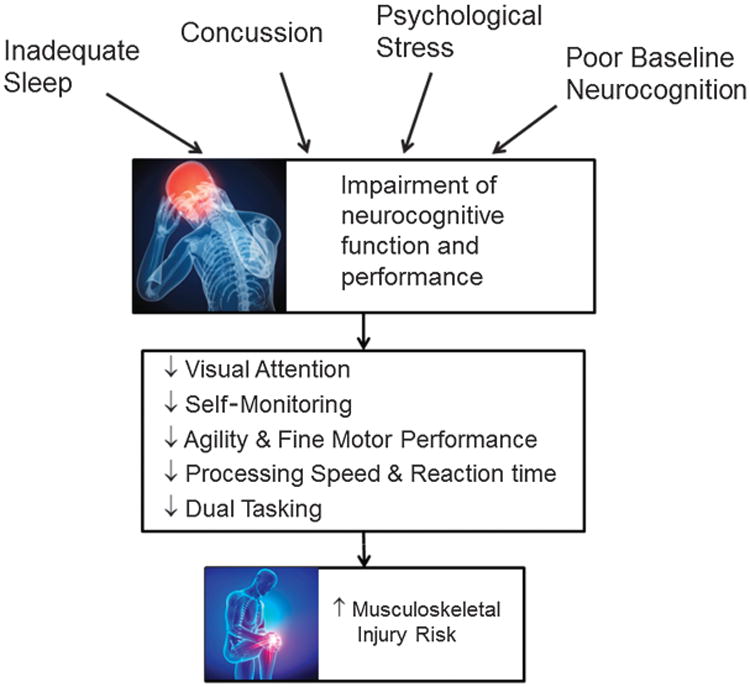
Proposed pathway by which perturbations to neuromuscular performance increase musculoskeletal injury risk.
Neurocognitive Performance and Musculoskeletal Injury Risk
Evidence supporting a relationship between neurocognitive performance and musculoskeletal injury risk can be found in the literature from both sports medicine and related fields from the past few decades.
Baseline Neurocognitive Performance
Support of this relationship may be observed in comparisons of injury rates between populations with different levels of baseline neurocognition. One population with generally lower levels of neurocognitive performance includes individuals with attention deficit hyperactivity disorder (ADHD). It is well-known that children with ADHD may demonstrate deficits in reaction time, fine and gross motor skills, and selected and sustained attention (20,22). Correspondingly, children with ADHD have been shown in multiple studies as having elevated risk for injuries ranging from fractures to sprains (17,27). Higher rates of positive results on screening tests for ADHD have been noted in patients with pediatric trauma compared with nontrauma patients (26). Patients with pediatric trauma with a diagnosis of ADHD also have greater severity of injury compared with patients without ADHD (27). Furthermore, the use of ADHD medications (in the absence of antidepression or other psychotropic medications) may be able to blunt this risk (41).
Given such evidence, it is reasonable to consider the effect of the normal spectrum of baseline neurocognition on injury risk. In a series of studies in the 1990s, Taimela et al. (36–38) analyzed psychometric characteristics in a variety of active populations and correlated those characteristics to injury risk. This research revealed that long reaction times were particularly correlated with history of accidental bone fractures in military recruits (37), musculoskeletal complaints such as leg and low back pain in adolescents (36), and musculoskeletal injuries in soccer players (38). Although these studies are hampered by a lack of rigorous detail regarding their measurements, injury definitions, and populations, these studies were among the first to identify an effect of neurocognitive performance on musculoskeletal injury risk.
Increased risk of musculoskeletal injury is also applicable to athletes with relatively lower levels of baseline neurocognitive performance. A landmark study by Swanik et al. (35) compared preseason baseline computerized neurocognitive performance scores among 80 collegiate athletes who had experienced a subsequent ACL injury and 80 uninjured collegiate athletes matched for height, weight, age, gender, sports, position, and years of experience. Neurocognitive performance was assessed using the Immediate Postconcussion Assessment and Cognitive Testing (ImPACT) software. The results demonstrated significantly worse neurocognitive performance among athletes with ACL injury across all four domains of the ImPACT test, including verbal memory, visual memory, visual motor speed, and reaction time (35). In another recent prospective study, poor reaction time as measured via the ImPACT test was predictive of lower extremity strains and sprains in college football players over one season, with 74% sensitivity and 51% specificity (44).
States of Neurocognitive Stress
Given these findings, it stands to reason that conditions featuring transient alterations in neurocognitive performance would similarly be associated with increased risk of injury. States that negatively impact neurocognitive performance include inadequate sleep, psychological stress, and concussion injury.
Investigations in the area of occupational health and performance using populations in states of stress featuring altered levels of neurocognition also have demonstrated evidence of this effect. States associated with relative neurocognitive impairment, such as after significant alterations in the timing of shift work and after periods of significantly restricted sleep, have been demonstrated on multiple occasions to negatively impact motor performance and increased risk for task error and musculoskeletal injuries in these circumstances (1,39,47). Similar studies have been performed in athletes under conditions of sleep restriction. These investigations have repeatedly noted significant decrements in various aspects of motor performance on tasks such as muscle power and throwing/shooting accuracy (10). To the best knowledge of the authors, no studies have yet demonstrated an effect of sleep restriction on athletic injury risk.
States of psychological stress also are well-known to blunt neurocognitive performance in athletic populations. Several studies have demonstrated that depression, anxiety, and other distressful conditions are associated with impaired performance on computer-based neurocognitive tests, particularly in the areas of visual performance and reaction time (3,5). Narrowing of peripheral vision and slowing of central vision reaction times occur in individuals with increased level of life event stress (45,46). Multiple studies also have demonstrated that athletes with high stress levels and personality traits as well as poor coping skills are at elevated risk of musculoskeletal injuries; furthermore, stress-reducing interventions such as cognitive-behavioral therapy may diminish this risk (9,11,18,30).
Perhaps the most prominent scenario of neurocognitive performance decrements is in the setting of a concussion injury. Concussions adversely affect attention, reaction time, and visuospatial skill (19). If deficits in these areas persist after return to competition, the athlete may be at elevated risk for subsequent injury. Although such cases are anecdotal, there are several instances of elite or professional athletes who have experienced serious musculoskeletal injury after return to play from a concussion.
Emerging scientific evidence indicates that history of concussion may indeed be a powerful factor in subsequent musculoskeletal injury risk. For example, Nordstrom et al. (28) studied 1,665 male professional European football players on 46 teams across 11 seasons, which yielded 71 concussions in 66 players. Relative to the year prior to a respective player's concussion injury, concussion was associated with significantly increased risk (hazard ratio (HR), of 1.47) of subsequent acute injury (28). Similarly, relative to nonconcussed players, concussion was associated with increased risk of acute and gradual-onset injuries in the year subsequent to the concussion. This risk compared with that in nonconcussed controls progressively increased with time. By 3 months, HR was 1.56 and had progressively increased to 4.07 by month 12. One limitation is that these data were not adjusted for number of exposures in each group.
A previous study by Makdissi et al. (25) prospectively followed 138 professional Australian football players with concussion after return to play and demonstrated an injury rate of 7.25 per 100 games in athletes with concussion compared with 3.25 per 100 games for team, position, and game-matched controls. This difference did not achieve statistical significance (HR, 2.23; range, 0.93 to 5.04). However, the authors acknowledged that the study was not powered to detect differences in injury rates and was further hampered by the relatively limited exposures during the follow-up period used for analysis (first three games upon return to play) (25).
More recently, Herman et al. (13) documented the risk of experiencing a time loss lower extremity musculoskeletal injury after return to play from a concussion in Division I athletes. Sixty-one athletes with concussion were retrospectively followed for up to 90 d after in-season return to play. Athletes with concussion were matched to up to three nonconcussed controls and followed over the same period. Concussed athletes had an odds ratio of incurring subsequent lower extremity musculoskeletal injury of 3.79 versus control athletes over a 90-d follow-up period (13). Similar results were noted by Pietrosimone et al. (31) who conducted an injury survey among more than 2,400 retired professional football players. Increased risk of injury was noted for multiple injury types, ranging from ACL injuries to ankle sprains and meniscus tears. Additionally, this risk was found to increase with increasing number of concussions, particularly lending support to the premise of increased musculoskeletal injury risk after concussion and to the overall relationship between neurocognitive performance and musculoskeletal injury risk.
Future Considerations for Injury Risk Stratification and Management
It is important to note that many of the investigations previously cited have significant limitations. Several studies supporting evidence for a relationship between neurocognitive performance and injury risk have been performed in nonathletic populations, are retrospective in design, and/ or include a relatively small number of subjects. Despite this being a relatively underdeveloped area of investigation, the results of these studies create several potential implications for sports medicine practice and opportunities for future investigation.
Assessment of Injury Risk
Sports injury prevention has been hampered partly by the lack of effective and easy-to-implement injury risk stratification algorithms. The studies by Swanik et al. (35) and Wilkerson (44) described in this article used a relatively affordable, clinically accessible, computer-based baseline neurocognitive performance assessment, which typically takes approximately 20 to 25 min to complete. This may make such testing a potentially time and cost effective means of identifying athletes at high risk for injury. Such baseline neurocognitive testing is becoming widespread for contact sports at the high school, collegiate, and professional levels. This testing affords an opportunity for programs to use existing infrastructure and protocols for injury risk assessment without significant additional effort or costs. Other opportunities for expanded injury risk screening may be to assess for high levels of life stress, as this may not only increase risk but be amenable to intervention (9,11,18,30). Additional research efforts are needed to determine optimal cut points for different testing instruments and domains to best identify athletes at high risk for injury. Further research is also needed to determine how such test results may inform a given athlete's specific exercise prescription to maximize the therapeutic benefit of their injury prevention program.
Injury Prevention and Rehabilitation Strategies
Current popular injury prevention programs tend to be focused on improving neuromuscular performance. Incorporating dimensions of neurocognitive performance may be feasible to implement and provide additive benefits. For example, the use of sports vision exercises to improve aspects such as visual attention and reaction time may aid in reducing injury risk (23). Neurocognitive performance aspects also could be directly integrated into existing neuromuscular performance exercises, such as requiring the completion of a second cognitive task during an exercise to improve dual tasking. Other practical interventions may include educating athletes regarding appropriate sleep requirements and practices during training/competition and with transmeridian travel.
Knowledge of a relationship between concussion and subsequent injury risk also may impact postconcussion rehabilitation. Current management often features relative rest with exercise and activity at the subsymptom exacerbation level, progressive exercise tolerance as the athlete improves, and a stepwise return-to-play protocol tailored to the demands of the athlete's sports. Rehabilitation efforts may need to be expanded to include additional focus on neurocognitive performance (particularly in the dimensions noted in the Table and in domains commonly associated with injury prevention programs, such as neuromuscular performance.
Reconsideration of Concussion Return-to-Play Criteria
The presence of increased risk for subsequent injury after concussion implies that current clinical treatment and postconcussion return-to-play benchmarks may be inadequate for the health and safety of the affected athlete. There is a growing body of evidence that neuromuscular impairments during gait are present during the postconcussion period, with persistent alterations noted even after the participating athletes had fulfilled standard clinical return-to-play criteria (24). These impairments have been observed in aspects including gait initiation, gait termination, and intersegmental coordination during tasks such as walking and obstacle navigation and can be particularly prominent when accompanied by a dual-attention task. It is compelling to note the relative simplicity of these tasks. Representative examples include performing normal self-selected walking, crossing an obstacle ranging from 4 cm to 10% body height off the ground, and performing unobstructed gait while reciting the months of the year in reverse order. While relatively functional with respect to activities of daily living, such tasks are far less demanding than those the athlete would be expected to complete at a faster pace and in a more complex and dynamic environment; thus, it is reasonable to consider the likelihood that these neuromuscular impairments are likely to be accentuated during high-demand athletic tasks and in states of physical and mental fatigue during competition. It can be surmised that the risk for injury may be significantly increased in complex sports that involve simultaneous tactical decision making, running, cutting, jump landing, throwing, and fast movement velocities.
When these results are combined with emerging evidence of increased risk for musculoskeletal injury and the potential for long-term postconcussion sequelae, current return-to-play guidelines might be called into question. Further research is necessary to determine 1) the magnitude and duration of vulnerability to musculoskeletal injury after concussion, 2) the concussion injury characteristics and other factors that may attenuate or amplify this risk (movement complexity or velocity), and 3) the most effective strategies for injury prevention.
Conclusions
Evidence supports the concept that poor baseline neurocognitive performance or impairments in neurocognitive performance via sleep deprivation, psychological stress, or concussion injury can increase the risk for subsequent musculoskeletal injury. Knowledge of the relationship between neurocognitive performance and musculoskeletal injury risk could augment the current neuromuscular-focused paradigm of injury risk screening, rehabilitation, and prevention. Such possibilities include improved preseason and postconcussion injury risk stratification and injury risk prevention/mitigation protocols. Improved identification of athletes at high risk for musculoskeletal injury via neurocognitive testing, potentially in conjunction with other injury risk identification measures, would aid in implementing injury prevention programs efficiently. Knowledge of the magnitude, duration, and modifying factors of musculoskeletal injury risk after concussion may help inform medical decision making, such as timing of return to play and postconcussion rehabilitation milestones and strategies. These strategies also could be applied to other populations in high-risk occupational settings such as the military and may help inform assessments of injury risk, duty readiness, and job performance. Thus, additional studies on this relationship have the potential to provide significant clinical impact in reducing the rate and impact of musculoskeletal injuries.
Acknowledgments
This work was partly supported by National Institutes of Health grant 5K12HD001097-18 (the Rehabilitation Medical Scientist Training Program).
References
- 1.Amirian I. The impact of sleep deprivation on surgeons' performance during night shifts. Dan Med J. 2014;61:B4912. [PubMed] [Google Scholar]
- 2.Andrew NE, Gabbe BJ, Wolfe R, Cameron PA. Trends in sport and active recreation injuries resulting in major trauma or death in adults in Victoria, Australia, 2001–2007. Injury. 2012;43:1527–33. doi: 10.1016/j.injury.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CM, Samples HL, Broshek DK, et al. The relationship between psychological distress and baseline sports-related concussion testing. Clin J Sport Med. 2010;20:272–7. doi: 10.1097/JSM.0b013e3181e8f8d8. [DOI] [PubMed] [Google Scholar]
- 4.Balazs GC, Pavey GJ, Brelin AM, et al. Risk of anterior cruciate ligament injury in athletes on synthetic playing surfaces: a systematic review. Am J Sports Med. 2014 doi: 10.1177/0363546514545864. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Covassin T, Elbin RJ, 3rd, Larson E, Kontos AP. Sex and age differences in depression and baseline sport-related concussion neurocognitive performance and symptoms. Clin J Sport Med. 2012;22:98–104. doi: 10.1097/JSM.0b013e31823403d2. [DOI] [PubMed] [Google Scholar]
- 6.Dai B, Herman D, Liu H, et al. Prevention of ACL injury, part II: effects of ACL injury prevention programs on neuromuscular risk factors and injury rate. Res Sports Med. 2012;20:198–222. doi: 10.1080/15438627.2012.680987. [DOI] [PubMed] [Google Scholar]
- 7.Dallinga JM, Benjaminse A, Lemmink KA. Which screening tools can predict injury to the lower extremities in team sports?: a systematic review. Sports Med. 2012;42:791–815. doi: 10.1007/BF03262295. [DOI] [PubMed] [Google Scholar]
- 8.Dragoo JL, Castillo TN, Braun HJ, et al. Prospective correlation between serum relaxin concentration and anterior cruciate ligament tears among elite collegiate female athletes. Am J Sports Med. 2011;39:2175–80. doi: 10.1177/0363546511413378. [DOI] [PubMed] [Google Scholar]
- 9.Edvardsson A, Ivarsson A, Johnson U. Is a cognitive-behavioural biofeed-back intervention useful to reduce injury risk in junior football players? J Sports Sci Med. 2012;11:331–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Fullagar HH, Skorski S, Duffield R, et al. Sleep and athletic performance: the effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2014;45:161–86. doi: 10.1007/s40279-014-0260-0. [DOI] [PubMed] [Google Scholar]
- 11.Galambos SA, Terry PC, Moyle GM, et al. Psychological predictors of injury among elite athletes. Br J Sports Med. 2005;39:351–4. doi: 10.1136/bjsm.2005.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold JR, Gold MM. Access for all: the rise of the Paralympic Games. J R Soc Promot Health. 2007;127:133–41. doi: 10.1177/1466424007077348. [DOI] [PubMed] [Google Scholar]
- 13.Herman D, Jones D, Harrison A, et al. Concussion increases the risk of subsequent lower extremity musculoskeletal injury in collegiate athletes. Clin J Sport Med. 2013;23:124. doi: 10.1007/s40279-016-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuro-muscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 15.Hewett TE, Myer GD, Ford KR, et al. The 2012 ABJS Nicolas Andry Award: the sequence of prevention: a systematic approach to prevent anterior cruciate ligament injury. Clin Orthop Relat Res. 2012;470:2930–40. doi: 10.1007/s11999-012-2440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42:311–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Hurtig T, Ebeling H, Jokelainen J, et al. The associationbetween hospital-treated injuries and ADHD symptoms in childhood and adolescence: a follow-up study in the northern Finland birth cohort 1986. J Atten Disord. 2013 doi: 10.1177/1087054713486699. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Ivarsson A, Johnson U, Podlog L. Psychological predictors of injury occurrence: a prospective investigation of professional Swedish soccer players. J Sport Rehabil. 2013;22:19–26. doi: 10.1123/jsr.22.1.19. [DOI] [PubMed] [Google Scholar]
- 19.Johnson EW, Kegel NE, Collins MW. Neuropsychological assessment of sport-related concussion. Clin Sports Med. 2011;30:73–88. doi: 10.1016/j.csm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser ML, Schoemaker MM, Albaret JM, Geuze RH. What is the evidence of impaired motor skills and motor control among children with attention deficit hyperactivity disorder (ADHD)? Systematic review of the literature. Res Dev Disabil. 2014;36c:338–57. doi: 10.1016/j.ridd.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Knowles SB, Marshall SW, Miller T, et al. Cost of injuries from a prospective cohort study of North Carolina high school athletes. Inj Prev. 2007;13(6):416–21. doi: 10.1136/ip.2006.014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kofler MJ, Rapport MD, Sarver DE, et al. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin Psychol Rev. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Knudsen D, Kluka D. Impact of vision and vision training on sports performance. J Phys Educ Recreation Dance. 1997;68:17–24. [Google Scholar]
- 24.Lee H, Sullivan SJ, Schneiders AG. The use of the dual-task paradigm in detecting gait performance deficits following a sports-related concussion: a systematic review and meta-analysis. J Sci Med Sport. 2013;16:2–7. doi: 10.1016/j.jsams.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Makdissi M, McCrory P, Ugoni A, et al. A prospective study of postconcussive outcomes after return to play in Australian football. Am J Sports Med. 2009;37:877–83. doi: 10.1177/0363546508328118. [DOI] [PubMed] [Google Scholar]
- 26.Maxson RT, Lawson KA, Pop R, et al. Screening for attention-deficit/ hyperactivity disorder in a select sample of injured and uninjured pediatric patients. J Pediatr Surg. 2009;44:743–8. doi: 10.1016/j.jpedsurg.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Merrill RM, Lyon JL, Baker RK, Gren LH. Attention deficit hyperactivity disorder and increased risk of injury. Adv Med Sci. 2009;54:20–6. doi: 10.2478/v10039-009-0022-7. [DOI] [PubMed] [Google Scholar]
- 28.Nordstrom A, Nordstrom P, Ekstrand J. Sports-related concussion increases the risk of subsequent injury by about 50% in elite male football players. Br J Sports Med. 2014;48:1447–50. doi: 10.1136/bjsports-2013-093406. [DOI] [PubMed] [Google Scholar]
- 29.Pakzad-Vaezi K, Singhal A. Trends in paediatric sport- and recreation-related injuries: an injury surveillance study at the British Columbia Children's Hospital (Vancouver, British Columbia) from 1992 to 2005. Paediatr Child Health. 2011;16:217–21. doi: 10.1093/pch/16.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perna FM, Antoni MH, Baum A, et al. Cognitive behavioral stress management effects on injury and illness among competitive athletes: a randomized clinical trial. Ann Behav Med. 2003;25:66–73. doi: 10.1207/S15324796ABM2501_09. [DOI] [PubMed] [Google Scholar]
- 31.Pietrosimone B, Golightly Y, Mihalik J, Guskiewicz K. Concussion frequency is associated with lower extremity musculoskeletal injury in retired national football league players. Med Sci Sports Exerc. 2014;46:760. doi: 10.1249/MSS.0000000000000684. [DOI] [PubMed] [Google Scholar]
- 32.Smith HC, Johnson RJ, Shultz SJ, et al. A prospective evaluation of the Landing Error Scoring System (LESS) as a screening tool for anterior cruciate ligament injury risk. Am J Sports Med. 2012;40:521–6. doi: 10.1177/0363546511429776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimoto D, Myer GD, Bush HM, et al. Compliance with neuromuscular training and anterior cruciate ligament injury risk reduction in female athletes: a meta-analysis. J Athl Train. 2012;47:714–23. doi: 10.4085/1062-6050-47.6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto D, Myer GD, Foss KD, Hewett TE. Dosage effects of neuromuscular training intervention to reduce anterior cruciate ligament injuries in female athletes: meta- and sub-group analyses. Sports Med. 2014;44:551–62. doi: 10.1007/s40279-013-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanik CB, Covassin T, Stearne DJ, Schatz P. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Am J Sports Med. 2007;35:943–8. doi: 10.1177/0363546507299532. [DOI] [PubMed] [Google Scholar]
- 36.Taimela S, Kujala UM. Reaction times with reference to musculoskeletal complaints in adolescence. Percept Mot Skills. 1992;75:1075–82. doi: 10.2466/pms.1992.75.3f.1075. [DOI] [PubMed] [Google Scholar]
- 37.Taimela S, Kujala UM, Osterman K. The relation of low grade mental ability to fractures in young men. Int Orthop. 1991;15:75–7. doi: 10.1007/BF00179701. [DOI] [PubMed] [Google Scholar]
- 38.Taimela S, Osterman L, Kujala U, et al. Motor ability and personality with reference to soccer injuries. J Sports Med Phys Fitness. 1990;30:194–201. [PubMed] [Google Scholar]
- 39.Valent F, Di Bartolomeo S, Marchetti R, et al. A case-crossover study of sleep and work hours and the risk of road traffic accidents. Sleep. 2010;33:349–54. doi: 10.1093/sleep/33.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valovich McLeod TC, Bay RC, Parsons JT, et al. Recent injury and health-related quality of life in adolescent athletes. J Athl Train. 2009;44:603–10. doi: 10.4085/1062-6050-44.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Ban E, Souverein P, Meijer W, et al. Association between ADHD drug use and injuries among children and adolescents. Eur Child Adolesc Psychiatry. 2014;23:95–102. doi: 10.1007/s00787-013-0432-8. [DOI] [PubMed] [Google Scholar]
- 42.Westerstahl M, Barnekow-Bergkvist M, Hedberg G, Jansson E. Secular trends in sports: participation and attitudes among adolescents in Sweden from 1974 to 1995. Acta Paediatr. 2003;92:602–9. doi: 10.1080/08035250310002713. [DOI] [PubMed] [Google Scholar]
- 43.Whitney DC, Sturnick DR, Vacek PM, et al. Relationship between the risk of suffering a first-time noncontact ACL injury and geometry of the femoral notch and ACL: a prospective cohort study with a nested case–control analysis. Am J Sports Med. 2014;42:1796–805. doi: 10.1177/0363546514534182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkerson G. Neurocognitive reaction time predicts lower extremity sprains and strains. IJATT. 2012;17:4–9. [Google Scholar]
- 45.Williams JM, Andersen MB. Psychosocial influences on central and peripheral vision and reaction time during demanding tasks. Behav Med. 1997;22:160–7. doi: 10.1080/08964289.1997.10543549. [DOI] [PubMed] [Google Scholar]
- 46.Williams JM, Tonymon P, Andersen MB. Effects of life-event stress on anxiety and peripheral narrowing. Behav Med. 1990;16:174–81. doi: 10.1080/08964289.1990.9934606. [DOI] [PubMed] [Google Scholar]
- 47.Wong IS, Smith PM, Mustard CA, Gignac MA. For better or worse? Changing shift schedules and the risk of work injury among men and women. Scand J Work Environ Health. 2014;40:621–30. doi: 10.5271/sjweh.3454. [DOI] [PubMed] [Google Scholar]
- 48.Woods B. Social Issues in Sport. Champaign (IL): Human Kinetics; 2011. [Google Scholar]


