Summary
Background
Traits such as behavioral inhibition and neuroticism have been linked to the development of mood and anxiety disorders. Hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, a manifestation of the stress response, is often seen in major depression and has also been demonstrated in animals and humans with inhibited temperaments. A recent study found HPA hyperactivity in adults with high levels of neuroticism. The present study investigated associations of temperament and HPA function in 31 healthy adults.
Methods and materials
Subjects completed diagnostic interviews, questionnaires, and the dexamethasone-/corticotropin-releasing hormone (Dex/CRH) test. Temperament was assessed using the Tridimensional Personality Questionnaire (TPQ).
Results
Novelty Seeking was inversely related to plasma cortisol concentrations in the Dex/CRH test. Harm Avoidance and Reward Dependence were not significantly associated with cortisol responses in the Dex/CRH test. The results were not accounted for by psychiatric symptoms or a history of stress or childhood maltreatment.
Conclusions
These findings are consistent with previous reports associating temperament factors with HPA axis hyperactivity. Further work is needed to replicate these observations and determine whether HPA axis dysfunction might account for some of the previously reported association of personality factors with mood and anxiety disorders.
Keywords: Cortisol, Dex/CRH test, HPA axis, Temperament, Personality, Inhibition
1. Introduction
Temperament and personality characteristics such as behavioral inhibition and neuroticism have been linked to mood and anxiety disorders. For example, prospective studies of non-depressed individuals have shown that neuroticism, which can be characterized by the tendency to experience negative affect, is a risk factor for the subsequent development of depression (Angst and Clayton, 1986; Hirschfeld et al., 1989; Kendler et al., 2004, 1993b). Moreover, findings from behavioral and molecular genetics studies indicate that neuroticism and major depression share common genetic risk factors (Fanous et al., 2002; Kendler et al., 1993a; Nash et al., 2004; Sen et al., 2004). Behavioral inhibition is another temperament factor that has been found predictive of anxiety disorders and depression (Fox et al., 2005). This trait, defined as the tendency to withdraw and avoid novel situations, demonstrates stability over time in both non-human primates (Kalin and Shelton, 2003) and children (Fox et al., 2005). Behavioral inhibition has been linked to anxiety disorders in family studies (Rosenbaum et al., 1988) and in prospective longitudinal studies of young children who have been followed through childhood (Hirshfeld et al., 1992; Rosenbaum et al., 1988) and into adolescence (Prior et al., 2000; Schwartz et al., 1999).
Stressful life experiences also play a prominent role in the development of major depression (Brown and Anderson, 1991; Browne and Finkelhor, 1986; Bryer et al., 1987; Kendler et al., 1993a; Lewinsohn et al., 1988) and anxiety disorders (Heim and Nemeroff, 2001; McFarlane et al., 2005). The neurobiological response to stress is coordinated in part by the hypothalamic–pituitary–adrenal (HPA) axis. Animal studies and recent human investigations indicate that early exposure to stress can induce enduring alterations in HPA axis function, alterations which mirror some of the neuroendocrine abnormalities that commonly occur in major depression and post-traumatic stress disorder (for reviews see Heim and Nemeroff (2001); Shea et al. (2005)).
The dexamethasone-/corticotropin-releasing hormone (Dex/CRH) test is a provocative challenge of HPA axis function that combines dexamethasone pretreatment with CRH administration on the following day. ACTH and cortisol responses to this test are exaggerated in depressed patients (Heuser et al., 1994), and elevated cortisol concentrations have also been shown in non-depressed individuals with a strong family history of mood disorders (Holsboer et al., 1995). The Dex/CRH test is thought to be sensitive to abnormalities of glucocorticoid receptor (GR) signaling at the level of the pituitary as well as to increased secretion of CRH and vasopressin that are hypothesized to result from impaired central GR signaling in major depression (Holsboer, 2000).
Several lines of research suggest the possibility that personality or temperament may account for some of the association between stress, depression, and HPA axis hyperactivity. There is evidence that personality can influence the likelihood of exposure to stressors (Kendler et al., 2003). In addition to altering exposure, personality or temperament may confer sensitivity to stressors (e.g., Bolger and Schilling, 1991; Kendler et al., 2004; Ormel and Wohlfarth, 1991; Rijsdijk et al., 2001; Van Os and Jones, 1999), and conversely, a temperament-based tendency to experience negative affect or to be socially inhibited may in part result from psychological or physiological sensitivity to stressors (Kagan et al., 1987).
Neuroticism reflects the tendency to experience negative affect, and individuals with this trait have elevated levels of anxiety, apprehension, and negative emotions (McCrae and Costa, 2003). Two groups of investigators have found a relationship between neuroticism and plasma cortisol response to the Dex/CRH test. In a small exploratory study of young adults selected for high or low neuroticism scores on the Eysenck Personality Questionnaire, McCleery and Goodwin (McCleery and Goodwin, 2001) found that high neuroticism was predictive of lower cortisol responses in the Dex/CRH test. In contrast, Zobel and colleagues (Zobel et al., 2004) conducted a large study of healthy adults with a wide age range and reported that neuroticism (measured by the NEO-FFI) and depressive temperament (measured by the Temperament Evaluation of Memphis) were independent positive predictors of the cortisol response to the Dex/CRH test. The discrepant results between these two studies may be explained by methodological differences. Perhaps most importantly, McCleery and Goodwin’s sample included subjects with a history of psychiatric disorders and a history of traumatic experiences. The high neuroticism group in their study had a large proportion of subjects with a history of one or more Axis I disorders (although only one had received treatment), as well as reports of poor parenting and a history of childhood parental separation. There is mounting evidence that early life stress and trauma may be linked to hypocortisolemia and a dampened cortisol response to stress (Carpenter et al., 2005; Gunnar and Vazquez, 2001; Heim et al., 2000), as well as to personality traits such as neuroticism (e.g., Rogosch and Cicchetti, 2004). The study by Zobel and colleagues excluded individuals with a history of treated psychiatric disorder, but did not contain information regarding early life stress or trauma history.
Another temperament trait that has been strongly associated with HPA axis dysfunction in children and non-human primates is behavioral inhibition. As mentioned above, behavioral inhibition is characterized by a tendency to withdraw and inhibit behavior in response to novel stimuli (Fox et al., 2005). Rhesus monkeys with behavioral inhibition have elevated concentrations of CRH in cerebrospinal fluid and increases in plasma cortisol (Kalin and Shelton, 2003). Similarly, social wariness and internalizing behaviors in children are positively associated with basal salivary cortisol concentrations (de Haan et al., 1998; Gunnar et al., 1997; Kagan et al., 1987; Scerbo and Kolko, 1994; Schmidt et al., 1997; Smider et al., 2002), and two studies have found an association between behavioral inhibition and genetic markers at the CRH locus (Smoller et al., 2003, 2005).
Conversely, individuals with extroversion or externalizing behaviors may have higher thresholds for physiological arousal. Consistent with this hypothesis, low salivary cortisol concentrations have been found in children with oppositional behavior (Tennes and Kreye, 1985; van Goozen et al., 1998) and conduct disorder (McBurnett et al., 1991). However, a small investigation in adults (Oswald et al., 2004) and a number of studies in children have also found extroversion to be positively associated with cortisol levels, particularly when cortisol is sampled in peer-group settings (Gunnar, 2001). A recent investigation indicates that this effect may be mediated by aggressive interactions leading to peer rejection, which can be a potent social stressor (Gunnar et al., 2003). Moreover, these authors showed that controlling for this effect can unmask a negative relationship between extroversion and cortisol levels.
The present study was designed to test the hypothesis that self-report measures of personality traits related to neuroticism and inhibition or introversion, which may reflect or confer sensitivity to stress, are associated with increased HPA axis reactivity in a sample of healthy adults.
2. Methods
2.1. Subjects
Participants were 17 females and 14 males, aged 18–50 years. Most participants were Caucasian (N = 24) and a few were Black (N = 2), Asian (N = 1), or did not identify their race (N = 4). Subjects were recruited via advertisements in=the community for a larger study of neuroendocrine function in relation to psychiatric symptoms. Participants underwent complete physical and neurological examinations. Electrocardiograms and laboratory studies for complete blood count, electrolytes, thyroid-stimulating hormone, urine toxicology, and urinalysis were conducted in order to rule out acute or unstable medical illness and substance use. Subjects were excluded if they met criteria for a current or past Axis I psychiatric disorder or if they had one or more of the following conditions: acute or unstable medical illness, endocrine disease, or ongoing treatment with drugs which might influence HPA axis function, including psychotropics, beta blockers, angiotensin-converting enzyme inhibitors, ketoconazole, metyrapone, and corticosteroids. Oral contraceptives were permitted. All subjects gave voluntary written informed consent to participate in this study, which was approved by the Butler Hospital Institutional Review Board.
2.2. Interviews and measures of personality and psychiatric symptomatology
Subjects completed the Structured Clinical Interview for Diagnostics (First et al., 1996) for determination of current and lifetime Axis I psychiatric diagnoses. In addition, participants reported on their current mood and anxiety symptoms, experiences of childhood abuse and neglect, and recent perceived stress. The following self-report questionnaires, all of which have demonstrated acceptable psychometric properties, were used: the Inventory for Depressive Symptomatology-Self Report (Rush et al., 1996), the State-Trait Anxiety Inventory (STAI; Spielberger, 1983), the Childhood Trauma Questionnaire (CTQ; Bernstein, 1998), and the Perceived Stress Scale (PSS; Cohen et al., 1983).
The Tridimensional Personality Questionnaire (TPQ; Cloninger et al., 1991) was used to assess temperament. This instrument has three scales: Harm Avoidance, Novelty Seeking, and Reward Dependence. The Novelty Seeking subscale includes dimensions of Exploratory Excitability vs. Stoic Rigidity, Impulsiveness vs. Reflection, Extra-vagance vs. Reserve, and Disorderliness vs. Regimentation. High levels of Novelty Seeking are correlated with Extraversion (De Fruyt et al., 2000) and low levels are indicative of a more introverted personality style. The Harm Avoidance scale is composed of the following subscales: Anticipatory Worry and Pessimism, Fear of Uncertainty, Shyness with Strangers, and Fatigability and Asthenia. Harm Avoidance is positively correlated with measures of neuroticism (De Fruyt et al., 2000; Stallings et al., 1996). In addition, Harm Avoidance is negatively correlated with Novelty Seeking (De Fruyt et al., 2000), and examination of their subscales shows that both are related to behavioral inhibition (i.e., low levels of Novelty Seeking and high Harm Avoidance). Because of their associations with negative affect and inhibition or introversion, high Harm Avoidance and low Novelty Seeking were hypothesized to be predictive of the cortisol response to the Dex/CRH test. In addition, the relationship between cortisol response and the TPQ scale Reward Dependence, composed of the sub-scales Sentimentality, Persistence, Attachment, and Dependence, was investigated in an exploratory fashion.
2.3. Dex/CRH test
On a separate day, subjects completed the Dex/CRH test as follows. Just prior to the test, subjects were queried about whether they had experienced any somatic symptoms, stressors, or changes in usual habits over the preceding week, and those who reported any significant aberrations had the visit rescheduled. For this test, participants took dexamethasone 1.5 mg orally at 23:00 h. The following day, they were given a balanced lunch, and an indwelling IV catheter was inserted at 13:00 h. Blood samples were drawn every 15 min from 14:45 to 16:30 h and at 17:00 h, immediately centrifuged, and stored at −80° C. At 15:00 h, CRH (corticorelin ovine triflutate, Acthrel®, Ferring Pharmaceuticals, Inc.) 100 μg reconstituted in 2 ml 0.9% NaCl was infused IV over 30 s. Throughout the procedure, the subjects rested in a semi-recumbent position. They were permitted to read or watch pre-selected films that did not involve emotionally charged material, and they were not allowed to sleep. During the test at 14:30, 15:30, and 16:30 h, subjects completed Visual Analogue Scales (VAS) that assessed the degree to which they felt a number of mood states, including anxious, depressed, fearful, irritable, nervous, and sad. All scales were anchored from 0 = not at all to 100 = most ever.
Plasma samples at baseline (immediately prior to infusion of CRH; 1500 h) and four time points after CRH infusion (15:30, 15:45, 16:00, and 16:15 h) were assayed in duplicate using the GammaCoat cortisol I-125 coated-tube radioimmunoassay (RIA) kit (INCSTAR Corp., Stillwater, Minn.). The intra-assay and inter-assay CVs observed for quality assessment samples (5 and 20 ug/dl) were less than 5% and 10%, respectively. Net area under the cortisol curve over time (netAUC) was calculated using the trapezoidal method, and the change in cortisol concentration from baseline to peak (peak Δ) was measured.
2.4. Statistical analyses
Analyses were conducted using SPSS 11.5.0 for Windows. All analyses were two-tailed with α set to .05. Correlations and t-tests were used to test for associations between the cortisol measures and age, sex, psychiatric symptoms and scores on the CTQ and PSS in order to determine whether these variables might confound a relationship between the personality variables and cortisol responses to the Dex/CRH test. Separate repeated-measures general linear models were used to test the main hypotheses regarding the relationship between cortisol concentrations over time with the Harm Avoidance and Novelty Seeking measures, and a repeated-measures general linear model was used to explore the relationship between cortisol response and Reward Dependence. Repeated-measures general linear models were also used to determine whether mood state reported on the VAS changed over time or in relation to the temperament scales. For the general linear models, Mauchly’s test of sphericity was used and the Huynh–Feldt test was applied when applicable. The TPQ scales were dichotomized via median split in order to depict their relationship with cortisol over time.
3. Results
3.1. Sample characteristics
Characteristics of the women and men in the sample are summarized in Table 1. Scores on the TPQ scales were similar to published norms (Cloninger et al., 1991, 1993). Females had higher cortisol concentrations than males at baseline (mean±SD: 46.49±37.96 versus 21.99±9.85 nmol/L; t(60.4) = 2.56, p<.05), but did not differ significantly from males at any other time point. There were no significant relationships between cortisol concentrations and age or symptoms of depression or anxiety in this healthy sample.
Table 1.
Sample characteristics.
| Mean±standard deviation |
||
|---|---|---|
| Women (N = 17) | Men (N = 14) | |
| Age (years) | 24.24±8.73 | 24.93±5.31 |
| Childhood Trauma Questionnaire | 7.81±2.64 | 6.20±1.20* |
| Perceived Stress Scale Total | 28.12±10.44 | 23.65±9.46 |
| State-Trait Anxiety Inventory-State | 30.06±5.72 | 28.36±7.12 |
| State-Trait Anxiety Inventory-Trait | 34.19±6.81 | 28.43±8.73* |
| Inventory for Depressive Symptoms | 8.94±4.66 | 6.93±4.46 |
| Novelty Seeking | 17.41±5.93 | 17.29±4.07 |
| Harm Avoidance | 13.47±5.41 | 9.36±7.85 |
| Reward Dependence | 14.76±3.90 | 15.57±4.27 |
p<.05.
3.2. Temperament and cortisol response to the Dex/CRH test
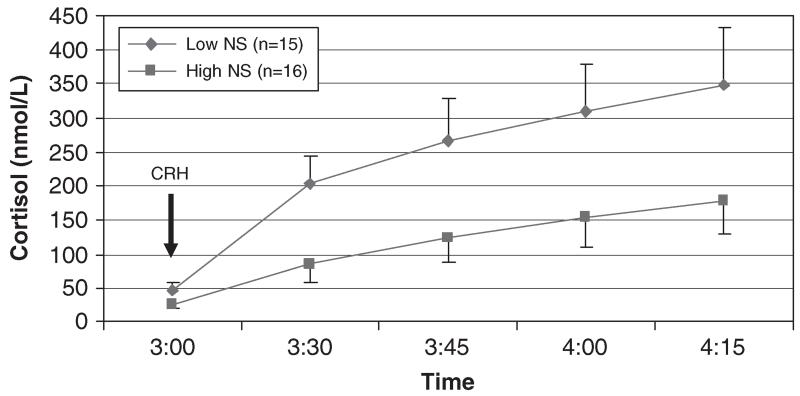
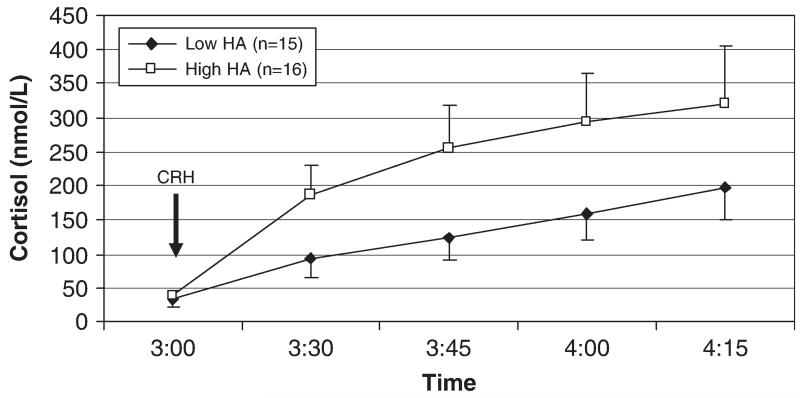
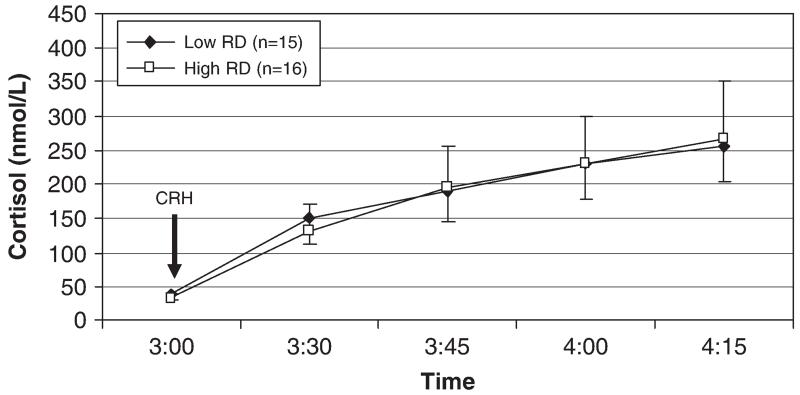
A repeated-measures general linear model testing the effect of Novelty Seeking on cortisol values over time and controlling for sex showed that in addition to a main effect of time (F(1.5, 41.5) = 6.74, p<.01), there was a main effect of Novelty Seeking (F(1, 28) = 5.14, p<.05), with lower levels of Novelty Seeking predicting higher cortisol concentrations (Fig. 1). This effect accounted for 16% of the variance in cortisol response (partial η2 = .16). The interaction of Novelty Seeking and time was not significant (p = .13). Partial correlations of Novelty Seeking with cortisol netAUC and peak Δ cortisol, controlling for gender, did not reach significance (r = −.34, p = 07 and r = −.29, p = .12, respectively). Harm Avoidance appeared to have a positive relationship with cortisol response to the Dex/CRH test (Fig. 2), but this was not significant in the repeated-measures model (F(1, 28) = 2.05, p = .16) or the partial correlations of Harm Avoidance with netAUC and peak Δ cortisol, controlling for gender (r = .27, p = .16 and r = .19, p = .32, respectively). Reward Dependence was not associated with cortisol response to the Dex/CRH test (Fig. 3).
Fig. 1.
Plasma cortisol response to the Dex/CRH test in subjects with low and high levels of Novelty Seeking. Note: Novelty Seeking had a significant main effect on cortisol concentrations (F(1, 28) = 5.14, p<.05). Low and high groups are defined by median split of the Novelty Seeking variable.
Fig. 2.
Plasma cortisol response to the Dex/CRH test in subjects with low and high levels of Harm Avoidance. Note: Harm Avoidance was not a significant predictor of cortisol concentrations (F(1, 28) = 2.05, p = .16). Low and high groups defined by median split of the Harm Avoidance variable.
Fig. 3.
Plasma cortisol response to the Dex/CRH test in subjects with low and high levels of Reward Dependence. Note: Reward Dependence was not a significant predictor of cortisol concentrations. Low and high groups defined by median split of the Reward Dependence variable.
In the repeated-measures models assessing mood state over time in relation to the temperament variables, there was no change in any of the VAS mood states over time, and none of the temperament scales had a significant effect on mood state during the test.
3.3. Stress and trauma
Scores on the PSS were positively correlated with baseline cortisol (r = .36, p<.05) but not with any of the other cortisol values. The general linear models assessing effects of the temperament scales on cortisol response to the Dex/CRH test were repeated with PSS as a covariate and the findings were unchanged.
Reports of childhood abuse and neglect assessed by the CTQ total score were not associated with scores on the Novelty Seeking or Harm Avoidance subscales, and the CTQ total score was not correlated with cortisol response to the Dex/CRH test in this sample.
4. Discussion
The findings of this study support the hypothesis that personality factors, which may reflect increased sensitivity to stimuli, are predictive of enhanced activation of the HPA axis. Low levels of Novelty Seeking, which reflect an introverted, rigid temperament, were predictive of greater cortisol responses to the Dex/CRH test in this study. In fact, the cortisol responses seen in subjects with low levels of Novelty Seeking were on the order of those reported for depressed patients (e.g., Lauer et al., 1997). Although Harm Avoidance appeared to be linked to increased cortisol responses to the Dex/CRH test, the association was not significant in this sample. Harm Avoidance is associated with neuroticism (De Fruyt et al., 2000) and is composed of subscales that are conceptually related to behavioral and social inhibition. Given the modest sample size, the power of the present study to detect a relationship between cortisol response and this trait was limited.
The inverse relationship between cortisol and Novelty Seeking is similar to findings of Rosenblitt et al. (2001) showing an inverse relationship between a measure of sensation seeking and a single basal measure of salivary cortisol in male, though not female, subjects. A number of other previous studies in adults have measured salivary or plasma cortisol under basal conditions or during a psychosocial stressor in relation to personality function. Results have been mixed, with several negative reports (Kirschbaum et al., 1992; Morgan et al., 2001; Roy et al., 2001; Schommer et al., 1999; Van Eck et al., 1996) in addition to findings of associations of cortisol concentrations with various personality measures (Grossi and Lundberg, 1998; Rosenblitt et al., 2001; Zorrilla et al., 1995). These studies varied widely in terms of sample characteristics, personality assessments, and measures of HPA axis function, so that almost none of the specific findings, negative or positive, have been replicated. Moreover, studies of cortisol concentrations under basal conditions or in response to psychosocial stress tests are open to the influence of additional psychological or environmental factors that may not have been accounted for in these investigations.
It has been posited that individuals with behavioral inhibition may have a lower threshold for activation of stress-sensitive physiological systems, so that the same level of stress would be expected to elicit more exaggerated physiological responses among inhibited subjects (Kagan et al., 1987). Alternatively, it is possible that inhibited individuals may experience a greater level of stress due to psychological sensitivity to stimuli and ineffective coping mechanisms. The same distinction can be made for the temperament traits we measured in the present study. Because the Dex/CRH test is a neurobiological challenge test that assesses the cortisol response to hormone administration under conditions that are designed to be psychologically neutral, there should be relatively less input from environmental or psychological factors. Consistent with this, we found no effect of the Dex/CRH test on negative mood states, and there was no relationship between Novelty Seeking or Harm Avoidance and mood over time in this test. The present findings therefore suggest that the trait of low Novelty Seeking may be directly associated with changes in the regulation of HPA axis function, rather than mediated by an association with affect state.
As mentioned above, behavioral inhibition in humans has recently been associated with genetic markers at the CRH locus (Smoller et al., 2003, 2005). Central administration of CRH in rodents and monkeys produces a variety of behavioral changes that resemble signs of mood and anxiety disorders as well as the temperament traits that have been associated with them. These effects include neophobia and the suppression of exploratory behavior, social withdrawal, and decreases in reproductive and appetitive behavior. Moreover, CRH administration increases physiological reactivity to novel or stressful stimuli (for reviews see Dunn and Berridge (1990); Owens and Nemeroff (1991)). These behavioral effects may be mediated through activation of the central noradrenergic system, with enhanced responsivity of amygdala and prefrontal cortex circuitry (Heim et al., 1997; Kalin and Shelton, 2003). The link between low levels of Novelty Seeking and HPA axis hyperactivity could therefore be explained by direct effects of CRH on both behavior and HPA axis function.
Our finding of an association between Novelty Seeking and cortisol response cannot be accounted for by symptoms of depression or anxiety or a history of a major psychiatric disorder such as post-traumatic stress disorder or major depression. Importantly, childhood maltreatment and adult stress, which have previously been linked to HPA axis dysfunction, also did not account for this effect.
This study is limited by a modest sample size, which precludes an analysis of the relationship between temperament and cortisol reactivity in subgroups based on sex or age, as proposed by Zobel et al. (2004), or an evaluation of possible effects of menstrual stage or oral contraceptive use in females. The sample included a wide age range, and although cortisol responses did not vary according to age, a larger study might identify age effects. In addition, the relatively invasive procedures in this study may have biased the sample toward those who are less harm avoidant. On the other hand, selection bias may also have occurred in response to the requirement that participants remain inactive throughout the testing. Overall, however, scores on the TPQ were within the range reported in the literature (Cloninger et al., 1991, 1993). While the findings provide further support for the notion that personality or temperament is associated with HPA reactivity, and extend this to the domain of inhibition/rigidity in adults, we are not able to directly replicate or refute the findings of the two previous studies on the Dex/CRH test because we used a different measure of temperament. Further investigations are needed to replicate these findings and determine whether hyperactivity of CRH and the HPA axis, which has previously been linked to major depression and anxiety disorders and produces behavioral signs of inhibition, enhanced stress-reactivity, and distress, might account for the association of personality factors with mood and anxiety disorders.
Acknowledgments
The authors gratefully acknowledge Sandra B. Tavares, R.N., B.S.N., for excellent clinical care to research subjects; Kelly Colombo, B.A., for data management, and John Carvalho, B.A., Lauren Wier, B.S., and Kobita Rikhye, Psy.D. for their assistance with the project.
This study was supported by Young Investigator Awards from NARSAD (ART, LLC), a Pfizer/Society of Women’s Health Research Scholar Award (LLC), and 1 K23 MH067947 (ART).
References
- Angst J, Clayton P. Premorbid personality of depressive, bipolar, and schizophrenic patients with special reference to suicidal issues. Compr. Psychiatry. 1986;27:511–532. doi: 10.1016/0010-440x(86)90055-6. [DOI] [PubMed] [Google Scholar]
- Bernstein DPFL. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. The Psychological Corporation; San Antonio: 1998. [Google Scholar]
- Bolger N, Schilling EA. Personality and the problems of everyday life: the role of neuroticism in exposure and reactivity to daily stressors. J. Pers. 1991;59:355–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. Am. J. Psychiatry. 1991;148:55–61. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- Browne A, Finkelhor D. Impact of child sexual abuse: a review of the research. Psychol. Bull. 1986;99:66–77. [PubMed] [Google Scholar]
- Bryer JB, Nelson BA, Miller JB, Krol PA. Childhood sexual and physical abuse as factors in adult psychiatric illness. Am. J. Psychiatry. 1987;144:1426–1430. doi: 10.1176/ajp.144.11.1426. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Carvalho JP, Wier L, Gagne GG, Mello AF, Mello MF, Anderson GM, Price LH. Decreased cortisol response to the Trier Social Stress Test in healthy adults with significant childhood adversity. Poster Presented at the Annual Meeting of the American College of Neuropsychopharmacology, Neuropsychopharmacology. 2005;30(Suppl. 1):S153. [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The Tridimensional Personality Questionnaire: US normative data. Psychol. Rep. 1991;69:1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch. Gen. Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- De Fruyt F, Van De Wiele L, Van Heeringen C. Cloninger’s psychobiological model of temperament and character and the five-factor model of personality. Pers. Individual Diff. 2000;29:441–452. [Google Scholar]
- de Haan M, Gunnar MR, Tout K, Hart J, Stansbury K. Familiar and novel contexts yield different associations between cortisol and behavior among 2-year-old children. Dev. Psychobiol. 1998;33:93–101. doi: 10.1002/(sici)1098-2302(199807)33:1<93::aid-dev8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Fanous A, Gardner CO, Prescott CA, Cancro R, Kendler KS. Neuroticism, major depression and gender: a population-based twin study. Psychol. Med. 2002;32:719–728. doi: 10.1017/s003329170200541x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu. Rev. Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Grossi G, Lundberg U. Psychological correlates of salivary cortisol secretion among unemployed men and women. Integr. Physiol. Behav. Sci. 1998;33:249–263. doi: 10.1007/BF02688666. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. The role of glucocorticoids in anxiety disorders: a critical analysis. In: Vasey MW, Dadds MR, editors. The Developmental Psychopathology of Anxiety. Oxford Press; New York: 2001. pp. 143–159. [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Dev. Psychobiol. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen MMH. Peer rejection, temperament, and cortisol activity in preschoolers. Dev. Psychobiol. 2003;43:346–358. doi: 10.1002/dev.10144. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. I. Endocrine factors in the pathophysiology of mental disorders. Psychopharmacol. Bull. 1997;33:185–192. [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Klerman GL, Lavori P, Keller MB, Griffith P, Coryell W. Premorbid personality assessments of first onset of major depression. Arch. Gen. Psychiatry. 1989;46:345–350. doi: 10.1001/archpsyc.1989.01810040051008. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic–pituitary–adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann. N Y Acad. Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. Am. J. Psychiatry. 1993a;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Arch. Gen. Psychiatry. 1993b;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Personality and the experience of environmental adversity. Psychol. Med. 2003;33:1193–1202. doi: 10.1017/s0033291703008298. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Bartussek D, Strasburger CJ. Cortisol responses to psychological stress and correlations with personality traits. Pers. Individual Diff. 1992;13:1352–1357. [Google Scholar]
- Lauer CJ, Bronisch T, Kainz M, Schreiber W, Holsboer F, Krieg JC. Pre-morbid psychometric profile of subjects at high familial risk for affective disorder. Psychol. Med. 1997;27:355–362. doi: 10.1017/s0033291796004400. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hoberman HM, Rosenbaum M. A prospective study of risk factors for unipolar depression. J. Abnorm. Psychol. 1988;97:251–264. doi: 10.1037//0021-843x.97.3.251. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, Christ MA, Hanson KS. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. J. Am. Acad. Child Adolesc. Psychiatry. 1991;30:192–196. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- McCleery JM, Goodwin GM. High and low neuroticism predict different cortisol responses to the combined dexamethasone–CRH test. Biol. Psychiatry. 2001;49:410–415. doi: 10.1016/s0006-3223(00)01056-8. [DOI] [PubMed] [Google Scholar]
- McFarlane A, Clark CR, Bryant RA, Williams LM, Niaura R, Paul RH, Hitsman BL, Stroud L, Alexander DM, Gordon E. The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. J. Integr. Neurosci. 2005;4:27–40. doi: 10.1142/s0219635205000689. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr. Personality in Adulthood: A Five-Factor Theory Perspective. Guildford Press; New York: 2003. [Google Scholar]
- Morgan CA, Wang S, Rasmusson A, Hazlett G, Anderson G, Charney DS. Relationship among plasma cortisol, catecholamines, neuropeptide Y, and human performance during exposure to uncontrollable stress. Psychosom. Med. 2001;63:412–422. doi: 10.1097/00006842-200105000-00010. [DOI] [PubMed] [Google Scholar]
- Nash MW, Huezo-Diaz P, Williamson RJ, Sterne A, Purcell S, Hoda F, Cherny SS, Abecasis GR, Prince M, Gray JA, Ball D, Asherson P, Mann A, Goldberg D, McGuffin P, Farmer A, Plomin R, Craig IW, Sham PC. Genome-wide linkage analysis of a composite index of neuroticism and mood-related scales in extreme selected sibships. Hum. Mol. Genet. 2004;13:2173–2182. doi: 10.1093/hmg/ddh239. [DOI] [PubMed] [Google Scholar]
- Ormel J, Wohlfarth T. How neuroticism, long-term difficulties, and life situation change influence psychological distress: a longitudinal model. J. Pers. Soc. Psychol. 1991;60:744–755. doi: 10.1037//0022-3514.60.5.744. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Mathena JR, Wand GS. Comparison of HPA axis hormonal responses to naloxone vs psychologically-induced stress. Psychoneuroendocrinology. 2004;29:371–388. doi: 10.1016/s0306-4530(03)00048-9. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol. Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, Oberklaid F. Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:461–468. doi: 10.1097/00004583-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC, Sterne A, Purcell S, McGuffin P, Farmer A, Goldberg D, Mann A, Cherny SS, Webster M, Ball D, Eley TC, Plomin R. Life events and depression in a community sample of siblings. Psychol. Med. 2001;31:401–410. doi: 10.1017/s0033291701003361. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D. Child maltreatment and emergent personality organization: perspectives from the five-factor model. J. Abnorm. Child Psychol. 2004;32:123–145. doi: 10.1023/b:jacp.0000019766.47625.40. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Gersten M, Hirshfeld DR, Meminger SR, Herman JB, Kagan J, Reznick JS, Snidman N. Behavioral inhibition in children of parents with panic disorder and agoraphobia. A controlled study. Arch. Gen. Psychiatry. 1988;45:463–470. doi: 10.1001/archpsyc.1988.01800290083010. [DOI] [PubMed] [Google Scholar]
- Rosenblitt JC, Soler H, Johnson SE, Quadagno DM. Sensation seeking and hormones in men and women: exploring the link. Horm. Behav. 2001;40:396–402. doi: 10.1006/hbeh.2001.1704. [DOI] [PubMed] [Google Scholar]
- Roy MP, Kirschbaum C, Steptoe A. Psychological, cardiovascular, and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendocrinology. 2001;26:375–391. doi: 10.1016/s0306-4530(00)00061-5. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol. Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: relationship to aggressive, hyperactive, and internalizing behaviors. J. Am. Acad. Child Adolesc. Psychiatry. 1994;33:1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Dev. Psychobiol. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Kudielka BM, Hellhammer DH, Kirschbaum C. No evidence for a close relationship between personality traits and circadian cortisol rhythm or a single cortisol stress response. Psychol. Rep. 1999;84:840–842. doi: 10.2466/pr0.1999.84.3.840. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am. J. Med. Genet. B. 2004;127:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M. Childhood maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30:162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: a prospective study. Child Dev. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, Laird NM, Kagan J, Snidman N, Hirshfeld-Becker D, Tsuang MT, Sklar PB, Slaugenhaupt SA. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol. Psychiatry. 2003;54:1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Kagan J, Snidman N, Faraone SV, Hirshfeld-Becker D, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol. Psychiatry. 2005;57:1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory STAI (Form Y) 1983. [Google Scholar]
- Stallings MC, Hewitt JK, Cloninger CR, Heath AC, Eaves LJ. Genetic and environmental structure of the Tridimensional Personality Questionnaire: three or four temperament dimensions? J. Pers. Soc. Psychol. 1996;70:127–140. doi: 10.1037//0022-3514.70.1.127. [DOI] [PubMed] [Google Scholar]
- Tennes K, Kreye M. Children’s adrenocortical responses to classroom activities and tests in elementary school. Psychosom. Med. 1985;47:451–460. doi: 10.1097/00006842-198509000-00005. [DOI] [PubMed] [Google Scholar]
- Van Eck MM, Nicolson NA, Berkhof H, Sulon J. Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biol. Psychiatry. 1996;43:69–84. doi: 10.1016/0301-0511(95)05159-7. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biol. Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Van Os J, Jones PB. Early risk factors and adult person–environment relationships in affective disorder. Psychol. Med. 1999;29:1055–1067. doi: 10.1017/s0033291799001026. [DOI] [PubMed] [Google Scholar]
- Zobel A, Barkow K, Schulze-Rauschenbach S, Von Widdern O, Metten M, Pfeiffer U, Schnell S, Wagner M, Maier W. High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic–pituitary–adrenocortical system in healthy volunteers. Acta Psychiatr. Scand. 2004;109:392–399. doi: 10.1111/j.1600-0447.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, DeRubeis FJ, Redei E. High self-esteem, hardiness, and affective stability are associated with higher basal pituitary-adrenal hormone levels. Psychoneuroendocrinology. 1995;20:591–601. doi: 10.1016/0306-4530(95)00005-9. [DOI] [PubMed] [Google Scholar]