Abstract
Objective
Gammadelta (γδ) T cells are a subset of pro-inflammatory innate-like T lymphocytes that serve as a bridge between innate and adaptive immunity. γδ T cells are highly enriched in cholesterol compared to αβ T cells. In this study, we aimed to identify the role of γδ T cells in atherosclerosis, a cholesterol and inflammation-driven disease.
Methods
We found that the percentages of γδ T cells are increased in ApoE−/− mice fed a Western diet. We generated TCRδ−/−ApoE−/− mice and fed them either rodent chow or a Western diet for ten weeks for the assessment of atherosclerosis.
Results
The atherosclerotic lesion size in diet-fed TCRδ−/−ApoE−/− mice was similar to that of diet-fed ApoE−/− mice. There were no differences in cytokine production or numbers of αβ T cells in aorta of TCRδ−/−ApoE−/− mice. Plasma lipoprotein profiles were unchanged by the absence of γδ T cells.
Conclusion
Our data suggest that γδ T cells do not contribute to early atherosclerotic plaque development.
Keywords: Gammadelta, Atherosclerosis, Cholesterol
1. Introduction
Different subsets of T cells play distinct roles in the development of atherosclerosis. For example, pro-inflammatory Th1 and possibly also Th17 cells are considered driving forces for atherosclerosis, and regulatory T cells are protective [1–3]. Most T cells express the αβ T cell receptor (TCR). However, a small subset expresses the γ and δ chains of TCRs. These γδ T cells represent ~3–5% of total CD3+ T cells in the blood and recognize non-peptide antigens such as lipids and phosphorylated nucleotides, and antigens that do not require processing and presentation by MHC molecules. Antigen-naive γδ T cells can react very fast, usually within hours as illustrated by pathogen infection, and thus serve a rapid innate immune role before the responses of adaptive αβ T cells could take place [4,5]. Given their distinct natures, the regulation of γδ T cells is different from conventional αβ T cells. We recently reported that γδ T cells contain a higher intracellular cholesterol content than conventional αβ T cells, and that the higher cholesterol content in γδ Tcells leads to enhanced TCR signaling and their hyper-active and proliferative nature [6].
Although only representing a small percentage of total CD3+ cells in the peripheral blood, γδ T cells may potentially play a significant role in the development of atherosclerosis. At least two individual studies reported significantly elevated proportions of γδ T cells in human atherosclerotic lesions [4,7,8]. The highest percentage of γδ T cells was found in aorta during the early stages of atherosclerotic lesion development, usually when the aorta contains relatively few CD3+ cells, implying participation of γδ T cells early during plaque development [4]. As γδ T cells possess rapid early, innate-like functions, the hypothesis that these cells would play a role in early atherosclerosis seems plausible. Based on their potent production of pro-inflammatory cytokines such as IFNγ and IL-17 [9,10], they are surmised to be pro-atherogenic. In fact, Smith et al. reported that approximately 50% or more IL-17+ CD3+ cells in the aorta and spleen are γδ T cells [11]. However, the exact, direct role that γδ T cells play in either driving or protecting against atherosclerosis is unknown. In this study, we directly tested whether the absence of γδ T cells impacted early atherogenesis using a TCRδ knockout mouse model of atherosclerosis in vivo.
2. Methods
Male ApoE−/− and TCRδ−/−ApoE−/− mice were used in this study. Flow cytometry gating strategies for γδ and conventional αβ lymphocytes are shown in Supplementary Fig. 1. Please refer to the supplementary data for detailed methods.
3. Results
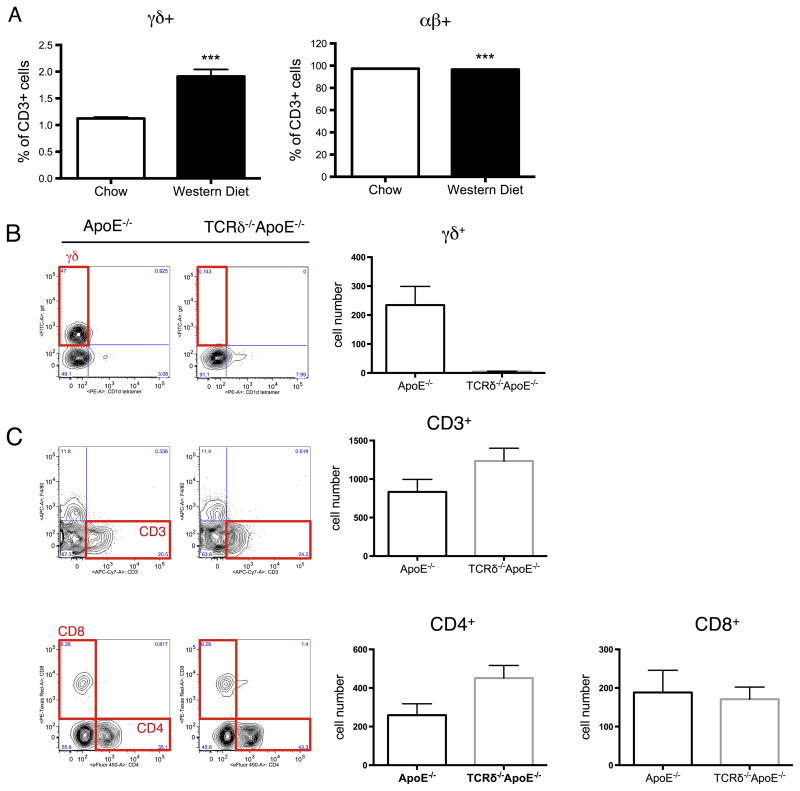
We have recently shown that γδ T cells are highly proliferative and activated comparing to conventional αβ T cells as a result of an increased intracellular cholesterol content in the γδ T cells [6]. Many cholesterol metabolism-related genes are differentially expressed in γδ T cells [6]. To investigate whether αβ and γδ T cells are differentially regulated by excess dietary cholesterol, ApoE−/− mice were fed with either normal chow (contains negligible cholesterol and 4% fat) or a Western diet (contains 0.2% cholesterol and 42% fat), and the percentages of αβ and γδ T cells were analyzed. Within two weeks of Western diet feeding, the percentages of γδ T cells increased approximately 2-fold (Fig. 1A, left panel), compared to chow-fed animals (P < 0.001), while the percentages of αβ T cells decreased (Fig. 1A, right panel). Given that γδ T cells are up-regulated by Western diet feeding, and that the percentage of aorta-infiltrating γδ T cells were found to be significantly elevated in early human atherosclerotic lesions [4,7], we decided to investigate the role of γδ T cells in the progression of atherosclerosis. TCRδ−/− mice, which were completely devoid of γδ T cells, provided a great tool for our study. To facilitate the development of atherosclerosis, we crossed TCRδ−/− mice to ApoE−/− mice. The γδ T cell population was confirmed to be completely absent in the resulting TCRδ−/−ApoE−/− mice (Supplementary Fig. 2A). Proportions of total CD3+, as well as CD8+ T cells were similar between TCRδ−/−ApoE−/− and ApoE−/− mice, while CD4+ cells were increased slightly in TCRδ−/−ApoE−/− mice (p < 0.05) (Supplementary Fig. 2B). To investigate the role of γδ T cells in atherosclerosis, age- and gender-matched ApoE−/− and TCRδ−/−ApoE−/− mice were fed a Western diet for 10 weeks [12,13]. Aortas of these mice were perfused to rid the tissue of blood cells, isolated, and used for either flow cytometric analysis or atherosclerotic lesion quantification. Flow cytometric analysis revealed that nearly 1/3 of the total aorta-infiltrated CD3+ cells were γδ T cells (29% ± 0.02), and that γδ T cells were similar in numbers to CD4+ and CD8+ cells in ApoE−/− mice after 10 weeks of Western diet feeding (Fig. 1B, C). The percentage of γδ T cells in aorta were much higher than other tissues, including spleen, lymph nodes, and blood (data not shown). However, despite the high proportion, loss of γδ T cells did not significantly affect the numbers of CD4+ and CD8+ T cell subsets in the aorta of TCRδ−/−ApoE−/− mice, compared to ApoE−/− mice (Fig. 1C).
Fig. 1.
Flow cytometric analysis of T cell populations. (A) γδ T cells are increased in mice fed with high fat diet. Percentages of γδ cells (left) are significantly higher while αβ cells (right) are significantly lower in ApoE−/− mice fed a high fat Western diet. Splenocytes were isolated from age- and gender-matched ApoE−/− mice fed a normal chow or Western diet for 2 weeks. Results are shown in mean ± SEM. N = 5–6 per group. (B)–(C) T cell populations are normal except the absence of gd T cells in the aorta of TCRδ−/−ApoE−/− mice fed Western diet. Age- and gender-matched ApoE−/− and TCRδ−/−ApoE−/− mice were fed Western diet for 10 weeks. After 10 weeks, aortas were isolated, digested, and analyzed by flow cytometry. (B) γδ T cells are absent in TCRδ−/−ApoE−/− mice. (C) Numbers of CD3+, CD4+, and CD8+ T cells are similar in ApoE−/− and TCRδ−/−ApoE−/− mice. Representative cytometric plots are shown on the left and averaged cell numbers are shown on the right. Results are shown in individual dots and mean ± SEM. N = 6 per group. ***P < 0.001.
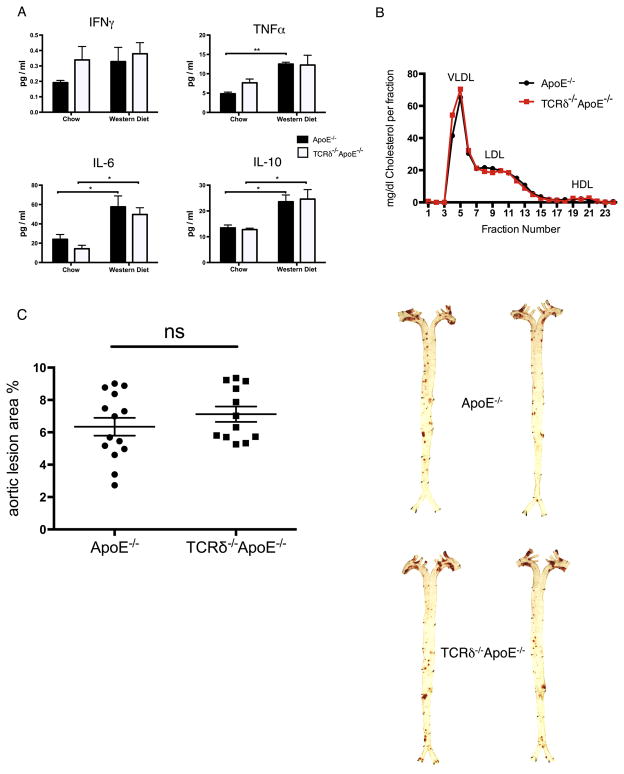
Plasma cytokines, especially those associated with T cell activation, were quantified by multiplex ELISA. We observed an increase in TNFα, IL-6, and IL-10 in the Western diet-fed group compared to chow-fed group in both genotypes. However, there was no significant difference between ApoE−/− and TCRδ−/−ApoE−/− mice fed the same diet (Fig. 2A). Other cytokines such as IL-1β, IL-2, IL-4, IL-12, and IL-17 were also quantified, but their plasma concentrations were essentially non-detectable, as they were lower than the range of detection of our assay (data not shown).
Fig. 2.
Similar plasma cytokine concentrations, lipoprotein profile, and atherosclerotic lesion size of ApoE−/− and TCRδ−/−ApoE−/− mice. (A) Plasma cytokine concentrations were similar in ApoE−/− (closed bar) and TCRδ−/−ApoE−/− (open bar) mice within the same diet group. Plasma cytokine were quantified with MSD multiplex assay with fasting plasma samples collected from mice fed a chow or 10 weeks of western diet. (B) Plasma cholesterol and lipoprotein fractions were the same in of ApoE−/− and TCRδ−/−ApoE−/− mice. Age- and gender-matched ApoE−/− and TCRδ−/−ApoE−/− mice were fed Western diet for 10 weeks. Fasting plasma were collected, and plasma lipids and lipoproteins were analyzed by FPLC. Each sample for FPLC analysis were pooled from plasma samples from 5 mice. Results are shown in mean ± SEM. N = 5 per group. (C) No significant difference in the development of atherosclerosis in TCRδ−/−ApoE−/− mice. Left: Quantification of plaque area as % of aortic surface in matched ApoE−/− and TCRδ−/−ApoE−/− mice after 10 weeks of Western diet feeding. Right: Representative Oil Red O staining (red) in aortic ApoE−/− and TCRδ−/−ApoE−/− mice after 10 weeks of Western diet feeding. Results are shown in individual dots and mean ± SEM. N = 12–14 per group. *P < 0.05, **P < 0.01, ns: not statistically significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Plasma lipoprotein concentrations are closely associated with the development of atherosclerosis [14]. FPLC analysis of pooled plasma from mice fed a Western diet for 10 weeks showed that the lipoprotein profiles (VLDL, LDL, HDL) are essentially the same in both ApoE−/− and TCRδ−/−ApoE−/− mice (Fig. 2B). Most importantly, histological quantification of atherosclerotic lesion area showed no differences between the two groups, suggesting that loss of γδ T cells did not impact the development of early atherosclerosis (Fig. 2C). Thus, our data indicate that deficiency of γδ T cells in mice did not appear to significantly contribute to the development of early atherosclerosis.
4. Discussion
In the current study, we report that γδ T cells are increased in ApoE−/− mice fed an atherogenic Western diet. The main focus of this study was to investigate whether the loss of γδ T cells affects the development of atherosclerosis in vivo. We found that although γδ T cells are increased in atherosclerotic lesions, deletion of γδ T cells from mice had no impact on the development of early atherosclerosis.
A previous study by Elhage et al. reported a slight, yet statistically insignificant, decrease in atherosclerotic lesion size of TCRδ−/−ApoE−/− mice at 18 weeks of age when fed a normal chow diet [15]. This 18-week chow-fed ApoE−/− model typically would represent an early atherosclerosis model. In the Elhage study, atherosclerosis quantification was not studied in TCRδ−/−ApoE−/− mice fed chow for a longer time period nor in mice fed an atherogenic diet. In our study, we used a high cholesterol and high fat-containing Western diet to facilitate the development of atherosclerotic lesions. We found no differences in atherosclerotic lesion sizes between TCRδ−/−ApoE−/− and ApoE−/− mice fed this 10-week Western diet (Fig. 2C). This 10-week Western diet feeding of ApoE−/− mice also reflects a model of early atherosclerosis development. Taking our findings and the report by Elhage et al. [15], both diet-facilitated and spontaneous atherogenesis mouse models illustrate that the absence of γδ T cells does not significantly impact the onset or the early progression of atherosclerosis. Given the rapid, innate-like role that these cells play in the immune response [5], and given that elevated levels of γδ T cells are present specifically in early atherosclerotic lesions [4,7,8], we surmised that these cells would primarily function to modulate the initiation or early progression of atherosclerosis, and we were surprised by this negative outcome. However, neither our study nor Elhage’s examined the possible role that γδ T cells may play in advanced stages of atherogenesis. Studies to address the role of γδ T cells in advanced atherosclerosis will need to be performed to answer this question.
We recently reported that resting, homeostatic γδ T cells have elevated intracellular cholesterol levels compared to αβ T cells [6]. In line with our recent findings that γδ T cells are highly proliferative due to this increased cholesterol content [6], the high γδ T cell accumulation in the aorta of atherosclerotic ApoE−/− mice suggests that these cells readily respond to increased cholesterol in specific regions such as aorta and may be proliferating locally there (Fig. 1B). An alternative hypothesis is that these cells are recruited to aorta, peri-aortic lymph nodes, and spleen by the inflammatory environment of the vasculature during the onset of atherogenesis. The question arises as to what the normal function of γδ cells is within the aortic wall. Our study shows that γδ T cell levels are normally very low in aorta and spleen in mice fed chow diets, yet γδ T cell levels substantially increase in these tissues during Western diet feeding. These findings suggest that γδ cells play a minor role in aortic tissue and peri-aortic lymph nodes under homeostatic conditions, and are likely either proliferating or being recruited to these tissues in response to the local inflammatory environment that ensues during atherosclerosis development. Importantly however, even though these γδ cells produce IL-1711, the increase in IL-17 production by these γδ T cells does not appear to modulate early atherogenesis. Perhaps the impact of γδ cells is masked by the dominant role played by macrophages and other immune cells (such as CD4+ effector T cells) that accumulate in the lesion over time. Our data certainly point to a minor role for γδ T cells in the aortic wall under both homeostatic conditions and during early atherogenesis.
In conclusion, we have shown that γδ T cells are up-regulated in Western diet-fed mice. However, the deficiency of γδ T cells in TCRδ−/−ApoE−/− mice does not modulate the overall T cell compartment, plasma lipoprotein profiles, cytokine profiles, or the degree of atherosclerosis in diet-fed mice. Our study is the first to directly test the role of γδ T cells in atherosclerosis using an atherogenic diet-fed mouse model, and our findings indicate that this lymphocyte subset does not play a key role in early atherosclerosis development.
Supplementary Material
Acknowledgments
Source of funding
This work was supported in part by NIH P01 HL55798, R01 HL085790, and R01 HL097368 (all to C.C.H.) and by an American Heart Association Postdoctoral Fellowship (to H-Y C).
Appendix A. Supplementary material
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.atherosclerosis.2014.03.007.
Footnotes
Disclosure
None.
Contributor Information
Hsin-Yuan Cheng, Email: hycheng@liai.org.
Runpei Wu, Email: hedrick@liai.org.
References
- 1.Xie JJ, Wang J, Tang TT, et al. The th17/treg functional imbalance during atherogenesis in apoE(−/−) mice. Cytokine. 2010;49:185–93. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson J, Wigren M, Shah PK. Regulatory t cells and the control of modified lipoprotein autoimmunity-driven atherosclerosis. Trends Cardiovasc Med. 2009;19:272–6. doi: 10.1016/j.tcm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Lichtman AH. Adaptive immunity and atherosclerosis: mouse tales in the AJP. Am J Pathol. 2013;182:5–9. doi: 10.1016/j.ajpath.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleindienst R, Xu Q, Willeit J, Waldenberger FR, Weimann S, Wick G. Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and t lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol. 1993;142:1927–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Kapsenberg ML. Gammadelta t cell receptors without a job. Immunity. 2009;31:181–3. doi: 10.1016/j.immuni.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Cheng HY, Wu R, Gebre AK, et al. Increased cholesterol content in gammadelta (gammadelta) t lymphocytes differentially regulates their activation. PloS One. 2013;8:e63746. doi: 10.1371/journal.pone.0063746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millonig G, Malcom GT, Wick G. Early inflammatory-immunological lesions in juvenile atherosclerosis from the pathobiological determinants of atherosclerosis in youth (pday)-study. Atherosclerosis. 2002;160:441–8. doi: 10.1016/s0021-9150(01)00596-2. [DOI] [PubMed] [Google Scholar]
- 8.Vanderlaan PA, Reardon CA. Thematic review series: the immune system and atherogenesis. The unusual suspects:An overview of the minor leukocyte populations in atherosclerosis. J Lipid Res. 2005;46:829–38. doi: 10.1194/jlr.R500003-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Jensen KD, Su X, Shin S, et al. Thymic selection determines gammadelta t cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. Gammadelta t cells: an important source of il-17. Curr Opin Immunol. 2008;20:353–7. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith E, Prasad KM, Butcher M, et al. Blockade of interleukin-17a results in reduced atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2010;121:1746–55. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostogrys RB, Franczyk-Zarow M, Maslak E, et al. Low carbohydrate, high protein diet promotes atherosclerosis in apolipoprotein e/low-density lipoprotein receptor double knockout mice (apoE/LDLR(−/−)) Atherosclerosis. 2012;223:327–31. doi: 10.1016/j.atherosclerosis.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Hanna RN, Shaked I, Hubbeling HG, et al. Nr4a1 (nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–27. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kones R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: Implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013;9:617–70. doi: 10.2147/VHRM.S37119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elhage R, Gourdy P, Brouchet L, et al. Deleting tcr alpha beta+ or cd4+ t lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein e-deficient mice. Am J Pathol. 2004;165:2013–8. doi: 10.1016/s0002-9440(10)63252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.