Abstract
Spinal cord injury results in permanent sensorimotor loss in mammals, in part due to a lack of injury-induced neurogenesis. The regeneration of neurons depends upon resident neural progenitors, which in zebrafish persist throughout the central nervous system as radial glia. However the molecular mechanisms regulating spinal cord progenitors remain uncharacterized. Wnt/ß-catenin signaling is necessary for the regenerative response of multiple tissues in zebrafish as well as other vertebrates, but it is not known whether the pathway has a role in spinal cord regeneration. Here we show that spinal radial glia exhibit Wnt/ß-catenin activity as they undergo neurogenesis following transection. We then use Cre-mediated lineage tracing to label the progeny of radial glia and show that Wnt/ß-catenin signaling is required for progenitors to differentiate into neurons. Finally, we show that axonal regrowth after injury also requires Wnt/ß-catenin signaling, suggesting coordinated roles for the pathway in functional recovery. Our data thus establish Wnt/ß-catenin pathway activation as a necessary step in spinal cord regeneration.
Keywords: zebrafish, spinal cord, injury, regeneration, Wnt, radial glia
Introduction
While spinal cord injury (SCI) is a permanent, chronic condition in humans, it is not permanent in other vertebrates such as the zebrafish Danio rerio (Becker et al., 1997; Kuscha et al., 2012a, b). Ascending and descending axons can regrow from surviving neurons far from the injury (Becker et al., 1997), but restoration of motoneuron and interneuron populations after injury relies upon local neural progenitor cells (Briona and Dorsky, 2014a; Hui et al., 2010; Reimer et al., 2008). In zebrafish, neural progenitors persist beyond embryogenesis throughout the CNS as a subpopulation of radial glia (Kroehne et al., 2011; Rothenaigner et al., 2011). However many of the signals regulating radial glial activation and differentiation remain uncharacterized.
In regenerative animal injury models, Wnt/ß-catenin signaling is activated in the regeneration blastema, and inhibition of pathway activity after injury arrests the regenerative process (Stoick-Cooper et al., 2007; Yokoyama et al., 2007). In the regenerating zebrafish caudal fin, Wnt/ß-catenin signaling functions to regulate blastemal cell proliferation and osteoblast differentiation (Wehner et al., 2014). Furthermore, in the zebrafish retina Wnt/ß-catenin activity is necessary for Müller glia to re-enter the cell cycle, dedifferentiate, and generate multiple cell types following injury (Meyers et al., 2012; Ramachandran et al., 2011). However, unlike Müller glia, radial glia of the spinal cord are not thought to undergo dedifferentiation following injury, and instead exhibit complex morphological and migratory behaviors that result in the creation of physical bridges that support axon regrowth (Goldshmit et al., 2012). The role of Wnt/ß-catenin signaling in any of the radial glial responses to SCI is completely unknown.
Here we show that Wnt/ß-catenin activity is initiated in radial glia following spinal cord transection. To characterize the lineage of radial glia following SCI, we generated a stable gfap:CreERT2 zebrafish line. Using this new tool, we show that a sub-population of cells from this lineage are GFAP+ radial glia which are minimally neurogenic in the uninjured spinal cord, but increase their rate of neurogenesis and contribute to regeneration in response to SCI. Finally, we show that when Wnt/ß-catenin signaling is inhibited there is a reduction of neurogenesis by converted radial glia, as well as a failure in axon regrowth. Together our data indicate that Wnt/ß-catenin signaling is required for the neurogenic response to SCI, and suggest that multiple functions of radial glia in regeneration require pathway activity.
Material & Methods
Zebrafish
Embryos were obtained from wild type (AB*), Tg(elavl3:EGFP)knu3 (Park et al., 2000), Tg(7xTCF-Xla.Siam:GFP)ia4 (Moro et al., 2012), Tg(hsp701:dkk1-GFP)w32 (Stoick-Cooper et al., 2007), Tg(ubi:loxP-eGFP-loxP-mCherry)cz1701 (Mosimann et al., 2011), and Tg(gfap:CreERT2,CY)zd16 zebrafish lines, raised in embryo medium with or without with 1-phenyl-2-thiourea (PTU), and staged according to Kimmel et al. (Kimmel et al., 1995). Zebrafish were raised and bred in the University of Utah core facility Centralized Zebrafish Animal Resource according to standard procedures. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee.
Construction of Tg(gfap:CreERT2,CY)zd16 transgenic zebrafish
To make p5E-gfap, a plasmid containing the zebrafish gfap enhancer/promoter (Bernardos and Raymond, 2006) was digested with XhoI and BamHI and ligated into p5E-MCS (Kwan et al., 2007), followed by PCR editing to remove a translation start codon within gfap exon 1. pME-CreERT2 (gift from Bruce Draper, U.C. Davis) was made by PCR amplification of a CreERT2 transgene (Matsuda and Cepko, 2007) adding attB sites, followed by BP cloning into pDONR221 (Life Technologies). pDestTol2CY (pCM326) was made by inserting an Asp718I-flanked PCR product of alpha-crystallin:YFP (Hesselson et al., 2009) into the Asp718I site in Tol2kit destination vector #394 (Kwan et al., 2007) in the same orientation as the Multisite Gateway cassette. The p5E-gfap, pME-CreERT2, and p3E-polyA (Kwan et al., 2007) entry vectors were recombined into the pDestTol2CY destination vector using LR cloning (Life Technologies).
To generate transgenic zebrafish lines, approximately 1nl of 30ng/μl plasmid DNA and 25ng/μl transposase RNA were co-injected into 1-cell stage wild-type embryos using a PLI-100 microinjector (Harvard Apparatus). Embryos were screened for YFP expression in the lens and raised to adulthood, when they were crossed with wild-type animals to screen for germline transmission. Four founders were identified.
Generation and treatment of embryos
Tg(gfap:CreERT2,CY)zd16 and Tg(ubi:loxP-eGFP-loxP-mCherry)cz1701 animals were crossed, embryos were screened for ubiquitous GFP expression, and raised in recovery media [E2 media + 0.2mM phenylthiourea (Sigma) + 10mg/L gentamycin sulfate (Amresco)] at 28.5°C. At 4 days post fertilization (dpf), larvae were transferred to fresh recovery media containing 10mM BrdU (Sigma) and 5μM 4-hydroxytaxmoxifen (4-OHT, Sigma) for 24 hours in the dark prior to surgery.
Spinal cord injury and Wnt pathway inhibition
Larvae were lesioned at 5dpf as described previously (Briona and Dorsky, 2014a, b). Briefly, microinjection pipettes were broken, beveled and used as glass scalpels. Larvae were anesthetized with 0.016% Tricaine (Sigma), and their spinal cords were transected at the level of the anal pore. Injured animals were allowed to recover in recovery media. For Wnt inhibition experiments, animals were allowed to recover for 6 hours, then transferred to recovery media containing DMSO or 35μM IWR1 (Sigma, #IO161-25MG). Media was made fresh and changed daily after feeding. For heat shock induction of Dkk1, Tg(hsp701:dkk1-GFP)w32 embryos were labeled with BrdU at 4dpf, and following transection were placed at 39C for one hour twice daily until the end of the experiment.
Immunohistochemistry
Larvae intended for cryosectioning before immunohistochemistry were fixed in fresh 4% PFA for 1 hour at room temperature, and washed and cryosectioned as described previously (Briona and Dorsky, 2014a). Larvae intended for whole mount immunohistochemistry were fixed overnight in fresh 4% PFA at 4°C, equilibrated in PTW, heat treated in 70°C Tris-HCl pH 9.0 and dehydrated with acetone as described previously (Inoue and Wittbrodt, 2011). For BrdU antigen retrieval, sections were incubated at room temperature in 2N HCl for 90 minutes. For PCNA antigen retrieval, sections were boiled in 10mM sodium citrate pH 6.0 for 20 minutes.
Primary antibodies used were: Rabbit anti-GFP (1:5000, Invitrogen #A-11122), chicken anti-GFP (1:1000, Aves #GFP-1020), mouse anti-HuC/D (1:500, Invitrogen #A-21271), chicken anti-BrdU (1:500, ICL #CBDU-65A-Z), mouse anti-PCNA (1:1000, Sigma #p8825), rabbit anti-Sox3 (1:200, Pierce Custom Antibodies and Peptides), rabbit anti-DsRed (1:200, Clontech #632496), mouse anti-acetylated tubulin (Sigma #T6793), and mouse anti-GFAP (1:200, Zebrafish International Resource Center #zrf1).
Secondary antibodies used were: goat anti-rabbit 488 (1:200, Invitrogen #A-11008), goat anti-rabbit 568 (1:200, Invitrogen #A-11041), goat anti-rabbit cy3 (1:200, Jackson ImmunoResearch #111-165-003), goat anti-mouse 633 (1:200, Invitrogen #A-21050), goat anti-mouse cy3 (1:200, Jackson ImmunoResearch #115-165-003), goat anti-chicken 488 (1:200, Invitrogen #A-11039), and goat anti-chicken 633 (1:200, Invitrogen #A-21103). Hoechst 33342 was used to visualize nuclei.
Confocal microscopy
Whole-mounted larvae or sections were imaged using an Olympus FV-1000XY or Nikon A1 confocal microscope housed in the University of Utah Cell Imaging Core Facility. Images were processed using ImageJ (rsbweb.nih.gov/ij) and GIMP (gimp.org).
Quantification
Analysis based on cryosections used five non-consecutive transverse sections each from five embryos per timepoint; analysis based on whole-mount laterally imaged larvae used five fish per timepoint. Antibody colocalization was scored using MaxZ projections generated with ImageJ and verified using single slices. To quantify axonal regrowth after SCI, the number of acetylated tubulin-labeled axons intersecting a dorsoventral line was counted from 3 non-consecutive confocal sections spanning the center of the spinal cord, then averaged between 3-5 animals (Lu et al., 2012).
Statistical analysis
All results were expressed as mean ± SEM. Two-tailed two-sample equal variance Student t-tests were calculated using Excel; differences were considered significant at p<0.05.
Results and Discussion
Wnt reporter expression is activated in response to SCI
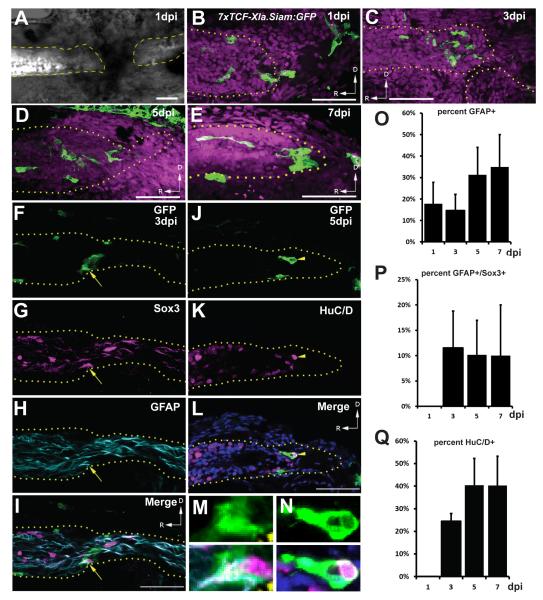
Canonical Wnt gene expression is detected at the leading edge of the blastema in the regenerating zebrafish caudal fin as early as 6 hours post amputation (hpa), while Wnt reporter activity is detectable by 12hpa (Stoick-Cooper et al., 2007; Wehner et al., 2014). Additionally, in the regenerating Xenopus limb bud, Xwnt3a is detectable in the blastema by 3 days post amputation (Yokoyama et al., 2007). To determine whether Wnt/ß-catenin signaling is active during regeneration after SCI, we performed spinal cord transection (Briona and Dorsky, 2014b) on Tg(7xTCF-Xla.Siam:GFP)ia4 larvae, which express GFP in cells with ß-catenin mediated transcription (Moro et al., 2012). We examined GFP expression daily from 1-7 days post-injury (dpi), when the two ends of the spinal cord have rejoined (Briona and Dorsky, 2014a). In contrast to uninjured spinal cord in which reporter expression was completely absent, GFP+ cells with the morphology of longitudinal radial glia (Goldshmit et al., 2012) were present in the blastema through 7dpi (Fig. 1B-E).
Figure 1.
Wnt reporter expression after SCI. (A) Lateral view of a transected spinal cord at 1dpi showing loss of neurons (white, labeled by elavl3:EGFP) at the injury site. (B-E) The 7xTCFXla.Siam:GFP Wnt reporter (green) is expressed in the regeneration blastema from 1-7dpi. Magenta channel is nuclear staining. (F-I) Wnt reporter-expressing cells express GFAP and Sox3 at 3dpi (arrow). (J-L) Other reporter-expressing cells are HuC/D+ neurons at 5dpi (arrowhead). Blue channel is nuclear staining. (M,N) High-magnification views of co-labeled cells from panels (I,L). Percentage of GFP+ cells co-expressing GFAP, GFAP and Sox3, and HuC/D. Image in (A) is merged from fluorescent and brightfield channels obtained with a compound microscope. Images in (B-N) are single confocal slices from lateral views of whole-mounted larvae. Scalebars = 50μm, dashed/dotted lines outline the spinal cord, and D/R arrows indicate dorsal and rostral, respectively. For (O-Q), n=5 sections at each timepoint; error bars = SEM.
To determine which cells express the Wnt reporter, we examined GFP colocalization with GFAP to label radial glia, Sox3 to identify neural progenitors (Kim and Dorsky, 2011), and HuC/D to label neurons. At all timepoints, 15-35% of the reporter-expressing cells were positive for GFAP (Fig. 1F-I, O). A smaller but consistent percentage of reporter-expressing GFAP+ radial glia were also Sox3+, but only after 1dpi (Fig. 1F-I, M, P). In addition, we found that a significant percentage of reporter-expressing cells at 3-7dpi were HuC/D+ (Fig. 1J-L, N, Q), which could either indicate active signaling in surviving neurons or perdurance of reporter activity in newly-generated neurons. Together these data show that Wnt/ß-catenin signaling is activated in response to spinal transection during the time period of regenerative neurogenesis, and that a significant proportion of responding cells are radial glial neural progenitors. The presence of Wnt/ß-catenin activity thus raised the hypothesis that this pathway has an important function in spinal cord regeneration.
A quiescent population of radial glia generates neurons after transection
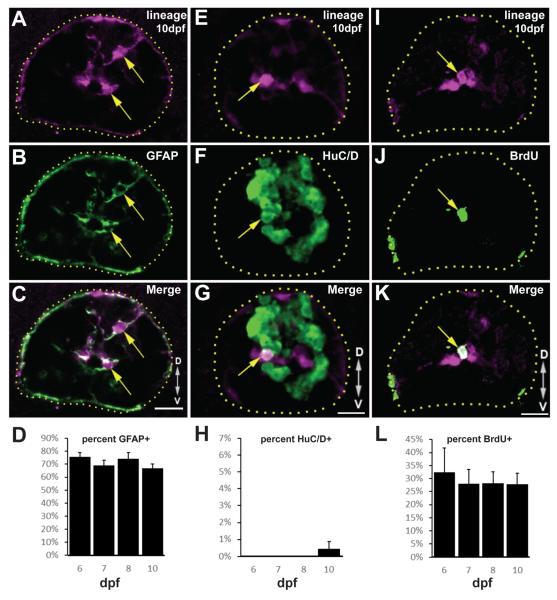
Radial glia throughout the zebrafish CNS persist as neural progenitors (Kroehne et al., 2011; Wan et al., 2012). In addition, our previous work showed that GFAP+ radial glia produce neurons during spinal cord regeneration (Briona and Dorsky, 2014a). To fully characterize the lineage of radial glia following SCI, we generated a stable transgenic line with CreERT2 expressed under the control of the zebrafish gfap promoter: Tg(gfap:CreERT2,CY)zd16. Progeny from a cross to Tg(ubi:loxP-eGFP-loxP-mCherry)cz1701 fish (Mosimann et al., 2011) were exposed to 5μM 4-hydroxy tamoxifen (4-OHT) from 4-5 dpf, then raised until 6-10dpf. At all timepoints a majority of converted mCherry+ cells were GFAP+ (Fig. 2A-D) indicating that the transgene effectively labels radial glia. We found that the conversion efficiency of GFAP+ cells following 4-OHT administration was variable between animals, ranging from 20-90% (not shown).
Figure 2.
Tg(gfap:CreERT2,CY)zd16 labels a quiescent population of radial glia. (A-D) Following 4-OHT treatment, most mCherry+ cells remain GFAP+ (arrows) through 10dpf. (E-H) In contrast, very few mCherry+ cells express HuC/D (arrow) beginning at 10dpf. (I-L) After incubation in BrdU from 4-5dpf, there is no significant expansion of the BrdU-labeled subset of mCherry+ cells (arrow). All images are single confocal slices of 12μM transverse cryosections. n=25 sections from 5 animals at each timepoint; error bars = SEM; scalebar = 10μM, dotted lines outline the spinal cord, and D/V arrows indicate dorsal and ventral, respectively.
We found that converted cells begin to generate neurons by 10dpf (Fig.2 E-H), but the low rate of neurogenesis indicates that the transgene labels a relatively quiescent population. To measure proliferative activity, we incubated embryos in 5μM 4-OHT and 10mM BrdU from 4-5dpf, then examined BrdU incorporation. Between 6-10dpf, the percentage of BrdU-labeled mCherry+ cells remained consistent (Fig. 2I-L). Together, these data indicate that labeled radial glia undergo few cell divisions and generate few neurons in the uninjured spinal cord. In addition, we conclude that our Tg(gfap:CreERT2,CY)zd16 line is an effective new tool for studying the radial glial lineage.
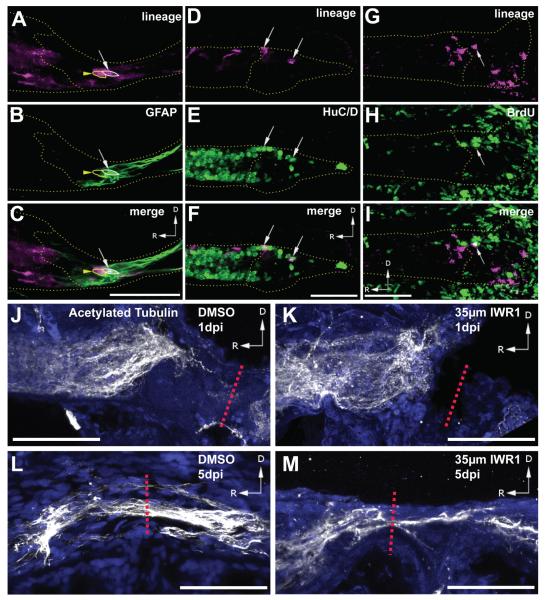
To determine whether radial glia labeled by our transgene actively contribute neurons to the regenerating spinal cord, double transgenic fish were incubated in 4-OHT and BrdU for 24 hours immediately preceding spinal cord transection at 5dpf. Analysis of the Cre-labeled lineage at 1-7dpi showed multiple GFAP+, HuC/D+, and BrdU-labeled progeny present in the blastema (Fig. 3). At 1dpi almost all converted cells in the blastema were GFAP+ (Fig. 4A). However by 3-5dpi, only 20-30% of converted cells expressed GFAP (Figs. 3A-C, 4A), suggesting that they had begun to undergo differentiation. Consistent with our previous findings (Briona and Dorsky, 2014a), we observed a significant percentage of mCherry+, HuC/D+ neurons in the blastema beginning at 3dpi (Fig. 4B). By 5dpi, mCherry+ neurons represented almost 40% of converted cells in the blastema (Figs. 3D-F, 4B).
Figure 3.
Representative images of gfap:CreERT2-labeled progeny in the regenerating blastema. (A-C) At 5dpi one mCherry+ cell is GFAP+ (arrow), while a neighboring cell is GFAP− (arrowhead). (D-F) At 5dpi, multiple HuC/D+ progeny (arrows) are present. (G-I) At 3dpi progeny labeled by BrdU incubation from 4-5dpf (arrow) are present. (J,K) At 1dpi, both DMSO and IWR1-treated larvae show a lack of axons, labeled with anti-acetylated tubulin, crossing the injury site (red dashed line). (L,M) At 5dpi, DMSO-treated larvae show robust axon recrossing, while in IWR-treated larvae only a few axons are present at the injury site (red dashed line). Images (A-I) are single confocal slices from lateral views of whole-mounted larvae, dotted lines outline the spinal cord and blastema. Images (J-M) are maximum intensity Z-projections from lateral views of whole-mounted larvae, blue channel is nuclear staining. Scalebars = 50μm.
Figure 4.
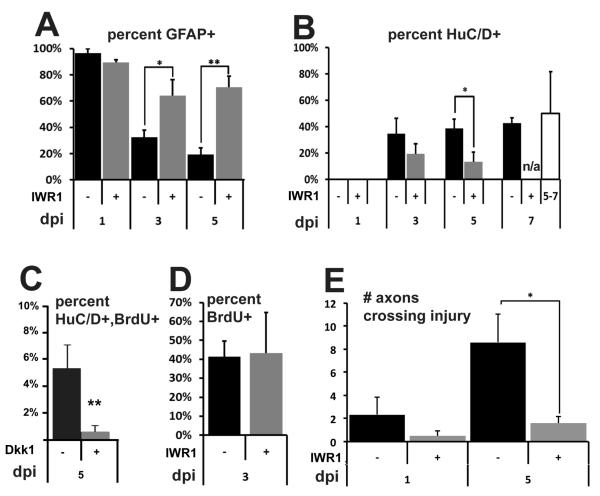
Radial glia contribute neurons to the blastema in a Wnt-dependent manner following SCI. (A) At 1 dpi, almost all lineage-labeled cells are GFAP+, but this fraction progressively decreases at 3dpi and 5dpi, In contrast, IWR1 treatment maintains a high proportion of GFAP+ cells in the lineage through 5dpi. (B) Lineage-labeled neurons are present in the blastema beginning at 3dpi, and this fraction is significantly decreased by IWR1 treatment at 5dpi. Treatment from 5-7dpi has no effect on neurogenesis. (C) Expression of Dkk1 twice daily after injury significantly reduces the fraction of newly born neurons in the blastema. (D) Following labeling from 4-5dpf, IWR1 treatment does not affect the number of BrdU+ cells at 3dpi. (E) The number of axons crossing a dorsoventral line through the injury site is significantly decreased at 5dpi following IWR1 treatment. For (A-D) n=5 sections each from 5 animals, for (E) n=3 sections each from 3-5 animals; error bars = SEM; *p<0.05, **p<0.005.
Inhibition of Wnt signaling blocks the neuronal differentiation of radial glia after transection
Inhibition of Wnt/ß-catenin signaling in the regenerating tail fin or limb bud blocks regeneration (Wehner et al., 2014; Yokoyama et al., 2007), suggesting that the pathway may be generally required for the regenerative response following injury. We therefore used the small molecule Wnt inhibitor IWR1, an Axin stabilizer that promotes ß-catenin degradation (Chen et al., 2009), to determine whether Wnt/ß-catenin pathway activity is required for neurogenesis following SCI. Our initial analysis determined that incubation in 35μM IWR1 was sufficient to inhibit Wnt reporter expression after SCI without being lethal (Supplementary Fig. 1). No difference in the percentage of GFAP+ converted cells was detectable at 1dpi following IWR1 treatment (Fig. 4A); however, at 3dpi and 5dpi we observed a significantly greater percentage of GFAP+ converted cells in the blastema of IWR1-treated larvae compared to controls (Fig. 4A). This result suggests that in the presence of IWR1, radial glial progenitors may be unable to differentiate as neurons and instead accumulate in the blastema as neural progenitors. Supporting this hypothesis, we observed a lower percentage of mCherry+ neurons in the blastema following IWR1 treatment, with a significant decrease observed at 5dpi (Fig 4B). To determine whether Wnt signaling was required later in the regeneration process, we placed transected animals in IWR1 from 5-7dpi. Following this treatment we did not observe a significant difference in the proportion of mCherry+ neurons in the blastema (Fig. 4B), suggesting that the critical window of Wnt/ß-catenin signaling for neurogenesis following SCI is before 5dpi.
As an independent confirmation of the requirement for Wnt/ß-catenin signaling, we performed spinal cord transections on Tg(hsp701:dkk1-GFP)w32 larvae at 5dpi (Stoick-Cooper et al., 2007). Following injury, larvae and controls were heat shocked at 39C for 1 hour twice daily, and Dkk1 expression was confirmed by the appearance of a fused GFP tag. Because the GFP-labeled transgene could not be detected in the presence of the ubi:loxP-eGFP-loxP-mCherry reporter, we detected newly born neurons using pre-injury BrdU labeling as in a previous study (Briona and Dorsky, 2014a). Consistent with our results from IWR1 treatment, we observed a significant reduction in the percentage of newly born neurons the blastema at 5dpi following Dkk1 expression, compared to controls (Fig. 4C). Thus two separate methods of Wnt pathway inhibition yielded similar results, indicating that Wnt/ß-catenin activity is required for regenerative neurogenesis.
IWR1 treatment does not affect proliferation of radial glia
Wnt/ß-catenin signaling has been shown to regulate the proliferation of multiple progenitor types, including retinal radial glia, after injury (Meyers et al., 2012; Ramachandran et al., 2011; Whyte et al., 2012), raising the possibility that pathway activity may also be required for the expansion of radial glial progeny following SCI. However, due to the stochastic nature of 4-OHT-induced recombination, we were unable to assess the effects of Wnt inhibition on the size of labeled clones. Instead, we labeled dividing cells by incubation in 10mM BrdU from 4-5dpf and examined BrdU incorporation in mCherry+ cells at 3dpi (Figs. 3G-I, 4D). Surprisingly, we found no significant effect of IWR1 treatment on the percentage of BrdU+ converted cells (Fig. 4D). Consistent with this result, we also found that incubation in 35μM IWR1 for 2 days or 9 days produced no significant effect on the number of PCNA+ cells in the uninjured spinal cord (Supplementary Fig. 2). Together these data indicate that inhibition of Wnt signaling does not modulate either homeostatic or regenerative cell division of spinal cord progenitors. This may reflect a fundamental variability in the regenerative behavior of distinct radial glial populations, such as the absence of dedifferentiation or the number of neural progenitors produced by a single glial cell.
Axon regrowth requires Wnt/ß-catenin signaling
Based on the presence of Wnt/ß-catenin activity in radial glia, and on the established role of glial bridges in facilitating axon regrowth (Goldshmit et al., 2012), we hypothesized that Wnt/ß-catenin signaling may also be required for this response to SCI. We therefore examined whether Wnt inhibition was sufficient to block the regrowth of axons across the injury site, which we have previously shown to occur by 5dpi (Briona and Dorsky, 2014a). Transected larvae were allowed to recover for 6 hours, and then transferred into recovery media containing 35μM IWR1. At 5dpi, IWR1-treated larvae showed a significant decrease in the number of axons re-crossing the injury site, counted from non-consecutive confocal slices using acetylated tubulin immunolabeling (Lu et al., 2012; Fig. 4J-M). While some axons are likely absent due to the loss of neurogenesis at the injury site, the magnitude of the effect we observe suggests that axons from more distant surviving neurons may fail to regrow as well. Along with other studies (Park et al., 2013; Suh et al., 2011), our data suggest that Wnt/ß-catenin signaling may promote axon regrowth either directly, or through induction of secondary pathways in radial glia.
Conclusions
Using a new transgenic line for lineage tracing, we have identified a quiescent GFAP+ spinal cord radial glia population that exhibits prolific neurogenesis in response to injury. Together our results support a model in which extrinsic Wnt signals are induced after spinal cord injury, and act directly on radial glia to promote their differentiation into neurons. However we cannot exclude an indirect function of the Wnt/ß-catenin pathway on radial glia, and other cell types including leukocytes have been shown to play an important role in CNS regeneration (Kyritsis et al., 2012). Identifying the source of Wnt signals, as well as the downstream cellular and molecular targets of Wnt/ß-catenin signaling involved in the neurogenic response will thus be informative in the pursuit of functional recovery after SCI in humans.
Supplementary Material
Supplementary Figure 1: IWR1 inhibits Wnt activity after SCI. (A) Uninjured spinal cord at 5dpi shows no significant Wnt reporter expression (green). (B) After 1 day in 10μM IWR1, Wnt reporter activity is still visible in the blastema. (C) After 1 day in 35μM IWR1, Wnt reporter activity is absent. Image (A) is from a compound microscope, and images (B,C) are single confocal slices, from lateral views of whole-mounted larvae. Magenta channel is nuclear staining, dotted lines outline the spinal cord. Scalebar = 50μm.
Supplementary Figure 2: IWR1 does not affect the endogenous proliferation index in uninjured spinal cord. PCNA+ cells were analyzed at either 7dpf (A,B, 2 days of inhibition) or 14dpf (C, D, 9 days of inhibition). (E) Quantitative analysis shows no effect on the percentage of PCNA+ cells. Images are single confocal slices of 12μM transverse cryosections. Scalebars = 10μM; n = 25 sections from 5 animals at each timepoint; error bars = SEM.
Highlights.
Radial glia in the larval zebrafish spinal cord initiate Wnt activity after injury.
Injury causes quiescent radial glia to undergo neurogenesis.
Inhibition of Wnt signaling blocks injury-induced neurogenesis and axon regrowth.
Acknowledgements
R.I.D. was supported by NIH R56NS053897, and L.K.B. was a predoctoral trainee supported by a University of Utah Graduate Research Fellowship. The pME-CreERT2 plasmid was a gift from D. Dranow and B. Draper at U.C. Davis, and the gfap enhancer/promoter plasmid was provided by Michael Barresi at Smith College. We thank D.A. Hutcheson and K.B. Moore for helpful discussion, the University of Utah CZAR core facility for animal husbandry and the Cell Imaging Core for help with imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997;377:577–595. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Briona LK, Dorsky RI. Radial glial progenitors repair the zebrafish spinal cord following transection. Exp Neurol. 2014a;256:81–92. doi: 10.1016/j.expneurol.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briona LK, Dorsky RI. Spinal cord transection in the larval zebrafish. Journal of visualized experiments : JoVE. 2014b doi: 10.3791/51479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Sztal TE, Jusuf PR, Hall TE, Nguyen-Chi M, Currie PD. Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:7477–7492. doi: 10.1523/JNEUROSCI.0758-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselson D, Anderson RM, Beinat M, Stainier DY. Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14896–14901. doi: 10.1073/pnas.0906348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui SP, Dutta A, Ghosh S. Cellular response after crush injury in adult zebrafish spinal cord. Dev Dyn. 2010;239:2962–2979. doi: 10.1002/dvdy.22438. [DOI] [PubMed] [Google Scholar]
- Inoue D, Wittbrodt J. One for all--a highly efficient and versatile method for fluorescent immunostaining in fish embryos. PLoS One. 2011;6:e19713. doi: 10.1371/journal.pone.0019713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Dorsky RI. Tcf7l1 is required for spinal cord progenitor maintenance. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:2256–2264. doi: 10.1002/dvdy.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–4841. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
- Kuscha V, Barreiro-Iglesias A, Becker CG, Becker T. Plasticity of tyrosine hydroxylase and serotonergic systems in the regenerating spinal cord of adult zebrafish. The Journal of comparative neurology. 2012a;520:933–951. doi: 10.1002/cne.22739. [DOI] [PubMed] [Google Scholar]
- Kuscha V, Barreiro-Iglesias A, Becker CG, Becker T. Plasticity of tyrosine hydroxylase and serotonergic systems in the regenerating spinal cord of adult zebrafish. J Comp Neurol. 2012b;520:933–951. doi: 10.1002/cne.22739. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338:1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1027–1032. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA. ss-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural development. 2012;7:30. doi: 10.1186/1749-8104-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro E, Ozhan-Kizil G, Mongera A, Beis D, Wierzbicki C, Young RM, Bournele D, Domenichini A, Valdivia LE, Lum L, Chen C, Amatruda JF, Tiso N, Weidinger G, Argenton F. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Developmental biology. 2012 doi: 10.1016/j.ydbio.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Mosimann C, Kaufman CK, Li P, Pugach EK, Tamplin OJ, Zon LI. Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development. 2011;138:169–177. doi: 10.1242/dev.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, Hong SK, Shin J, Yoo KW, Hibi M, Hirano T, Miki N, Chitnis AB, Huh TL. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol. 2000;227:279–293. doi: 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- Park JH, Min J, Baek SR, Kim SW, Kwon IK, Jeon SR. Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fibroblasts. Acta neurochirurgica. 2013;155:809–816. doi: 10.1007/s00701-013-1663-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15858–15863. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, Sorensen I, Kuscha V, Frank RE, Liu C, Becker CG, Becker T. Motor neuron regeneration in adult zebrafish. J Neurosci. 2008;28:8510–8516. doi: 10.1523/JNEUROSCI.1189-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenaigner I, Krecsmarik M, Hayes JA, Bahn B, Lepier A, Fortin G, Gotz M, Jagasia R, Bally-Cuif L. Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development. 2011;138:1459–1469. doi: 10.1242/dev.058156. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta neurochirurgica. 2011;153:1003–1010. doi: 10.1007/s00701-011-0945-1. [DOI] [PubMed] [Google Scholar]
- Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Developmental cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner D, Cizelsky W, Vasudevaro MD, Ozhan G, Haase C, Kagermeier-Schenk B, Roder A, Dorsky RI, Moro E, Argenton F, Kuhl M, Weidinger G. Wnt/beta-catenin signaling defines organizing centers that orchestrate growth and differentiation of the regenerating zebrafish caudal fin. Cell reports. 2014;6:467–481. doi: 10.1016/j.celrep.2013.12.036. [DOI] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Helms JA. Wnt signaling and injury repair. Cold Spring Harbor perspectives in biology. 2012;4:a008078. doi: 10.1101/cshperspect.a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Ogino H, Stoick-Cooper CL, Grainger RM, Moon RT. Wnt/beta-catenin signaling has an essential role in the initiation of limb regeneration. Dev Biol. 2007;306:170–178. doi: 10.1016/j.ydbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: IWR1 inhibits Wnt activity after SCI. (A) Uninjured spinal cord at 5dpi shows no significant Wnt reporter expression (green). (B) After 1 day in 10μM IWR1, Wnt reporter activity is still visible in the blastema. (C) After 1 day in 35μM IWR1, Wnt reporter activity is absent. Image (A) is from a compound microscope, and images (B,C) are single confocal slices, from lateral views of whole-mounted larvae. Magenta channel is nuclear staining, dotted lines outline the spinal cord. Scalebar = 50μm.
Supplementary Figure 2: IWR1 does not affect the endogenous proliferation index in uninjured spinal cord. PCNA+ cells were analyzed at either 7dpf (A,B, 2 days of inhibition) or 14dpf (C, D, 9 days of inhibition). (E) Quantitative analysis shows no effect on the percentage of PCNA+ cells. Images are single confocal slices of 12μM transverse cryosections. Scalebars = 10μM; n = 25 sections from 5 animals at each timepoint; error bars = SEM.